Abstract
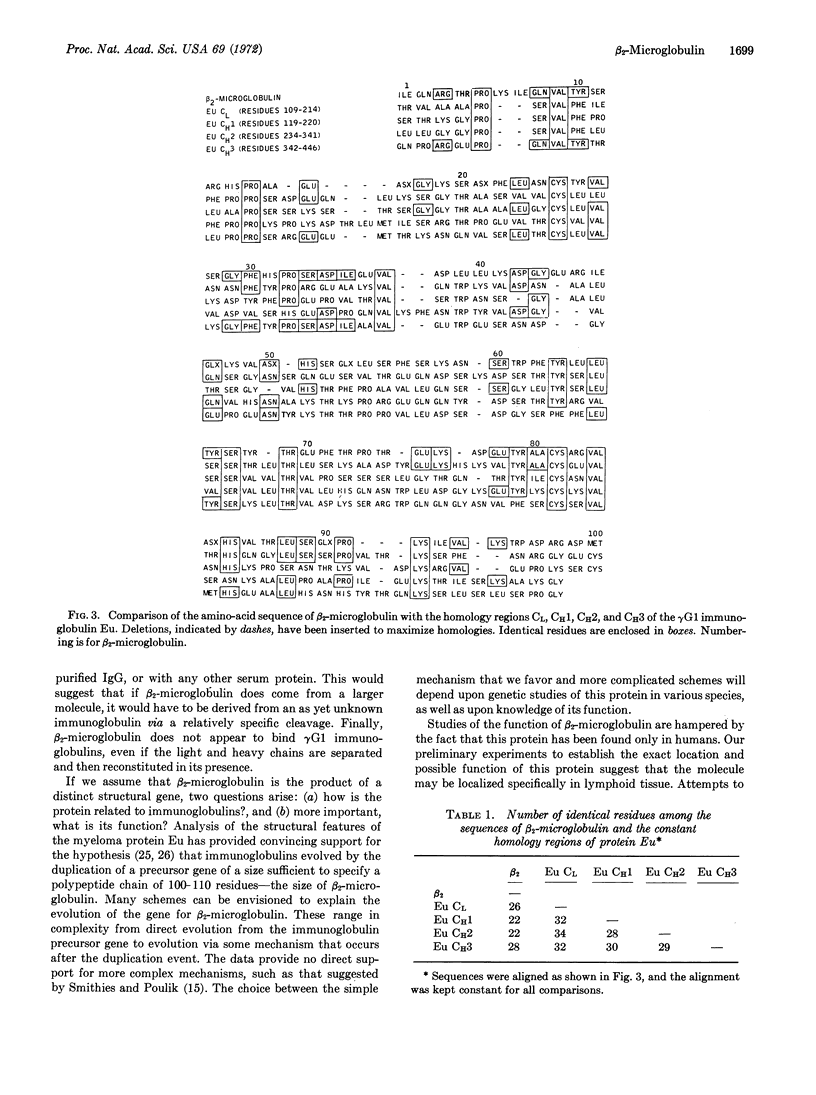
Analysis of the primary structure of β2-microglobulin indicates that this human protein is homologous in sequence to the constant portion of immunoglobulin light chains (CL), and to the homology regions (CH1, CH2, and CH3) of the constant portion of γ1 (heavy) chains of immunoglobulin G. Homology with the CH3 region is particularly striking. No convincing homology could be demonstrated by similar comparisons with the variable regions of immunoglobulin light and heavy chains. β2-Microglobulin contains an intrachain disulfide loop of 57 amino-acid residues that is similar in size to disulfide loops found in the constant regions of immunoglobulin G. These findings suggest that β2-microglobulin is a free immunoglobulin domain, possibly serving an effector function similar to that of the CH3 domain of γ1 chains of immunoglobulin G.
Keywords: light and heavy chain, amino-acid sequence
Full text
PDF

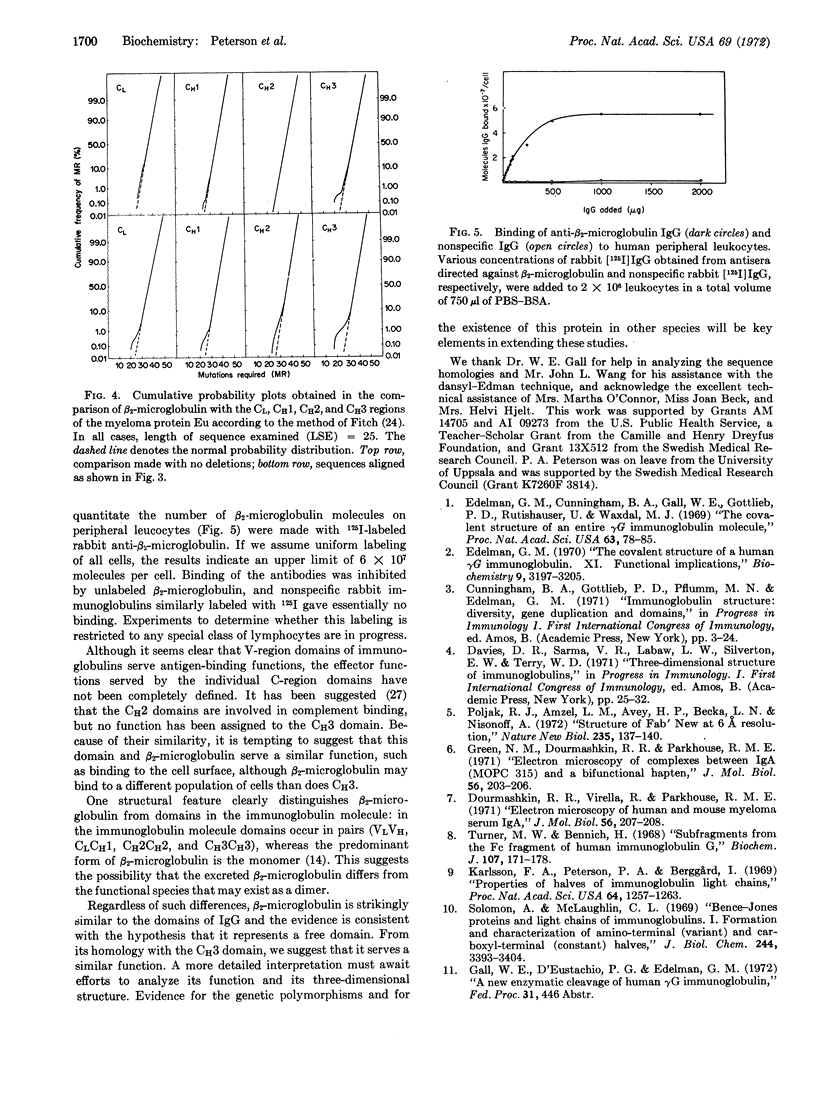

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berggård I., Bearn A. G. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem. 1968 Aug 10;243(15):4095–4103. [PubMed] [Google Scholar]
- Björk I., Karlsson F. A., Berggård I. Independent folding of the variable and constant halves of a lambda immunoglobulin light chain. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1707–1710. doi: 10.1073/pnas.68.8.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk I., Tanford C. Gross conformation of free polypeptide chains from rabbit immunoglobulin G. I. Heavy chain. Biochemistry. 1971 Apr 13;10(8):1271–1280. doi: 10.1021/bi00784a001. [DOI] [PubMed] [Google Scholar]
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- Cummingham B. A., Gottlieb P. D., Konigsberg W. H., Edelman G. M. The covalent structure of a human gamma G-immunoglobulin. V. Partial amino acid sequence of the light chain. Biochemistry. 1968 May;7(5):1983–1994. doi: 10.1021/bi00845a049. [DOI] [PubMed] [Google Scholar]
- Dourmashkin R. R., Virella G., Parkhouse R. M. Electron microscopy of human and mouse myeloma serum IgA. J Mol Biol. 1971 Feb 28;56(1):207–208. doi: 10.1016/0022-2836(71)90097-0. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. The covalent structure of a human gamma G-immunoglobulin. XI. Functional implications. Biochemistry. 1970 Aug 4;9(16):3197–3205. doi: 10.1021/bi00818a012. [DOI] [PubMed] [Google Scholar]
- Fitch W. M. An improved method of testing for evolutionary homology. J Mol Biol. 1966 Mar;16(1):9–16. doi: 10.1016/s0022-2836(66)80258-9. [DOI] [PubMed] [Google Scholar]
- Gottlieb P. D., Cunningham B. A., Rutishauser U., Edelman G. M. The covalent structure of a human gamma G-immunoglobulin. VI. Amino acid sequence of the light chain. Biochemistry. 1970 Aug 4;9(16):3155–3161. doi: 10.1021/bi00818a007. [DOI] [PubMed] [Google Scholar]
- Green N. M., Dourmashkin R. R., Parkhouse R. M. Electron microscopy of complexes between IgA (MOPC 315) and a bifunctional hapten. J Mol Biol. 1971 Feb 28;56(1):203–206. doi: 10.1016/0022-2836(71)90096-9. [DOI] [PubMed] [Google Scholar]
- Hill R. L., Delaney R., Fellows R. E., Lebovitz H. E. The evolutionary origins of the immunoglobulins. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1762–1769. doi: 10.1073/pnas.56.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson F. A., Peterson P. A., Berggard I. Properties of halves of immunoglobulin light chains. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1257–1263. doi: 10.1073/pnas.64.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. M., Fougereau M. Immunoglobulin peptide with complement fixing activity. Nature. 1969 Dec 20;224(5225):1212–1213. doi: 10.1038/2241212a0. [DOI] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Milstein C., Clegg J. B., Jarvis J. M. C-terminal half of immunoglobulin lambda-chains. Nature. 1967 Apr 15;214(5085):270–272. doi: 10.1038/214270a0. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Becka L. N. Structure of Fab' New at 6 A resolution. Nat New Biol. 1972 Feb 2;235(57):137–140. doi: 10.1038/newbio235137a0. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Doolittle R. F. Antibody active sites and immunoglobulin molecules. Science. 1966 Jul 1;153(3731):13–25. doi: 10.1126/science.153.3731.13. [DOI] [PubMed] [Google Scholar]
- Smithies O., Poulik M. D. Initiation of protein synthesis at an unusual position in an immunoglobulin gene? Science. 1972 Jan 14;175(4018):187–189. doi: 10.1126/science.175.4018.187. [DOI] [PubMed] [Google Scholar]
- Solomon A., McLaughlin C. L. Bence-Jones proteins and light chains of immunoglobulins. I. Formation and characterization of amino-terminal (variant) and carboxyl-terminal (constant) halves. J Biol Chem. 1969 Jun 25;244(12):3393–3404. [PubMed] [Google Scholar]
- Turner M. W., Bennich H. Subfragments from the Fc fragment of human immunoglobulin G. Isolation and physicochemical charaterization. Biochem J. 1968 Mar;107(2):171–178. doi: 10.1042/bj1070171. [DOI] [PMC free article] [PubMed] [Google Scholar]


