Abstract
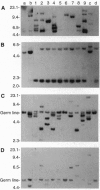
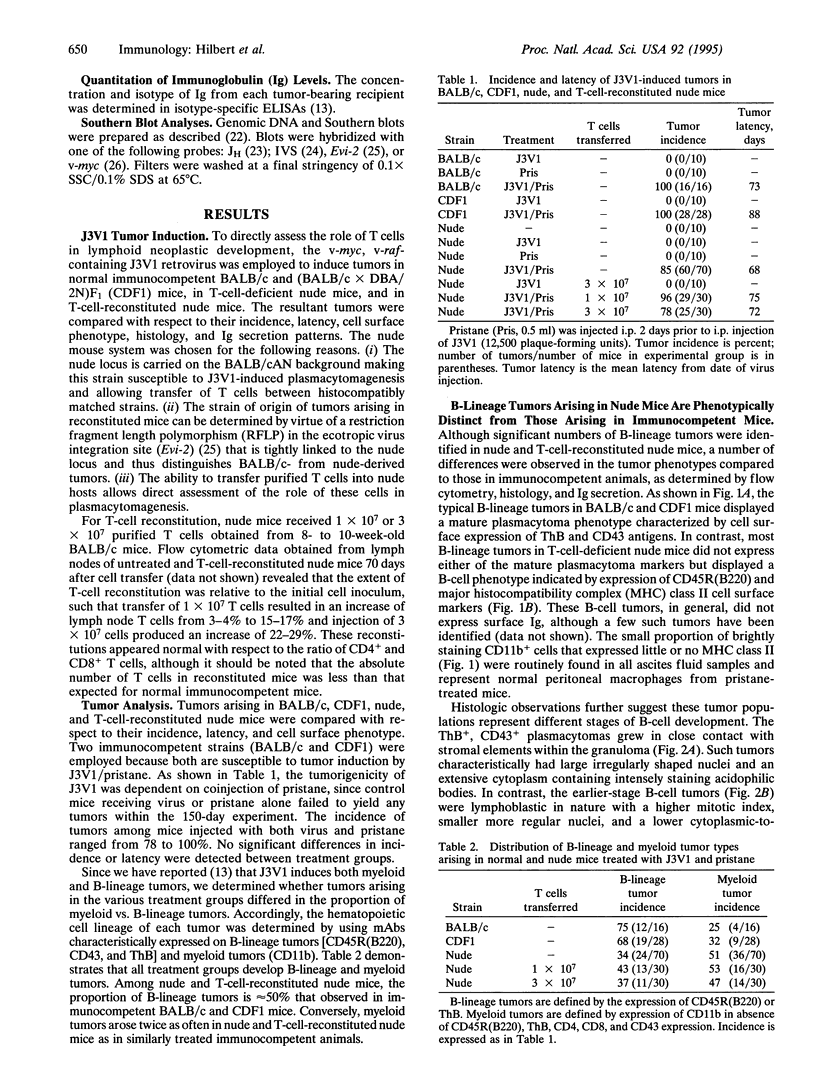
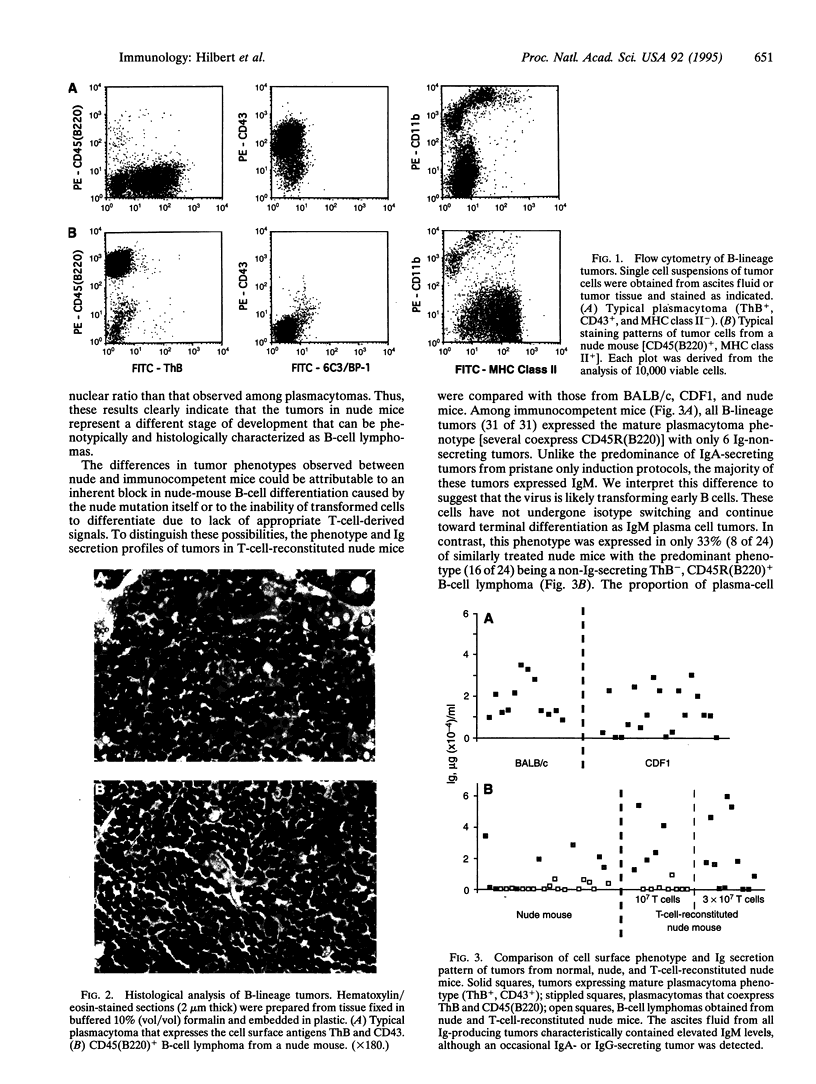
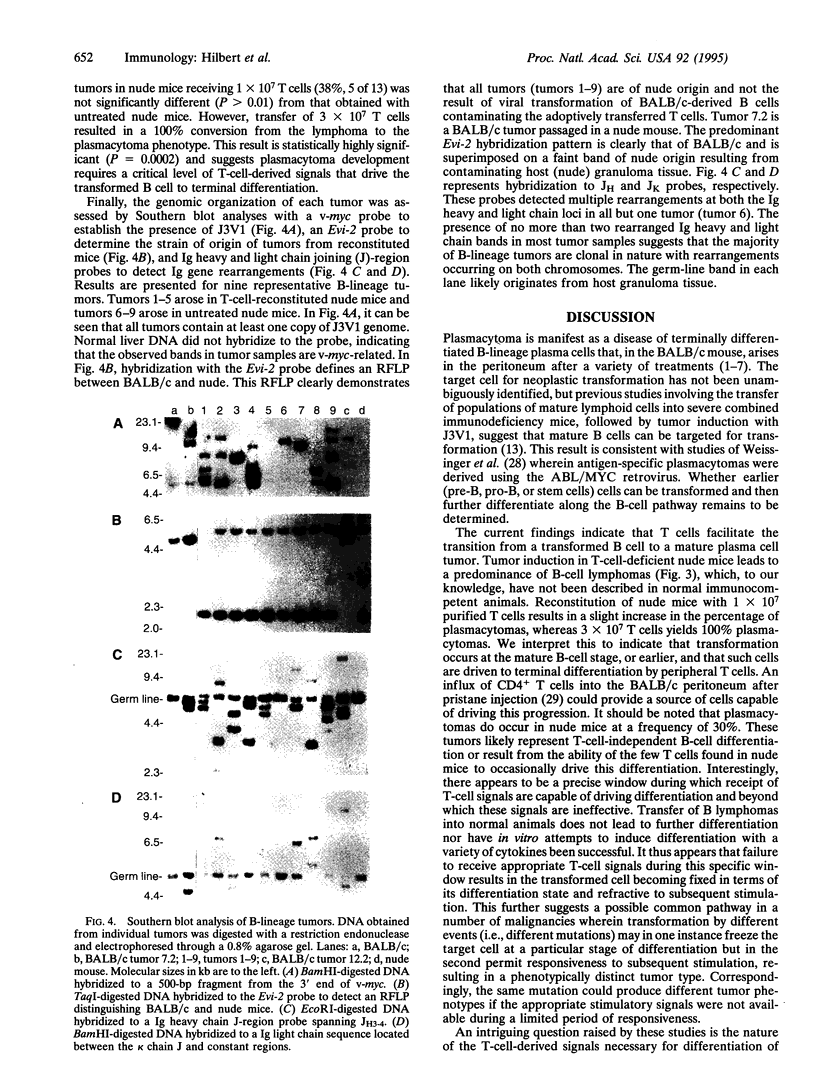
Major interest in the analysis of mature plasma cell neoplasias of mice and humans has focused on identification of precursor cells that give rise to mature malignant plasma cells. Although several laboratories have recently suggested that such cells are present in the granulomas of pristane-treated mice and the bone marrow of some multiple myeloma patients, the in vivo cellular interactions required for their differentiation into mature plasma cell tumors remains unclear. Given the extensive interactions of peripheral T cells and normal B cells, we assessed the potential role of T cells in plasma-cell tumor development, by using a myc, raf-containing retrovirus, J3V1, to induce plasmacytomas in normal BALB/c mice, T-cell-deficient nude mice, and T-cell-reconstituted nude mice. The B-lineage tumors arising in normal BALB/c mice were uniformly mature plasmacytomas, most of which secreted immunoglobulin. In contrast, nude mice yielded predominantly non-immunoglobulin-secreting B-cell lymphomas with a phenotype characteristic of peripheral B cells. T-cell reconstitution of nude mice prior to tumor induction resulted in a shift from B-cell lymphomas to plasmacytomas. These results imply that transformation can occur prior to terminal differentiation of B cells and that such transformed cells can be driven to terminal differentiation by peripheral T cells. These findings further suggest that, in human multiple myeloma, the ability of T cells to influence the differentiation state of transformed B cells may provide a mechanism by which malignant plasma cells found in the bone marrow could arise from clonotypically related less-mature B cells found in both the bone marrow and periphery.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballester O. F., Moscinski L. C., Lyman G. H., Chaney J. V., Saba H. I., Spiers A. S., Klein C. High levels of interleukin-6 are associated with low tumor burden and low growth fraction in multiple myeloma. Blood. 1994 Apr 1;83(7):1903–1908. [PubMed] [Google Scholar]
- Bast E. J., van Camp B., Reynaert P., Wiringa G., Ballieux R. E. Idiotypic peripheral blood lymphocytes in monoclonal gammopathy. Clin Exp Immunol. 1982 Mar;47(3):677–682. [PMC free article] [PubMed] [Google Scholar]
- Bergui L., Schena M., Gaidano G., Riva M., Caligaris-Cappio F. Interleukin 3 and interleukin 6 synergistically promote the proliferation and differentiation of malignant plasma cell precursors in multiple myeloma. J Exp Med. 1989 Aug 1;170(2):613–618. doi: 10.1084/jem.170.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billadeau D., Ahmann G., Greipp P., Van Ness B. The bone marrow of multiple myeloma patients contains B cell populations at different stages of differentiation that are clonally related to the malignant plasma cell. J Exp Med. 1993 Sep 1;178(3):1023–1031. doi: 10.1084/jem.178.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billadeau D., Quam L., Thomas W., Kay N., Greipp P., Kyle R., Oken M. M., Van Ness B. Detection and quantitation of malignant cells in the peripheral blood of multiple myeloma patients. Blood. 1992 Oct 1;80(7):1818–1824. [PubMed] [Google Scholar]
- Buchberg A. M., Bedigian H. G., Taylor B. A., Brownell E., Ihle J. N., Nagata S., Jenkins N. A., Copeland N. G. Localization of Evi-2 to chromosome 11: linkage to other proto-oncogene and growth factor loci using interspecific backcross mice. Oncogene Res. 1988;2(2):149–165. [PubMed] [Google Scholar]
- Byrd L. G., McDonald A. H., Gold L. G., Potter M. Specific pathogen-free BALB/cAn mice are refractory to plasmacytoma induction by pristane. J Immunol. 1991 Nov 15;147(10):3632–3637. [PubMed] [Google Scholar]
- Cassel A., Leibovitz N., Hornstein L., Quitt M., Aghai E. Evidence for the existence of circulating monoclonal B-lymphocytes in multiple myeloma patients. Exp Hematol. 1990 Dec;18(11):1171–1173. [PubMed] [Google Scholar]
- Clynes R., Wax J., Stanton L. W., Smith-Gill S., Potter M., Marcu K. B. Rapid induction of IgM-secreting murine plasmacytomas by pristane and an immunoglobulin heavy-chain promoter/enhancer-driven c-myc/v-Ha-ras retrovirus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6067–6071. doi: 10.1073/pnas.85.16.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini P., Boccadoro M., Voena C., Pileri A. Evidence for a bone marrow B cell transcribing malignant plasma cell VDJ joined to C mu sequence in immunoglobulin (IgG)- and IgA-secreting multiple myelomas. J Exp Med. 1993 Sep 1;178(3):1091–1096. doi: 10.1084/jem.178.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degrassi A., Hilbert D. M., Rudikoff S., Anderson A. O., Potter M., Coon H. G. In vitro culture of primary plasmacytomas requires stromal cell feeder layers. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2060–2064. doi: 10.1073/pnas.90.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J., Barlogie B., Katzmann J., Alexanian R. Phenotypic heterogeneity in aneuploid multiple myeloma indicates pre-B cell involvement. Blood. 1988 Apr;71(4):861–865. [PubMed] [Google Scholar]
- Grogan T. M., Durie B. G., Lomen C., Spier C., Wirt D. P., Nagle R., Wilson G. S., Richter L., Vela E., Maxey V. Delineation of a novel pre-B cell component in plasma cell myeloma: immunochemical, immunophenotypic, genotypic, cytologic, cell culture, and kinetic features. Blood. 1987 Oct;70(4):932–942. [PubMed] [Google Scholar]
- Hata H., Xiao H., Petrucci M. T., Woodliff J., Chang R., Epstein J. Interleukin-6 gene expression in multiple myeloma: a characteristic of immature tumor cells. Blood. 1993 Jun 15;81(12):3357–3364. [PubMed] [Google Scholar]
- Hilbert D. M., Cancro M. P. Characterization of an inbred mouse exhibiting low responsiveness to phosphorylcholine. Antibodies from low-responder mice suggest gene conversion events within the S107VH gene family. J Immunol. 1988 Jun 15;140(12):4364–4371. [PubMed] [Google Scholar]
- Hilbert D. M., Pumphrey J. G., Troppmair J., Rapp U. R., Rudikoff S. Susceptibility and resistance to J3V1 retrovirus-induced murine plasmacytomagenesis in reconstituted severe combined immunodeficient mice. Oncogene. 1993 Jul;8(7):1993–2000. [PubMed] [Google Scholar]
- Jensen G. S., Belch A. R., Mant M. J., Ruether B. A., Yacyshyn B. R., Pilarski L. M. Expression of multiple beta 1 integrins on circulating monoclonal B cells in patients with multiple myeloma. Am J Hematol. 1993 May;43(1):29–36. doi: 10.1002/ajh.2830430108. [DOI] [PubMed] [Google Scholar]
- Jensen G. S., Mant M. J., Belch A. J., Berenson J. R., Ruether B. A., Pilarski L. M. Selective expression of CD45 isoforms defines CALLA+ monoclonal B-lineage cells in peripheral blood from myeloma patients as late stage B cells. Blood. 1991 Aug 1;78(3):711–719. [PubMed] [Google Scholar]
- Jensen G. S., Mant M. J., Pilarski L. M. Sequential maturation stages of monoclonal B lineage cells from blood, spleen, lymph node, and bone marrow from a terminal myeloma patient. Am J Hematol. 1992 Nov;41(3):199–208. doi: 10.1002/ajh.2830410311. [DOI] [PubMed] [Google Scholar]
- Jernberg H., Nilsson K., Zech L., Lutz D., Nowotny H., Scheirer W. Establishment and phenotypic characterization of three new human myeloma cell lines (U-1957, U-1958, and U-1996). Blood. 1987 Jun;69(6):1605–1612. [PubMed] [Google Scholar]
- Jernberg H., Pettersson M., Kishimoto T., Nilsson K. Heterogeneity in response to interleukin 6 (IL-6), expression of IL-6 and IL-6 receptor mRNA in a panel of established human multiple myeloma cell lines. Leukemia. 1991 Mar;5(3):255–265. [PubMed] [Google Scholar]
- Kawano M. M., Huang N., Harada H., Harada Y., Sakai A., Tanaka H., Iwato K., Kuramoto A. Identification of immature and mature myeloma cells in the bone marrow of human myelomas. Blood. 1993 Jul 15;82(2):564–570. [PubMed] [Google Scholar]
- King M. A., Nelson D. S. Tumor cell heterogeneity in multiple myeloma: antigenic, morphologic, and functional studies of cells from blood and bone marrow. Blood. 1989 May 15;73(7):1925–1935. [PubMed] [Google Scholar]
- Kubagawa H., Vogler L. B., Capra J. D., Conrad M. E., Lawton A. R., Cooper M. D. Studies on the clonal origin of multiple myeloma. Use of individually specific (idiotype) antibodies to trace the oncogenic event to its earliest point of expression in B-cell differentiation. J Exp Med. 1979 Oct 1;150(4):792–807. doi: 10.1084/jem.150.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. B., Stanton L. W., Marcu K. B. On immunoglobulin heavy chain gene switching: two gamma 2b genes are rearranged via switch sequences in MPC-11 cells but only one is expressed. Nucleic Acids Res. 1982 Jan 22;10(2):611–630. doi: 10.1093/nar/10.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largaespada D. A., Kaehler D. A., Mishak H., Weissinger E., Potter M., Mushinski J. F., Risser R. A retrovirus that expresses v-abl and c-myc oncogenes rapidly induces plasmacytomas. Oncogene. 1992 Apr;7(4):811–819. [PubMed] [Google Scholar]
- MERWIN R. M., ALGIRE G. H. Induction of plasma-cell neoplasms and fibrosarcomas in BALB/c mice carrying diffusion chambers. Proc Soc Exp Biol Med. 1959 Jul;101(3):437–439. doi: 10.3181/00379727-101-24970. [DOI] [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Bennich H., Johansson S. G., Pontén J. Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp Immunol. 1970 Oct;7(4):477–489. [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N., Ogata A., Shima Y., Tani Y., Ogawa H., Nakagawa M., Sugiyama H., Yoshizaki K., Kishimoto T. Oncostatin M, leukemia inhibitory factor, and interleukin 6 induce the proliferation of human plasmacytoma cells via the common signal transducer, gp130. J Exp Med. 1994 Apr 1;179(4):1343–1347. doi: 10.1084/jem.179.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordan R. P., Potter M. A macrophage-derived factor required by plasmacytomas for survival and proliferation in vitro. Science. 1986 Aug 1;233(4763):566–569. doi: 10.1126/science.3726549. [DOI] [PubMed] [Google Scholar]
- Okuno Y., Takahashi T., Suzuki A., Ichiba S., Nakamura K., Okada T., Okada H., Imura H. In vitro growth pattern of myeloma cells in liquid suspension or semi-solid culture containing interleukin-6. Int J Hematol. 1991 Feb;54(1):41–47. [PubMed] [Google Scholar]
- Osterborg A., Steinitz M., Lewin N., Bergenbrant S., Holm G., Lefvert A. K., Mellstedt H. Establishment of idiotype bearing B-lymphocyte clones from a patient with monoclonal gammopathy. Blood. 1991 Nov 15;78(10):2642–2649. [PubMed] [Google Scholar]
- POTTER M., BOYCE C. R. Induction of plasma-cell neoplasms in strain BALB/c mice with mineral oil and mineral oil adjuvants. Nature. 1962 Mar 17;193:1086–1087. doi: 10.1038/1931086a0. [DOI] [PubMed] [Google Scholar]
- Potter M., Morrison S., Wiener F., Zhang X. K., Miller F. W. Induction of plasmacytomas with silicone gel in genetically susceptible strains of mice. J Natl Cancer Inst. 1994 Jul 20;86(14):1058–1065. doi: 10.1093/jnci/86.14.1058. [DOI] [PubMed] [Google Scholar]
- Potter M., Sklar M. D., Rowe W. P. Rapid viral induction of plasmacytomas in pristane-primed BALB-c mice. Science. 1973 Nov 9;182(4112):592–594. doi: 10.1126/science.182.4112.592. [DOI] [PubMed] [Google Scholar]
- Potter M., Wax J. S. Peritoneal plasmacytomagenesis in mice: comparison of different pristane dose regimens. J Natl Cancer Inst. 1983 Aug;71(2):391–395. [PubMed] [Google Scholar]
- Rapp U. R., Cleveland J. L., Fredrickson T. N., Holmes K. L., Morse H. C., 3rd, Jansen H. W., Patschinsky T., Bister K. Rapid induction of hemopoietic neoplasms in newborn mice by a raf(mil)/myc recombinant murine retrovirus. J Virol. 1985 Jul;55(1):23–33. doi: 10.1128/jvi.55.1.23-33.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scibienski R. J., Paglieroni T. G., MacKenzie M. R. Establishment and characterization of a CD5 positive, immunosuppressive human myeloma cell line. Leukemia. 1990 Nov;4(11):775–780. [PubMed] [Google Scholar]
- Shimizu S., Yoshioka R., Hirose Y., Sugai S., Tachibana J., Konda S. Establishment of two interleukin 6 (B cell stimulatory factor 2/interferon beta 2)-dependent human bone marrow-derived myeloma cell lines. J Exp Med. 1989 Jan 1;169(1):339–344. doi: 10.1084/jem.169.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva S., Sugiyama H., Babonits M., Wiener F., Klein G. Differential susceptibility of BALB/c and DBA/2 cells to plasmacytoma induction in reciprocal chimeras. Int J Cancer. 1991 Sep 9;49(2):224–228. doi: 10.1002/ijc.2910490214. [DOI] [PubMed] [Google Scholar]
- Troppmair J., Potter M., Wax J. S., Rapp U. R. An altered v-raf is required in addition to v-myc in J3V1 virus for acceleration of murine plasmacytomagenesis. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9941–9945. doi: 10.1073/pnas.86.24.9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme J., Opdenakker G., Simpson R. J., Rubira M. R., Cayphas S., Vink A., Billiau A., Van Snick J. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J Exp Med. 1987 Mar 1;165(3):914–919. doi: 10.1084/jem.165.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Riet I., Heirman C., Lacor P., De Waele M., Thielemans K., Van Camp B. Detection of monoclonal B lymphocytes in bone marrow and peripheral blood of multiple myeloma patients by immunoglobulin gene rearrangement studies. Br J Haematol. 1989 Nov;73(3):289–295. doi: 10.1111/j.1365-2141.1989.tb07742.x. [DOI] [PubMed] [Google Scholar]
- Vennström B., Moscovici C., Goodman H. M., Bishop J. M. Molecular cloning of the avian myelocytomatosis virus genome and recovery of infectious virus by transfection of chicken cells. J Virol. 1981 Aug;39(2):625–631. doi: 10.1128/jvi.39.2.625-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissinger E. M., Mischak H., Largaespada D. A., Kaehler D. A., Mitchell T., Smith-Gill S. J., Risser R., Mushinski J. F. Induction of plasmacytomas secreting antigen-specific monoclonal antibodies with a retrovirus expressing v-abl and c-myc. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8735–8739. doi: 10.1073/pnas.88.19.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. G., Gu J. J., Lu Z. Y., Yasukawa K., Yancopoulos G. D., Turner K., Shoyab M., Taga T., Kishimoto T., Bataille R. Ciliary neurotropic factor, interleukin 11, leukemia inhibitory factor, and oncostatin M are growth factors for human myeloma cell lines using the interleukin 6 signal transducer gp130. J Exp Med. 1994 Apr 1;179(4):1337–1342. doi: 10.1084/jem.179.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]