Abstract
Background: The pathophysiology of breast cancer-related lymphedema (BCRL) is poorly understood. The present study evaluated the lymphatic collectors in the arms of patients with BCRL.
Methods and Results: In total, 123 patients with ipsilateral BCRL who had undergone magnetic resonance lymphangiography using gadobenate dimeglumine as a contrast agent were enrolled in this study. Morphological changes and the numbers of collecting lymphatic vessels were recorded. Associations between the number of visualized lymphatic collectors and edema accumulation, subcutis thickness, and the BCRL duration and latency were analyzed. Tortuous and significantly dilated lymphatic collectors were visualized in the lymphedematous arms of 104 patients (85%). The median number of visualized lymphatic collectors was four. The duration of BCRL was weakly but significantly correlated with the number of lymphatic collectors (rs=0.2054, p=0.0226). The differences in the tissue water content and thickness of the subcutis between the bilateral arms demonstrated moderate correlations with the number of collecting lymphatics (rs=0.31 and 0.35, respectively; p<0.01). More lymphatic collectors tended to be seen in more advanced cases. There was no statistical difference in the amount of lymphatic vessels among different breast cancer treatment methods.
Conclusions: The number of functional remaining lymphatic collectors increases with the prolongation and severity of BCRL. This may imply persistent reactions of lymphatic collectors in response to lymphostasis.
Introduction
Lymphedema is one of the most serious complications of breast cancer. According to the World Health Organization, 1.38 million women worldwide are diagnosed with breast cancer every year.1 Among them, more than one in five women who survive breast cancer will develop arm lymphedema.2 Breast cancer-related lymphedema (BCRL) occurs as a consequence of axillary lymph node removal and damage to the lymphatic vessels of the arm. BCRL is usually accompanied by erysipelas and progressive fibrosis and seriously affects patients' quality of life.
The pathophysiology of BCRL is complicated.3 Previous studies have demonstrated that radiation, obesity, time after surgery, and other risk factors are significantly correlated with the development of lymphedema.2 Studies on the pathophysiology of BCRL have shown that edema, tissue fibrosis, fat deposition, and thickened skin are pathological consequences of lymphatic blockage.4 The collecting lymphatics play a central role in the onset of lymphedema. Previous studies of both collecting lymphatic vessels and skin lymphatics have focused on the local histological changes of the vessels.5,6 Little is known about the outcome and pathogenesis (morphological and functional) of lymphatic collector obstruction in patients with BCRL. Whether the lymphatics become gradually or acutely occluded and whether the numbers of functional lymph vessels decrease with the progression of the lymphedema remain to be elucidated. A thorough investigation of the lymphatic collectors in the arms of patients with BCRL is important to predict prognosis and guide therapy. Our understanding of the pathophysiology of lymphatic collector obstruction has been limited by the technical (methodological) difficulties in assessing human lymphatics. Lymphatic channels in the affected arm cannot be clearly demonstrated with the commonly used method of lymphoscintigraphy because of its insufficient resolution.7 High-resolution magnetic resonance lymphangiography (MRL) was recently shown to be useful in the diagnosis of peripheral lymphatic system disorders.8,9 This technique is particularly useful for studying the structural changes of peripheral lymphatic channels in both primary10 and secondary lymphedema.11 Intracutaneously injected paramagnetic contrast material has been found to be easily absorbed by the initial lymph vessels, and this procedure permits dynamic and real-time observation of contrast enhancement in lymphatic vessels and drainage nodes.
A study of the lymphatic collectors in the arms of 123 patients with BCRL was carried out in the author's clinic with the use of MRL. The visualized vessels were considered to be functional lymphatics because enhancement of the lymphatic channels on MRL imaging is the result of absorption and transportation of the intradermal injected contrast agent by the lymphatics. The associations between the number of visualized lymphatic collectors and edema accumulation, subcutis thickness, and BCRL duration and latency were analyzed to verify the pathophysiology of BCRL.
Materials and Methods
Patients
The present study included 123 patients diagnosed with ipsilateral BCRL (mean age, 57 years; range, 33–84 years) after axillary surgery for unilateral breast cancer. Of these patients, 42 underwent an axillary operation combined with chemotherapy and radiotherapy, 33 underwent axillary lymph node dissection followed by radiation, 12 underwent axillary surgery and chemotherapy, 32 underwent axillary lymph node dissection only, 3 underwent axillary radiotherapy only, and 1 underwent chemotherapy only. The median duration of lymphedema (lymphedema morbidity period) among all patients was 24 months (range, 1–240 months; interquartile range, 60). Among the 123 patients with BCRL, 27 developed arm lymphedema immediately after breast cancer treatment. The median latent phase in the remaining patients was 22 months (range, 4–233 months; interquartile range, 54). In 28 of the 123 patients, lymphedema occurred immediately after excessive use of the affected arm (e.g., carrying a baby, doing heavy housework, lifting heavy bags, or exercising excessively). The clinical manifestations of the patients are summarized in Table 1.
Table 1.
Characteristics of the 123 BCRL Patients in the Study
| Characteristics | Value |
|---|---|
| Age (yr) | |
| Median | 57 |
| Range | 33–84 |
| Breast cancer treatment | |
| axillary operation+chemotherapy+radiotherapy | 42 |
| axillary operation+radiotherapy | 33 |
| axillary operation+chemotherapy | 12 |
| axillary operation only | 32 |
| radiotherapy only | 3 |
| chemotherapy only | 1 |
| Duration of disease (month) | |
| Median | 24 |
| Range | 1–240 |
| Latent phase | |
| Number of patient without latent phase | 27 (22%) |
| Number of patients with latent phase | 96 (78%) |
| Median of latent phase (month) | 22 |
| Range | 4–233 |
Magnetic resonance lymphangiography (MRL)
MRL was performed as previously described8,9 with a 3.0 T MR unit (Philips Medical Systems, Best, The Netherlands). First, three-dimensional heavily T2-weighted MRI was performed. After intracutaneous injection of the paramagnetic contrast agent gadobenate dimeglumine into the digital web spaces of upper limbs at 0.7 to 0.8 mL per point (MultiHance®; Bracco, Milan, Italy), three-dimensional fast spoiled gradient-recalled echo T1-weighted images were acquired with a fat-saturation technique at consecutive time points for dynamic observation and mapping of lymphatic vessels of both arms at the same time. Contrast-enhanced lymph flow in the collecting vessels could be visualized approximately 7 to 8 min after intradermal contrast injection.
Before contrast injection, the thickness of the subcutaneous layer of the edematous arm and nonedematous contralateral upper arm was measured at four points (ulnar, radial, dorsal, and ventral) from the skin surface to the muscular fascia of the forearm on the cross section of a T2-weighted MR image (Fig. 1). The thickness of the subcutis was defined as the average of the four measurements. The percentage difference in the thickness of the subcutis between the ipsilateral lymphedematous and contralateral nonedematous limbs (%) was calculated using the following formula:
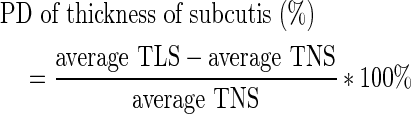 |
FIG. 1.

The thickness of the subcutaneous layer was measured at four points (ulnar, radial, dorsal, and ventral) from the skin surface to the muscular fascia of the forearm of lymphedematous and nonedematous contralateral arm on the cross section of a T2-weighted MR image.
(TLS, thickness of ipsilateral lymphedematous subcutis; TNS, thickness of nonedematous contralateral subcutis)
The contrast-enhanced lymphatic collectors were evaluated on T1-weighted dynamic MRL imaging. The morphological changes and number of visualized lymphatic collectors in each limb were recorded.
Tissue water measurement
Tissue water in the arm was quantified with bioimpedance spectroscopy in body composition analysis (InBody 720; Biospace Co., Ltd., Seoul, Korea) for direct measurement of differences in extracellular fluid between the edematous and nonedematous upper limbs. The percentage difference in tissue water between the edematous and nonedematous upper limbs (%) was calculated using the following formula:
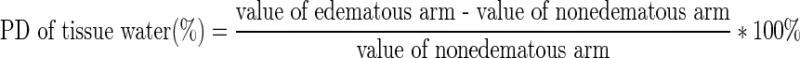 |
Statistical analysis
The nonparametric Spearman rank correlation test was used for statistical analysis to assess the relationships between the numbers of lymphatic collectors with respect to the duration and latency of lymphedema, percentages of differences in tissue water, and thickness of the subcutis, respectively. The effects of various breast cancer treatment methods on the number of lymphatic collectors in the lymphedematous limbs were analyzed with Wilcoxon's rank sum test. Statistical analyses were performed using SAS software version 9.3 (SAS Institute, Cary, N.C.), and a value of p<0.05 was considered statistically significant.
Results
Tissue water
The tissue water in the bilateral limbs was measured with bioimpedance spectroscopy in 100 patients. The differences in the water content between the lymphedematous and nonedematous limbs ranged from 0.10 to 4.43 kg. The median of the comparative ratio between the bilateral arms was 37.98 (interquartile range, 41.52) (Table 2).
Table 2.
MR Lymphangiographic Findings of Lymph Collectors in 123 BCRL Patients
| Visualization of Lymphatic collector | |
| Number of patients without visualized lymphatic collector | 19 (15%) |
| Number of patients with visualized lymphatic collector | 104 (85%) |
| Number of patients with visualized lymphatic collector in the contralateral arm | 60 (49%) |
| Number of lymphatic collector in lymphedematous arm | |
| Median | 4 |
| Range | 1–35 |
| Number of patients with numerous lymphatic communication branches | 9 |
| Morphology of lymphatic collectors | |
| Tortuous and significantly dilated lymphatic collectors were visualized | 104 patients |
| Obvious damage to lymphatic collector (cystic dilatation, disruption, lymph leakage, regeneration of small lymphatics between the broken vessels) | 15 patients |
Thickness of subcutis
Stagnant water in the subcutaneous layer of the arm could be clearly observed on the cross-sectional T2-weighted MR images. Edema fluid superficially encircled the limb, was dispersed throughout the whole layer of subcutis, or was present between the fibrotic and fat tissue in lymphedematous arms, resulting in thickening of the subcutis. The difference in the subcutis thickness between the lymphedematous and nonedematous arms was determined in 114 patients. No intramuscular edema was observed in the examined arms. The median comparative ratio of the average thickness of the subcutis between the edematous and nonedematous arms was 111.1 (interquartile range, 112.64) (Table 2). “Dermal backflow” that is lymphedematous skin (epidermis) massively diffused with contrast on the MRL image was seen in 23 patients.7,11
Morphology of lymphatic collectors
The contrast-enhanced lymph collectors were visualized in the lymphedematous arms in 104 of the 123 patients (85%). The lymphatic collectors in the lymphedematous arms were tortuous and significantly dilated with a diameter ranging from 0.5 to 5.0 mm on MRL imaging. Obvious damage to the collecting lymphatic vessels of the forearm was seen in 15 patients and included cystic dilatation, disruption, lymph leakage, “lymph lake” formation, and regeneration of small lymphatics between the broken vessels (Fig. 2a, b). The lymphatics were observed in the contralateral nonedematous limb in 60 patients with much weaker signal intensity and discontinued in shape, that could be easily contrasted with vein. No deep lymphatics were observed in the tested arms.
FIG. 2.
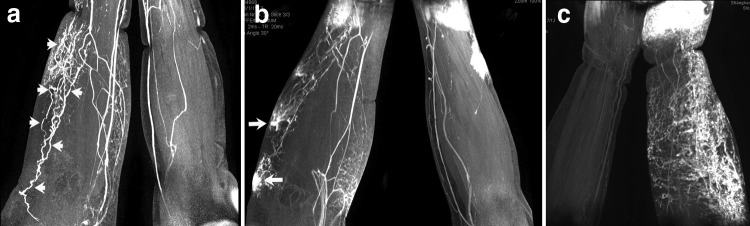
Morphological changes of lymphatic collectors on MRL imaging of BCRL arm. (a) Tortuous and significantly dilated collecting lymphatics (arrows). (b) Lymph collector disruption and lymphorrhea (arrows) in the forearm. (c) Significantly dilated lymphatic collectors with extensive opening of numerous communication branches.
Numbers of lymphatic collectors
The median number of contrast-enhanced lymphatic collectors in the lymphedematous arm was 4, with a very wide range of 1 to 35 (interquartile range, 9). The lymphatic collectors were significantly dilated with extensive opening of numerous communication branches in nine patients (Fig. 2c). Interestingly, numerous lymphatic branches were seen in a patient with a 15-year history of chronic lymphedema and in a patient with a 1-month history of acute lymphedema and 14-year latent period. These findings indicate that a similar lymphatic reaction to lymphedema can occur in both the early (acute) and late (chronic) stages of the disease. The MRL findings of lymphatic collectors are summarized in Table 2.
Correlation of duration and latency of BCRL with number of lymphatic collectors
The duration of BCRL was weakly but significantly correlated with the number of lymphatic collectors (rs=0.2054, p=0.0226) (Fig. 3). The increase in the number of functional lymphatics with the prolongation of BCRL was unexpected. However, the latent phase (time since breast cancer treatment) was not associated with the number of lymphatics in the affected arm (p=0.5243) (Fig. 3). This means that there was no significant difference in the number of functional lymphatic collectors between patients with a latent phase of 0 months (lymphedema developed immediately after breast cancer treatment) and patients with a latent phase of 4 to 233 months.
FIG. 3.
Scatter plot of the number of lymphatics with respect to duration, latency of lymphedema, PD of tissue water, and PD of the thickness of subcutis. There are significant correlations between number of lymphatics and three clinical characteristics: duration of lymphedema, percentage difference of tissue water, and percentage difference of the thickness of subcutis (all the p values are less than 0.05), except latency of lymphedema (p=0.5243). *PD of tissue water: Percentage difference of tissue water between the edematous and nonedematous upper limbs (%); *PD of thickness of subcutis: Percentage difference of thickness of subcutis between the ipsilateral lymphedematous and contralateral nonedematous limbs (%).
Correlation of number of functional lymphatics with tissue fluid and with subcutis thickness of affected arm
Additional unanticipated results were the differences in the tissue water content and thickness of the subcutis between the bilateral arms. The correlation of each of these parameters with the number of lymphatic collectors was moderate (rs=0.31 and 0.35, respectively; p<0.01) (Fig. 3). These results indicate that more lymphatics tend to be present in more advanced cases.
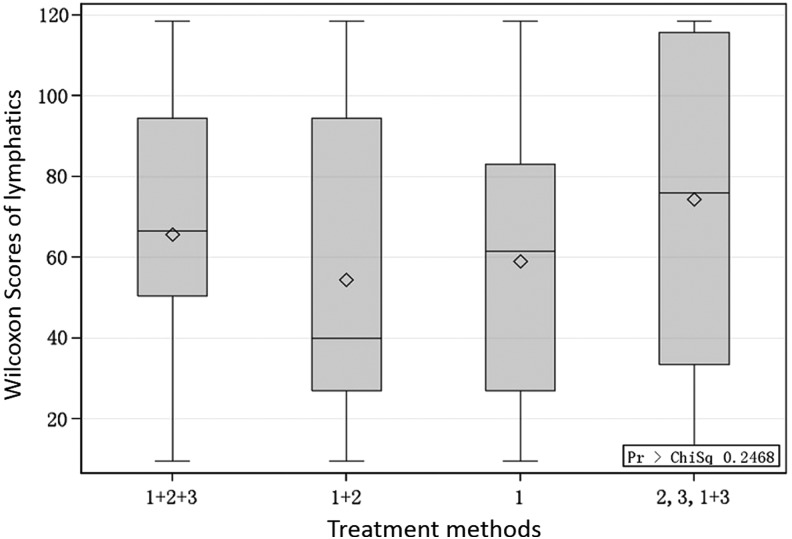
Relationship between number of lymphatics and breast cancer treatment method
Figure 4 shows that there were no statistical differences in the amount of lymphatic vessels (as indicated by the Wilcoxon scores) among the different breast cancer treatment methods (axillary lymph node dissection plus radiotherapy and chemotherapy, axillary node extirpation plus chemotherapy, and axillary surgery alone; p=0.2468).
FIG. 4.
The effects of various breast cancer treatment methods on the number of lymphatic collectors in BCRL arms. 1: underwent axillary lymph node dissection only; 2: underwent chemotherapy only; 3: underwent axillary radiotherapy only; 1+2: underwent axillary lymph node dissection followed by radiation; 1+3: underwent axillary surgery and chemotherapy; 1+2+3: underwent axillary operation combined with chemotherapy and radiotherapy.
Discussion
Damage to the axillary lymphatic system secondary to surgery and radiotherapy is the primary cause of BCRL. The lymphatic pathology that develops because of lymphatic outflow impairment may have a profound impact on the development of BCRL and vice versa. The total number of collecting lymphatics in the upper extremity is normally between 20 and 30.12 These lymphatic vessels are not always filled with lymph; therefore, they may not be visualized on imaging in cases involving patent vessels. When the upstream lymph circulation is interrupted after breast cancer treatment, the lymphatic collectors may undergo lymph stagnancy, ectasis, stretching,13 and disruption11,14 under high pressure within the lumen; subsequent fibrosis and sclerosis of the wall; narrowing; and eventually obstruction of the lumen.11 Therefore, it seems that the number of functional collecting lymph vessels would decrease with development of the disease.15 However, it seems that this speculation may not be accurate.
In the present study, MRL revealed upstream blockage-induced damage to the lymphatic collectors in the form of vascular dilatation, tortuosity, disorder, breakage, and lymph leakage. The unexpected finding is that the lymphatic collectors had not declined in number with the development of the disease, but showed a trend of increasing numbers with the course of the disease. This result is counterintuitive to the conventional understanding of the pathology of BCRL. Furthermore, the number of collecting lymphatics was significantly associated with the severity of subcutaneous edema and the thickness of the subcutis of the affected arm. It seems that the stagnant water in the subcutaneous layer and increased thickness of the subcutis did not lead to the collapse or obliteration of more lymphatic collectors, but resulted in the persistent opening and filling of the lymphatics, allowing of transport the stagnant lymph. The underlying mechanism of this phenomenon is currently unclear.
Possible explanations for this phenomenon may be as follows. First, the collecting lymphatics may retain their physiological reaction of counteracting the impending lymphostasis13 by increases in the number of dilated vessels in the BCRL arm. Second, the pathophysiology of BCRL might differ to some extent from what was seen in previous experimental studies of dogs10 and secondary lymphedema of the human lower extremity;15 these studies showed that severe lymphatic damage caused by very high intralumen pressure may result in early obliteration of obstructed lymphatic collectors. This may account for the more severe clinical symptom of elephantiasis in the lymphedematous lower extremity.
Axillary node extirpation increases the resistance to lymph drainage from the arm and hence raises pressure in the lymphatics, which may lead to an increase and subsequent fall in lymphatic contraction;13,16 the lymphatics may even stop pulsating after a period of time.17 How long the pulsating function of the lymphatics in BCRL-affected arms persists remains to be clarified. These lymphatic collectors may exhibit valvular insufficiency due to dilatation under persistent high intraluminal pressure. In this study, contrast-enhanced lymph flow in the collecting vessels could be visualized approximately 7 to 8 min after intradermal contrast injection during MRL examination. This indicates that both the initial and collecting lymphatics maintained their basic function of absorbing and transporting lymph and did not become paralyzed in chronic BCRL (longest duration was 18 years). Furthermore, the number of these functionally impaired but still working lymphatics was significantly correlated with the duration of BCRL. One of the important reactions of lymphatic collectors in response to lymphostasis is the opening of collateral pathways. This functional phenomenon of lymphatic collecting vessels was seen in BCRL-affected arms with both acute (1-month) and chronic (180-month) disease in the present study. Therefore, the reaction of lymphatic collecting vessels in response to lymphedema by opening their communication branches could be fast and persistent. The lymphatic collectors were visualized in the contralateral arm in nearly half of the tested patients in this study, and lymph flow was increased in the contralateral arm in others;18 these findings indicate that the reaction to the axillary surgery may have been systemic, not just regional.
One study showed that muscular lymph drainage always exceeded subcutis drainage in BCRL-affected arms and that muscular lymph drainage in the ipsilateral arm was unimpaired relative to the contralateral arm.19 This may be consistent with the present study, which showed that edema was located only within the subcutis and not in the muscle of the affected arm of all 123 patients. Why the axillary node extirpation does not interfere with deep lymphatic outflow remains unknown because the axillary region is the main pathway of both the superficial and deep systems. What happened to the connection between the deep and superficial system in BCRL-affected arms is also unclear. Normally, lymph from the superficial system (superficial to the fascia) drains into the deep lymphatic system across fascial connections, and these connections can be visualized.20 The epifascial and subfascial compartments communicate at the wrist and elbow.21 However, there was no evidence of a connection between the two systems in this study because no contrast-enhanced deep lymphatic channel or cubital node was visualized on MRL imaging. Possible explanations for the noninvolvement of the deep lymphatics in BCRL may be as follows. First, the deep lymphatics travel to infraclavicular lymph nodes or take other pathways through communication branches to maintain patency of the deep lymphatics and prevent muscle swelling. Second, the connecting pathway between the deep and superficial systems closes when edema develops;22 thus, the stagnant lymph could not flow out through the deep vessel. This hypothesis could partially explain the pathophysiology of BCRL, and if so, patients with a poor fascial connection between the deep and superficial (superficial to the fascia) systems might be predisposed to BCRL after breast cancer treatment.
The fact that lymphatic collectors were seen in a considerable number of patients with BCRL raises the question of whether these lymphatic collectors should be the targets in the treatment of chronic arm lymphedema. Because these contrast-enhanced collecting lymphatic vessels retained their basic function of absorbing and transporting lymph, any treatment that could cause further damage to the collectors should be avoided. The reconstructive approach to restoration of the collecting vessel pathway23 with the surgical approach may be considered not only in the early stage of BCRL, but also in the late stage.
Conclusion
In this study, contrast-enhanced collecting lymphatics were visualized in the lymphedematous arms of 104 of 123 patients with BCRL. The number of functional remaining lymphatics increased with the prolongation of BCRL. The number of these lymphatics was significantly associated with the severity of subcutaneous edema and thickness of the subcutis of the affected arm. These results may imply persistent reactions of lymphatic collectors to lymphostasis in BCRL-affected arms. These findings may help to elucidate the pathophysiology of BCRL and develop proper treatment.
Acknowledgments
Funding Sources: Chinese Nature Science Foundation (grant no.81272146); Shanghai Science and Technology Committee (grant no. 12401900504)
Author Disclosure Statement
The authors declare no conflicting financial interests.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer statistics. CA Cancer J Clin 2011;61: 69–90 [DOI] [PubMed] [Google Scholar]
- 2.Disipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol 2013;14:500–515 [DOI] [PubMed] [Google Scholar]
- 3.Mortimer P. Arm lymphoedema after breast cancer. Lancet Oncol 2013;14:442–443 [DOI] [PubMed] [Google Scholar]
- 4.Gardner GC, Nickerson JP, Watts R, Nelson L, Dittus KL, O'Brien PJ. Quantitative and morphologic change associated with breast cancer-related lymphedema. Comparison of 3.0T MRI to external measures. Lymphat Res Biol 2014;12:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X, Li R, Liu N. Microscopic analysis of lymphatic vessels in primary lymphedematous skin. Phlebologie 2012;41:13–17 [Google Scholar]
- 6.Koshima I. Kawada S, Moriguchi T, Kajiwara Y. Ultrastructural observations of lymphatic vessels in lymphedema in human extremities. Plast Reconstr Surg 1996;97:397–405 [DOI] [PubMed] [Google Scholar]
- 7.Liu NF, Lu Q, Liu PA, Wu XF, Wang BS. Comparison of radionuclide lymphoscintigraphy and dynamic magnetic resonance lymphangiography for investigating extremity lymphedema. Br J Surg 2010;97:359–365 [DOI] [PubMed] [Google Scholar]
- 8.Liu NF, Lu Q, Jiang ZH. Anatomic and functional evaluation of the lymphatics and lymph nodes in diagnosis of lymphatic circulation disorders with contrast magnetic resonance lymphangiography. J Vasc Surg 2009;49:980–987 [DOI] [PubMed] [Google Scholar]
- 9.Lu Q, Xu J, Liu NF. Chronic lower extremity lymphedema: A comparative study of high-resolution interstitial MR lymphangiography and heavily T2-weighted MRI. Eur J Radiol 2010;73:365–373 [DOI] [PubMed] [Google Scholar]
- 10.Liu NF. Yan ZX. Classification of lymphatic system malformations in primary lymphoedema based on MR lymphangiography. Eur J Vascu Endo Surg 2012;44:345–349 [DOI] [PubMed] [Google Scholar]
- 11.Liu NF, Yan ZX, Wu XF, Luo Y. Magnetic resonance lymphangiography demonstrates spontaneous lymphatic disruption and regeneration in obstructive lymphedema. Lymphology 2013;46:56–63 [PubMed] [Google Scholar]
- 12.Foeldi M, Foeldi E. Anatomy of the lymphatic system. In: Földi M, Földi E, Kubik S. (eds). Textbook of Lymphology: For Physicians and Lymphoedema Therapists. San Francisco CA, Urban and Fischer, 2003, pp.1–164 [Google Scholar]
- 13.Clodius L. Secondary arm lymphoedema. In: Lymphedema, Clodius L. (ed). Georg Thieme, Stuttgart,1977, pp. 147–174 [Google Scholar]
- 14.Mihara M1, Hara H, Hayashi Y, Narushima M, Yamamoto T, Todokoro T, Iida T, Sawamoto N, Araki J, Kikuchi K, Murai N, Okitsu T, Kisu I, Koshima I. Pathological steps of cancer-related lymphedema: Histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One 2012;7:e41126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olszewski WL. Pathophysiological and clinical observations of obstructive lymphedema of the limbs. In: Clodius L, (ed). Lymphedema. Stuttgart: Georg Thieme; 1977,pp. 79–102 [Google Scholar]
- 16.Stanton AWB, Modi S, Mellor RH, Levick JR, Mortimer PS. Recent advances in breast cancer-related lymphedema of the arm: Lymphatic pump failure and predisposing factors. Lymphat Res Biol 2009;7:29–45 [DOI] [PubMed] [Google Scholar]
- 17.Modi1 S, Stanton1 AWB, Svensson WE, Peters AM, Mortimer PS, Levick JR. Human lymphatic pumping measured in healthy and lymphoedematous arms by lymphatic congestion lymphoscintigraphy. J Physiol 2007;583:271–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen MR, Simonsen L, Karlsmark T, Bulow J. Microvascular filtration is increased in the forearms of patients with breast cancer-related lymphedema. J Appl Physiol 2013;114:19–27 [DOI] [PubMed] [Google Scholar]
- 19.Stanton AW, Modi S, Bennett Britton TM, Purushotham AD, Peters AM. Levick JR, Mortimer PS. Lymphatic drainage in the muscle and subcutis of the arm after breast cancer treatment. Breast Cancer Res Treat 2009;117:549–557 [DOI] [PubMed] [Google Scholar]
- 20.Foeldi M, Foeldi E. Physiology and pathophsiology of the lymphatic system. In: Földi M, Földi E, Kubik S. (eds). Textbook of Lymphology: For Physicians and Lymphoedema Therapists. San Francisco CA: Urban and Fischer, 2003, pp.196–230 [Google Scholar]
- 21.Gray H. Lymphatic drainage of the upper limbs. In: Williams PL. (ed), Gray's Anatomy. Churchill Livingstone, Edinburgh, 1995, pp. 1613–1615 [Google Scholar]
- 22.Mortimer PS. The pathophysiology of lymphedema. Cancer Supplement 1998;83:2798–2802 [DOI] [PubMed] [Google Scholar]
- 23.Suami H, Chang DW. Overview of surgical treatments for breast cancer related lymphoedema. Plast Reconstr Surg 2010;126:1853–1863 [DOI] [PubMed] [Google Scholar]