Summary
Cre-mediated conditional gene targeting has been shown to be successful in many cell and tissue types. However, gene recombination in the uterus with heterogeneous cell types by Cre activation is not yet well established. Using recombinant adenoviruses expressing a functional Cre (ADV-Cre) and ROSA26 reporter mice, we show here that ADV-Cre infused intraluminally in a small volume (10 μl) conditionally excises the loxP site, resulting in lacZ expression in uterine luminal epithelial cells without significantly affecting pregnancy. In contrast, a similar intraluminal infusion of ADV-Cre in a larger volume (50 μl) damages the normal architecture and integrity of the luminal epithelium, inducing gene recombination in the underneath stromal cells, with disruption of pregnancy. Further, decidualizing stromal cells at the implantation sites can be targeted by ADV-Cre after intravenous administration on days 5–6. This route of administration also elicits Cre activity in other tissues, including the liver, spleen, ovary, and, more remarkably, in the adrenal cortex. These findings demonstrate the feasibility of achieving conditional expression or deletion of specific genes in uterine cells at desired times and physiological states.
Keywords: conditional gene targeting, adenovirus, Cre, uterus
Over the past 20 years, conventional transgenic technologies to direct gene overexpression or gene silencing have greatly advanced our knowledge of the genetic basis of many biological processes and diseases. However, on many occasions genetic manipulations using conventional strategies result in early embryonic lethality, leaving no chance to study the function of a gene of interest during adult life. Furthermore, many genes are widely expressed in multiple cell and tissue types; thus, the phenotypes manifested from a universal gene alteration may not reflect the function of that gene in a particular tissue or cell type. Recent evidence shows that an acute silencing of a gene in mice presents different phenotypic consequences from those exhibited by germline loss of the same gene (Sage et al., 2003). Therefore, an experimental approach for conditional gene targeting is emerging as a powerful tool to examine the precise function of a particular gene in the context of cell type and physiological status in mouse models.
Currently, the Cre/loxP system is the most widely used approach to induce site-specific DNA recombination (Sauer, 1998). Cre is a site-specific DNA recombinase derived from the P1 bacteriophage that recognizes 34 basepair sequences called loxP sites. Strategies to target cell specific or inducible expression of the Cre recombinase have been developed in mice (Gu et al., 1994; Metzger et al., 1995; Tsien et al., 1996). These systems fulfill the purpose of generating somatic mutations of a target gene at a given time during the animal's life-span and in a specific cell type. In this respect, there are few studies that have shown Cre-mediated gene recombination in uterine cells (Jamin et al., 2003; Soyal et al., 2005; Wen et al., 2003). For example, a progesterone receptor Cre (PR-Cre) knockin mouse line has been shown to be able to target various progesterone-responsive tissues including the uterus, ovary, oviduct, pituitary gland, and mammary gland (Soyal et al., 2005). To our knowledge, no Cre transgenic mouse line is currently available that specifically targets uterine expression. This is due to our inability to identify a well-characterized uterine cell-specific gene promoter. Furthermore, some of the frequently used chemical inducers, such as tamoxifen and RU486 for turning on Cre expression in vivo under the control of estrogen receptor (ER) or PR, by themselves are likely to influence uterine functions, questioning the suitability of these chemically induced Cre mouse lines in studies related to early pregnancy (Wen et al., 2003). In addition, some of the Cre transgenetic mouse lines, such as AMHR2-Cre mice, induce Cre activity during midgestational embryo development, excluding their potential application in exploring the function of developmental genes in the adult uterus (Jamin et al., 2003). These shortcomings in studying uterine functions motivated us to look for other methods for introducing the Cre recombinase in mice carrying loxP-flanked genes.
Recombinant adenoviruses are being used as an alternative approach for the delivery of transgenes into specific target tissues (Akagi et al., 1997; Davidson et al., 1993; Kaartinen and Nagy, 2001; Tallone et al., 2001; Wang et al., 1996). There is evidence that nonreplicating adenoviruses infect a variety of cell types in vivo, irrespective of their cellular proliferation and differentiation status (Brody and Crystal, 1994). Using recombinant adenoviruses expressing a functional Cre (ADV-Cre) and the ROSA26 mice carrying an activatable floxed LacZ reporter gene (Soriano, 1999), we show here that ADV-Cre can conditionally excise a loxP inserted gene in uterine luminal epithelial cells after intraluminal infusion and in decidualizing stromal cells during early pregnancy following intravenous (i.v.) administration. This route of administration also elicits Cre activity in other tissues including the liver, spleen, ovary and, more remarkably, in the adrenal cortex. These findings demonstrate the feasibility of achieving conditional expression or deletion of specific genes in uterine cells at desired times.
Results and Discussion
Intraluminal Adenoviral Cre Infusion Induces Conditional Gene Recombination in the Uterine Luminal Epithelium
One of the key factors that determine the efficacy of adenovirus-mediated gene transfer is the degree of transfection of the target cells by the viral vectors. Previous studies in rodents have shown that the route of administration is a major determinant for the transfection efficiency in somatic tissues by adenoviral recombinants (Huard et al., 1995). However, limited information is currently available for in vivo adenoviral gene delivery in the uterus (Tan et al., 2004). To explore the potential route for ADV-Cre delivery into uterine cells during early pregnancy, we first examined whether a direct infusion of the virus into the uterine lumen is suitable for conditionally targeting uterine cells.
ROSA26loxPLacZ homozygous mice were intraluminally infused with either ADV-Cre (1010 plaque-forming units (pfu) in 10 μl saline) or empty viral vectors (1010 pfu in 10 μl saline) on day 4 of pseudopregnancy. When examined 48 h later, successful viral infection with the resulting Cre activity in excising the loxP flanked gene was detected by LacZ staining in uteri infused with ADV-Cre, but not in those with empty viral vectors (Fig. 1a). The conditional gene recombination was restricted to the uterine luminal epithelium. No LacZ staining was visualized in the glandular epithelium or stroma cells (Fig. 1a). These results show that delivery of adenovirus locally in the uterus is effective in infecting specifically luminal epithelial cells. Both coxsackie adenovirus receptors (CAR) receptors and integrins are critical for adenoviral infection (Bergelson et al., 1997; Wickham et al., 1993). Thus, our findings are consistent with previous observations of higher expression of CAR as well as αvβ3 and αvβ5 integrins in uterine epithelial cells with only lower levels detected in stromal cells (Aplin et al., 1996; Beauparlant et al., 2004). However, it is also possible that the epithelial basement membrane functions as a barrier in preventing viral particles to travel to the underneath stromal cells.
Fig. 1.
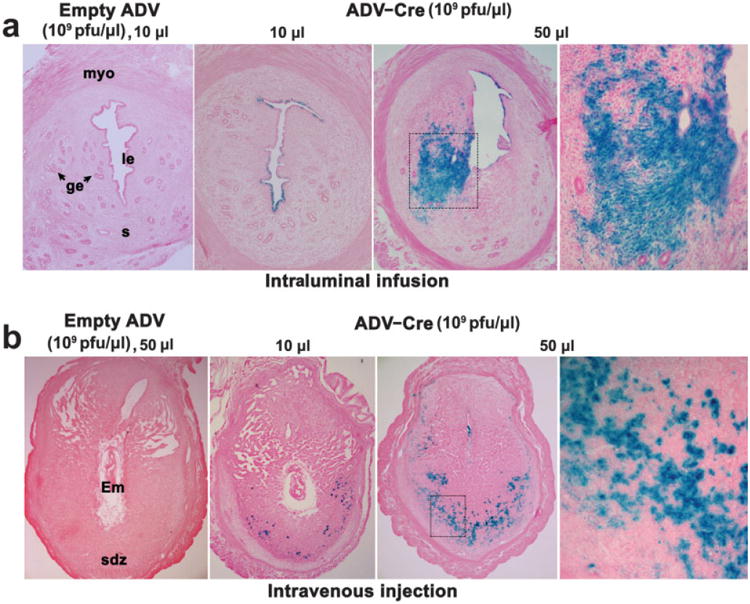
Conditional gene targeting in the uterus through intraluminal (a) or i.v. (b) administration of ADV-Cre. Photomicrographs of lacZ staining in representative cross sections of uteri from homozygous ROSA26loxpLacZ mice are shown at 100× (panels 1–3) and 200× (panel 4) in (a) and at 40× (panels 1–3) and 200× (panel 4) in (b). The conditional gene recombination visualized by LacZ staining was restricted to the uterine luminal epithelium in mice intraluminally infused with 10 μl of ADV-Cre, but not in those with empty viral vectors. However, prominent blue staining was detected in uterine stromal cells in 50 μl ADV-Cre infused mice (a). When pregnant homozygous ROSA26loxPLacZ females were i.v. administered ADV-Cre on days 5–6 and examined on day 8, gene recombination was noted in many decidual cells located in the secondary decidual zone, especially in those receiving higher amounts of ADV-Cre. Numerous small nuclear blue spots visible at higher magnification indicated Cre-driven gene recombination in these cells (b). le, luminal epithelium; ge, glandular epithelium; s, stroma; myo, myometrium; Em, embryo; sdz, secondary decidual zone.
To test whether increasing the load of ADV-Cre helps to target the uterine glandular epithelium and stroma cells, ROSA26 reporter mice were intraluminally infused with 5-fold more ADV-Cre particles (5 × 1010 pfu) in 50 μl saline. As expected, gene recombination visualized by LacZ staining was observed in the uterine luminal epithelium (Fig. 1a). However, intraluminal infusion of this large volume damages the normal architecture and integrity of the luminal epithelial layer, leading to localized gene recombination from diffusion of ADV-Cre underneath stromal cells (Fig. 1a). ADV-Cre infection and activity was rarely observed in glandular epithelium with low or high viral load.
Since one of the major goals of our research program is to explore the molecular road map to embryo implantation, it was felt necessary to examine whether uterine virus load by the intraluminal infusion approach would interfere with normal blastocyst implantation. Therefore, we addressed this issue using a physiologically relevant embryo transfer and delayed implantation model. Pseudopregnant ROSA26loxPLacZ homozygous mice were intraluminally infused with the same amount of viral vectors on day 4 (1010 pfu in 10 μl saline). Mice were ovariectomized at the same time of virus administration to induce delayed implantation. This condition was maintained by daily injections of progesterone (P4, 2 mg per mouse) from days 5–7. On day 7, normal blastocysts from day 4 pregnant females were transferred into P4-primed virus-infused uterine lumens. All recipients received an injection of E2 (10 ng/mouse) immediately after the transfer. When examined 24 h after E2 injection, normal implantation sites demarcated by blue bands by Chicago Blue dye solution were noted in all five tested mice examined, indicating that the uterine viral load apparently had no detrimental effect on blastocyst function and uterine preparation for implantation. We also performed experiments in normal pregnant mice. For example, females were intraluminally infused with ADV-Cre in 10 or 50 μl saline on day 2 of pregnancy and implantation sites were examined on midmorning of day 5 by the blue dye method. A comparable number of implantation sites was detected when mice were infused with 10 μl ADV-Cre (n =7, 7.4 ± 1.1) or empty vector (n = 5, 8.1 ± 0.8). Thus, ADV-Cre delivery through intraluminal infusion is a feasible approach to achieve conditional gene overexpression or deletion in the uterine luminal epithelium. However, implantation failure in pregnant mice infused with 50 μl of ADV-Cre (data not shown) raises a cautionary note against those experiments using a large volume for intraluminal infusion to influence embryo implantation.
Intravenous Adenoviral-Cre Administration Induces Conditional Gene Recombination in Uterine Decidual Cells at the Site of Implantation in a Dose-Dependent Manner
Our failure to achieve conditional transgene delivery in uterine stromal cells without impairing uterine capacity for implantation by intraluminal infusion of ADV-Cre led us to consider developing an alternative route of administration for targeting these cells. Previous studies have shown that ADV-Cre after systemic delivery is capable of mediating efficient loxP-dependent recombination in vivo (Akagi et al., 1997; Wang et al., 1996). Therefore, we next examined whether an i.v. injection of ADV-Cre would lead to successful gene targeting in uterine cells during early pregnancy.
Homozygous ROSA26loxPLacZ females were i.v. injected with ADV-Cre though a tail vein on day 4 of pseudopregnancy. Mice were sacrificed 48 h after virus administration. The uterus was examined for LacZ staining to monitor the efficacy of ADV-Cre delivery. To our surprise, no sign of gene recombination was noticed in any uterine cell types (data not shown). This failure to achieve ADV-Cre transfer into uterine cells may be due to the intact vascular endothelium, which represents a barrier to distribution of vectors via the circulation. There is now evidence that experimentally induced acute permeabilization of the peripheral microvasculature can significantly enhance the systemic gene transduction by adeno-associated viral vectors (Gregorevic et al., 2004). Therefore, we speculated that an increase in uterine stromal vascular permeability would allow for achieving a higher efficacy for ADV-Cre delivery in the uterine endometrial bed.
In mice, initiation of blastocyst attachment with the uterine luminal epithelium coincides with a localized increased stromal vascular permeability at the site of the blastocyst. This hallmark event of embryo implantation led us to ask whether ADV-Cre would be more effective in targeting the decidual cells when introduced with the initiation of implantation. Indeed, when pregnant homozygous ROSA26loxPLacZ females were i.v. administered with various amounts of ADV-Cre (1010 or 5 × 1010 pfu/mouse) on days 5–6 and examined on day 8, conditional gene recombination was noted in numerous decidual cells located in the secondary decidual zone, which is more prominent in mice treated with a higher load of ADV-Cre (Fig. 1b). This conditional gene targeting by ADV-Cre is consistent with the induced expression of CAR receptors in the deciduum (S.K. Das, unpubl. obs.). Furthermore, no apparent detrimental impact of the viral load on postimplantation embryonic growth was noticeable, since mice treated with ADV-Cre maintained normal term pregnancy. Therefore, the systemic delivery of ADV-Cre through i.v. administration is a practical approach for studying the acute gene function during uterine decidualization.
We also examined the status of Cre-mediated gene recombination in other tissues after i.v. injection of ADV-Cre. Consistent with previous reports (Akagi et al., 1997; Wang et al., 1996), a strong LacZ staining in hepatocytes was observed throughout the liver (Fig. 2). Furthermore, many interstitial cells surrounding the lymphoid follicles in the spleen receiving higher loads of ADV-Cre were also positive for LacZ staining (Fig. 2). Interestingly, a remarkable gene recombination was detected in cells located in the zona fasiculata and zona reticularis within the adrenal cortex, although no sign of recombination was evident in the adrenal medulla (Fig. 2). This presents a great opportunity for investigators who are interested in exploring adrenal cortical functions via conditional gene targeting. The newly formed corpora lutea in the ovary are also targets for ADV-Cre during early pregnancy (Fig. 2). In addition, we noted that glomerulus and some tubular epithelial cells in the kidney cortex also show low levels of LacZ staining (data not shown). In contrast, Cre activity was not detected in many other tissues, such as the brain, heart and lung (data not shown). This could be due to restricted delivery of ADV-Cre by this route of administration (Huard et al., 1995) or because these tissues lack or express very low levels of adenoviral receptors (Tomko et al., 2000).
Fig. 2.
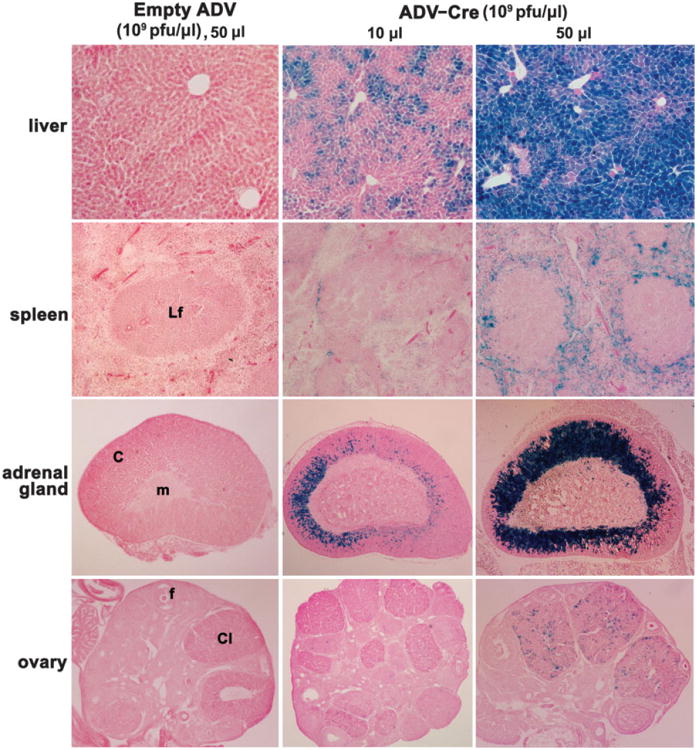
Conditional gene recombination in a range of somatic tissues after i.v. administration of ADV-Cre. Representative photomicrographs of lacZ staining in somatic tissues are shown at 1003 for the liver and spleen and at 40× for the adrenal gland and ovary. Hepato-cytes, interstitial cells surrounding the lymphoid follicles, and fasiculata and reticularis in the adrenal cortex showed strong LacZ staining in a dose-dependent manner. In addition, ADV-Cre also targeted the newly formed ovarian corpora lutea during early pregnancy. However, recombination was completely absent in similar tissue sections derived from mice injected i.v. with empty viral vectors. Lf, lymphoid follicle; c, cortex; m, medulla; f, follicle, Cl, corpus luteum.
Collectively, we provide experimental evidence here that ADV-Cre-mediated gene delivery is a viable approach for achieving conditional overexpression or deletion of specific genes in the uterus. This approach will be useful to study potential acute function of genes in the uterus during early pregnancy.
Materials and Methods
Mice
LacZ ROSA26 reporter mice originally developed by Dr. Soriano's group were housed in the Institutional Animal Care Facility according to National Institutes of Health and institutional guidelines for laboratory animals. Cre expression in this mouse line results in the removal of a loxP-flanked DNA segment that restrains the expression of a lacZ gene (Soriano, 1999). Homozygous females were mated with fertile or vasectomized males of the same strain to induce pregnancy or pseudopregnancy, respectively (day 1 = vaginal plug).
Construction of Recombinant Adenoviral Vectors for Cre Gene
Generation of the replication-defective adenoviral vector for bacteriophage P1 Cre gene (ADV-Cre) followed the technique described by us (Tan et al., 2004). In brief, a 1.2-kb fragment (Xba1/SmaI) of a Cre gene containing the N-terminal nuclear localization signal (NLS) was derived from an expression clone, pBS/RIP-Cre-GH (generously provided by Holger Kulessa and originally developed by Christopher Wright, Vanderbilt University) and subsequently subcloned into XbaI/ECoRV sites of the shuttle vector, pAdTrack-CMV. The selected clone for the sense direction of the gene based on CMV promoter was sequenced to verify the clone identity. The resultant plasmid was linearized with PmeI and subsequently cotransfected with pAdEasy-1 into E. coli BJ5183 for the selection of recombinant plasmid. The generation of the empty recombinant plasmid (Empty ADV) has previously been described by us (Tan et al., 2004). The viral packaging of these vectors was carried out by transfecting 293 cells (Tan et al., 2004). Viral particles were purified through CsCl density gradient centrifugation and stored at –70°C. Titers of the purified viral stock were determined by measuring OD260 with each reading of 1.0 equivalent to 5 × 1010 plaque-forming units (pfu)/ml.
Administration of the Recombinant Adenovirus
In order to achieve successful gene delivery through virus infection in the uterine luminal epithelium, ADV-Cre (1010 pfu in 10 μl or 5 × 1010 pfu in 50 μl) or empty (Empty ADV, 1010 pfu in 10 μl) particles were directly infused into the uterine lumens of day 4 pseudopregnant homozygous ROSA26 reporter mice. Uterine tissues were collected 48 h after virus infusion for LacZ staining. For examining adenovirus delivery into the uterine cells at the site of implantation, pregnant homozygous ROSA26 reporter females were injected with viral vectors (1010 or 5 × 3 1010 pfu diluted in 200 μl of saline) through tail veins on days 5 and 6 of pregnancy. After sacrificing the mice on day 8, implantation sites as well as other maternal tissues, such as brain, lung, heart, kidney, liver, adrenal gland, spleen, and ovary were collected for LacZ staining.
LacZ Staining
To examine the ADV-Cre infection and activity, harvested tissues from ADV-Cre or mice administered empty virus were subjected to lacZ staining as previously described (Ma et al., 2001). In brief, small pieces of tissues were fixed in 0.2% paraformaldehyde solution followed by infusion in 30% sucrose at 4°C overnight. Tissues were embedded in OCT and snap-frozen. Frozen sections were mounted onto glass slides and stained overnight at 37°C using 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside as a substrate. Sections were counterstained with eosin.
Acknowledgments
We thank Yi Tan for initially growing the viral vectors in 293 cells. Su.K.D. is the recipient of Method to Extend Research in Time (MERIT) Awards from the National Institute on Drug Abuse (NIDA) and the National Institute of Child Health and Human Development (NICHD). H.W. is the recipient of the Solvay-Mortola Research Award from the Society for Gynecologic Investigation. H.X. is a Lalor Foundation postdoctoral fellow.
Contract grant sponsor: National Institutes of Health; Contract grant numbers: DA06668, HD12304, HD33994, CA77839 (to Su.K.D.) and ES07814, HD37830 (to Sa.K.D.).
Literature Cited
- Akagi K, Sandig V, Vooijs M, Van der Valk M, Giovannini M, Strauss M, Berns A. Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res. 1997;25:1766–1773. doi: 10.1093/nar/25.9.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin JD, Spanswick C, Behzad F, Kimber SJ, Vicovac L. Integrins beta 5, beta 3 and alpha v are apically distributed in endometrial epithelium. Mol Hum Reprod. 1996;2:527–534. doi: 10.1093/molehr/2.7.527. [DOI] [PubMed] [Google Scholar]
- Beauparlant SL, Read PW, Di Cristofano A. In vivo adenovirus-mediated gene transduction into mouse endometrial glands: a novel tool to model endometrial cancer in the mouse. Gynecol Oncol. 2004;94:713–718. doi: 10.1016/j.ygyno.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Brody SL, Crystal RG. Adenovirus-mediated in vivo gene transfer. Ann N Y Acad Sci. 1994;716:90–101. doi: 10.1111/j.1749-6632.1994.tb21705.x. discussion 101–103. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Allen ED, Kozarsky KF, Wilson JM, Roessler BJ. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993;3:219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, Russell DW, Chamberlain JS. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- Huard J, Lochmuller H, Acsadi G, Jani A, Massie B, Karpati G. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995;2:107–115. [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Genetic studies of the AMH/MIS signaling pathway for Mullerian duct regression. Mol Cell Endocrinol. 2003;211:15–19. doi: 10.1016/j.mce.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Nagy A. Removal of the floxed neo gene from a conditional knockout allele by the adenoviral Cre recombinase in vivo. genesis. 2001;31:126–129. doi: 10.1002/gene.10015. [DOI] [PubMed] [Google Scholar]
- Ma W, Tan J, Matsumoto H, Robert B, Abrahamson DR, Das SK, Dey SK. Adult tissue angiogenesis: evidence for negative regulation by estrogen in the uterus. Mol Endocrinol. 2001;15:1983–1992. doi: 10.1210/mend.15.11.0734. [DOI] [PubMed] [Google Scholar]
- Metzger D, Clifford J, Chiba H, Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci U S A. 1995;92:6991–6995. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage JR, Anagnostaras SG, Mitchell S, Bronstein JM, De Salles A, Masterman D, Knowlton BJ. Analysis of probabilistic classification learning in patients with Parkinson's disease before and after pallidotomy surgery. Learn Mem. 2003;10:226–236. doi: 10.1101/lm.45903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Inducible gene targeting in mice using the Cre/lox system. Methods. 1998;14:381–392. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, Demayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- Tallone T, Malin S, Samuelsson A, Wilbertz J, Miyahara M, Okamoto K, Poellinger L, Philipson L, Pettersson S. A mouse model for adenovirus gene delivery. Proc Natl Acad Sci U S A. 2001;98:7910–7915. doi: 10.1073/pnas.141223398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol. 2004;265:181–195. doi: 10.1016/j.ydbio.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomko RP, Johansson CB, Totrov M, Abagyan R, Frisen J, Philipson L. Expression of the adenovirus receptor and its interaction with the fiber knob. Exp Cell Res. 2000;255:47–55. doi: 10.1006/excr.1999.4761. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Krushel LA, Edelman GM. Targeted DNA recombination in vivo using an adenovirus carrying the cre recombinase gene. Proc Natl Acad Sci U S A. 1996;93:3932–3936. doi: 10.1073/pnas.93.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen F, Cecena G, Munoz-Ritchie V, Fuchs E, Chambon P, Oshima RG. Expression of conditional cre recombinase in epithelial tissues of transgenic mice. genesis. 2003;35:100–106. doi: 10.1002/gene.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]


