Abstract
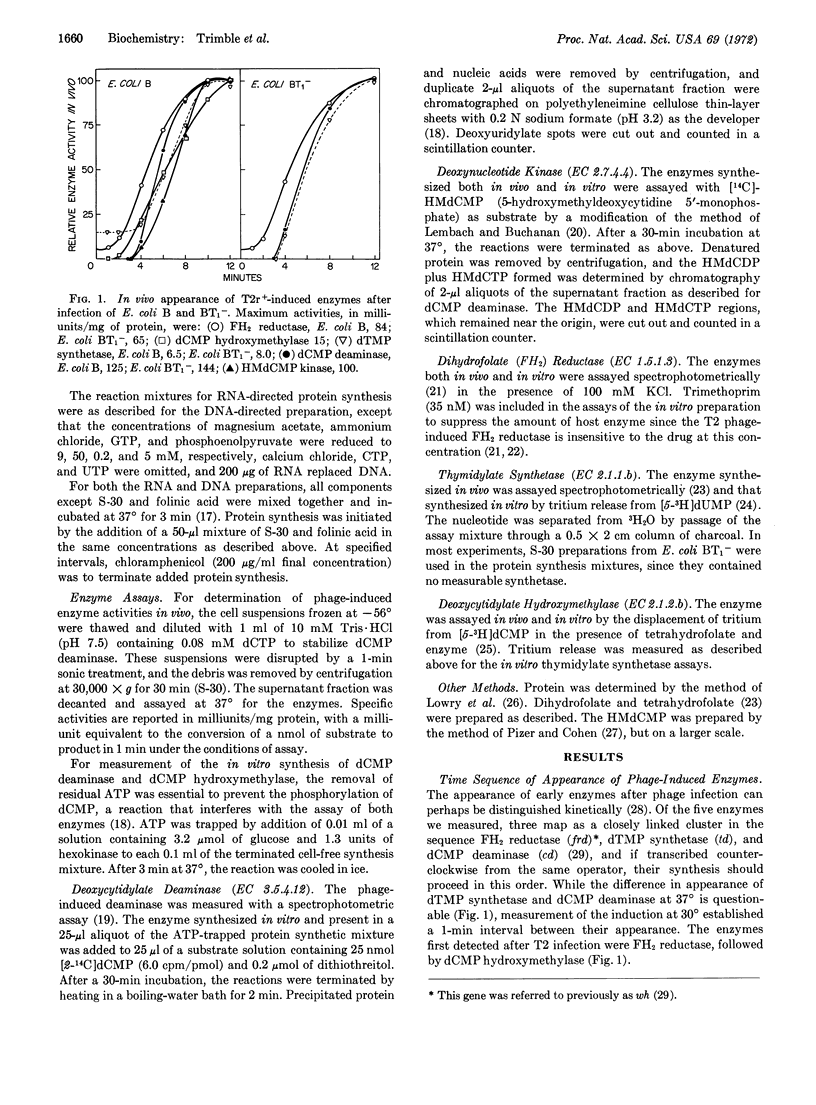
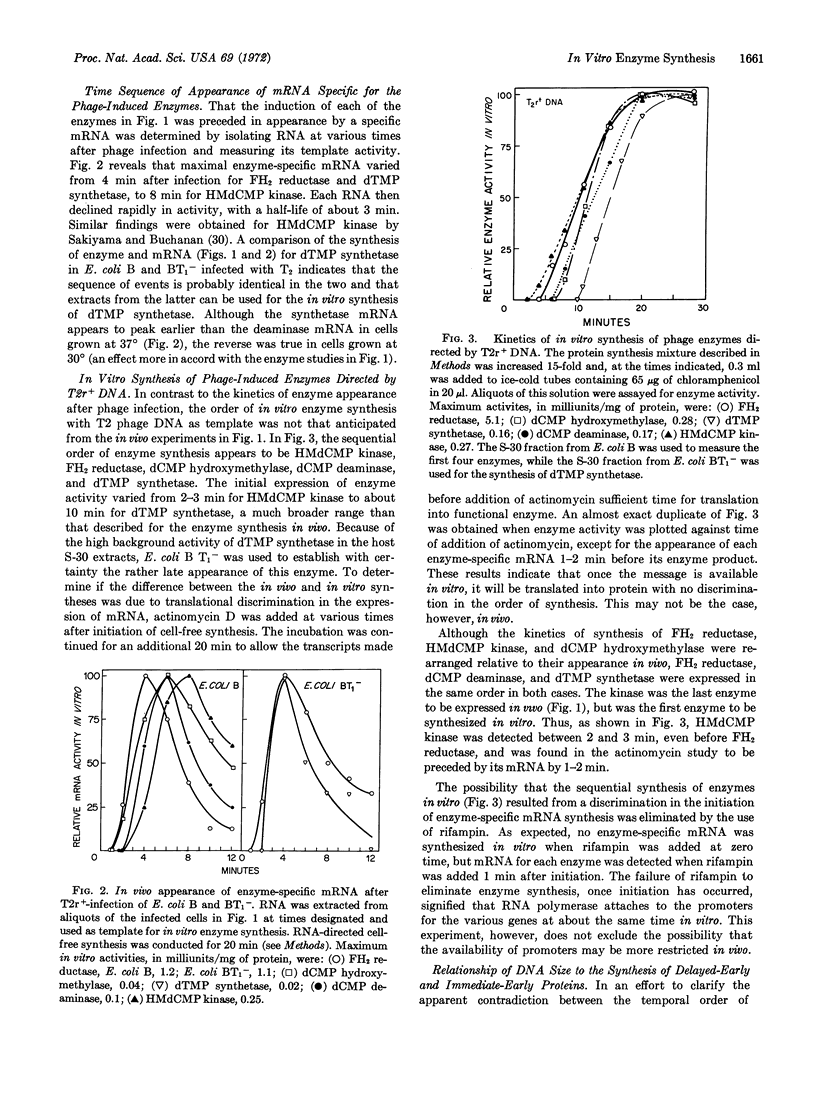
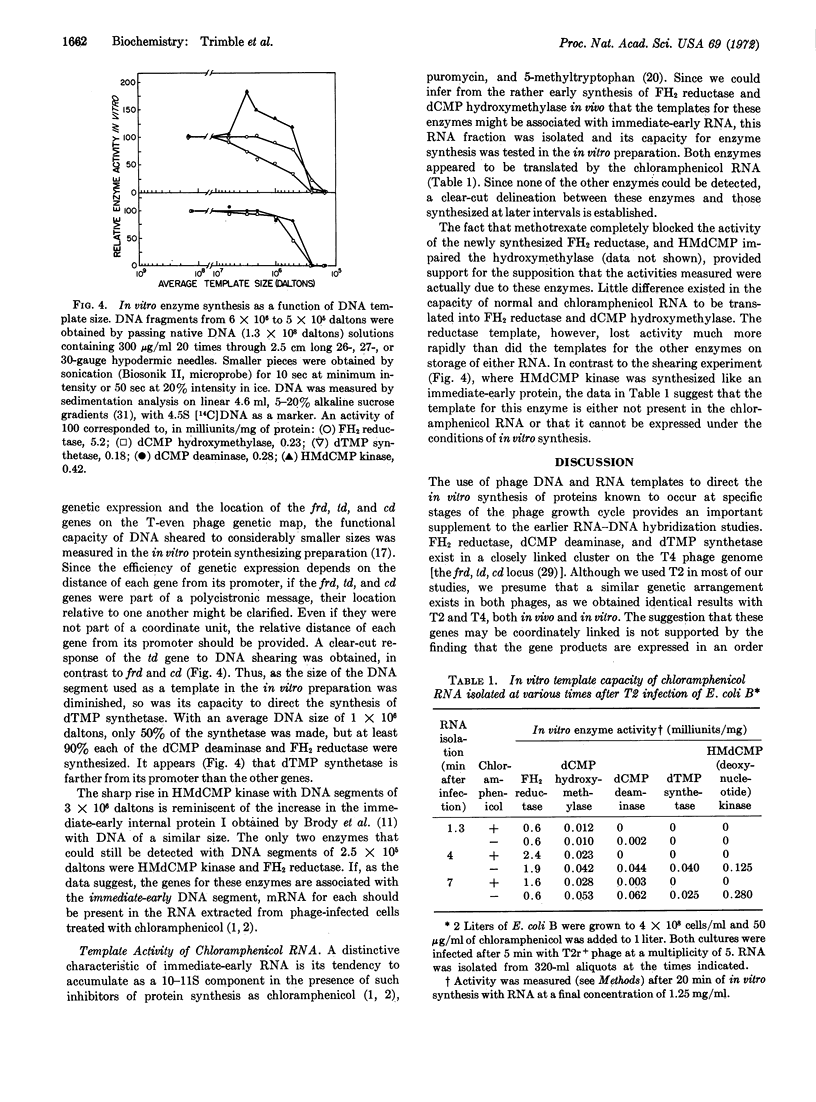
The kinetic order of synthesis of deoxycytidylate deaminase (EC 3.5.4.12), deoxycytidylate hydroxymethylase (EC 2.1.2.b), dihydrofolate reductase (EC 1.5.1.3), 5-hydroxymethyldeoxycytidylate kinase (EC 2.7.4.4), and thymidylate synthetase (EC 2.1.1.b) after infection of Escherichia coli with T2r+ bacteriophage was found not to correlate with their order of synthesis in an in vitro protein-synthesizing preparation. The in vivo and in vitro synthesis of enzyme-specific messenger RNA measured in the protein-synthesizing preparation preceded each enzyme by about 1 min. Through the use of sheared DNA, it was shown that the thymidylate synthetase gene was most susceptible to a loss in template activity, which suggests that this gene is further removed from its promoter than the other genes are from theirs. With a DNA segment of 2.5 × 105 daltons, the synthesis of dihydrofolate reductase alone was obtained, but at a much reduced rate. Translation of the RNA from phage-infected cells treated with chloramphenicol yielded amounts of dihydrofolate reductase and deoxycytidylate hydroxymethylase activities similar to those obtained with RNA from untreated infected cells. These results suggest that the chloramphenicol RNA, which consists primarily of immediate-early RNA, may contain most, if not all, of the information required for the synthesis of phage dihydrofolate reductase and deoxycytidylate hydroxymethylase.
Keywords: template, initiation, enzyme synthesis, chloramphenicol RNA
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B. R. Differential binding to the hydrophobic bonding region of T2 phage induced, Escherichia coli B, and pigeon liver dihydrofolic reductases. J Med Chem. 1967 Sep;10(5):912–917. doi: 10.1021/jm00317a035. [DOI] [PubMed] [Google Scholar]
- Bautz E. K., Bautz F. A., Dunn J. J. E. coli sigma factor: a positive control element in phage T4 development. Nature. 1969 Sep 6;223(5210):1022–1024. doi: 10.1038/2231022a0. [DOI] [PubMed] [Google Scholar]
- Black L. W. Ahmad-Zadeh C,+AHMADAAZADEH C: Internal proteins of bacteriophage T4D: their characterization and relation to head structure and assembly. J Mol Biol. 1971 Apr 14;57(1):71–92. doi: 10.1016/0022-2836(71)90120-3. [DOI] [PubMed] [Google Scholar]
- Black L. W., Gold L. M. Pre-replicative development of the bacteriophage T4: RNA and protein synthesis in vivo and in vitro. J Mol Biol. 1971 Sep 14;60(2):365–388. doi: 10.1016/0022-2836(71)90300-7. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Brody E. N., Gold L. M., Black L. W. Transcription and translation of sheared bacteriophage T4 DNA in vitro. J Mol Biol. 1971 Sep 14;60(2):389–393. doi: 10.1016/0022-2836(71)90301-9. [DOI] [PubMed] [Google Scholar]
- DeVries J. K., Zubay G. Characterization of a beta-galactosidase formed between a complementary protein and a peptide synthesized de novo. J Bacteriol. 1969 Mar;97(3):1419–1425. doi: 10.1128/jb.97.3.1419-1425.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso R. J., Buchanan J. M. Synthesis of early RNA in bacteriophage T4-infected Escherichia coli B. Nature. 1969 Nov 29;224(5222):882–885. doi: 10.1038/224882a0. [DOI] [PubMed] [Google Scholar]
- Guha A., Szybalski W., Salser W., Geiduschek E. P., Pulitzer J. F., Bolle A. Controls and polarity of transcription during bacteriophage T4 development. J Mol Biol. 1971 Jul 28;59(2):329–349. doi: 10.1016/0022-2836(71)90054-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lembach K. J., Buchanan J. M. The relationship of protein synthesis to early transcriptive events in bacteriophage T4-infected Escherichia coli B. J Biol Chem. 1970 Apr 10;245(7):1575–1587. [PubMed] [Google Scholar]
- Lomax M. I., Greenberg G. R. A new assay of thymidylate synthetase activity based on the release of tritium from deoxyuridylate-5-3-H. J Biol Chem. 1967 Jan 10;242(1):109–113. [PubMed] [Google Scholar]
- Lorenson M. Y., Maley G. F., Maley F. The purification and properties of thymidylate synthetase from chick embryo extracts. J Biol Chem. 1967 Jul 25;242(14):3332–3344. [PubMed] [Google Scholar]
- Maley G. F., Guarino D. U., Maley F. T2r + bacteriophage-induced enzymes. I. The purification and properties of deoxycytidylate deaminase. J Biol Chem. 1972 Feb 10;247(3):931–939. [PubMed] [Google Scholar]
- Maley G. F., Maley F. Regulatory properties and subunit structure of chick embryo deoxycytidylate deaminase. J Biol Chem. 1968 Sep 10;243(17):4506–4512. [PubMed] [Google Scholar]
- Mathews C. K. Evidence that bacteriophage-induced dihydrofolate reductase in a viral gene product. J Biol Chem. 1967 Sep 25;242(18):4083–4086. [PubMed] [Google Scholar]
- Milanesi G., Brody E. N., Grau O., Geiduschek E. P. Transcriptions of the bacteriophage T4 template in vitro: separation of "delayed early" from "immediate early" transcription. Proc Natl Acad Sci U S A. 1970 May;66(1):181–188. doi: 10.1073/pnas.66.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I., COHEN S. S. Virus-induced acquisition of metabolic function. V. Purification and properties of the deoxycytidylate hydroxymethylase and studies on its origin. J Biol Chem. 1962 Apr;237:1251–1259. [PubMed] [Google Scholar]
- Peterson R. F., Cohen P. S., Ennis H. L. Properties of phage T4 messenger RNA synthesized in the absence of protein synthesis. Virology. 1972 Apr;48(1):201–206. doi: 10.1016/0042-6822(72)90127-4. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sakiyama S., Buchanan J. M. In vitro synthesis of deoxynucleotide kinase programmed by bacteriophage "T4-RNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1376–1380. doi: 10.1073/pnas.68.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W., Bolle A., Epstein R. Transcription during bacteriophage T4 development: a demonstration that distinct subclasses of the "early" RNA appear at different times and that some are "turned off" at late times. J Mol Biol. 1970 Apr 28;49(2):271–295. doi: 10.1016/0022-2836(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Schachner M., Seifert W., Zillig W. A correlation of changes in host and T 4 bacteriophage specific RNA synthesis with changes of DNA-dependent RNA polymerase in Escherichia coli infected with bacteriophage T 4 . Eur J Biochem. 1971 Oct 26;22(4):520–528. doi: 10.1111/j.1432-1033.1971.tb01572.x. [DOI] [PubMed] [Google Scholar]
- Schachner M., Zillig W. Fingerprint maps of tryptic peptides from subunits of Escherichia coli and T 4 -modified DNA-dependent RNA polymerases. Eur J Biochem. 1971 Oct 26;22(4):513–519. doi: 10.1111/j.1432-1033.1971.tb01571.x. [DOI] [PubMed] [Google Scholar]
- Schweiger M., Gold L. M. Escherichia coli and Bacillus subtilis phage deoxyribonucleic acid-directed deoxycytidylate deaminase synthesis in Escherichia coli extracts. J Biol Chem. 1970 Oct 10;245(19):5022–5025. [PubMed] [Google Scholar]
- Travers A. A. Bacteriophage sigma factor for RNA polymerase. Nature. 1969 Sep 13;223(5211):1107–1110. doi: 10.1038/2231107a0. [DOI] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Lewis N. The synthesis of deoxycytidylate deaminase and dihydrofolate reductase and its control in Escherichia coli infected with bacteriophage T4 and T-4 amber mutants. Virology. 1966 May;29(1):172–175. doi: 10.1016/0042-6822(66)90208-x. [DOI] [PubMed] [Google Scholar]
- Wilhelm J. M., Haselkorn R. In vitro synthesis of T4 proteins: the products of genes 9, 18, 19, 23, 24, and 38. Virology. 1971 Jan;43(1):198–208. doi: 10.1016/0042-6822(71)90237-6. [DOI] [PubMed] [Google Scholar]
- Witmer H. J. In vitro transcription of T4 deoxyribonucleic acid by Escherichia coli ribonucleic acid polymerase. Sequential transcription of immediate early and delayed early cistrons in the absence of the release factor, rho. J Biol Chem. 1971 Sep 10;246(17):5220–5227. [PubMed] [Google Scholar]
- Yeh Y. C., Dubovi E. J., Tessman I. Control of pyrimidine biosynthesis by phage T4: mutants unable to catalyze the reduction of cytidine diphosphate. Virology. 1969 Apr;37(4):615–623. doi: 10.1016/0042-6822(69)90279-7. [DOI] [PubMed] [Google Scholar]
- Yeh Y. C., Greenberg G. R. Tetrahydrofolate-dependent labilization of the hydrogen atom on carbon 5 of 5'-deoxycytidylate, a step in the deoxycytidylate hydroxymethylase reaction. J Biol Chem. 1967 Mar 25;242(6):1307–1313. [PubMed] [Google Scholar]



