Abstract
In Parkinson's disease, the long-term use of dopamine replacing agents is associated with the development of motor complications; therefore, there is a need for non-dopaminergic drugs. This study evaluated the potential therapeutic impact of six different NR2B and A2A receptor antagonists given either alone or in combination in unilateral 6-OHDA-lesioned rats without (monotherapy) or with (add-on therapy) the co-administration of L-Dopa: Sch-58261+ Merck 22; Sch-58261+Co-101244; Preladenant + Merck 22; Preladenant + Radiprodil; Tozadenant + Radiprodil; Istradefylline + Co-101244. Animals given monotherapy were assessed on distance traveled and rearing, whereas those given add-on therapy were assessed on contralateral rotations. Three-way mixed ANOVA were conducted to assess the main effect of each drug separately and to determine whether any interaction between two drugs was additive or synergistic. Additional post hoc analyses were conducted to compare the effect of the combination with the effect of the drugs alone. Motor activity improved significantly and was sustained for longer when the drugs were given in combination than when administered separately at the same dose. Similarly, when tested as add-on treatment to L-Dopa, the combinations resulted in higher levels of contralateral rotation in comparison to the single drugs. Of special interest, the activity observed with some combinations could not be described by a simplistic additive effect and involved more subtle synergistic pharmacological interactions. The combined administration of A2A/NR2B-receptor antagonists improved motor behaviour in 6-OHDA rats. Given the proven translatability of this model such a combination may be expected to be effective in improving motor symptoms in patients.
Introduction
The progressive loss of dopaminergic neurons from the substantia nigra pars compacta (SNc) leads to striatal dopamine (DA) deficiency and the emergence of the cardinal motor symptoms of Parkinson's disease (PD): bradykinesia, resting tremor, rigidity and postural instability [1]. While DA replacement therapy is the gold standard for treating patients with PD, the use of L-Dopa or DA agonists is associated with motor complications such as dyskinesia, dystonia, wearing-off and on/off phenomenon [2]–[4].
The emergence of significant motor complications associated with dopaminergic agents and the fact that such side-effects can become severely disabling highlights the need to develop innovative therapies able to circumvent the severe complications associated with deleterious neuro-adaptations resulting from dopaminergic neurodegeneration and pulsatile dopaminergic therapy [5], [6]. As direct modulation of the dopaminergic system eventually leads to serious side effects and, in the long term, becomes ineffective, significant effort has been invested to find non-dopaminergic targets. Two targets which have shown great promise in preclinical disease models are the adenosine A2A receptor and the NR2B subunit of the NMDA receptor.
Adenosine 2A (A2A) receptors are abundant in the striatum, of both rodent and human brains [7] and are specifically expressed in GABAergic striatopallidal neurons (i.e. indirect output pathway) [8]. Within these neurons they co-localize with dopamine D2 receptors [9] and are able to form A2A-D2 heterodimeric complexes [10]. Mechanistically, activation of the GS coupled A2A receptors will antagonize signaling of the Gi coupled D2 receptor at the level of cAMP, while stimulation of the A2A receptor reduces the ability of dopamine to bind to the D2 receptor by means of an intra-membrane A2A–D2 receptor interactions [11]. The observation that A2A receptors functionally oppose the actions of D2 receptors on GABAergic striatopallidal neurons, led to the hypothesis that A2A antagonists could enhance the activity of dopaminergic agents in alleviating parkinsonian motor symptoms [12] and also act by themselves to reduce the over-activity of the indirect pathway and the severe motor inhibition associated with it [13]. In rodent or primate models, when A2A antagonists are given alone (i.e. as monotherapy) to severely DA-depleted animals they show only marginal activity [14]–[16], however, they are able to significantly potentiate dopaminergic treatment [17]–[21]. In the clinic, when the A2A antagonist Istradefylline was given as monotherapy (i.e. without L-Dopa) to de- novo PD patients, it did not produce statistically significant benefits [22]. However, when combined with L-Dopa, Istradefylline, and other A2A antagonists, demonstrated significant efficacy [23]–[25]. In fact, Istradefylline is now approved in Japan as add-on treatment to L-Dopa because of its ability to counteract wearing-off phenomena in fluctuating PD patients [26].
Striatal dopamine depletion is also associated with over activation of the glutamatergic NMDA receptors [27]. A number of studies have examined the efficacy of NMDA antagonists in animal models of PD. These studies showed that NMDA receptor blockade alleviates the parkinsonian motor symptoms, augments the effectiveness of dopaminergic therapy and can even prevent or reverse the induction of involuntary movements induced by L-Dopa [28], [29]. However, non-selective NMDA receptor antagonists have limited therapeutic value due to mechanism based side-effects. Accordingly, the modulation of specific receptor subtypes might provide a better alternative to modulate glutamatergic input to the basal ganglia [28]. In particular, NR2B receptor antagonists have been proposed as promising alternatives for the treatment of the motor symptoms of PD [30]–[32] and have been shown to be effective in alleviating experimental parkinsonism in both rodent and non-human primate models of PD [33]–[36]. NR2B antagonists have been shown to potentiate the therapeutic effect of L-Dopa [34], [37], [38] and to be effective in reducing L-Dopa-induced dyskinesia [33], [39]. In both rat and human brains, NR2B receptors are expressed throughout the brain, with high expression in the cortex, hippocampus, striatum, thalamus and olfactory bulb [31]. In situ hybridization studies identified intense NR2B signals in all striatal neuronal populations in human brains [40]. While the selective NR2B antagonist, CP-101,606 demonstrated efficacy in counteracting L-Dopa-induced dyskinesia in a randomized double-blind placebo-controlled clinical trial [41], a single clinical study with the selective MK-0657 gave negative results when administered as monotherapy [42].
There is an increasing body of evidence which suggests that the NMDA and A2A receptors interact, at least, within the striatum. Indeed, it has been suggested that these two receptors share a common intracellular signaling pathway, whereby NMDA receptor signaling increases the activity of the A2A receptors [43]. Therefore the efficacy of an A2A antagonist should be enhanced by the co-inhibition of the NMDA receptor. Interestingly, activation of the Gs coupled A2A receptor will increase protein kinase A (PKA), and PKA is known to increase the functionality of the NMDA receptor [44]–[46]. Therefore, within the striatum, the A2A and NMDA receptors may act to mutually stimulate each other, suggesting that inhibiting both receptors could provide significant benefit.
The objective of this study was to explore the potential therapeutic benefit of combining A2A and NR2B antagonists for the treatment of the motor symptoms of PD. The efficacy of six different combinations was assessed in a classic preclinical model of PD, i.e. the unilateral 6-OHDA-lesioned rat model [47], [48]. To explore the effect of this new potential treatment on motor symptoms, A2A/NR2B receptor antagonist combinations were tested without L-Dopa (monotherapy) and as adjunctive treatment with a low active dose of L-Dopa (add-on therapy). The A2A and NR2B receptor antagonist drugs used were selected on the basis of their specific selectivity and affinity for the two respective receptors (see Table 1 and Table 2 ). To explain superior level of motor activity, statistical analysis, three-way mixed ANOVA, first, aimed at investigating the overall effect of the A2A and NR2B receptor antagonists and second, explored the effect of their interaction. The nature of the interaction, whether additive or synergistic was evaluated and any pharmacokinetic interaction using two combinations as examples were ruled out.
Table 1. Adenosine A2A antagonists and their affinity for the receptor: in vitro binding.
| A2A antagonists | Pharmaceutical Company | Ki a A2A | Ki A1 | Ki A2B | Ki A3 | References |
| Istradefylline | Kyowa Hakko Kirin Co Ltd | 12 | 841 | >10,000 | 4,470 | [71] |
| Sch-58261 | Schering-Plough | 0.6 | 287 | 5,011 | >10,000 | [71], [72] |
| Merck & Co Inc | 5 | 725 | 1110 | 1200 | ||
| Preladenant | Merck & Co Inc | 1.1 | >1000 | >1,700 | >1,000 | [73] [71] |
| 0.9 | >1000 | >1000 | ||||
| Tozadenant | Biotie Therapies | 4.9 | 1,320 | ND | ND | [71] |
Ki is expressed in nM (rat or human).
ND: no data available.
Table 2. NR2B antagonists and their affinity for the receptor: in vitro and functional binding.
| NR2B antagonists | Pharmaceutical Company | NR2B | NR2A | NR2C | Assays | References |
| Merck 22 | Merck & Co Inc | Ki = 0.88 nM | ND | ND | In vitro Binding | [49] |
| Radiprodil | Gedeon Richter | IC50 = 3–10 nM | IC50>10 µM | ND | In vitro Binding | [69] |
| UCB | ||||||
| Co-101,244 | Parke-Davis | IC50 = 0.026 µM | IC50 = 230 µM | IC50 = 590 µM | TEVC recording | [33], [74] |
| Pfizer | IC50 = 0.043 µM | IC50>100 µM | IC50>100 µM | |||
| IC50 = 4 nM | In vitro binding | [75] |
ND: no data available.
Materials and Methods
Animals and ethic statement
All animal experiments were performed according to the Helsinki declaration and conducted in accordance with the guidelines of the European Community Council directive 86/609/EEC. The ethical committee from UCB BioPharma SPRL (LA1220040 and LA2220363) approved the experimental protocols. Male Sprague-Dawley rats (Janvier, France) were housed in cages (4 rats per cage) for one week before experimentation. They were kept on a 12∶12 light/dark cycle with light on at 06:00 h and at a temperature maintained at 20–21°C and at humidity of approximately 40%. All animals had free access to standard pellet food and water before assignment to experimental groups. The animals weighed 250–275 g at the time of surgery and 400–450 g at the time of drug testing. Additional enrichment and welfare were provided (Enviro-dri, PharmaServ) before and after the surgery. Animal health was monitored daily by the animal care staff. Surgeries were performed under ketamine and xylazine or under isoflurane anesthesia, and all efforts were made to minimize suffering. Sacrifice were done with CO2 or when necessary by exsanguination.
6-OHDA lesion
To protect norepinephrinergic neurons, animals were administered imipramine HCl (Sigma) 15 minutes before surgery. They were subsequently anesthetized with ketamine (Ceva, 75 mg/kg) and xylazine (Bayer, 10 mg/kg) and placed in stereotaxic frame (David Kopf Instrument). 6-OHDA was injected into the right ascending medial forebrain bundle at the following coordinates (in mm) relative to bregma and surface of the dura, AP = −3.5, ML = −1.5, DV = −8.7. Each rat received one injection of 6-OHDA (4 µg/µl) over a period of 5 minutes (0.5 µl/min) for a total of 10 µg per rat. Animals were monitored for 3 weeks to ensure full recovery and habituation to the environment and experimenters.
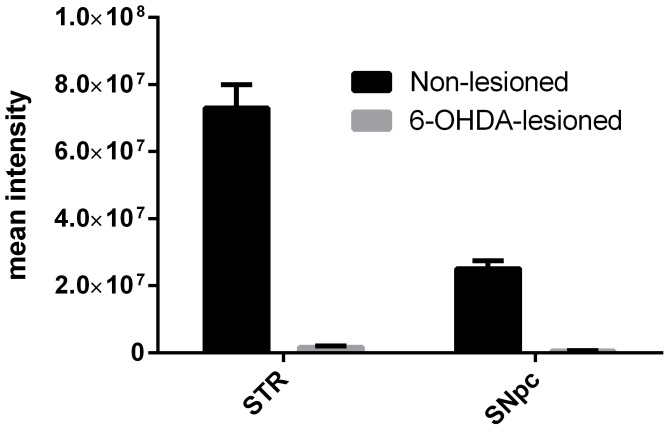
On day 21 post surgery, all rats were challenged with a small dose of subcutaneously administered apomorphine (Sigma, 0.05 mg/kg). Rats showing more than 90 contralateral rotations (360°) over a 45-minute recording period were included in the study. Rats meeting this criteria have a unilateral loss of dopaminergic neurons and a unilateral depletion of striatal DA of over 95% and this was demonstrated with the vehicle-treated 6-OHDA-lesioned rats which showed strong degeneration of the right dopaminergic nigrostriatal system. Internal quantification of vehicle-treated controls groups demonstrated a loss of 98% of TH immunostaining within both the striatum and the SNc ( Fig. 1 ). For each experiment, rats were allocated to the different experimental groups in order to get the same mean level of basal activity measured at the apomorphine challenge amongst the different experimental groups.
Figure 1. Quantification of TH and NeuN in the dopaminergic system after 6-OHDA lesioning.
Intensity of TH and NeuN positive immunostaining of the substantia nigra pars compacta (left) and striatum (right) in rats (n = 24) following administration of 10 µg of 6-OHDA in the medial forebrain bundle of the right hemisphere. Immunostaining was performed in rats having received vehicle treatment only.
Drugs
A2A and NR2B antagonist reference compounds were dissolved in a volume of 5 ml of vehicle per kg. L-Dopa methyl ester (Sigma) was dissolved in physiological saline solution at a volume of 5 ml/kg. The vehicle solution of reference compounds was made of 5% dimethyl sulfoxide (DMSO) and 95% distilled water containing 1% methyl cellulose. 6-OHDA-HBr (Sigma) was dissolved in a 0.02% ascorbic acid -distilled water at a concentration of 4 µg 6-OHDA per µl.
All compounds were obtained from commercially available standard suppliers with the exception of compound ‘Merck 22’, which was synthesized based on methodology described by [49]. The A2A antagonists were Sch-58261 (Synthelabo), Preladenant (Endotherm), Tozadenant (Pharmablock) and Istradefylline (Diverchim). The NR2B antagonists were Radiprodil (Axon), Co-101244 (Key Organics) and Merck 22 (UCB synthesis).
Behavioural recording apparatus
General activity in actometers (distance and rearing)
Drug-naïve separate rats were tested individually in clear acrylic chambers (LE8811 IR, Panlab). Locomotion was detected and measured by 16 infrared light-beam sensors located on each side of the enclosure at a height of 3 cm. Sensors were spaced such that the light beams formed a matrix of 15×15 squares over the surface. Activity counts were recorded on a computer and Acti-Track software (Actitrack, Panlab) was used to convert raw data into “distance traveled” and “rearing counts” for analysis as relevant dependent variables.
Rotational activity in rotometers (ipsi/contralateral rotations)
The rats tested one week earlier for general activity (actometers) were once again placed in clear acrylic individual chambers (50×45 cm) with the same treatment (drug and dose) than that previously received plus L-Dopa. Eight chambers were placed on a level, clear glass bottom, held by a robust frame, and were subdivided into four individual test cages of 25×22 cm. A removable acrylic plate served as a lid.
Rotational behaviour was recorded using a home-made computerized system. Rats were fixed in a harness and linked to mechanical sensors connected directly to a computer. Each 360° clock-wise or counter clock-wise turn was automatically recorded for up to 120 minutes at the maximum. Throughout the experiments, rats were allocated to individual test cages.
Pharmacokinetics
Satellite lesioned rats (400–450 g) were anesthetized with isoflurane and sacrificed by exsanguination. Blood samples were collected in EDTA centrifuged 10 min at 3000 rpm at 4°C to obtain plasma and stored at −20°C prior the analysis. Brains were perfused by saline collected and immediately stored at −20°C prior the analysis. Brain samples were added to 4 volumes of water before being homogenized. A structural analog compound was added as internal standard to the samples and proteins were crashed using 8 volumes of acetonitrile. Compounds were analyzed on an API5000 mass spectrometer (ABSciex, Framingham, MA, USA.) equipped with a turbo ion spray source and interfaced with an Agilent 1290 Infinity LC system (Agilent Technologies, Waldbroon, Germany). The mass spectrometer was operated in the positive ionization mode with Multiple Reactions Monitoring used to quantify the various analytes (407 to 292 for Tozadenant; 398 to 109 for Radiprodil, 346 to 105 for Sch-58261 and 342 to 100 for Co-101244). A Waters HSS T3 column (2.1×30 mm, 1.8 µm) was used. The mobile phase consisted of 100% water with 0.1% formic acid (phase A) and 100% acetonitrile with 0.1% formic acid (phase B). The flow rate was 1 mL/min. A linear gradient was performed from 0 to 1 min (95 to 30% phase A), a jump to 90% B in 0.01 min, a hold period of 1.29 min at 90% phase B followed by equilibration at the initial condition was applied before the following injection.
Experimental design
NR2B and A2A receptor antagonist combinations as ‘monotherapy’
Six different combinations of A2A/NR2B antagonists were tested without L-Dopa. For each combination, there were four different procedures: vehicle only; A2A antagonist followed by vehicle; vehicle followed by the NR2B antagonist and A2A antagonist followed by the NR2B antagonist. The drugs, doses and combinations are described in Table 3. After drug administration, rats were placed in actometers (open-field) for monitoring the general level of motor activity (distance traveled and rearing counts). The hemilesioned rats were assessed for motor recovery in these apparatus and not in the usual rotometers (i.e. monitoring the contralateral recordings), because the behaviour displayed under the A2A/NR2B combination was entirely devoid of any asymmetrical bias.
Table 3. A2A and NR2B receptor antagonist combinations tested either alone or in combination in monotherapy (i.e. without L-Dopa) in 6-OHDA-lesioned rats.
| A2A receptor antagonist drugs | NR2B receptor antagonist drugs |
| Sch-58261 1 mg/kg (ip) | Merck 22 1 mg/kg (ip) |
| Sch-58261 1 mg/kg (ip) | Co-101244 1 mg/kg (ip) |
| Preladenant 0.1 mg/kg (ip) | Merck 22 0.3 mg/kg (ip) |
| Preladenant 0.1 mg/kg (ip) | Radiprodil 1 mg/kg (ip) |
| Tozadenant 30 mg/kg (po) | Radiprodil 3 mg/kg (po) |
| Istradefylline 0.3 mg/kg (ip) | Co-101244 1 mg/kg (ip) |
Before undertaking the study, each A2A and NR2B antagonist was tested individually. The doses were selected on the basis of preliminary dose-response curves performed separately for each drug and submaximal dose (i.e. response observed is not at its maximal level) of each drug was selected for the combination studies. The two drugs were administered separately via an intraperitoneal (ip) injection with a 2-minute interval between the two drugs. Five minutes after the second injection, animals were placed in the testing cage for behavioural recording. Due to formulation and exposure issues, Tozadenant and Radiprodil had to be administered orally and therefore recording was started 60 minutes post dosing at this corresponds to maximum plasma concentrations. Eight drug-naïve animals were used for each of the four procedures; 32 in total for each combination. All behavioral experiments were conducted between 8.00 AM and 2.00 PM at the latest. Preliminary pharmacokinetic assays investigated the plasma and brain disposition of selected drugs administered alone or in combination (ie. Tozadenant/Radiprodil, and Sch-58261/Co-101244; 3 animals per group). Tozadenant and Radiprodil were measured in samples obtained 60 minutes after single oral administration at 30 mg/kg. Sch-58261 and Co-101244 were measured in samples collected 90 min after single intraperitoneal administration at 3 mg/kg.
NR2B and A2A receptor antagonist combinations as ‘add-on therapy’ to L-Dopa
Four different combinations were administered as add-on treatment to a low active dose of L-Dopa (25 mg/kg, ip) without any dopa decarboxylase inhibitor to avoid any additional pharmacokinetic interaction. The doses of the compounds were selected on the basis of preliminary dose-response curves performed separately for each drug. To avoid ceiling effect with L-Dopa, only submaximal doses of each drug were selected for testing (as it was done previously for monotherapy). Eight L-Dopa naïve animals were tested for each of the four pharmacological condition: vehicle and L-Dopa (25 mg/kg); A2A antagonist followed by vehicle and L-Dopa; vehicle followed by NR2B antagonist and L-Dopa; A2A antagonist followed by NR2B and L-Dopa. Due to the dominance of the L-Dopa-effect on the lesioned striatum, the unilateral 6-OHDA-lesioned rats rotated after the drugs administration and behavioral activity was recorded in rotometers (i.e. level of contralateral rotations).
All drugs were administered i.p. 25 minutes before behavioural recording, except for the Tozadenant and Radiprodil combination which was administered orally 60 minutes before behavioural recording. As Radiprodil was administered orally, L-Dopa was given 45 minutes after the Radiprodil injection whereas for the other combinations L-Dopa was given 15 minutes after the NR2B drug. The drugs, the doses and the combinations given in co-administration to L-Dopa are described in Table 4 .
Table 4. A2A and NR2B receptor antagonist combinations tested either alone or in combination as add-on therapy to L-Dopa in 6-OHDA-lesioned rats.
| A2A receptor antagonist drugs | NR2B receptor antagonist drugs |
| Sch-58261 0.3 mg/kg (ip) | Co-101244 1 mg/kg (ip) |
| Preladenant 0.03 mg/kg (ip) | Radiprodil 0.3 mg/kg (ip) |
| Preladenant 0.03 mg/kg (ip) | Merck 22 1 mg/kg (ip) |
| Tozadenant 30 mg/kg (po) | Radiprodil 3 mg/kg (po) |
All behavioral experiments were conducted between 8.00 AM and 2.00 PM at the latest.
Statistics
The effects of A2A and NR2B antagonists on the level of motor activity (i.e. distance traveled and rearing counts) were assessed with three-way mixed ANOVA, combining the A2A receptor antagonist (2 levels, vehicle and the A2A drug) and the NR2B antagonist (2 levels, vehicle and the NR2B drug) as between-group factors with the time as within-subjects factor (6 or 9 levels). The interaction between the two drugs was carefully explored, including the time effect, in order to assess whether the interaction was additive (i.e. non-significant) or synergistic (i.e. significant) to explain the increased motor activity observed overtime. To determine whether administration of the combination had any advantage over administration of single drugs, additional post hoc tests were conducted taking into account the two independent factors only. The multiple pairwise comparisons among the four different means were performed by Newman-Keuls post hoc test.
The impact of treatment with A2A or NR2B receptor antagonists, given alone or in combination, on the effect of L-Dopa on contralateral rotations was also analysed with a three-way mixed ANOVA. The ANOVA included the A2A and the NR2B receptor antagonists (both, 2 levels, vehicle and the drug) as between-group factors combined with time (12 levels) as within-subjects factor. Here again, the effect of the interaction between both drugs was carefully examined including the time effect to determine whether the effects observed on L-Dopa resulted from an additive (non-significant interaction between A2A and NR2B drug) or a synergistic effect (significant interaction). The ability of the combination to increase and prolong the activity of L-Dopa was also explored. Consequently, drug effects were analyzed for every 10-min time interval. The reliability of the between-mean differences within the time intervals was assessed with planned contrasts using an F statistic. Data are expressed as averages +/- standard error of the mean. Statistical analyses were performed using the Statistica software (StatSoft Inc., OK, USA). For each test, statistical significance was assumed if P<0.05.
For every statistical analysis, a synergistic effect was concluded for the A2A/NR2B the combination if (1) a significant effect of the “A2A xNR2B” statistical interaction was measured and, (2) if a superior effect of the group treated with the combination over the groups treated with the drugs alone was demonstrated.
Results
Tozadenant and Radiprodil exposure
Tozadenant and Radiprodil demonstrated similar plasma and brain exposure whether administered alone or in combination ( Table 5 ). The absence of a pharmacokinetic interaction was also demonstrated for Sch-58261 and Co-101244 combination ( Table 6 ). Based on literature data, the other combinations were considered unlikely to produce pharmacokinetic interactions and were thus not further assessed.
Table 5. Plasma and brain exposure of Tozadenant and Radiprodil administered alone or in combination.
| Sample | Dosing regimena | Tozadenant (µg/mL) | Radiprodil (µg/mL) |
| Plasma | Tozadenant | 6.05±0.95 | - |
| Radiprodil | - | 4.96±0.85 | |
| Tozadenant + Radiprodil | 5.04±0.14 | 5.59±2.93 | |
| Brainb | Tozadenant | 0.18±0.04 | - |
| Radiprodil | - | 2.63±0.56 | |
| Tozadenant + Radiprodil | 0.11±0.04 | 2.59±2.48 |
Results expressed as mean ± standard deviation of three animals.
Both compounds were delivered orally at 30 mg/kg with samples obtained 60 min after dosing.
Concentrations expressed per g tissue.
Table 6. Plasma and brain exposure of Sch-58261 and Co-101244 administered alone or in combination.
| Sample | Dosing regimena | Sch-58261 (ng/mLb) | Co-101244 (ng/mL) |
| Plasma | Sch-58261 | 97.2±10.1 | - |
| Co-101244 | - | 54.7 (n = 2) | |
| Sch-58261+Co-101244 | 112±4.16 | 55.4±8.32 | |
| Brainb | Sch-58261 | 246±34.7 | - |
| Co-101244 | - | 443 (n = 2) | |
| Sch-58261+Co-101244 | 264±38.3 | 436±74.0 |
Results expressed as mean ± standard deviation of three animals.
Both compounds were delivered ip at 3 mg/kg with samples obtained 90 min after dosing.
Concentrations expressed per g tissue.
Impact of A2A/NR2B combinations on motor activity in 6-OHDA-lesioned rats
Six different combinations were tested as monotherapy (i.e. without L-Dopa): Sch-58261 + Co-101244; Preladenant + Radiprodil; Preladenant + Merck 22; Tozadenant + Radiprodil; Sch-58261 + Merck; Istradefylline + Co-101244.
A consistent, strong and lasting increase in motor activity (distance traveled and rearing counts) was observed in animals that received the combination compared with those that received single drugs, even though single drugs led to increased activity. The statistical results showed that both A2A and NR2B receptor antagonist drugs had a very significant effect on the level of distance traveled and rearing counts. The analysis of the interaction of the effects between the two drugs showed that, for some combinations, the effects were only additive, when no significant interaction was found between the A2A and the NR2B drugs. Whereas for other combinations, where the interaction was significant, the presence of a synergistic effect between the two compounds might be concluded.
Additional post hoc analyses, done after variance analysis, highlighted the differences between the controls (vehicle-treated rats), the rats treated with the A2A antagonist or the NR2B antagonist alone, or the rats treated with the A2A/NR2B combination. These second-step analyses clearly demonstrated that for all the combinations, the rats treated with the A2A/NR2B combination had a very significantly higher level of locomotor activity than those treated with the drug alone. These results clearly demonstrated the superiority of combination treatment in hemilesioned rats.
Effect on distance traveled
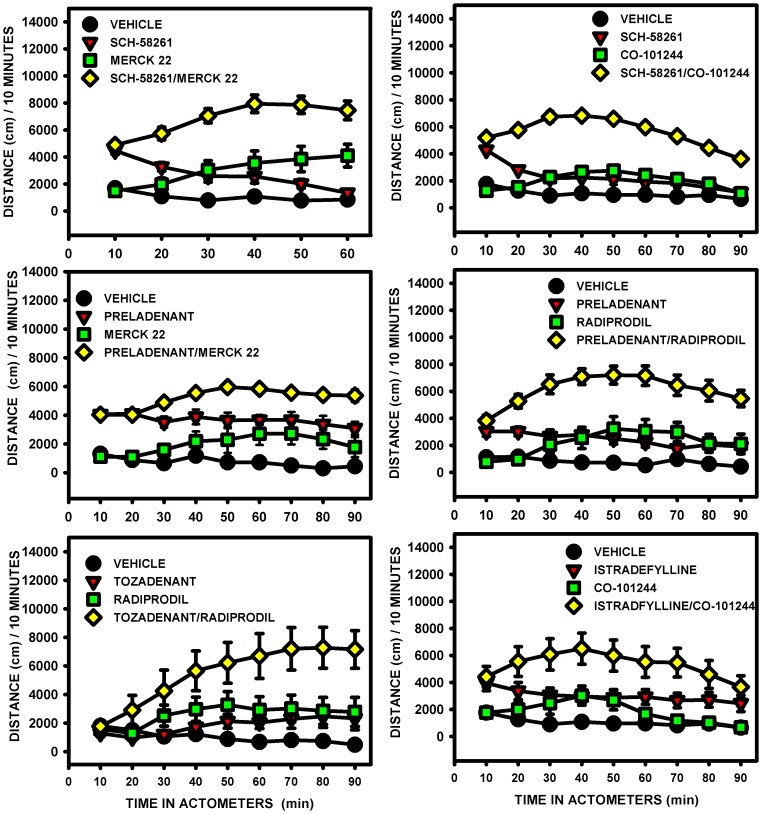
All A2A and NR2B receptor antagonists combinations significantly increased distance traveled by the animals compared with the drug administered alone at the same dose (Fig. 2). The three-way mixed ANOVA showed a significant main effect of the A2A antagonist drug, of the NR2B antagonist drug and, of time for all the combinations. For some combinations, significant “A2A×NR2B” interaction was also found: Sch-58261+ Merck 22; Sch-58261+ Co-101244; Preladenant + Radiprodil (Table 7). For these three specific combinations, the A2A and the NR2B antagonist drugs interacted in a synergistic manner whereas in the absence of statistical significance, the interaction could be considered additive.
Figure 2. Distance traveled in unilateral 6-OHDA-lesioned rats.
The effects of the A2A/NR2B receptor antagonist combinations, given without l-Dopa, were compared with those of the drugs alone for the distance traveled: Sch-58261 (1 mg/kg) + Merck 22 (1 mg/kg); Sch-58261 (1 mg/kg) + Co-101244 1(mg/kg); Preladenant (0.1 mg/kg) + Merck 22 (0.3 mg/kg); Preladenant (0.1 mg/kg) + Radiprodil (1 mg/kg); Tozadenant (30 mg/kg) + Radiprodil (3 mg/kg); Istradefylline (0.3 mg/kg) + Co-101244 (1 mg/kg). Administration of the combinations resulted in significantly greater distance traveled than administration of the drugs alone (Table 7; Three-way mixed ANOVA followed by Newman-Keuls post hoc test).
Table 7. Statistical analysis for the distance traveled following administration of A2A and NR2B receptor antagonist combinations.
| A2A antagonist | NR2B antagonist | A2A×NR2B interaction | Comment | |
| Sch-58261 | Merck 22 | Combination | Synergistic effect | |
| Drugs main effecta | p<0.001 | p<0.001 | p<0.05 | between |
| Drugs × Time effect a | Not significant | p<0.001 | p<0.05 | Sch-58261 |
| Combination vs drugs aloneb | Both p<0.001 | and | ||
| Drugs alone vs vehicleb | Both p<0.01 | Merck 22 | ||
| Sch-58261 | Co-101244 | Combination | Synergistic effect | |
| Drugs main effecta | p<0.001 | p<0.001 | p<0.001 | between |
| Drugs × Time effect a | p<0.001 | p<0.001 | Not significant | Sch-58261 |
| Combination vs drugs aloneb | Both p<0.001 | and | ||
| Drugs alone vs vehicleb | Both p<0.01 | Co-101244 | ||
| Preladenant | Merck 22 | Combination | Additive effect | |
| Drugs main effecta | p<0.001 | p<0.001 | Not significant | between |
| Drugs × Time effect a | Not significant | p<0.001 | Not significant | Preladenant |
| Combination vs drugs aloneb | Both p<0.01 | and | ||
| Drugs alone vs vehicleb | Preladenant: p<0.001 | Merck 22 | ||
| Merck 22: p<0.05 | ||||
| Preladenant | Radiprodil | Combination | Synergistic effect | |
| Drugs main effecta | p<0.001 | p<0.001 | p<0.05 | between |
| Drugs × Time effect a | Not significant | p<0.001 | Not significant | Preladenant |
| Combination vs drugs aloneb | Both p<0.001 | and | ||
| Drugs alone vs vehicleb | Both p<0.05 | Radiprodil | ||
| Tozadenant | Radiprodil | Combination | Additive effect | |
| Drugs main effecta | p<0.05 | p<0.01 | Not significant | between |
| Drugs × Time effect a | p<0.001 | p<0.001 | Not significant | Tozadenant |
| Combination vs drugs aloneb | Both p<0.01 | and | ||
| Drugs alone vs vehicleb | Not significant | Radiprodil | ||
| Istradefylline | Co-101244 | Combination | Additive effect | |
| Drugs main effecta | p<0.001 | p<0.05 | Not significant | between |
| Drugs × Time effect a | Not significant | p<0.001 | Not significant | Istradefylline |
| Combination vs drugs aloneb | Both p<0.05 | and | ||
| Drugs alone vs vehicleb | Not significant | Co-101244 |
Primary statistical analyses conducted via Three-way mixed ANOVA;
Post hoc analyses conducted via Newman-Keuls test.
Post hoc analyses were conducted to compare the difference between the mean of the total overall scores of the four groups: vehicle, A2A antagonist, NR2B antagonist and the A2A/NR2B combination. The results showed that, for all combinations, the effects of drugs when administered in combination were greater than when administered alone. However, not all drugs when administered alone improved distance traveled compared with vehicle, these included Istradefylline, Tozadenant, Radiprodil (po) and Co-101244 (in one data set only).
Effect on rearing counts
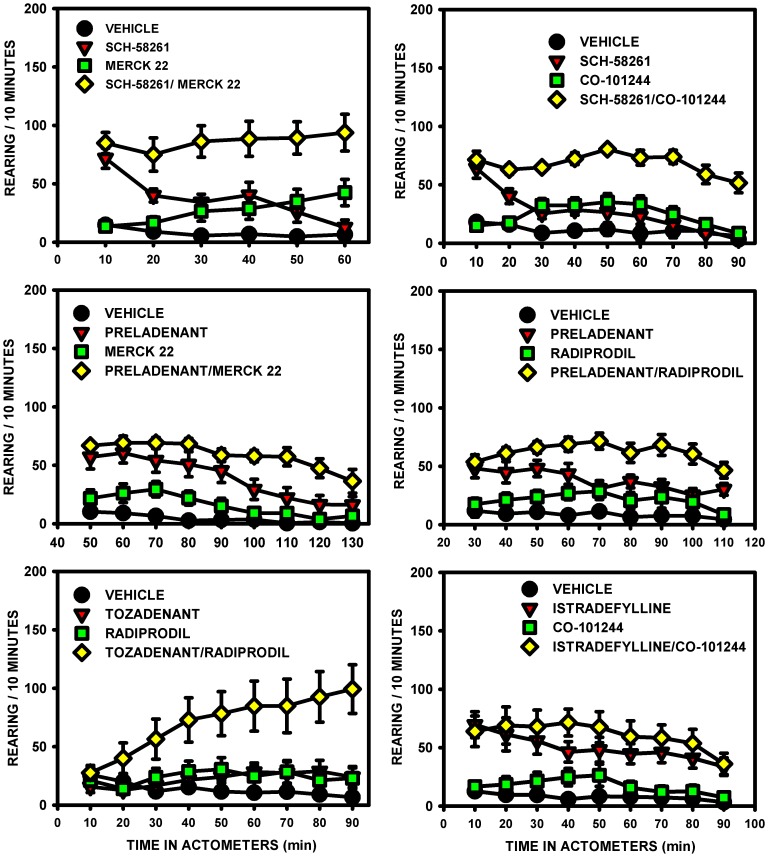
The combination of A2A and NR2B antagonist drugs was always more efficacious on the rearing counts than any individual compound administered at the same dose (Fig. 3).
Figure 3. Rearing counts in unilateral 6-OHDA-lesioned rats.
The effects of the A2A/NR2B receptor antagonist combinations, given without l-Dopa, were compared with those of the drugs alone for the number of rearing counts: Sch-58261 (1 mg/kg) + Merck 22 (1 mg/kg); Sch-58261 (1 mg/kg) + Co-101244 (1 mg/kg); Preladenant (0.1 mg/kg) + Merck 22 (0.3 mg/kg); Preladenant (0.1 mg/kg) + Radiprodil (1 mg/kg); Tozadenant (30 mg/kg) + Radiprodil (3 mg/kg); Istradefylline (0.3 mg/kg) + Co-101244 (1 mg/kg). Administration of the combinations resulted in significantly greater rearing counts than administration of the drugs alone (Table 8; Three-way mixed ANOVA followed by Newman-Keuls post hoc test).
Three-way mixed ANOVA showed significant main effect of A2A antagonists, of NR2B antagonists and, of time ( Table 8 ). However, a significant “A2A×NR2B” interaction was only found for the Sch-58261+ Co-101244 combination and for the triple “Preladenant × Merck 22× Time” interaction. Marginally significant effects of the interaction was also reported for two additional combinations: Sch-58261+ Merck 22 (p = 0.07) and Tozadenant + Radiprodil (p = 0.06). As above, lack of statistical significance indicates potential additive effects between the two drugs in increasing the level of rearing counts.
Table 8. Statistical analysis for the rearing counts following administration of A2A and NR2B receptor antagonist combinations.
| A2A antagonist | NR2B antagonist | A2A×NR2B interaction | Comments | |
| Sch-58261 | Merck 22 | Combination | Additive effect | |
| Drugs main effecta | p<0.001 | p<0.001 | Not significant (p = 0.07) | between |
| Drugs × Time effect a | p<0.001 | p<0.001 | Not significant | Sch-58261 and |
| Combination vs drugs aloneb | Both p<0.001 | Merck 22 | ||
| Drugs alone vs vehicleb | Sch-58261 p<0.05 | |||
| Merck 22, not significant | ||||
| Sch-58261 | Co-101244 | Combination | Synergistic effect | |
| Drugs main effecta | p<0.001 | p<0.001 | p<0.001 | between |
| Drugs × Time effect a | p<0.001 | p<0.001 | p<0.05 | Sch-58261 |
| Combination vs drugs aloneb | Both p<0.001 | and Co-101244 | ||
| Drugs alone vs vehicleb | Both p<0.05 | |||
| Preladenant | Merck 22 | Combination | Synergistic effect | |
| Drugs main effecta | p<0.001 | p<0.01 | Not significant | between |
| Drugs × Time effect a | p<0.001 | Not significant | p<0.01 | Preladenant |
| Combination vs drugs aloneb | Both p<0.01 | and Merck 22 | ||
| Drugs alone vs vehicleb | Preladenant p<0.001 | when | ||
| Merck 22, not significant | time included | |||
| Preladenant | Radiprodil | Combination | Additive effect | |
| Drugs main effecta | p<0.001 | p<0.001 | Not significant | between |
| Drugs × Time effect a | Not significant | p<0.01 | Not significant | Preladenant |
| Combination vs drugs aloneb | Both p<0.01 | and Radiprodil | ||
| Drugs alone vs vehicleb | Preladenant p<0.001 | |||
| Radiprodil, not significant | ||||
| Tozadenant | Radiprodil | Combination | Additive effect | |
| Drugs main effecta | p<0.01 | p<0.01 | Not significant (p = 0.06) | between |
| Drugs × Time effect a | p<0.001 | p<0.001 | Not significant | Tozadenant |
| Combination vs drugs aloneb | Both p<0.01 | and | ||
| Drugs alone vs vehicleb | Not significant | Radiprodil | ||
| Istradefylline | Co-101244 | Combination | Additive effect | |
| Drugs main effecta | p<0.001 | Not significant | Not significant | between |
| Drugs × Time effect a | p<0.01 | p<0.01 | Not significant | Istradefylline |
| Combination vs drugs aloneb | Co-101244, p<0.01 | and | ||
| Drugs alone vs vehicleb | Istradefylline, p<0.01 | Co-101244 | ||
| Co-101244, not significant |
Primary statistical analyses conducted via Three-way mixed ANOVA;
Post hoc analyses conducted via Newman-Keuls test.
After the variance analyses, Newman-Keuls post hoc tests were conducted on the total rearing scores to compare the effects of the four different treatments: vehicle, A2A receptor antagonist drug, NR2B receptor antagonist drug and the combination. Results showed that the effects of all drugs were greater when administered in combination than when administered alone, with the exception of the “Istradefylline + Co-101244” combination, where its effect was only superior to Co-101244 but not to Istradefylline ( Table 8 ).
Results also showed that not all NR2B receptor antagonists tested alone (Merck 22, Radiprodil and Co-101244 in one set of experiment) increased the number of rearing counts in comparison to vehicle rats, whereas all the A2A receptor antagonists did, with the exception of Tozadenant.
A2A/NR2B combination prolongs L-Dopa effect on contralateral rotations
Four different combinations were tested as add-on therapy to L-Dopa: Sch-58261+ Co-101244; Preladenant + Radiprodil; Preladenant + Merck 22; Tozadenant + Radiprodil ( Fig. 4 ). Due to the dominance of L-Dopa in the lesioned striatum, the hemilesioned rats rotated and contralateral rotations were monitored in rotometers to evaluate drug effects. To avoid the ceiling effect with L-Dopa, only a sub-maximal dose of A2A and NR2B receptor antagonists was used (doses were determined by previous dose-response investigations using the antagonists alone).
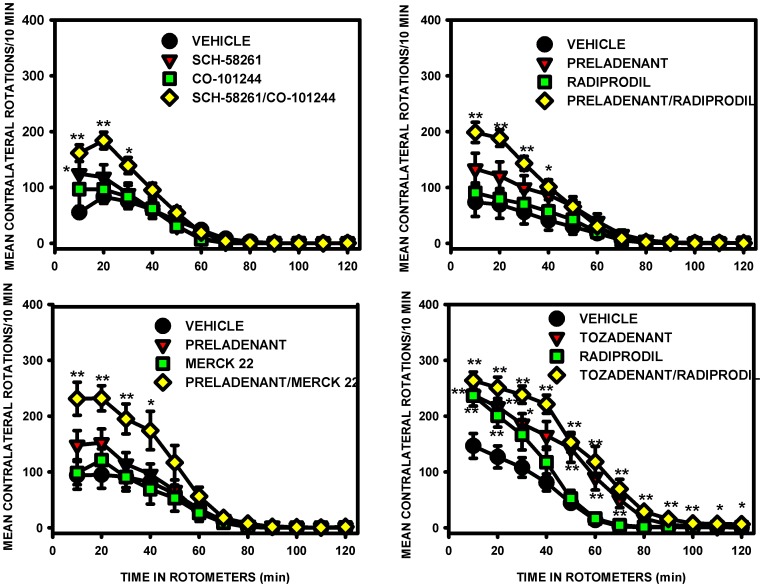
Figure 4. L-Dopa-induced contralateral rotations in unilateral 6-OHDA-lesioned rats.
The effects of the A2A/NR2B receptor antagonist combinations, given with L-Dopa, were compared with those of the antagonists alone for the number of contralateral rotations: Sch-58261 (0.3 mg/kg) + Co-101244 (1 mg/kg); Preladenant (0.03 mg/kg) + Radiprodil (0.3 mg/kg); Preladenant (0.03 mg/kg) + Merck 22 (1 mg/kg); Tozadenant (30 mg/kg) + Radiprodil (3 mg/kg). All combinations showed significant greater contralateral rotations than vehicle for the respective time intervals (Table 9; Three-way ANOVA and planned contrasts [*p<0.05 and **p<0.01 versus vehicle group]).
Three-way mixed ANOVA showed that, when given in combination with NR2B receptor antagonist, all A2A receptor antagonists showed significant overall effect on the level of contralateral rotations induced by a low active dose of L-Dopa. In contrast, with the exception of Radiprodil in combination with Tozadenant, other NR2B antagonist drugs did not show any significant main effect when administered in combination. The analysis of the “A2A× NR2B” interaction across the four different combinations did not show any significance, suggesting that the improvement observed with the two drugs administered together, corresponded to a summation of their effect on L-Dopa rather than to a synergistic phenomenon. However, a very strong overall effect of the time was observed for the four combinations and this time effect also strongly interacted with all the A2A and NR2B antagonists tested. Planned comparisons aimed at evaluating the effects of the combination with the vehicle or the effect of the drugs alone with the vehicle for each 10-min time interval. These analyses showed that the combination significantly increased and prolonged the level of L-Dopa-induced contralateral rotations compared with the drugs alone. Results also showed that the A2A or NR2B antagonist, when given separately, did not increase or prolong L-Dopa effect with the exception of Tozadenant and Radiprodil which were found to be active for 80 and 20 minutes respectively ( Table 9 ).
Table 9. Statistical analysis for the contralateral rotations following administration of A2A and NR2B receptor antagonist combinations as add-on treatment to l-Dopa.
| A2A antagonist | NR2B antagonist | A2A×NR2B interaction | Comments | |
| Sch-58261 | Co-101244 | Combination | Additive effect | |
| Drugs main effecta | p<0.05 | Not significant | Not significant | between |
| Drugs × Time effecta | p<0.001 | p<0.001 | Not significant | Sch-58261 |
| Combination vs vehicleb | p<0.05 for 30′ | And | ||
| Drugs alone vs vehicleb | Sch-58261, p<0.05 for 10′ | Co-101244 | ||
| Preladenant | Merck 22 | Combination | Additive effect | |
| Drugs main effecta | p<0.05 | Not significant | Not significant | between |
| Drugs × Time effecta | p<0.001 | p<0.01 | Not significant | Preladenant |
| Combination vs vehicleb | p<0.05 for 40′ | and | ||
| Drugs alone vs vehicleb | Not significant | Merck 22 | ||
| Preladenant | Radiprodil | Combination | Additive effect | |
| Drugs main effecta | p<0.01 | Not significant | Not significant | between |
| Drugs × Time effecta | p<0.001 | p<0.01 | Not significant | Preladenant |
| Combination vs vehicleb | p<0.05 for 40′ | and | ||
| Drugs alone vs vehicleb | Not significant | Radiprodil | ||
| Tozadenant | Radiprodil | Combination | Additive effect | |
| Drugs main effecta | p<0.001 | p<0.01 | Not significant | between |
| Drugs × Time effecta | p<0.001 | p<0.001 | Not significant | Tozadenant |
| Combination vs vehicleb | p<0.05 for 120′ | and | ||
| Drugs alone vs vehicleb | Tozadenant p<0.05 for 80′ | Radiprodil | ||
| Radiprodil p<0.05 for 20′ |
Primary statistical analyses conducted via Three-way mixed ANOVA;
A priori Planned Contrasts done on each.
10-min time interval of rotations recording.
Discussion
A2A and NR2B receptor antagonists both show anti-parkinsonian activity in preclinical models; however, the effects are weaker in magnitude compared with dopamine agonists or L-Dopa [14]–[16], [34]. A2A antagonists have also demonstrated weak efficacy in clinical trials when tested as monotherapy in PD patients [22]. Given the localization of A2A and NR2B receptors within the basal ganglia and the numerous lines of evidence which suggest that they interact on a molecular level, we wanted to test if NR2B and A2A antagonist combination treatment might indeed provide increased efficacy in PD models. The results obtained show that concomitant administration of a NR2B receptor antagonist with an A2A receptor antagonist substantially increased the quantity of motor activity in hemi-lesioned rats in comparison to the effects measured with the compounds alone. Interestingly, the observed motor activity did not consist of a rotational response as seen with dopaminergic treatment but rather evoked straight displacement in the absence of any body torsion which suggests a normalization of the movement trajectory and of the body position. Furthermore, the activity observed with some combinations could not be described by a simple additive effect and might involve more subtle pharmacodynamics synergistic effect since significant interaction was found between some A2A and the NR2B antagonist drugs.
Each of the A2A antagonists tested alone gave a moderate increase in the distance traveled by the lesioned rats although this did not always reach statistical significance. For example, rats treated with Sch-58261 and Preladenant showed significant increase of the distance traveled in comparison to vehicle-treated controls but not those treated with Tozadenant or Istradefylline. Several studies have shown the ability of A2A antagonists to ameliorate the parkinsonian motor symptoms when given as monotherapy (i.e. without L-Dopa) in preclinical models. A2A antagonists have been shown to be efficacious against haloperidol-induced catalepsy [16], [20], [50] and showed positive effects in the forepaw stepping test in unilaterally 6-OHDA lesioned rats [51] and in the rotarod test [52]. However, most studies failed to demonstrate a significant effect of A2A receptor antagonists on the level of contralateral rotation in lesioned rats in the absence of L-Dopa [14]–[16], [53]. To our knowledge, this is the first time that the efficacy of A2A receptor antagonists on motor activity has been systematically evaluated in unilaterally 6-OHDA-lesioned rats by measuring the general level of motor activity. Most remarkably, A2A antagonists not only (in some instances markedly) increased the motor activity of the animals without inducing rotation, but also, with the exception of Tozadenant, increased the level of rearing, a behaviour which is typically lost following administration of MPTP and 6-OHDA [54].
The NR2B antagonists only weakly increased motor activity, with only Merck 22, Co-101244 and Radiprodil (ip) increasing the distance traveled to a statistically significant level. NR2B receptor antagonists did not increase the level of rearing in unilateral 6-OHDA-lesioned rats with the exception of Co-101244.
These data are in line with reports in the literature showing that NR2B antagonists are able to improve some parkinsonian symptoms. However, different tests were used and to our knowledge no data exist demonstrating the activity of such a treatment in the open-field. The non-selective NMDA antagonists CPP and dizocilpine have been shown to ameliorate the stepping deficit in rats with a unilateral lesion of the medial forebrain bundle [55]. The NR2B selective antagonist Ifenprodil was shown to counteract Reserpine-induced akinesia at the highest dose tested [35]. Loschmann et al. reported the efficacy of Ro 25–6981 in inducing contralateral rotations in unilaterally 6-OHDA-lesioned rats when given as monotherapy and tested in a rotometer [34]. In agreement with this finding, we also observed increased contralateral rotations with some NR2B receptor antagonists when administered as monotherapy and tested for rotations in rotometers [56].
Careful observation of the animals in different environments led us to pursue the investigations in the open-field instead of narrow rotometers, when the drugs where given without L-Dopa (i.e. in monotherapy). By allowing the animals to move freely in a larger space, NR2B or A2A receptor antagonists were found to be more active than previously reported, with the animals showing exploratory and thigmotactic behaviour with straight movements. More importantly, as opposed to what is typically observed in unilaterally-lesioned rats receiving a dopaminergic treatment (L-Dopa or dopamine agonists), no behavioural asymmetry was observed with these drug treatments.
Although the locomotor activities induced by the individual compounds were significant in some cases, combined treatment with A2A and NR2B receptor antagonists clearly showed major additional improvement in motor function in unilateral 6-OHDA-lesioned rats. This increased efficacy might be attributed to an additive effect or to a synergistic effect using different combinations of the different compounds. In any case, the observed improvements were strong, highly reproducible and long-lasting. For every group treated with the A2A/NR2B combination, we consistently observed a significant increase in both the distance traveled and the number of rearing counts compared with vehicle-treated animals but also with animals treated with the same dose of each drug alone. In addition to the quantity of movement quantified automatically, visual observation of the rats in the activity chambers showed a surprising richness of behaviour. Furthermore, besides the strong increase of motor activity, the animals did not exhibit the typical contralateral rotations observed with stimulant drugs such as dopaminergic agents [57], [58]. They also did not show solely ipsilateral rotations as observed with dopamine transporter blockers [59], the dopamine releaser amphetamine [48] and with 5-HT1A agonists [60]. The locomotor activity was accompanied by thigmotactic behaviour, as well as centre exploration. During movements, the animals switched from one direction to the other (left to right and right to left) spontaneously and covered the entire surface of the open-field while exploring. In addition, all these behaviours were performed with an adequate quadrupedal and straight body position seemingly quite similar to normal body positioning and movement (data on file, detailed report in preparation). No abnormal involuntary or stereotypic movements were observed. These are all behavioural characteristics that had never been observed together with dopaminergic drugs. In addition, this behavioral assessment would never have been possible with the traditional measure of motor recovery, i.e.contralateral rotations, which only records the motor stimulation produced by dopaminergic drugs.
Previously, one report demonstrated that the co-administration of CGP37849, a competitive, and Dizocilpine, a non-competitive, NMDA receptor antagonist could potentiate the effects of a threshold dose of Theophylline, a non-selective adenosine receptor antagonist, in the model of haloperidol-induced catalepsy [61]. In addition, the potential benefits on the restoration of a more complex behaviour could not be monitored since the behavioural assessment only corresponded to a measure of the latency time for catalepsy.
Preliminary investigations were performed to determine whether the improvement in motor activity following administration of the combinations might be due to pharmacokinetic interactions between the two drugs. When co-administered, the respective plasma and brain levels of Tozadenant and Radiprodil were not different from those observed when the compounds were administered alone. Lack of pharmacokinetic interaction was also demonstrated for the Sch-58261 and Co-101244 combination. Although not assessed, pharmacokinetic interaction with the other combinations is unlikely based on literature data. Preladenant was reported to be devoid of in-vitro risks of drug interactions with cytochrome P-450 (CYP) and P-glycoprotein (Pgp) substrates, a finding confirmed in clinical studies [62]. Absence of in-vitro interactions with CYPs and Pgp was also reported for the NR2B antagonist Merck 22 compound [49]. Finally, in human subjects, Istradefylline does not impair CYP3A-mediated drug metabolism, its effects being restricted to modest inhibition of P-gp [63]. Overall, it is reasonable to conclude that the synergistic activity observed with some of the A2A and NR2B combinations is unlikely to result from changes in drug disposition.
To interpret the behavioural observations, one might speculate that the improvement and richness of the locomotor activity observed under the A2A and NR2B combination, including the absence of behavioral asymmetry, is due to a restoration of the balance of the dysregulated circuits of the basal ganglia. This could be due to a differential but unique stimulation of both the direct and indirect pathways by the two compounds, mediated by the specific receptor distributions and their effects at different sites of the circuits. That re-equilibration might be expected to mainly result from the direct inhibition of the overactive indirect striatopallidal pathway. However, NR2B antagonism could also modulate the glutamatergic cortical input to the striatum [29] while inhibition of pre-synaptic A2A receptors present in glutamatergic terminals of cortico-striatal afferents to the direct MSN could further reduce the release of glutamate into the striatum [64] . Dampening this activity would additionally decrease the over-activity of the indirect striatopallidal pathway. These mechanistic hypotheses strongly deserve further investigation.
In addition to the effects of the combined administration of an NR2B receptor antagonist and an A2A receptor antagonist on the locomotor activity in unilateral 6-OHDA lesioned rats, our data also showed that co-administration of the A2A/NR2B combination is able to potentiate L-Dopa efficacy (add-on therapy). Due to the dominance of L-DOPA in the basal ganglia, the animals rotated and were monitored in rotometers in contrast to the previous investigations. Surprisingly, the addition of either an A2A receptor antagonist or of an NR2B receptor antagonist alone did not increase and/or prolong the effect of L-Dopa on the level of contralateral rotation. These observations contrast with literature reports [34], [37], [65] but may reflect the fact that lower doses of A2A and NR2B antagonists were used in our study. Despite the lack of efficacy when the compounds were administered alone with L-Dopa, the combined administration of an A2A and a NR2B receptor antagonist to the same dose of L-DOPA significantly increased the magnitude of the L-Dopa effect on the level of contralateral rotations. This observation suggests that the drug effects should be translatable to the clinical condition: in that, the addition of A2A antagonists to L-DOPA has been shown to enhance the efficacy of L-DOPA as measured as a reduction in off-time. Current reviews confirm that the level of contralateral rotations and the ability of compounds to increase this parameter are a reliable index for the clinical activity of the antiparkinsonian potential of newly developed drugs [66].
In conclusion, in the present study we have demonstrated that the combined administration of an NR2B receptor antagonist with an A2A receptor antagonist in monotherapy (i.e. without L-Dopa) significantly increased both the quantity and the quality of movements in unilateral 6-OHDA-lesioned rats. Furthermore, the NR2B/A2A receptor antagonist combination also significantly potentiated the efficacy of L-Dopa when given as add-on therapy. Given the accepted translatability of the 6-OHDA model we believe that such combination therapy could have a profound effect on the motor symptoms of PD patients. Our aim was to study the effect of the combination on the motor symptoms, however, it is worth mentioning that both NR2B and A2A antagonists could modify non-motor symptoms. Indeed A2A antagonists have been proposed to improve several non-motor symptoms such as excessive daytime sleepiness [67], cognition and depression [68], while NR2B antagonists could improve depression and anxiety [69]. Furthermore, both NR2B antagonists [70] and A2A receptor antagonists [12] have been suggested to be neuro-protective through different mechanisms so, the combined use could theoretically also modify disease progression. Given the data presented here, we propose that monotherapy with a drug combining both A2A and NR2B receptor antagonist properties could provide significant benefit in early or de novo PD patients while this combination given as add-on therapy to L-DOPA could be used in advanced and fluctuating Parkinson's disease patients.
Acknowledgments
The authors warmly thank Azita Tofighy (UCB Pharma SA) and Sarah Salvage (KCL) for the critical reading of the manuscript, Sandra Tribolo (ALQAN SAS, France) for the image analysis. From UCB BioPharma the authors are very grateful to Pierre Bonnaillie for analytical assistance, Catherine De Wolf, Melina Caruso, Fabian Hustadt and Catherine Thissen for their technical assistance, Peter Colman for the critical review of the statistical analysis.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Our data are available upon request because of legal restriction, as the data cannot be published as per our UCB policy on confidentiality. Nevertheless, the excel files will be made available to readers upon direct request to anne.michel@ucb.com.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Dauer W, Przedborski S (2003) Parkinson's disease: mechanisms and models. Neuron 39:889–909. [DOI] [PubMed] [Google Scholar]
- 2. Bargiotas P, Konitsiotis S (2013) Levodopa-induced dyskinesias in Parkinson's disease: emerging treatments. NeuropsychiatrDisTreat 9:1605–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramirez-Zamora A, Molho E (2014) Treatment of motor fluctuations in Parkinson's disease: recent developments and future directions. ExpertRevNeurother 14:93–103. [DOI] [PubMed] [Google Scholar]
- 4. Gallagher DA, O'Sullivan SS, Evans AH, Lees AJ, Schrag A (2007) Pathological gambling in Parkinson's disease: risk factors and differences from dopamine dysregulation. An analysis of published case series. Mov Disord 22:1757–1763. [DOI] [PubMed] [Google Scholar]
- 5. Jenner P (2008) Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci 9:665–677. [DOI] [PubMed] [Google Scholar]
- 6. Nadjar A, Gerfen CR, Bezard E (2009) Priming for l-dopa-induced dyskinesia in Parkinson's disease: a feature inherent to the treatment or the disease? Prog Neurobiol 87:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S (2007) Adenosine A2A receptors and basal ganglia physiology. ProgNeurobiol 83:277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schiffmann SN, Jacobs O, Vanderhaeghen JJ (1991) Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem 57:1062–1067. [DOI] [PubMed] [Google Scholar]
- 9. Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, et al. (1992) Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain ResMolBrain Res 14:186–195. [DOI] [PubMed] [Google Scholar]
- 10. Fuxe K, Ferre S, Canals M, Torvinen M, Terasmaa A, et al. (2005) Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. JMolNeurosci 26:209–220. [DOI] [PubMed] [Google Scholar]
- 11. Quiroz C, Gomes C, Pak AC, Ribeiro JA, Goldberg SR, et al. (2006) Blockade of adenosine A2A receptors prevents protein phosphorylation in the striatum induced by cortical stimulation. JNeurosci 26:10808–10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M (2006) Targeting adenosine A2A receptors in Parkinson's disease. Trends Neurosci 29:647–654. [DOI] [PubMed] [Google Scholar]
- 13. Ferre S, Popoli P, Gimenez-Llort L, Rimondini R, Muller CE, et al. (2001) Adenosine/dopamine interaction: implications for the treatment of Parkinson's disease. ParkinsonismRelat Disord 7:235–241. [DOI] [PubMed] [Google Scholar]
- 14. Bibbiani F, Oh JD, Petzer JP, Castagnoli N Jr, Chen JF, et al. (2003) A2A antagonist prevents dopamine agonist-induced motor complications in animal models of Parkinson's disease. ExpNeurol 184:285–294. [DOI] [PubMed] [Google Scholar]
- 15. Pinna A, Fenu S, Morelli M (2001) Motor stimulant effects of the adenosine A(2A) receptor antagonist SCH 58261 do not develop tolerance after repeated treatments in 6-hydroxydopamine-lesioned rats. Synapse 39:233–238. [DOI] [PubMed] [Google Scholar]
- 16. Shook BC, Rassnick S, Osborne MC, Davis S, Westover L, et al. (2010) In vivo characterization of a dual adenosine A2A/A1 receptor antagonist in animal models of Parkinson's disease. JMedChem 53:8104–8115. [DOI] [PubMed] [Google Scholar]
- 17. Pinna A, Di CG, Wardas J, Morelli M (1996) Blockade of A2a adenosine receptors positively modulates turning behaviour and c-Fos expression induced by D1 agonists in dopamine-denervated rats. EurJNeurosci 8:1176–1181. [DOI] [PubMed] [Google Scholar]
- 18. Pinna A, Volpini R, Cristalli G, Morelli M (2005) New adenosine A2A receptor antagonists: actions on Parkinson's disease models. EurJPharmacol 512:157–164. [DOI] [PubMed] [Google Scholar]
- 19. Tronci E, Simola N, Borsini F, Schintu N, Frau L, et al. (2007) Characterization of the antiparkinsonian effects of the new adenosine A2A receptor antagonist ST1535: acute and subchronic studies in rats. EurJPharmacol 566:94–102. [DOI] [PubMed] [Google Scholar]
- 20. Hodgson RA, Bertorelli R, Varty GB, Lachowicz JE, Forlani A, et al. (2009) Characterization of the potent and highly selective A2A receptor antagonists preladenant and SCH 412348 [7-[2-[4-2,4-difluorophenyl]-1-piperazinyl]ethyl]-2-(2-furanyl)-7H-pyrazol o[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine] in rodent models of movement disorders and depression. J Pharmacol Exp Ther 330:294–303. [DOI] [PubMed] [Google Scholar]
- 21.Atack JR, Shook BC, Rassnick S, Jackson PF, Rhodes K, et al. (2014) JNJ-40255293, A Novel Adenosine A2A/A1 Antagonist With Efficacy In Preclinical Models of Parkinson's Disease. ACS ChemNeurosci. [DOI] [PubMed]
- 22. Fernandez HH, Greeley DR, Zweig RM, Wojcieszek J, Mori A, et al. (2010) Istradefylline as monotherapy for Parkinson disease: results of the 6002-US-051 trial. ParkinsonismRelat Disord 16:16–20. [DOI] [PubMed] [Google Scholar]
- 23. Hauser RA, Olanow CW, Kieburtz KD, Pourcher E, Docu-Axelerad A, et al. (2014) Tozadenant (SYN115) in patients with Parkinson's disease who have motor fluctuations on levodopa: a phase 2b, double-blind, randomised trial. Lancet Neurol 13:767–776. [DOI] [PubMed] [Google Scholar]
- 24. Stacy M, Silver D, Mendis T, Sutton J, Mori A, et al. (2008) A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology 70:2233–2240. [DOI] [PubMed] [Google Scholar]
- 25. Lewitt PA, Guttman M, Tetrud JW, Tuite PJ, Mori A, et al. (2008) Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson's disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). AnnNeurol 63:295–302. [DOI] [PubMed] [Google Scholar]
- 26.Co.Ltd KHK (2013) Launch of NOURIAST tablets 20 mg, in Japan, a novel antiparkinsonian agent. In: http://kyowa-kirin.com/news_releases/2013/e20130529_01.html, editors.
- 27. Blandini F, Porter RH, Greenamyre JT (1996) Glutamate and Parkinson's disease. MolNeurobiol 12:73–94. [DOI] [PubMed] [Google Scholar]
- 28. Hallett PJ, Standaert DG (2004) Rationale for and use of NMDA receptor antagonists in Parkinson's disease. Pharmacol Ther 102:155–174. [DOI] [PubMed] [Google Scholar]
- 29. Duty S (2012) Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson's disease. CNSDrugs 26:1017–1032. [DOI] [PubMed] [Google Scholar]
- 30. Marino MJ, Valenti O, Conn PJ (2003) Glutamate receptors and Parkinson's disease: opportunities for intervention. Drugs Aging 20:377–397. [DOI] [PubMed] [Google Scholar]
- 31. Loftis JM, Janowsky A (2003) The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. PharmacolTher 97:55–85. [DOI] [PubMed] [Google Scholar]
- 32. Marino MJ, Awad H, Poisik O, Wittmann M, Conn PJ (2002) Localization and physiological roles of metabotropic glutamate receptors in the direct and indirect pathways of the basal ganglia. AminoAcids 23:185–191. [DOI] [PubMed] [Google Scholar]
- 33. Blanchet PJ, Konitsiotis S, Whittemore ER, Zhou ZL, Woodward RM, et al. (1999) Differing effects of N-methyl-D-aspartate receptor subtype selective antagonists on dyskinesias in levodopa-treated 1-methyl-4-phenyl-tetrahydropyridine monkeys. J Pharmacol Exp Ther 290:1034–1040. [PubMed] [Google Scholar]
- 34. Loschmann PA, De Groote C, Smith L, Wullner U, Fischer G, et al. (2004) Antiparkinsonian activity of Ro 25–6981, a NR2B subunit specific NMDA receptor antagonist, in animal models of Parkinson's disease. Exp Neurol 187:86–93. [DOI] [PubMed] [Google Scholar]
- 35. Nash JE, Hill MP, Brotchie JM (1999) Antiparkinsonian actions of blockade of NR2B-containing NMDA receptors in the reserpine-treated rat. Exp Neurol 155:42–48. [DOI] [PubMed] [Google Scholar]
- 36. Nash JE, Fox SH, Henry B, Hill MP, Peggs D, et al. (2000) Antiparkinsonian actions of ifenprodil in the MPTP-lesioned marmoset model of Parkinson's disease. Exp Neurol 165:136–142. [DOI] [PubMed] [Google Scholar]
- 37. Steece-Collier K, Chambers LK, Jaw-Tsai SS, Menniti FS, Greenamyre JT (2000) Antiparkinsonian actions of CP-101,606, an antagonist of NR2B subunit-containing N-methyl-d-aspartate receptors. ExpNeurol 163:239–243. [DOI] [PubMed] [Google Scholar]
- 38. Nash JE, Ravenscroft P, McGuire S, Crossman AR, Menniti FS, et al. (2004) The NR2B-selective NMDA receptor antagonist CP-101,606 exacerbates L-DOPA-induced dyskinesia and provides mild potentiation of anti-parkinsonian effects of L-DOPA in the MPTP-lesioned marmoset model of Parkinson's disease. Exp Neurol 188:471–479. [DOI] [PubMed] [Google Scholar]
- 39. Wessell RH, Ahmed SM, Menniti FS, Dunbar GL, Chase TN, et al. (2004) NR2B selective NMDA receptor antagonist CP-101,606 prevents levodopa-induced motor response alterations in hemi-parkinsonian rats. Neuropharmacology 47:184–194. [DOI] [PubMed] [Google Scholar]
- 40. Kuppenbender KD, Standaert DG, Feuerstein TJ, Penney JB Jr, Young AB, et al. (2000) Expression of NMDA receptor subunit mRNAs in neurochemically identified projection and interneurons in the human striatum. J Comp Neurol 419:407–421. [DOI] [PubMed] [Google Scholar]
- 41. Nutt JG, Gunzler SA, Kirchhoff T, Hogarth P, Weaver JL, et al. (2008) Effects of a NR2B selective NMDA glutamate antagonist, CP-101,606, on dyskinesia and Parkinsonism. Mov Disord 23:1860–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Addy C, Assaid C, Hreniuk D, Stroh M, Xu Y, et al. (2009) Single-dose administration of MK-0657, an NR2B-selective NMDA antagonist, does not result in clinically meaningful improvement in motor function in patients with moderate Parkinson's disease. J Clin Pharmacol 49:856–864. [DOI] [PubMed] [Google Scholar]
- 43. Nash JE, Brotchie JM (2000) A common signaling pathway for striatal NMDA and adenosine A2a receptors: implications for the treatment of Parkinson's disease. J Neurosci 20:7782–7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aman TK, Maki BA, Ruffino TJ, Kasperek EM, Popescu GK (2014) Separate intramolecular targets for protein kinase A control of N-methyl-D-aspartate receptor gating and Ca2+ permeability. JBiolChem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, et al. (2006) Protein kinase A regulates calcium permeability of NMDA receptors. NatNeurosci 9:501–510. [DOI] [PubMed] [Google Scholar]
- 46. Chen BS, Roche KW (2007) Regulation of NMDA receptors by phosphorylation. Neuropharmacology 53:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ungerstedt U (1971) Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl 367 69–93. [DOI] [PubMed] [Google Scholar]
- 48. Ungerstedt U, Arbuthnott GW (1970) Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res 24:485–493. [DOI] [PubMed] [Google Scholar]
- 49. Layton ME, Kelly MJ III, Rodzinak KJ, Sanderson PE, Young SD, et al. (2011) Discovery of 3-substituted aminocyclopentanes as potent and orally bioavailable NR2B subtype-selective NMDA antagonists. ACS ChemNeurosci 2:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mihara T, Mihara K, Yarimizu J, Mitani Y, Matsuda R, et al. (2007) Pharmacological characterization of a novel, potent adenosine A1 and A2A receptor dual antagonist, 5-[5-amino-3-(4-fluorophenyl)pyrazin-2-yl]-1-isopropylpyridine-2(1H)-one (ASP5854), in models of Parkinson's disease and cognition. JPharmacolExpTher 323:708–719. [DOI] [PubMed] [Google Scholar]
- 51. Kelsey JE, Langelier NA, Oriel BS, Reedy C (2009) The effects of systemic, intrastriatal, and intrapallidal injections of caffeine and systemic injections of A2A and A1 antagonists on forepaw stepping in the unilateral 6-OHDA-lesioned rat. Psychopharmacology (Berl) 201:529–539. [DOI] [PubMed] [Google Scholar]
- 52. Lundblad M, Vaudano E, Cenci MA (2003) Cellular and behavioural effects of the adenosine A2a receptor antagonist KW-6002 in a rat model of l-DOPA-induced dyskinesia. J Neurochem 84:1398–1410. [DOI] [PubMed] [Google Scholar]
- 53. Acquas E, Fenu S, Loddo P, Di CG (1999) A within-subjects microdialysis/behavioural study of the role of striatal acetylcholine in D1-dependent turning. BehavBrain Res 103:219–228. [DOI] [PubMed] [Google Scholar]
- 54. Laloux C, Petrault M, Lecointe C, Devos D, Bordet R (2012) Differential susceptibility to the PPAR-gamma agonist pioglitazone in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 6-hydroxydopamine rodent models of Parkinson's disease. PharmacolRes 65:514–522. [DOI] [PubMed] [Google Scholar]
- 55. Kelsey JE, Mague SD, Pijanowski RS, Harris RC, Kleckner NW, et al. (2004) NMDA receptor antagonists ameliorate the stepping deficits produced by unilateral medial forebrain bundle injections of 6-OHDA in rats. Psychopharmacology (Berl) 175:179–188. [DOI] [PubMed] [Google Scholar]
- 56.Michel A, Christophe B (2008) Comparison of the activity of three NR2B antagonists, Ro 25–6981, Co 101244 and 20J in experimental models of Parkinson's disease. Society for Neuroscience, Abstract 742.
- 57. Schwarting RK, Huston JP (1996) The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol 50:275–331. [DOI] [PubMed] [Google Scholar]
- 58. Delfino MA, Stefano AV, Ferrario JE, Taravini IR, Murer MG, et al. (2004) Behavioral sensitization to different dopamine agonists in a parkinsonian rodent model of drug-induced dyskinesias. Behav Brain Res 152:297–306. [DOI] [PubMed] [Google Scholar]
- 59. Janhunen S, Tuominen RK, Piepponen TP, Ahtee L (2005) Nicotine and epibatidine alter differently nomifensine-elevated dopamine output in the rat dorsal and ventral striatum. EurJPharmacol 511:143–150. [DOI] [PubMed] [Google Scholar]
- 60. Mignon L, Wolf WA (2007) Postsynaptic 5-HT1A receptor stimulation increases motor activity in the 6-hydroxydopamine-lesioned rat: implications for treating Parkinson's disease. Psychopharmacology (Berl) 192:49–59. [DOI] [PubMed] [Google Scholar]
- 61. Hauber W, Munkle M (1996) The adenosine receptor antagonist theophylline induces a monoamine-dependent increase of the anticataleptic effects of NMDA receptor antagonists. Naunyn Schmiedebergs ArchPharmacol 354:179–186. [DOI] [PubMed] [Google Scholar]
- 62. Udo de Haes J, Xuan F, Kumar B, Triantafyllou I, Wang Z, et al. (2013) Lack of pharmacokinetic (PK) effect of preladenant as a perpetrator of probe drug-drug interactions. Clinical Pharmacology & Therapeutics 93 supplement 1: S111. [Google Scholar]
- 63. Rao N, Dvorchik B, Sussman N, Wang H, Yamamoto K, et al. (2008) A study of the pharmacokinetic interaction of istradefylline, a novel therapeutic for Parkinson's disease, and atorvastatin. JClinPharmacol 48:1092–1098. [DOI] [PubMed] [Google Scholar]
- 64. Orru M, Bakesova J, Brugarolas M, Quiroz C, Beaumont V, et al. (2011) Striatal pre- and postsynaptic profile of adenosine A(2A) receptor antagonists. PLoSOne 6:e16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pinna A (2014) Adenosine A Receptor Antagonists in Parkinson's Disease: Progress in Clinical Trials from the Newly Approved Istradefylline to Drugs in Early Development and Those Already Discontinued. CNSDrugs. [DOI] [PubMed]
- 66. Duty S, Jenner P (2011) Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease. BrJPharmacol 164:1357–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fox SH, Brotchie JM, Lang AE (2008) Non-dopaminergic treatments in development for Parkinson's disease. Lancet Neurol 7:927–938. [DOI] [PubMed] [Google Scholar]
- 68. Schapira AH (2005) Present and future drug treatment for Parkinson's disease. JNeurolNeurosurgPsychiatry 76:1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mony L, Kew JN, Gunthorpe MJ, Paoletti P (2009) Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. BrJPharmacol 157:1301–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Johnson KA, Conn PJ, Niswender CM (2009) Glutamate receptors as therapeutic targets for Parkinson's disease. CNSNeurolDisordDrug Targets 8:475–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Armentero MT, Pinna A, Ferre S, Lanciego JL, Muller CE, et al. (2011) Past, present and future of A(2A) adenosine receptor antagonists in the therapy of Parkinson's disease. PharmacolTher 132:280–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ongini E, Dionisotti S, Gessi S, Irenius E, Fredholm BB (1999) Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 359:7–10. [DOI] [PubMed] [Google Scholar]
- 73. Neustadt BR, Hao J, Lindo N, Greenlee WJ, Stamford AW, et al. (2007) Potent, selective, and orally active adenosine A2A receptor antagonists: arylpiperazine derivatives of pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines. Bioorg Med Chem Lett 17:1376–1380. [DOI] [PubMed] [Google Scholar]
- 74. Zhou ZL, Cai SX, Whittemore ER, Konkoy CS, Espitia SA, et al. (1999) 4-Hydroxy-1-[2-(4-hydroxyphenoxy)ethyl]-4-(4-methylbenzyl)piperidine: a novel, potent, and selective NR1/2B NMDA receptor antagonist. J Med Chem 42:2993–3000. [DOI] [PubMed] [Google Scholar]
- 75. Barta-Szalai G, Borza I, Bozo E, Kiss C, Agai B, et al. (2004) Oxamides as novel NR2B selective NMDA receptor antagonists. Bioorg Med Chem Lett 14:3953–3956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Our data are available upon request because of legal restriction, as the data cannot be published as per our UCB policy on confidentiality. Nevertheless, the excel files will be made available to readers upon direct request to anne.michel@ucb.com.