Abstract
Background: Less than half of breast cancer survivors with lymphedema perform self-care as directed. Effective lymphedema self-care is required to obtain acceptable health outcomes. Self-Regulation Theory suggests that objective self-measurement of physiological conditions is necessary to promote self-regulation/self-care. Bioelectric Impedance Spectroscopy (BIS) represents a potential self-measurement method for arm lymphedema. The purpose of this pilot study was to examine the impact of arm self-measurement on daily self-care activities and health outcomes in breast cancer survivors with lymphedema.
Methods and Results: A pilot randomized clinical trial compared outcomes between breast cancer survivors with lymphedema who self-monitored for 3 months and breast cancer survivors with lymphedema who did not self-monitor. Data were collected at baseline, months 1, 2, 3, and 4. Eighty-six women with lymphedema were screened: 62 were eligible, 50 were enrolled, 10 withdrew, and 1 had incomplete data, thus N=39. No between group differences were noted in participant characteristics. The self-monitored group had higher days of garment use (p=0.005) that remained stable after self-monitoring stopped. The median number of days of simple manual lymphatic drainage increased in the intervention group (p=0.004) with a downward trend after self-monitoring ceased.
Conclusions: Objective self-monitoring of arms using BIS is possible. Self-monitoring may positively impact self-care behaviors. Highly symptomatic patients may require coaching or other psychological support to improve their self-care. Studies that combine a cognitive behavioral therapy component along with self-measurement should be considered as potential interventions to impact lymphedema self-care. Other applications of self-monitoring warrant investigation.
Introduction
There are approximately 2.3 million breast cancer survivors (BCS) in the United States.1 Lymphedema—swelling in the arm, a breast, or chest related to cancer treatment—is a chronic effect of breast cancer treatment for which no cure exists. The current standard treatment for lymphedema onset and swelling exacerbations is a two-phase complete decongestive therapy (CDT).2 During Phase 1 of professionally administered CDT, patients are treated with manual lymphatic drainage (MLD), compression garments, bandaging, and meticulous skin care. During Phase 2, lifelong self-care consisting of compression garments, self-administered simple-MLD, skin care, and self-monitoring of arm volume and condition is required. This helps maintain the therapeutic gains from Phase 1 and reduces exacerbations of swelling and infection.
Evidence supports that breast cancer treatment-related lymphedema is costly in terms of symptom burden (physical and psychological), economic outlay, and reduced quality of life (QOL).3,4 Altered sensations in the limb and impaired shoulder mobility are only two examples of physical difficulties BCS with lymphedema experience.5–7 Psychological distress and depression also occur.8–10 Comparison of medical costs between BCS with lymphedema and those without reveal significantly higher medical costs within the 2 years following breast cancer treatment in the former group ($22,153, p≤0.001).4 Higher cost items were outpatient prescription drugs, physical therapy, and lymphedema treatment supplies. QOL studies reflect similar findings (e.g., one study found that lymphedema patients experienced significantly more arm-related problems and scored lower on the FACT-B+4 subscale (p<0.05) than those without lymphedema).11
Effective lymphedema self-care is required to reduce the impact of lymphedema on well-being and disease progression.12 Previous work by this team has found that less than 50% of BCS with lymphedema complete Phase 2 self-care as directed.9,13 Contributing factors are the lack of perceived results and feelings of helplessness in managing the condition.13 To improve outcomes in chronic disease patient populations, such as individuals with diabetes and hypertension, emphasis has been placed on patient education,14,15 disease monitoring,16,17 and support for self-care.15,18 A component of many self-care management programs is objective monitoring of the physiological conditions (e.g., glucose levels, blood pressure). Such monitoring helps patients determine the effectiveness of self-care, set self-care goals, and reinforce continuation of self-care. There is no equivalent standard self-measurement tool for lymphedema patients to use for monitoring their lymphedema.
This team, based upon patients' requests to use single frequency bioimpedance spectroscopy (BIS) for home self-monitoring, previously developed and tested a lymphedema self-monitoring protocol using BIS.19 The Food and Drug Administration (FDA) has approved a BIS device to measure changes in extracellular fluid associated with lymphedema.20 This device is small, portable, and gives numerical output (L-Dex readings) informing the patient if extracellular fluid is above normal ranges.21 Guided by Self-Regulation Theory,22 a pilot, randomized clinical trial (RCT) was conducted with aims to examine the impact lymphedema self-measurement, using a lymphedema BIS self-monitoring protocol, on 1) self-care adherence (garment use, skin care, simple-MLD) and 2) select health outcomes (symptoms, productivity/activity, self-management/self-efficacy, QOL, treatment days, number of arm infections, and number of antibiotic prescriptions).
Materials and Methods
Participants
Scientific approval for this pilot study was obtained from the Vanderbilt-Ingram Cancer Center Scientific Review Committee, Nashville, TN, USA. Institutional Review Board (IRB) approval was granted by Vanderbilt University prior to commencing study activities. During the informed consent process, all participants were made aware that their monthly self-report forms would be reviewed by a study nurse and that they would be contacted by the nurse if potential warning signs of skin deterioration, infection, or increasing lymphedema were identified.
This community-based study successfully recruited the targeted number of 50 participants from: 1) an existing Vanderbilt University IRB approved registry of breast cancer survivors who have given permission to be contacted for future studies; 2) the Middle Tennessee, USA, region; and 3) flyers posted on national websites (Lymphatic Education and Research Network and National Lymphedema Network). Recruitment took place from August 2011 until August 2012. Inclusion criteria were: 1) a history of breast cancer and diagnosis of lymphedema in one arm only; 2) age 21 or older; 3) ability to stand upright; 4) no conditions that could cause swelling such as pregnancy, congestive heart failure, or liver failure; 5) no open sores on arms or known sensitivity to electrodes; 6) no pacemaker or internal defibrillator; and 7) no use of laxatives or diuretics to lose weight. Exclusion criteria included bilateral arm lymphedema, active cancer, and undergoing chemotherapy or radiation. Participants received token compensation for participation up to a total of $90 for completing the study and all follow up measures.
Design
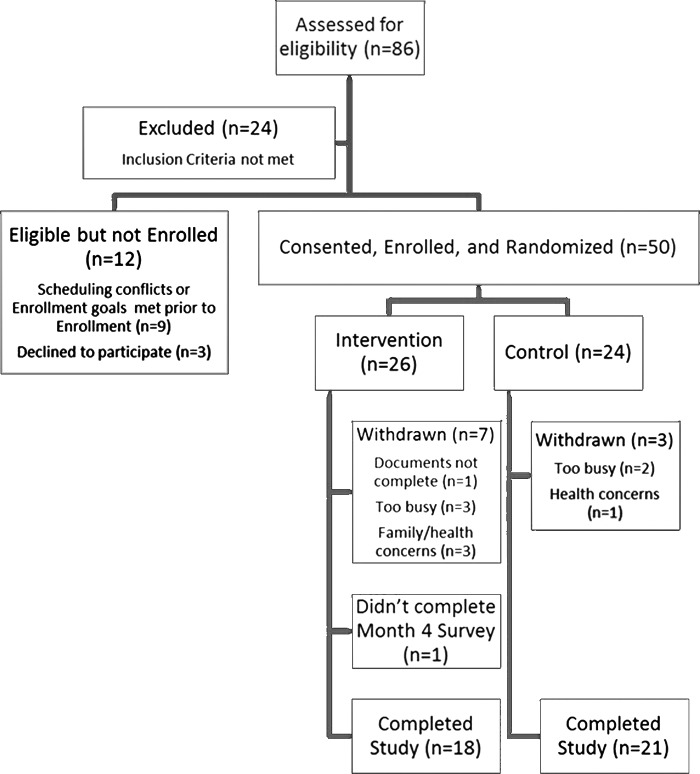
This pilot RCT tested a previously developed self-measurement protocol.19 After completion of baseline assessments, participants were randomized (1:1 allocation) into either a BIS self-measurement intervention or standard care control group using a computer-generated, permuted block randomization scheme developed prior to implementation of the study. (Fig. 1). The study principal investigator was blinded to assignments.
FIG. 1.
Consort diagram.
Data collection
Data collected during a baseline nurse interview from all participants included self-report demographics, medical history, and lymphedema disease and treatment questionnaires. Additional outcome instruments were completed by participants either with pen and paper or online via the REDCap data capture system and included:
Weekly lymphedema self-care checklist
A 20-item weekly self-report survey of frequency of performed self-care (e.g., days wearing a compression sleeve, days of self-MLD).13
Skin assessment
A 20-point yes/no, standardized assessment of skin condition was performed for both the affected and unaffected arms by trained staff who examined the arms at baseline and used as a self-report form by participants during the remainder of the study.9,13
Protocol observation checklist
A checklist corresponding to protocol steps was used by staff to document participant's completion of each step.
BIS
Arm measurements were made using a single frequency BIS device manufactured by Impedimed (Mansfield, Australia) that provided output in units of L-Dex, or lymphedema index, scores. An L-Dex reading of greater than 10 is indicative of lymphedema.21
BIS measurement log
Date, time, person completing the measurement (lab version only), menstrual status, L-Dex scores and comments (home version only) were recorded.
Perceived Medical Condition Self-Management Scale (PMCSMS)
The PMCSMS, an 8-item generic scale that can be adapted to any specific medical condition (e.g., lymphedema), was used to assess perceived ability to complete self-care. Cronbach's alpha of 0.84 has been reported.23
Lymphedema Symptom Intensity and Distress Survey-Arm (LSIDS-A)
The 36-item checklist required participants to indicate the presence of a symptom (“yes” or “no”) and if “yes” rate intensity and associated distress on two separate 10 point scales. In two previous studies, a Cronbach's alpha of 0.95 were noted.24
Work Productivity and Activity Impairment (WPAI) Questionnaire
This 9-item questionnaire was used to evaluate lost hours of productivity and reduced activity seven days prior to administration.25,26 When compared to the SF-36® Health Survey, it correlates with general health scores (r=0.52), and physical role (r=0.52).
Upper Limb Lymphedema (ULL-27)
This is a 27-item self-report QOL scale specific to arm lymphedema.27 Cronbach's alphas were 0.92 and 0.93 for the ULL-27 in previous studies.3,9
Resource Utilization and Economic Burden Questionnaire (RUEBQ)
This 25-item pilot instrument captured self-reported information specific to lymphedema treatment expense and was generated based upon previous work by this team.4
Procedures
Intervention group
Participants randomized to this group were taught the self-measurement protocol by study staff. They practiced self-measurement, recording all readings on the BIS Measurement Log. Once participants demonstrated the ability to measure their arms with staff present in the lab, they were knowingly observed through a one-way mirror for ability to follow the protocol unassisted. The Protocol Observation Checklist was used by staff to determine if the participants were able to complete the major steps of the protocol. If problems were noted at any time, additional training and re-assessment took place. Intervention participants who achieved an observation checklist score of 90% were given a copy of the protocol, a BIS Measurement Log, and loaned a BIS device for home use for 3 months. They were instructed to measure their arms every morning, before eating or drinking and after voiding, for 5 days, then once every 3 days for a total of 3 months (approximately 26 total measurements). These participants completed weekly self-care checklists and monthly follow-up questionnaires for 4 months. Study staff called participants the day of their first self-measurement to ensure understanding of the protocol and device use as well as the document completion schedule. Participants were encouraged to call or email staff with any questions that arose. Study staff picked up the equipment after completion of the third month.
Standard care control group
Reflective of the current standard of lymphedema self-care, participants in this group did not conduct any self-measurement. These participants completed weekly self-care checklists and monthly follow-up questionnaires for 4 months. They were called by study staff the day after their baseline visit to ensure understanding of the document completion schedule. Participants were encouraged to call or email staff with any questions that arose.
Per IRB protocol, self-reported data collected monthly were reviewed by a study nurse to identify potential warning signs of skin deterioration, infection, or increasing lymphedema. Participants were contacted by a study nurse for a phone assessment and therapeutic referrals were made, if needed, should infection be indicated.
Statistical analysis
Statistical analyses were conducted using SPSS (version 21.0) and STATA (version 12). Nominal demographic and study measures were summarized using counts and percentages. Age and education were summarized using mean and standard deviation; however, all of the remaining continuous data distributions were heavily skewed. Therefore, median and 25th to 75th interquartile range were used to describe them. Study group demographic and clinical history variables were compared using Chi-Square Tests of Independence (nominal) and independent t-tests (age, education). Differential effectiveness of the study groups was tested specifically by the interaction effect of study group and time of assessment within general linear models using Generalized Estimating Equations (GEE) to account for the lack of independence of observations (repeated assessments). Given a statistically significant interaction effect, contrast terms (representing both between groups and time-related contrasts) were used to determine how the response curves differed between the groups. A Bonferroni-corrected alpha value was used for interpreting these post-hoc contrasts. Otherwise an alpha of 0.05 (p<0.05) was used for determining statistical significance.
Results
Sample characteristics
Eighty-six individuals were screened for the study, 50 individuals enrolled and were randomized. All participants randomized to the intervention group scored ≥90% on their observed self-measurement in the laboratory and received a device. A final sample of 39 completed the study with seven withdrawals from the intervention group and three withdrawals from the control group (Fig. 1). Compared to those who completed the study (N=39), those who withdrew (n=10) or did not complete the final set of assessments (n=1) were closer to their last chemotherapy treatment (median=0.8 years, min=1, max=7 vs. median=5.3 years, min=1, max=18; p=0.024), more recently diagnosed with lymphedema (median=1.2 years, min=0, max=8 vs. median=5.4 years, min=0, max=16; p=0.040), reported fewer days per week of keeping skin clean (<7 days per week: 2 of 11, 18.2% vs. 0 of 39, 0.0%, p=0.007), lower scores on the ULL-27 (median=74.1, min=67, max=80 vs. median=86.7, min=39, max=99; p=0.002), and greater symptom intensity and distress (medians=1.2 and 1.2, min=0 and 0, max=3 and 2 vs. medians=0.8 and 0.7, min=0 and 0, max=4 and 3; p=0.021 and 0.028).
Demographic characteristics of the 39 participants who completed the study are summarized in Table 1. There were no statistically significant differences between the groups in terms of those characteristics. Nor were there demographic differences in those who withdrew and those who remained in the study.
Table 1.
Sample Demographic Characteristics
| Total N=39 N (%) | Intervention N=18 N (%) | Control N=21 N (%) | P value (Likelihood Ratio) | |
|---|---|---|---|---|
| Race | 0.247 | |||
| White | 28 (71.8) | 13 (72.2) | 15 (71.4) | |
| African American | 9 (23.1) | 5 (27.8) | 4 (19.0) | |
| Asian | 2 (5.1) | 0 (0.0) | 2 (9.5) | |
| Nation of origin | 0.262 | |||
| USA | 38 (97.4) | 18 (100.0) | 20 (95.2) | |
| Bangladesh | 1 (2.6) | 0 (0.0) | 1 (4.8) | |
| Marital status | 0.963 | |||
| Married | 22 (56.4) | 11 (61.1) | 11 (52.4) | |
| Single | 11 (28.2) | 4 (22.2) | 7 (33.3) | |
| Single, living with partner | 2 (5.1) | 1 (5.6) | 1 (4.8) | |
| Other | 2 (5.1) | 1 (5.6) | 1 (4.8) | |
| Widowed | 2 (5.1) | 1 (5.6) | 1 (4.8) | |
| Employment status | 0.263 | |||
| Employed | 26 (66.7) | 14 (77.8) | 12 (57.1) | |
| Unemployed | 12 (30.8) | 4 (22.2) | 8 (38.1) | |
| Other | 1 (2.6) | 0 (0.0) | 1 (4.8) | |
| Area of residence | 0.182 | |||
| City | 29 (76.3) | 15 (83.3) | 14 (70.0) | |
| Country | 8 (21.1) | 2 (11.1) | 6 (30.0) | |
| Other | 1 (2.6) | 1 (5.6) | 0 (0.0) | |
| Insurance coverage (Primary) | 0.076 | |||
| Private insurance | 29 (74.4) | 16 (88.9) | 13 (61.9) | |
| Government | 8 (20.5) | 1 (5.6) | 7 (33.3) | |
| Other | 2 (5.1) | 1 (5.6) | 1 (4.8) | |
| Insurance coverage (Secondary) | N=6 | N=2 | N=4 | 0.105 |
| Annual household income | 0.364 | |||
| ≤30,000 | 5 (12.8) | 2 (11.1) | 3 (14.3) | |
| 30,001–50,000 | 14 (35.9) | 5 (27.8) | 9 (42.9) | |
| Over 50,000 | 16 (41.0) | 10 (55.6) | 6 (28.6) | |
| Do not care to respond | 4 (10.3) | 1 (5.6) | 3 (14.3) | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 58.2 (8.4) | 56.4 (6.2) | 59.8 (9.7) | 0.207 |
| Highest grade completed | 14.8 (2.0) | 15.3 (1.9) | 14.3 (1.9) | 0.095 |
Adverse events
No adverse events related to the study were noted. No referrals for lymphedema treatment were indicated at the time of the monthly reviews of the self-report data and measurement logs.
Aim 1
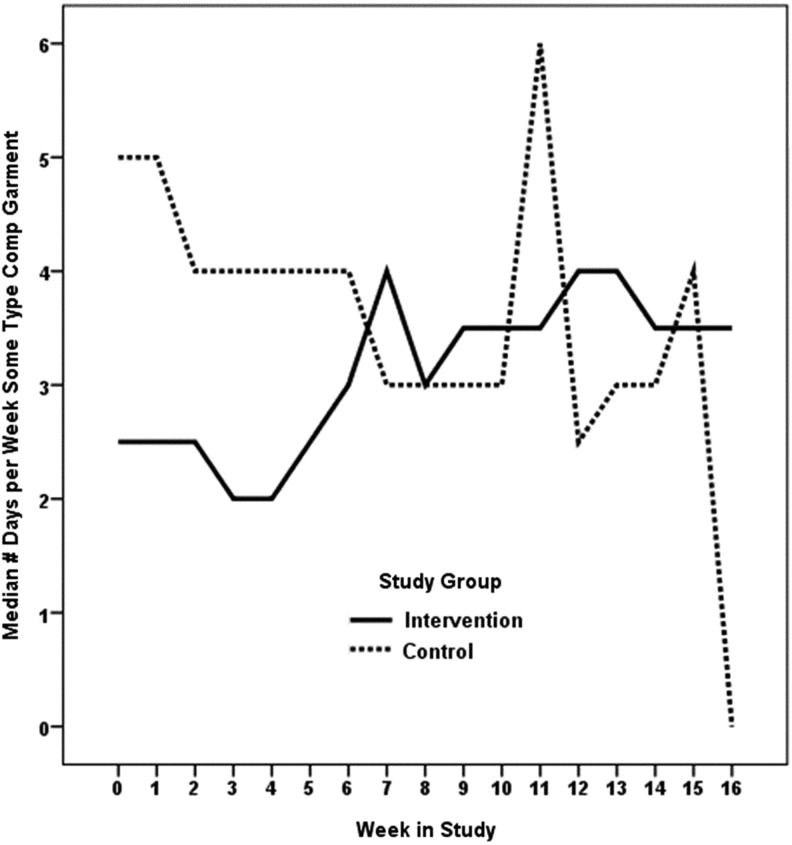
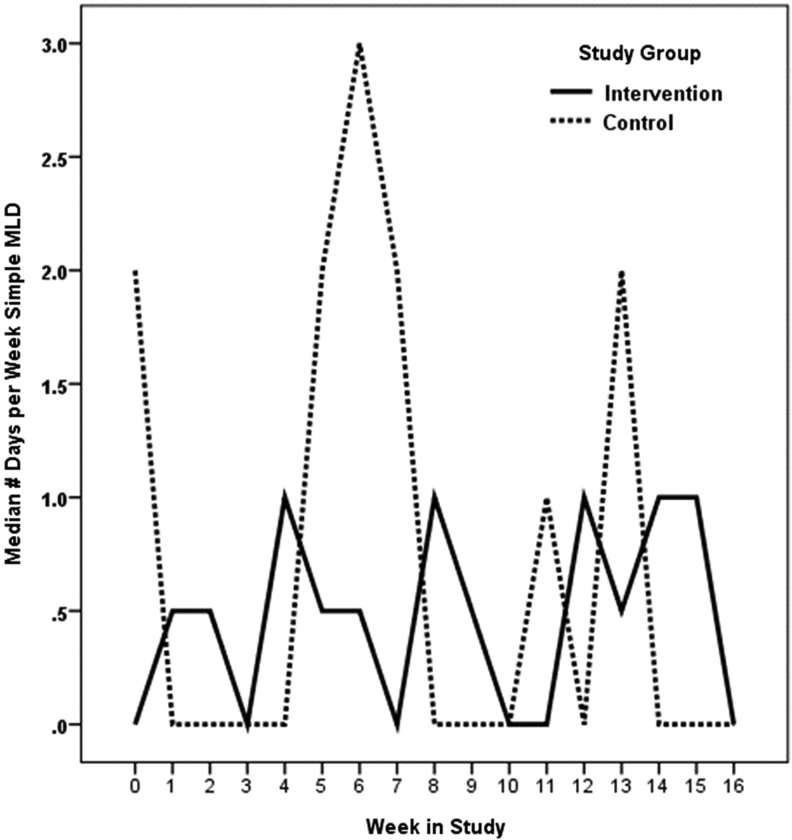
Summaries of self-care activities at baseline, week 12, and week 16 are shown in Table 2. We hypothesized that compared to participants who are not self-monitoring, those who self-monitor limb volume would report more days of garment use, skin care, and simple-MLD. Our hypothesis was partially supported. Statistically significant (p=0.005) differences in patterns of change were noted in garment usage in favor of the intervention group during the 3 months of self-monitoring. Median days of garment use improved from 2.5 days to 4 days during the 3 months of monitoring and maintained at approximately this level during the 30-day post self-monitoring period (Fig. 2). Statistically significant (p=0.004) differences in patterns of change were also noted in self-MLD in favor of the intervention group during the 3 months of self-monitoring (Table 2). Median days of self-MLD changed from 0 to 1 during the week. This dropped back to baseline levels during the 30-day post self-monitoring period (Fig. 3). No statistically significant group differences were noted in time spent per day doing self-care and number of days of skin care. In the group that received the devices, there were no statistically significant changes in L-Dex values over the course of the study; thus, arm volume remained stable in the intervention group.
Table 2.
Summaries of Self-care Activities
| Overall Median [IQR] (Min,Max) | Intervention Median [IQR] (Min,Max) | Control Median [IQR] (Min,Max) | |
|---|---|---|---|
| Minutes each day taking care of lymphedema | N=38 | n=18 | n=20 |
| Baseline | 30.0 [10,34] (3,180) | 17.5 [5,34] (3,60) | 30.0 [10,42] (5,180) |
| Week 12 (N=37)* | 25.0 [11,40] (0,180) | 15.0 [7,38] (0,128) | 30.0 [16,44] (4,180) |
| Week 16 | 25.0 [10,45] (0,120) | 15.0 [5,34] (0,76) | 30.0 [15,57] (3,120) |
| Days per week using some type of compression garment | N=39 | n=18 | n=21 |
| Baseline | 3.0 [0,7] (0,7) | 2.5 [0,5] (0,7) | 5.0 [0,7] (0,7) |
| Week 12 (N=37)* | 4.0 [0,7] (0,7) | 4.0 [0,7] (0,7) | 2.5 [0,7] (0,7) |
| Week 16 (N=38)** | 3.0 [0,7] (0,7) | 3.5 [0,7] (0,7) | 0.0 [0,7] (0,7) |
| Days per week performing Simple MLD | |||
| Baseline | 1.0 [0,5] (0,7) | 0.0 [0,4] (0,7) | 2.0 [0,7] (0,7) |
| Week 12 (N=37)* | 0.0 [0,5] (0,7) | 1.0 [0,5] (0,7) | 0.0 [0,6] (0,7) |
| Week 16 (N=38)** | 0.0 [0,5] (0,7) | 0.0 [0,5] (0,6) | 0.0 [0,7] (0,7) |
| Days per week performing skin care | |||
| Baseline | 7.0 [7,7] (2,7) | 7.0 [7,7] (2,7) | 7.0 [7,7] (7,7) |
| Week 12 (N=37)* | 7.0 [7,7] (3,7) | 7.0 [7,7] (3,7) | 7.0 [7,7] (4,7) |
| Week 16 (N=38)** | 7.0 [7,7] (0,7) | 7.0 [7,7] (0,7) | 7.0 [7,7] (5,7) |
Intervention n=17, Control n=20; **Control n=20.
FIG. 2.
Compression garment. Wald X2(df=16)=34.37, p=0.005.
FIG. 3.
Self-manual lymphatic drainage. Wald X2(df=16)=35.21, p=0.004.
Aim 2
We hypothesized that when compared to participants who are not self-monitoring, participants who self-monitor limb volume would have fewer, less distressful, less intense symptoms; better productivity/activity; report higher perceived self-management/self-efficacy and QOL; experience fewer missed days of work, lymphedema treatment days, arm infections; and have a smaller number of antibiotic prescriptions. The overall number of reported symptoms and levels of intensity and distress from symptoms of lymphedema in this study were quite low. As such, there were no statistically significant differences between the groups in the patterns of change in those measures over the course of the study (p>0.05).
Of the cases with baseline and end-of-study employment information, 64.7% (22 of 34) were employed at the time of the study (Intervention: 12 of 17, 70.6%; Control: 10 of 17, 58.8%; p=0.473). No statistically significant group differences or differences between the groups in patterns of change over time were identified in the impact of health on work or non-work activities.
As shown in Table 3, perceived self-management and QOL scores were quite high for participants in this study. There were no statistically significant modifications in those scores throughout the course of the study. Nor were statistically significant differences noted in resource utilization, number of treatment days, number of arm infections, and number of antibiotic prescriptions, all of which were very low (Table 4). No reports of health expenditures related to lymphedema were noted.
Table 3.
Perceived Medical Condition Self-Management Scale and Upper Limb Lymphedema
| Overall Median [IQR] (Min,Max) | Intervention Median [IQR] (Min,Max) | Control Median [IQR] (Min,Max) | |
|---|---|---|---|
| PMCSMS Total Score | N=35 | N=16 | N=19 |
| Baseline | 32.0 [28,39] (8,40) | 33.0 [29,38] (8,40) | 32.0 [25,40] (8,40) |
| Month 3 | 32.0 [24,39] (8,40) | 32.0 [26,39] (8,40) | 32.0 [24,39] (12,40) |
| Month 4 | 33.0 [25,38] (12,40) | 35.0 [24,38] (16,40) | 31.0 [25,40] (12,40) |
| ULL-27 Overall Score | N=22 | N=11 | N=11 |
| Baseline | 86.3 [81,93] (39,99) | 84.4 [82,93] (65,94) | 87.4 [77,95] (39,99) |
| Month 3 (N=19)* | 88.2 [83,92] (45,99) | 87.4 [80,92] (68,96) | 88.5 [74,95] (45,99) |
| Month 4 | 87.0 [80,93] (44,100) | 86.7 [83,92] (60,94) | 90.4 [71,95] (44,100) |
| ULL-27 Physical | N=26 | N=11 | N=15 |
| Baseline | 89.3 [85,95] (25,100) | 89.3 [86,96] (67,100) | 89.3 [80,95] (25,99) |
| Month 3 (N=23)** | 93.3 [82,98] (44,100) | 96.0 [86,98] (75,99) | 90.0 [82,97] (44,100) |
| Month 4 | 92.0 [85,98] (37,100) | 90.7 [86,96] (77,100) | 92.0 [92,98] (37,100) |
| ULL-27 Psychological | N=35 | N=17 | N=18 |
| Baseline | 82.9 [71,92] (34,100) | 82.9 [71,89] (37,100) | 84.3 [68,92] (34,100) |
| Month 3 (N=34)*** | 80.0 [70,92] (40,100) | 80.0 [71,92] (43,97) | 77.1 [70,93] (40,100) |
| Month 4 | 80.0 [68,86] (31,100) | 80.0 [68,85] (31,100) | 78.6 [67,92] (34,100) |
| ULL-27 Social | N=33 | N=16 | N=17 |
| Baseline | 92.0 [82,100] (48,100) | 90.0 [72,99] (56,100) | 96.0 [84,100] (48,100) |
| Month 3 (N=32)**** | 88.0 [73,99] (32,100) | 90.0 [72,92] (52,100) | 88.0 [81,100] (32,100) |
| Month 4 | 88.0 [76,98] (44,100) | 80.0 [73,96] (48,100) | 92.0 [82,100] (44,100) |
Intervention n=9, Control n=10; **Intervention n=9, Control n=14; ***Control n=17; ****Control n=16.
Table 4.
Resource Utilization
| Overall Median [IQR] (Min,Max) | Intervention Median [IQR] (Min,Max) | Control Median [IQR] (Min,Max) | |
|---|---|---|---|
| Office visits or hospital admissions | N=37 | N=17 | N=20 |
| Baseline | 0 [0,20] (0,125) | 0 [0,20] (0,125) | 0 [0,17.5] (0,93) |
| % no medical care in the past week | 68% | 65% | 70% |
| Week 12 (N=36)* | 0 [0,20] (0,500) | 0 [0,22.5] (0,400) | 0 [0,10] (0,500) |
| % no medical care in the past week | 72% | 69% | 75% |
| Week 16 (N=36)** | 0 [0,0] (0,2200) | 0 [0,0] (0, 85) | 0 [0,15] (0, 2200) |
| % no medical care in the past week | 78% | 82% | 74% |
| Supplies | N=36 | N=18 | N=18 |
| Baseline | 0 [0,4.5] (0,20) | 0 [0,0] (0,20) | 0 [0, 5] (0,20) |
| % no expenses related to medical supplies in the past week | 69% | 78% | 61% |
| Week 12 (N=35)*** | 0 [0,7] (0,7000) | 0 [0,8] (0,150) | 0 [0,4.5] (0,7000) |
| % no expenses related to medical supplies in the past week | 69% | 67% | 70% |
| Week 16 (N=37)** | 0 [0,0] (0,291) | 0 [0,0] (0,291) | 0 [0,0] (0, 65) |
| % no expenses related to medical supplies in the past week | 84% | 88% | 80% |
Intervention n=16, Control n=20; **Intervention n=17, Control n=19; *** Intervention n=15, Control n=20; **** Intervention n=17, Control n=20.
Discussion
This is the first known study to test the self-monitoring of lymphedema in the home setting using BIS. As this was a pilot study, findings should be considered in light of its limitations, which include a small sample size, limitation of self-measurement to 3 months, only a 30 day follow-up period, and no end of study BIS measurements for those in the control group. Despite these limitations, several valuable findings were generated.
Self-monitoring with BIS appears to be feasible, as patients willingly monitored their arms as directed and reported no problems with the equipment. There were no noted differences between those who withdrew from the intervention vs. the control groups in terms of demographic characteristics. The device was durable, as there was no equipment failure during the study. Most participants in the intervention expressed a desire to have the device available to them for home use on a consistent basis.
In terms of self-care activities, almost all participants were conducting skin care 7 days a week at baseline and throughout the study. This likely accounts for the lack of change noted in this self-care activity and also suggests that patients are more adherent to skin care than other, more difficult to complete components of Phase 2 CDT, such as self-MLD and donning of compression garments. Improvement in garment use and self-MLD in the intervention group suggests that, as Self-Regulation Theory would support, objective monitoring of lymphedema may enable patients to determine the effectiveness of self-care, set self-care goals, and provide reinforcement for continuation of self-care.22 Given the findings that there were no statistically significant changes in L-Dex values over the course of the study in the group that received the devices, it is possible that self-monitoring and/or the improvement in self-MLD and compression garment may assist patients in maintaining a stable volume level.
There are some possible explanations for the lack of improvement in several health outcomes, despite statistically significant improvement in self-care. First and foremost, this pilot study was underpowered to detect significant differences in many health outcomes that were examined; however, participants did complete 100% of the outcome measures, establishing that is it feasible to follow such outcomes. Therefore, findings from this study can be used to power larger future studies that evaluate these outcomes. Second, it is possible that the self-care dose (increased garment use and increased self-MLD), despite some improvement, may not have been high enough to facilitate more outcome improvement. Third, a longer observation and follow-up period may be needed. Finally, those who withdrew had significantly poorer reported QOL and a more severe symptom profile compared to those who remained in the study. Thus, those with the greatest room for improvement are not accounted for in these findings. An additional intervention component such as motivational training or coaching, coupled with self-monitoring, may be needed to increase the self-care dose to a level that a broader clinical relevance is established in those with poor QOL and high symptom burden.
There was little utilization of healthcare resources by either group during the course of this study for either an acute issue, such as cellulitis, or lymphedema therapy by a healthcare professional. It is possible that by asking for participants to report healthcare utilization in the past week only instead of from the time of the last assessment, all utilization patterns may not have been captured. In addition, items included in the RUEBQ may not have accounted for all categories of actual lymphedema-related health care expense; however, the lack of change in work productivity supports that participants in this study as a group did not experience health issues that lead to missed work or extensive medical care.
These findings taken together support that a longer observation and follow-up period may be needed, but this was prohibited due to the pilot nature of the study. A 12-month monitoring period should be considered in future self-monitoring studies, as this would account for potential seasonal variation in lymphedema volume and associated symptoms. Findings from this study support a larger BIS trial with longer follow-up periods and that compare this method to other self-measurement options.
There are other potential implications from this study beyond those for self-monitoring alone. For example, participants in lymphedema intervention trials could be given devices to self-measure changes in limb status at home over time. This would reduce the need for and cost of researchers to travel to homes to conduct measurements, or, alternatively, for research participants to come to laboratories or clinics for measurements. Additionally, prospective self-monitoring with these devices for breast cancer survivors who are at high risk for lymphedema, such as those with elevated BMI, postoperative wound infections, and axillary dissection, is clearly possible.28,29 This is critical given findings that early intervention can potentially prevent subclinical swelling from progressing to chronic swelling, and that early identified clinical swelling treated with compression alone produces clinically significant results.30,31 Such prospective use could lead to early detection of swelling and trigger immediate interventions that could lead to better patient outcomes and less intensive treatment for those developing chronic lymphedema. Theoretically, this could reduce the overall cost of lymphedema after breast cancer treatment to patients and society.
Conclusions
Objective self-monitoring of arms using BIS is possible. Self-monitoring appears to positively impact lymphedema self-care behaviors. However, self-monitoring alone may not be sufficient to promote high levels of self-care activities in highly symptomatic patients, such as those who withdrew from the study and who may require coaching or other forms of psychological support to improve their self-care. Studies that combine a cognitive behavioral therapy component along with self-measurement should be considered as potential interventions to impact lymphedema self-care. Additionally, further studies exploring other potential applications of self-monitoring are indicated.
Acknowledgments
The authors wish to thank Candace Bonner, Emily Galford, and Jessica Harbison for their assistance with this project.
Author Disclosure Statement
No competing financial interests exist.
Primary funding for this study was provided by grant 1 R21 NR012271-01A1 from the National Institutes of Health. Bioelectrical impedance devices used in this study were partially supported through this grant as well as through the Cancer Survivorship Research Core. The Cancer Survivorship Research Core is supported in part by Grant 1UL 1rr024975 from the National Center for Research Resources, National Institutes of Health. Study data were collected and managed using REDCap electronic data capture tools hosted at Vanderbilt University. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources. REDCap is supported by Vanderbilt Institute for Clinical and Translational Research grant UL1 TR000011 from NCATS/NIH.
References
- 1.Institute of Medicine of the National Academies. Meeting the Psychosocial Needs of Women with Breast Cancer. In: Hewitt M, Herdman R, Holland J, eds. Washington, D. C.2004: http://www.nap.edu/catalog.php?record_id=10909#orgs Accessed December/18/2013 [Google Scholar]
- 2.National Lymphedema Network. Position Statement of the National Lymphedema Network: Treatment. 2009; http://www.lymphnet.org/pdfDocs/nlntreatment.pdf Accessed December/18/2013
- 3.Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer 2005;13:904–911 [DOI] [PubMed] [Google Scholar]
- 4.Shih Y-CT, Xu Y, Cormier JN, Giordano S, Ridner SH, Buchholz TA, Perkins GH, Elting LS. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: A 2-year follow-up study. J Clin Oncol 2009;27:2007–2014 [DOI] [PubMed] [Google Scholar]
- 5.Kwan W, Jackson J, Weir LM, Dingee C, McGregor G, Olivotto IA. Chronic arm morbidity after curative breast cancer treatment: Prevalence and impact on quality of life. J Clin Oncol 2002;20:4242–4248 [DOI] [PubMed] [Google Scholar]
- 6.Nesvold I-L, Dahl AA, Løkkevik E, Marit Mengshoel A, Fosså SD. Arm and shoulder morbidity in breast cancer patients after breast-conserving therapy versus mastectomy. Acta Oncol 2008;47:835–842 [DOI] [PubMed] [Google Scholar]
- 7.Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol 2000;39:393–397 [DOI] [PubMed] [Google Scholar]
- 8.Ramos SM, O'Donnell LS, Knight G. Edema volume, not timing, is the key to success in lymphedema treatment. Am J Surg 1999;178:311–315 [DOI] [PubMed] [Google Scholar]
- 9.Ridner SH. Symptoms Clusters Occurring With Lymphedema After Breast Cancer [Dissertation]. Nashville: Nursing Science Department Vanderbilt University; 2003 [Google Scholar]
- 10.Woods M. Patients' perceptions of breast-cancer-related lymphoedema. Eur J Cancer Care 1993;2:125–128 [DOI] [PubMed] [Google Scholar]
- 11.Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat 2001;68:273–282 [DOI] [PubMed] [Google Scholar]
- 12.Sneddon MC, Lewis M. Lymphoedema: A female health issue with implications for self care. Br J Nurs 2006;16:76. [DOI] [PubMed] [Google Scholar]
- 13.Ridner SH, Dietrich M, Kidd N. Breast cancer treatment-related lymphedema self-care: Education, practices, symptoms, and quality of life. Support Care Cancer 2011;19:631–637 [DOI] [PubMed] [Google Scholar]
- 14.Ni H, Nauman D, Burgess D, Wise K, Crispell K, Hershberger RE. Factors influencing knowledge of and adherence to self-care among patients with heart failure. Arch Intern Med 1999;159:1613–1619 [DOI] [PubMed] [Google Scholar]
- 15.Strömberg A, Mårtensson J, Fridlund B, Levin L-Å, Karlsson J-E, Dahlström U. Nurse-led heart failure clinics improve survival and self-care behaviour in patients with heart failure. Eur Heart J 2003;24:1014–1023 [DOI] [PubMed] [Google Scholar]
- 16.Cappuccio FP, Kerry SM, Forbes L, Donald A. Blood pressure control by home monitoring: Meta-analysis of randomised trials. BMJ 2004;329:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl SM, Kelley CR, Neill PJ, Grim CE, Mamlin J. Effects of home blood pressure measurement on long-term BP control. Am J Public Health 1984;74:704–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klapper B, Kuhne H. Patient self-management by telehealth using the Bosch model of care. J Telemed Telecare 2010;16:193–195 [DOI] [PubMed] [Google Scholar]
- 19.Ridner SH, Bonner CM, Doersam JK, Rhoten BA, Schultze B, Dietrich MS. Bioelectrical impedance self-measurement protocol development and daily variation between healthy volunteers and breast cancer survivors with lymphedema. Lymphat Res Biol 2014;12:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U. S. Food and Drug Administration. FDA Approval of ImpediMed Device for Monitoring of Extremity Lymphedema. Washington, D.C.: U. S. Food and Drug Administration; 2008 [Google Scholar]
- 21.ImpediMed, Ltd. L-Dex™ XCA. 2010; http://www.impedimed.com/products/l-dex-xca/xca-for-usa.htm Accessed December/18/2013
- 22.Johnson JE. Self-regulation theory and coping with physical illness. Res Nurs Health 1999;22:435–448 [DOI] [PubMed] [Google Scholar]
- 23.Wallston KA, Rothman RL, Cherrington A. Psychometric properties of the Perceived Diabetes Self-Management Scale (PDSMS). J Behav Med 2007;30:395–401 [DOI] [PubMed] [Google Scholar]
- 24.Ridner SH, Dietrich MS. Development of the Lymphedema Symptoms Intensity and Distress Survey—Arm J Clin Oncol (Meeting Abstracts). 2010;28:9125 [Google Scholar]
- 25.Bolge SC, Doan JF, Kannan H, Baran RW. Association of insomnia with quality of life, work productivity, and activity impairment. Qual Life Res 2009;18:415–422 [DOI] [PubMed] [Google Scholar]
- 26.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353–365 [DOI] [PubMed] [Google Scholar]
- 27.Launois R, Megnigbeto AC. A specific quality of life scale in upper limb lymphoedema: The ULL-27 questionnaire. Qual Life Res 2001:261 [Google Scholar]
- 28.Kilbreath SL, Refshauge KM, Ward LC, Kastanias K, Yee J, Koelmeyer LA, Beith JM, French JR, Ung OA, Black D. Factors affecting the preoperative and postoperative extracellular fluid in the arm on the side of breast cancer: A cohort study. Lymphat Res Biol 2013;11:66–71 [DOI] [PubMed] [Google Scholar]
- 29.Ugur S, Arici C, Yaprak M, Mesci A, Arici GA, Dolay K, Ozman V. Risk factors of breast cancer-related lymphedema. Lymphat Res Biol 2013;11:72–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dayes IS, Whelan TJ, Julian JA, Parpia S, Pritchard KO, D'Souza DP, Kligman L, Reise D, LeBlanc L, McNeely ML, Manchul L, Wiernikowski J, Levine MN. Randomized trial of decongestive lymphatic therapy for the treatment of lymphedema in women with breast cancer. J Clin Oncol 2013;31:3758–3763 [DOI] [PubMed] [Google Scholar]
- 31.Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008;112:2809–2819 [DOI] [PubMed] [Google Scholar]