Abstract
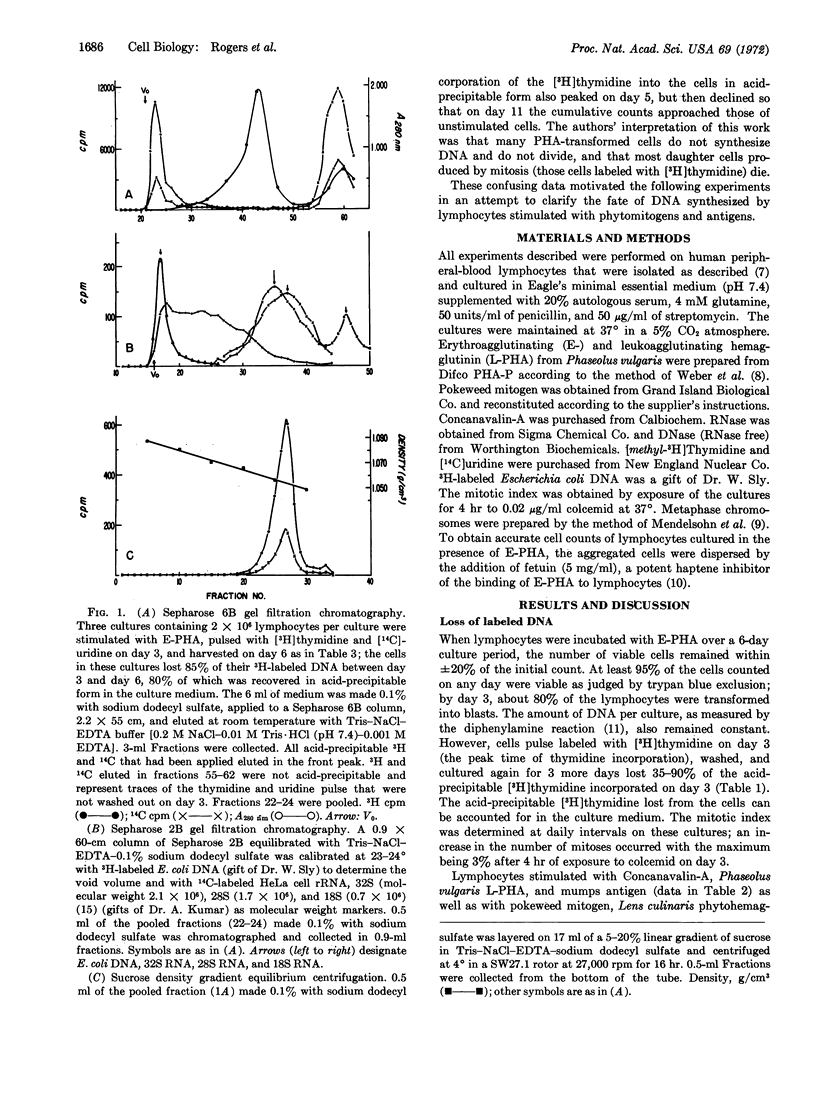
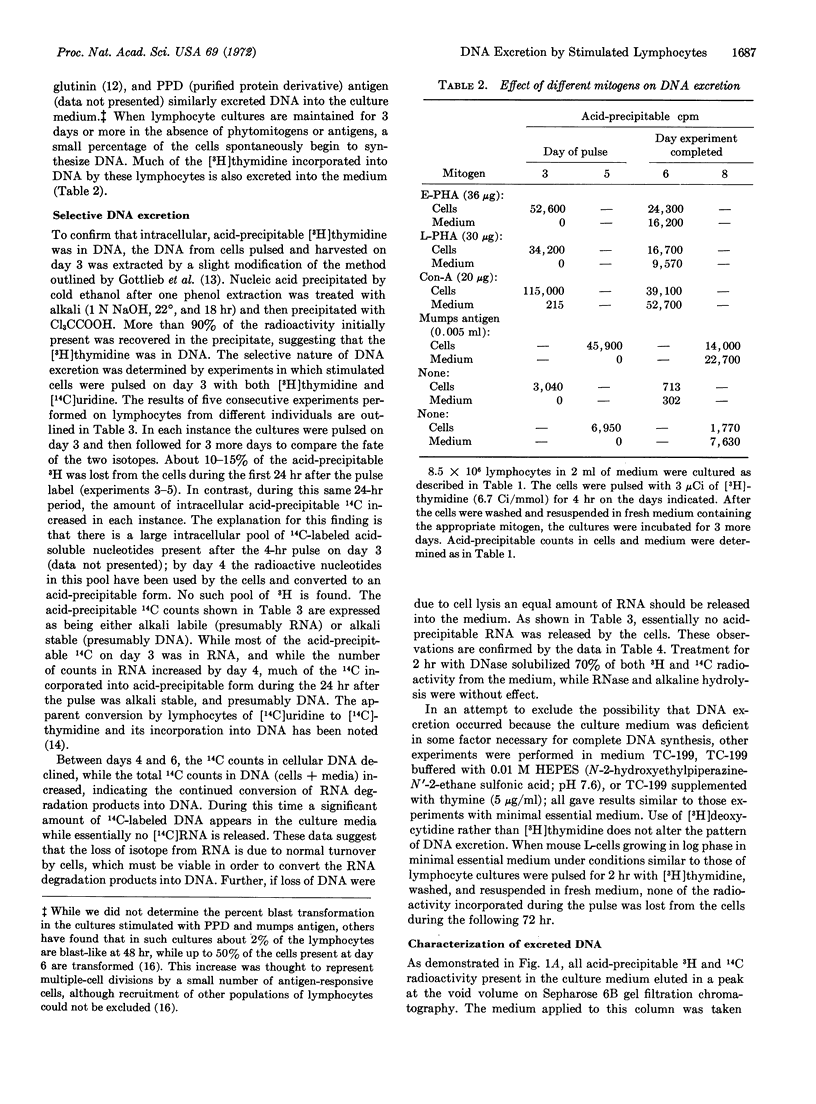
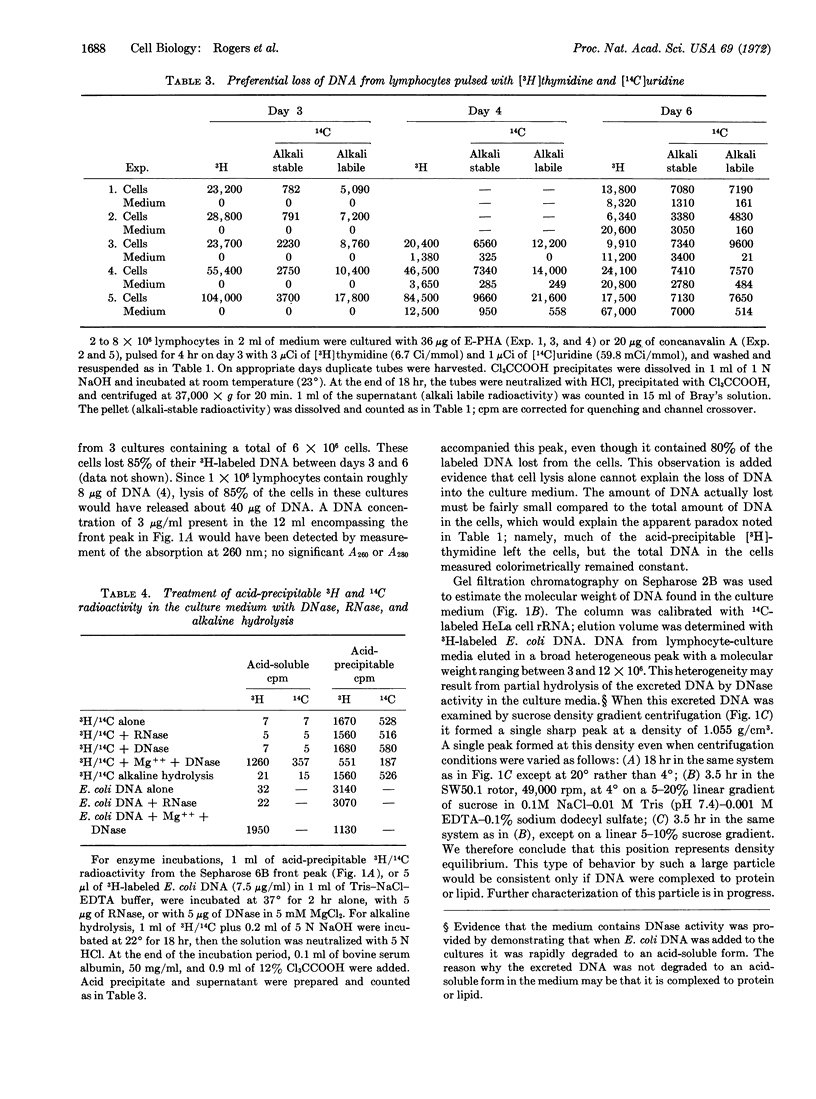
When human lymphocytes are cultured in the presence of phytomitogens, 70-90% of the cells undergo blast transformation and synthesize DNA. However, less than 40% of these lymphocytes actually undergo mitosis while 35-90% of the newly synthesized DNA is excreted into the media. The release of DNA by the cells is selective since experiments with [14C]uridine indicate that RNA is not lost into the culture media. DNA excretion occurs under many culture conditions. The excreted DNA has an estimated molecular weight of 3 to 12 × 106 as determined by gel filtration on Sepharose 2B. It forms a single sharp peak at a density of 1.055 g/cm3 when examined by sucrose density gradient centrifugation, suggesting that the DNA is complexed to protein or lipid.
Keywords: mitogenesis, pulse labeling, density gradient centrifugation, DNase
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. D., Blackler A. W. Gene amplification proceeds by a chromosome copy mechanism. J Mol Biol. 1972 Jan 14;63(1):75–83. doi: 10.1016/0022-2836(72)90522-0. [DOI] [PubMed] [Google Scholar]
- Donahue H. A., Moore V. G., Little J. R. Ribonucleic acid synthesis in rabbit lymph node cells. J Immunol. 1970 Sep;105(3):592–603. [PubMed] [Google Scholar]
- Gottlieb A. A., Taylor L., Seinsheimer F., 3rd Replication of deoxyribonucleic acid in lymphoid cells. An unusual effect of bromodeoxyuridine. Biochemistry. 1970 Oct 27;9(22):4322–4328. doi: 10.1021/bi00824a012. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Grossman J., Troll W., Weissmann G. The effect of epsilon amino caproic acid and other inhibitors of proteolysis upon the response of human peripheral blood lymphocytes to phytohemagglutinin. J Clin Invest. 1971 Jun;50(6):1206–1217. doi: 10.1172/JCI106598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard I. K., Sage H. J. Isolation and characterization of a phytohemagglutinin from the lentil. Biochemistry. 1969 Jun;8(6):2436–2441. doi: 10.1021/bi00834a028. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. The structure of a phytohemagglutinin receptor site from human erythrocytes. J Biol Chem. 1970 May 25;245(10):2536–2545. [PubMed] [Google Scholar]
- Kumar A., Warner J. R. Characterization of ribosomal precursor particles from HeLa cell nucleoli. J Mol Biol. 1972 Jan 28;63(2):233–246. doi: 10.1016/0022-2836(72)90372-5. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Meinke W., Goldstein D. A. Membrane-associated DNA in the cytoplasm of diploid human lymphocytes. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1212–1216. doi: 10.1073/pnas.68.6.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. R., Donahue H. A. Altered nucleic acids of antibody-forming tissues. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1299–1306. doi: 10.1073/pnas.67.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Agarwal S. S. DNA polymerase. Correlation with DNA replication during transformation of human lymphocytes. Exp Cell Res. 1971 Jun;66(2):299–304. doi: 10.1016/0014-4827(71)90681-1. [DOI] [PubMed] [Google Scholar]
- Marshall W. H., Valentine F. T., Lawrence H. S. Cellular immunity in vitro. Clonal proliferation of antigen-stimulated lymphocytes. J Exp Med. 1969 Aug 1;130(2):327–343. doi: 10.1084/jem.130.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn J., Moore D. E., Salzman N. P. Separation of isolated Chinese hamster metaphase chromosomes into three size-groups. J Mol Biol. 1968 Feb 28;32(1):101–112. doi: 10.1016/0022-2836(68)90148-4. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- Pelc S. R. Turnover of DNA and function. Nature. 1968 Jul 13;219(5150):162–163. doi: 10.1038/219162a0. [DOI] [PubMed] [Google Scholar]
- Polgar P. R., Kibrick S. Origin of small lymphocytes following blastogenesis induced by short-term PHA stimulation. Nature. 1970 Feb 28;225(5235):857–858. doi: 10.1038/225857a0. [DOI] [PubMed] [Google Scholar]
- Schellekens P. T., Eijsvoogel V. P. Lymphocyte transformation in vitro. I. Tissue culture conditions and quantitative measurements. Clin Exp Immunol. 1968 Jul;3(6):571–584. [PMC free article] [PubMed] [Google Scholar]
- Tedesco T. A., Mellman W. J. Desoxyribonucleic acid assay as a measure of cell number in preparations from monolayer cell cultures and blood leucocytes. Exp Cell Res. 1967 Jan;45(1):230–232. doi: 10.1016/0014-4827(67)90126-7. [DOI] [PubMed] [Google Scholar]
- Weber T., Nordman C. T., Gräsbeck R. Separation of lymphocyte-stimulating and agglutinating activities in phytohaemagglutinin (PHA) from Phaseolus vulgaris. Scand J Haematol. 1967;4(1):77–80. doi: 10.1111/j.1600-0609.1967.tb01601.x. [DOI] [PubMed] [Google Scholar]



