Abstract
Members of the transforming growth factor beta (TGFβ) superfamily of secreted factors play essential roles in nearly every aspect of cartilage formation and maintenance. However, the mechanisms by which TGFβs transduce their effects in cartilage in vivo remain poorly understood. Mutations in several TGFβ family members, their receptors, extracellular modulators, and intracellular transducers have been described, and these usually impact the development of the cartilaginous skeleton. Furthermore, genome-wide association studies have linked components of the (TGFβ) superfamily to susceptibility to osteoarthritis. This review focuses on recent discoveries from genetic studies in the mouse regarding the regulation of TGFβ signaling in developing growth plate and articular cartilage, as well as the different modes of crosstalk between canonical and noncanonical TGFβ signaling. These new insights into TGFβ signaling in cartilage may open new prospects for therapies that maintain healthy articular cartilage.
Keywords: TGFβ signaling, cartilage, development, maintenance
Introduction
Members of the transforming growth factor beta (TGFβ) superfamily of secreted factors play essential roles in nearly every aspect of development, from the generation of germ cells, through gastrulation and organ formation, and into postnatal life. Mutations in several TGFβ family members, their receptors, extracellular modulators, and intracellular transducers have been described, and these commonly impact the development of the cartilaginous skeleton. Furthermore, genome-wide association studies have linked components of the (TGFβ) superfamily to susceptibility to osteoarthritis. This review focuses on recent discoveries from genetic studies in the mouse regarding the regulation of TGFβ signaling in developing growth plate and articular cartilage, as well as the different modes of crosstalk between canonical and noncanonical TGFβ signaling. These new insights into TGFβ signaling in cartilage may open new prospects for therapies that maintain healthy articular cartilage.
ENDOCHONDRAL BONE FORMATION
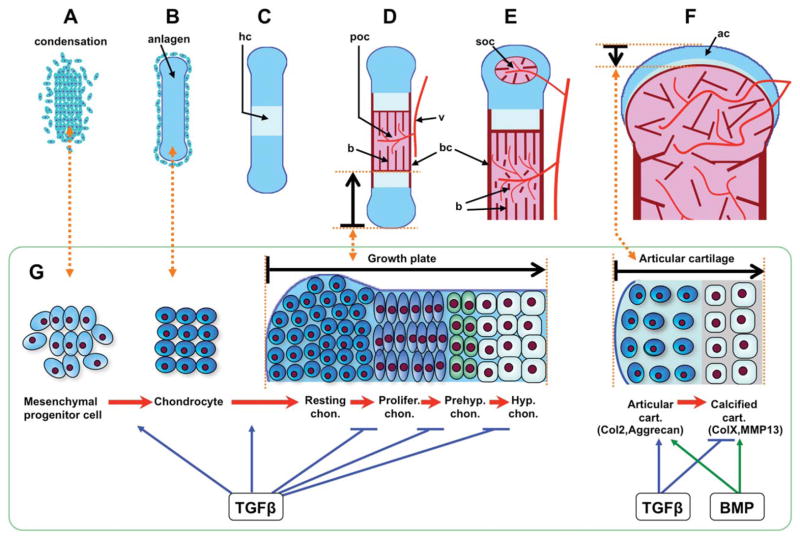
The skeleton is composed primarily of cartilage and bone. Throughout the axial and appendicular skeleton, with the exception of the skull, the skeleton is formed from a hyaline cartilage template. During development, cells from three distinct lineages (sclerotome, paraxial mesoderm, and neural crest) undergo chondrogenesis through a similar sequence of events to form the cartilage of the embryonic skeleton (Long and Ornitz, 2013; Pitsillides and Beier, 2011) (Fig. 1). The first overt sign of chondrogenesis is aggregation of mesenchymal chondroprogenitor cells into condensations. This process is mediated by elevated expression of various cell adhesion molecules, such as neural cadherin (N-cadherin) and neural cell adhesion molecule (NCAM). These molecules mediate crucial cell–cell interactions and are critical for maintenance of the expression of Sox9, the transcription factor currently known to act earliest in the chondrogenic program (Akiyama and Lefebvre, 2011).
FIGURE 1.
Process of endochondral bone formation and TGFβs role during cartilage development. (A) Mesenchymal progenitor cells condense. (B) Cells of condensations become chondrocytes that synthesize a collagen II and aggrecan-rich extracellular matrix (ECM), constructing a cartilage template (anlagen) for endochondral ossification. (C) Chondrocytes at the center of condensation stop proliferating and become hypertrophic chondrocytes that synthesize a collagen X-rich ECM, the hypertrophic cartilage (hc) is calcified and provides the niche for vascular invasion. (D) Hypertrophic chondrocytes undergo apoptosis and attract blood vessels (v) that bring in precursors of osteoblasts, which secret collagen I into ECM, and build the calcified bone (b) in the primary ossification center (poc). Perichondrial cells adjacent to hypertrophic chondrocytes also become osteoblasts, forming bone collar (bc). Chondrocytes continue to proliferate and differentiate in the growth plate that drives the bone elongation. Chondrocytes differentiate into four different cell subtypes, including resting (Rc), proliferative (Plc), prehypertrophic (Phc), and hypertrophic chondrocyte (Hc). (E) At the end of the bone, the secondary ossification center (soc) forms through cycles of chondrocyte hypertrophy, vascular invasion, and osteoblast activity. The soc separates the epiphyseal cartilage from the growth plate cartilage. (F) Epiphyseal cartilage becomes articular cartilage (ac) at the end of bone. In humans the primary and secondary ossification centers fuse after puberty, whereas in adult mice a narrow region of growth plate cartilage remains. (G) During cartilage development, TGFβ signaling promotes mesenchymal cell condensation and differentiation into chondrocytes, and maintains resting chondrocytes in a quiescent stage. It prevents chondrocytes from further differentiating into proliferative cells, and inhibits prehypertrophic and hypertrophic differentiation. In articular cartilage, TGFβ signaling cooperates with BMP signaling to stimulate anabolic function of chondrocytes, for example, enhancing collagen II (Col2) and aggrecan expression. On the contrary, TGFβ signaling antagonizes BMP signaling to prevent calcification and catabolic gene expression, such as collagen X (ColX) and matrix metallopeptidase 13 (MMP13).
GROWTH PLATE CARTILAGE FORMATION
Cells at the core of the condensations differentiate into chondrocytes. This involves a change in morphology from fibroblast-like to more spherical, along with a significant increase in synthesis of specific extracellular matrix (ECM) molecules. The Sox9 mediated transcriptional program continues, with the collaboration of the structurally related transcription factors Sox5 and 6, driving the expression of collagen types II, IX, and XI, and the major proteoglycan of cartilage, aggrecan (Karsenty et al., 2009; Kronenberg, 2003; Long and Ornitz, 2013). Cells at the periphery of the condensations retain a fibroblastic morphology, and continue to express type I collagen, giving rise to the structure known as the perichondrium (for review see, Karsenty and Kronenberg, 2009).
Chondrocytes in the cores of the condensations initially undergo rapid proliferation that leads to linear growth of the developing skeletal element. Subsequently, chondrocytes in the centers of the elements exit the cell cycle and execute a well-coordinated program of maturation. This ordered process of proliferation and differentiation leads to the formation of stratified zones of cells at different stages of the cell cycle, with continued expression of Sox9 throughout the resting and proliferating chondrocytes. From the ends of the element to the center, these zones include a layer of relatively quiescent cells (resting zone) that exhibit a round cell morphology, a zone of rapidly proliferating cells that have a more flattened morphology and form stacks (columnar or proliferative zone), a zone of postmitotic cells that begin to enlarge and are characterized by the expression of Indian Hedgehog (Ihh) and decreased expression of Sox9 (prehypertrophic zone), and a zone of terminal enlarged chondrocytes (hypertrophic zone). Most of these undergo cell death, leaving an ECM that is replaced by bone-forming osteoblasts (Shapiro et al., 2005). Hypertrophic chondrocytes produce a unique mineralized ECM containing type X collagen. These cells also produce matrix metalloproteinase 13 (MMP-13), which modifies the ECM to facilitate vascular invasion. The invading vasculature permits the entry of osteoprogenitors, which differentiate into osteoblasts. These cells build the bone matrix and subsequently replace the cartilage.
ARTICULAR CARTILAGE FORMATION AND MAINTENANCE
Unlike growth plate cartilage, which is eventually replaced by bone in most species, articular cartilages are permanent structures. Articular cartilage is formed during embryonic stages at sites of joint formation, but is not replaced by bone and instead remains and develops during postnatal stages of growth (Chan et al., 2012). Articular cartilage is distinct from growth plate cartilage in terms of ECM content, cellular organization, and mechanical properties (Iwamoto et al., 2013). Briefly, there are fewer cells in this structure, and they are embedded as solitary cells within a distinct ECM that contains more collagen crosslinks than in growth plate cartilage. Mature articular cartilage has a zonal organization that is divided into a superficial layer, a mid layer, the deep layer, and the calcified layer, in order from the surface of articular cartilage toward the bone (Las Heras et al., 2012; Poole, 2003).
Within this cartilage, through mechanisms that are not well understood, the nonhypertrophic Sox trio (Sox9/5/6) program is maintained and chondrocyte differentiation is blocked, resulting in a permanent cartilage residing at the end of the long bones. However, during osteoarthritis, articular chondrocytes lose their inactive phenotype, and undertake hypertrophic chondrocyte terminal differentiation, thus expressing markers ColX and MMP-13.
The different morphologies and functions of cartilage across the lifespan are supported by the differences in proliferation and differentiation of chondrocytes, tightly controlled by many cytokines and their intracellular signaling pathways. These important cytokines include the transforming growth factor-beta (TGFβ) superfamily, Wnts, Hedgehog, Notch, and FGFs (Gao et al., 2013; Long and Ornitz, 2013; Pan et al., 2008).
OVERVIEW OF BMP/TGFβ SIGNALING
The TGFβ superfamily consists of two subfamilies. The TGFβ subfamily includes TGFβs (1, 2, and 3), Activin (A and B), Nodals, myostatin (GDF-8), and Mullerian inhibiting substance. The bone morphogenetic protein (BMP) subfamily consists of BMPs 2, 4–10, and the growth and differentiation factors (GDFs) (Gordon and Blobe, 2008; Guo and Wang, 2009; Hinck, 2012). Ligands are usually assigned to either of these subfamilies based on the utilization of the downstream signaling mediators known as the receptor-regulated Smad proteins (R-Smads). Members of the TGFβ subfamily usually transduce signals through R-Smads 2 and 3, while members of the BMP subfamily transduce signals through R-Smads 1, 5, and 8 (Burks and Cohn, 2011; Weiss and Attisano, 2013). Smad4 is a cofactor that forms a complex with the activated R-Smads from both groups; it is thought to be essential for canonical signaling. The third group includes the inhibitory Smads 6 and 7 (I-Smads), which act as inhibitors on the BMP and TGFβ signaling cascade by various mechanisms (Song et al., 2009).
TGFβ ligands initiate signaling cascades across the cell membrane by binding and assembling a receptor complex on the cell surface (Fig. 2). These complexes are assembled from serine/threonine kinase types I and II receptors (Hinck, 2012; Massague, 2012). Upon ligand binding, the type II receptor is activated and transphosphorylates the type I receptor (Song et al., 2009). The type I receptors are termed ALKs (activin receptor-like kinases), of which seven have been discovered (Hinck, 2012; ten Dijke et al., 1994; van der Kraan et al., 2009). ALK 1, 2, 3, and 6 bind BMPs and signal via the R-Smads1/5/8; ALK 4, 5, and 7 bind activins and TGFβs and signal through R-Smads2/3 (Hinck, 2012; Massague, 2012b; Weiss and Attisano, 2013). ALK5 is the canonical type I receptor for TGFβs and activates Smads2/3. However, TGFβ can also bind to ALK1 and ALK2 in some cell types to activate R-Smads 1,5,8, thus activating the BMP pathway (van der Kraan et al., 2009). There are five type II receptors, including TβRII, ActRII, ActRIIb, BMPRII, and MISRII (Hinck, 2012; Weiss and Attisano, 2013).
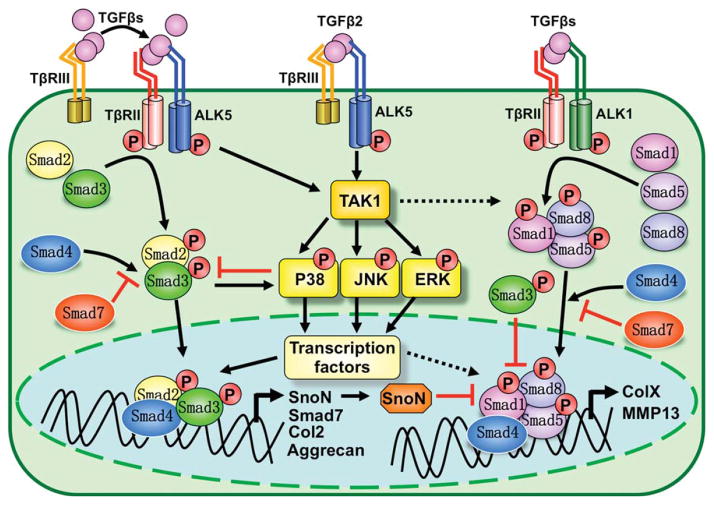
FIGURE 2.
Canonical and noncanonical TGFβ signaling pathways in cartilage formation and maintenance. Canonical Smad-dependent TGFβ signaling is initiated by TGFβ ligand binding to receptors and assembly of heteromeric complexes of type II (TβRII) and type I (ALK5) receptors. Type III (TβRII) receptor stabilizes TGFβs, particularly TGFβ2, at the cell membrane, and facilitates presentation of TGFβs to TβRII and ALK5, which are activated and then transduce signals through Smad2 and 3. Activated Smads form a complex with Smad4 and then translocate into the nucleus, where they interact with other transcription factors to regulate target gene expression. Activated ALK5 can relay signaling to noncanonical TAK1-mediated pathways, which are also the primary pathways activated by TβRIII/ALK5 complexes that do not contain TβRII. TAK1 pathways act either by converging with or repressing Smad2/3, depending on the cell context. TGFβs can also activate Smad1/5/8 (BMP) pathways through ALK1 receptor. The balance of signaling through Smad2/3 versus Smad1/5/8 thus depends on ratios of ALK1 and ALK5 expression. Smad2/3-mediated TGFβ signaling generally counteracts Sma1/5/8 (BMP) pathways through inhibiting transcriptional activity by forming mixed Smad3/Smad1/5 complexes, or though SnoN, and blocking Smad1/5/8 interaction with Smad4. TAK1 can activate and cooperate with Smad1/5/8 to regulate cartilage development, but whether and how TAK1 mediated TGFβ signaling interacts with Smad1/5/8 is still not clear.
Upon receptor activation, TGFβ/BMPs can signal through canonical and noncanonical pathways (Qiao et al., 2005; ten Dijke et al., 2002; Yoon et al., 2004). In the canonical pathway, activated type I receptors trigger phosphorylation of specific R-Smads, which then complex with Smad4, and translocate into the nucleus to direct transcriptional responses in combination with other gene specific transcription factors (Song et al., 2009). In addition, TGFβ/BMPs signal through a variety of noncanonical, Smad-independent avenues, utilizing MAP kinases, TAK1, RhoA, and mTOR pathways (Moustakas and Heldin, 2005; Mu et al., 2012; Yamaguchi et al., 1995; Yonekura et al., 1999; Zhang, 2009).
TGFβ Signaling in Cartilage Development
TGFβs play critical roles in regulating chondrocyte differentiation from early to terminal stages, including condensation, proliferation, terminal differentiation, and maintenance of articular chondrocytes (Li et al., 2005; Serra and Chang, 2003; Serra et al., 1997; van der Kraan et al., 2009; Yang et al., 2001). All three TGFβ isoforms are expressed in mesenchymal condensations. TGFβ3 is highly expressed in ribs and vertebral cartilage, whereas TGFβ1 and TGFβ2 expression is barely detected (Pelton et al., 1991). Levels of expression of all of these ligands are reduced at later stages of development in cartilage (Pelton et al., 1990, 1991). In the perichondrium, TGFβ3 is expressed at higher levels than other TGFβs (Pelton et al., 1990, 1991). In appendicular growth plates, TGFβ1 and TGFβ3 are expressed mainly in the proliferative and hypertrophic zones, whereas TGFβ2 is expressed in all zones, but at its highest levels in the hypertrophic zone (Horner et al., 1998; Millan et al., 1991; Sandberg et al., 1988; Thorp et al., 1992).
TGFβS IN PRECHONDROCYTE CONDENSATION
There is a considerable amount of in vitro evidence to indicate that TGFβ signaling pathways promote mesenchymal condensation. In vitro data demonstrate that TGFβ1 induces mesenchymal cell condensation via up-regulation of N-cadherin and fibronectin (FN) (Song et al., 2007; Tuli et al., 2003). TGFβ1 treatment initiates chondrogenesis of mesenchymal progenitor cells (Tuli et al., 2003). TGFβ2 and TGFβ3 are even more effective, causing a twofold greater accumulation of glycosaminoglycan (Barry et al., 2001). However, knockouts for individual TGFβ ligands do not exhibit phenotypes that support an essential role for TGFβs in condensation in vivo. Tgfb1 null mice that survive to birth do not exhibit any skeletal defects, but die from diffuse inflammation (Shull et al., 1992). However, at least 50% of Tgfβ1−/− embryos die prior to the onset of skeletal development (Dunker et al., 2001), raising the possibility that this ligand could play a role in early skeletal patterning on specific genetic backgrounds. This possibility can now be addressed because a conditional allele for TGFβ1 has been generated (Azhar et al., 2009).
If TGFβs play an essential role in condensation in vivo, this should be seen in mice deficient for the TGFβ receptor ALK5, as this is the primary receptor that transduces TGFβ signals. Alk5−/− mice die at midgestation, exhibiting severe defects in vascular development in the yolk sac and placenta that preclude an analysis of skeletal formation (Larsson et al., 2001). Conditional ablation of Alk5 using Dermo1-Cre, which targets skeletal progenitor cells prior to condensation, results in cartilage malformation and short limbs (Matsunobu et al., 2009). In these mice, chondrocytes proliferate and differentiate, but ectopic cartilaginous tissues protrude into the perichondrium, a phenotype related to the abnormally thin perichondrial layer. These and other studies in which the impact of TGFβ signaling has been investigated at early stages of chondrogenesis in the mouse are discussed in greater detail below, but in vivo genetic data demonstrating an essential role for TGFβ signaling in condensation stages are lacking.
TGFβ SIGNALING IN JOINT FORMATION
Several studies have revealed an essential role for TGFβ signaling through the type II receptor TβRII in appendicular and axial joint formation. Constitutive deletion of Tgfbr2 causes defects in yolk sac hematopoiesis and vasculogenesis, resulting in embryonic lethality around 10.5 days of gestation (Oshima et al., 1996). In Tgfbr2;Prx-1Cre mice, where the Prx-1 limb enhancer drives Cre recombinase expression in limb mesenchyme beginning at E9.5 and prior to condensation, loss of TβRII signaling resulted in the absence of interphalangeal joints (Spagnoli et al., 2007). The chemokine MCP-5 was recently identified as a key target for TβRII in the joint; TGFβ signaling was shown to be required to down-regulate MCP5 expression in joint interzone cells, to prevent the acquisition of a chondrogenic fate (Longobardi et al., 2012).
There are some similarities in defects in Alk5;Dermo1-Cre and Tgfbr2;Prx1-Cre conditional knockout mice. Both Dermo1-Cre and Prx1-Cre are expressed in mesenchymal progenitors, and in each case, the mice develop short-limbed dwarfism, abnormal sternums, and defects in joint formation (Seo and Serra, 2007; Spagnoli et al., 2007), suggesting that TGFβRII and ALK5 work together, likely in a complex, in mesenchymal progenitor cells. However, there is a distinction as to where joint fusion defects arise in these two mutants. Tgfbr2;Prx1-Cre mice develop fused phalangeal joints, while Alk5;Dermo1-Cre mice showed normal phalangeal joints, but partially fused knee joints (Matsunobu et al., 2009). In addition, there was a decrease in chondrocyte proliferation and a delay of late hypertrophic differentiation in Tgfbr2;Prx1-Cre mice that was not seen in Alk5;Dermo1-Cre growth plates (Matsunobu et al., 2009). The phenotypic differences in these two mouse models may result from the differences in Prx1-Cre and Dermo1-Cre expression patterns. Prx1-Cre is expressed earlier than Dermo1-Cre in limb mesenchymal progenitors (Rodda and McMahon, 2006). It is also possible that the expression levels of Dermo1-Cre and Prx1-Cre differ in mesenchyme progenitors, perichondrial cells, and chondrocytes. Finally, as discussed below, the differences between Alk5 and Tgfbr2 mutant mice may reflect the fact that these two receptors do not have to work together in all tissues to transduce TGFβ signals.
TGFβ SIGNALING IN THE GROWTH PLATE
As discussed above, mice lacking TGFβ1 do not exhibit an obvious growth plate phenotype (Kaartinen and Heisterkamp, 1995; Shull, 1992). Similarly, while loss of TGFβ3 leads to perinatal lethality, defects in chondrogenesis are not observed (Kaartinen and Heisterkamp, 1995; Proetzel, 1995). However, mice lacking TGFβ2 present with generalized chondrodysplasia that appears to have an onset at late gestation stages (Sanford, 1997). Furthermore, it has been shown that loss of TGFβ2 (but not loss of TGFβ3) prevents the ability of exogenous Shh (sonic hedgehog) to block hypertrophic differentiation in ex vivo metatarsal cultures (Alvarez, 2002). In summary, of the three TGFβ ligands, the evidence suggests that TGFβ2 may be the predominant one impacting chondrogenesis in vivo.
The evidence for a role for TGFβ signaling in the growth plate is clearer at the level of the receptors, although these roles are still not well understood. The majority of loss-of-function studies have been conducted using targeted deletions or overexpression of dominant-negative forms of Tgfbr2 (the gene encoding TβRII). Tgfbr2;Prx1-Cre growth plates display elevated Indian hedgehog (Ihh) expression, along with a delay in the onset of hypertrophy and decrease in levels of Col10a1 expression in hypertrophic cells (Spagnoli et al., 2007). This result is more severe than but consistent with the phenotype resulting from blockade of TGFβ signaling by the overexpression of a dominant negative form of TβRII (dnTβRII) in condensing mesenchymal cells and chondrocytes; this resulted in absence of hypertrophic chondrocytes at 14.5 dpc, suggesting impaired hypertrophic chondrocyte differentiation (Hiramatsu et al., 2011).
Interestingly, in contrast to the above results where deletion or transgenic overexpression of TβRII occurs prior to the onset of formation of differentiated (Col2a1-expressing) chondrocytes, conditional deletion of the TβRII in Col2a1-expressing chondrocytes did not lead to obvious defects in appendicular elements (Baffi et al., 2004). These findings strongly suggest that TβRII transduces TGFβ signaling at pre-chondrogenic stages, but may not have as substantial a role at later stages. Consistent with this speculation, defects in Tgfbr2;Col2a1-Cre mice were restricted to axial elements, where defective segmentation and formation of intervertebral discs was found (Baffi et al., 2004). In axial elements, Col2a1 is activated in somites, considerably earlier than the onset of Col2a1 expression in chondrocytes within appendicular elements.
Given the lack of severe cartilage phenotypes in appendicular elements when Tgfbr2 is ablated in differentiated chondrocytes, the question arises as to whether TGFβ signaling plays a substantial role in growth plate chondrocytes in vivo. This would be best addressed by studies in which the type I receptor ALK5 is ablated. ALK5 clearly has an important role in the formation of the perichondrium, as revealed in the Alk5;Dermo1-Cre mice discussed above (Matsunobu et al., 2009). However, the role, if any, of ALK5 in growth plate chondrocytes has not yet been investigated in vivo.
Investigating the role of TGFβ signaling at the level of ALK5 gains importance because it was shown recently that TβRII may be dispensable for TGFβ signaling in some settings; TGFβ ligands are able to elicit signals in TβRII (Tgfbr2) mutant mice (Iwata et al., 2012). Loss of Tgfbr2 in cranial neural crest cells results in elevated expression of TGFβ2 and the type III TGFβ receptor (TβRIII; also known as betaglycan). TβRIII can keep TGFβ at the cell surface and can promote signaling by presenting ligand to the TβRI/TβRII complex (Shi and Massague, 2003). However, Iwata et al. (2012) demonstrated the existence of an ALK5/TβRIII-mediated, TβRII-independent signaling pathway that was essential for proliferation in the palatal mesenchyme. In palatal mesenchyme, TβRI/TβRII guides TGFβ signaling through Smad2 and Smad3, while TβRI/TβRIII transduces a signal through the noncanonical TAK1/p38 pathway (Bernabeu et al., 2009; Iwata et al., 2012). Of interest, TβRIII has high affinity for TGFβ2, but not for TGFβ1 or TGFβ3. Thus, utilization of this TβRI/TβRIII pathway may explain why skeletal phenotypes are observed in TGFβ2-deficient mice, but not in mice lacking TGFβ1 or TGFβ3, and why loss of TβRII does not exhibit a strong phenotype in growth plate chondrocytes. Finally, it has been reported that ALK5 can form a complex with other type II receptors, such as ACTRII (Andersson et al., 2006; Rebbapragada et al., 2003; Tsuchida et al., 2008; Wu et al., 2003). Whether TGFβ relays signals through ALK5/TβRIII or ALK5/ACTRII complexes during chondrogenesis warrants further investigation.
TGFβ SIGNALING IN POSTNATAL/ARTICULAR CARTILAGE
In postnatal cartilage homeostasis, TGFβs act as inhibitors of terminal hypertrophic differentiation in chondrocytes. TGFβ1 arrests differentiation at an early stage of hypertrophy in bovine synovial explants (Shintani et al., 2013), and TGFβ3 inhibits terminal differentiation of chondrocytes from cultured mesenchymal stem cells (Mueller et al., 2010; Mueller and Tuan, 2008). In micromass culture using mouse limb bud cells, TGFβ treatment delayed chondrocyte maturation and hypertrophy, and in accordance, inhibited expression of type X collagen, VEGF, MMP13, and osteocalcin (Zhang et al., 2004). TGFβ1 prevents the terminal differentiation of epiphyseal chondrocytes into hypertrophic cells (Ballock et al., 1993). These data along with the previously discussed data showing that TβRII is required for hypertrophy at early stages, indicate that TGFβ promotes the initial stages of chondrocyte differentiation, but represses terminal hypertrophic differentiation.
TβRII has profound roles in maintaining cartilage integrity. TβRII inhibits terminal differentiation and hypertrophy in articular chondrocytes. Deletion of Tgfbr2 in early post-natal chondrocytes, achieved using tamoxifen-inducible;Tgfbr2;Col2-CreER mice, results in up-regulation of Runx2, Mmp13, and Adamts5 expression in articular cartilage tissue, leading to progressive development of an osteoarthritis (OA)-like phenotype (Shen et al., 2013). One caveat of these studies is that the ablation was carried out in 2-week old mice. No significant changes were reported in growth plate chondrocytes, strongly suggesting that the effects seen on formation of articular cartilage were direct. However, studies in which TGFβ signaling is ablated in adult articular cartilage will be needed to investigate the impact of these pathways on maintenance of articular cartilage.
Whether and how ALK5 acts in postnatal articular cartilage development and maintenance are not well understood and should be further investigated. ALK5 is expressed in murine and human cartilage, but its expression level decreases in normal aging and OA cartilage (Blaney Davidson et al., 2009). In vitro knockdown of ALK5 in articular chondrocytes leads to elevated expression of MMP13, a marker of terminally differentiated chondrocytes and a major cartilage-degrading enzyme in OA (Billinghurst et al., 1997; Blaney Davidson et al., 2009). These results strongly suggest that ALK5 plays an important role in inhibiting chondrocyte terminal differentiation in articular cartilage, but this remains to be confirmed in vivo.
It has been shown that TGFβ can activate canonical BMP pathways through engagement of ALK1 (Goumans et al., 2002), and that this pathway leads to activation of Smads1/5/8 in articular cartilage (van der Kraan et al., 2009). Moreover, the ALK1/ALK5 ratio is elevated in aged and OA articular chondrocytes as a consequence of an age-related decline in ALK5 expression (Blaney Davidson et al., 2009; van der Kraan et al., 2012). The increased ALK1/ALK5 ratio is correlated with a shift from Smad2/3 to Smad1/5/8 signaling during aging and OA in murine cartilage (Blaney Davidson et al., 2009; van der Kraan et al., 2012). Moreover, there is a significant correlation between ALK1 and MMP13 mRNA expression in the cartilage of human OA knee joints (Blaney Davidson et al., 2009). The effects of ALK1/Smad1/5/8 signaling on expression of many genes are opposite to those of ALK5/Smad2/3 in cartilage; thus, the outcome of TGFβ treatment will differ depending on the constellation of receptors in cartilage. The above discussion focuses on the role of TGFβ in articular chondrocytes. It is important to bear in mind that TGFβ signaling through Smad2/3 and Smad1/5 affects multiple joint tissues, including ligament, meniscus, subchondral bone, and synovium, and signaling through either pathway (Smad2/3) versus (Smad1/5/8) can have both protective and harmful effects (Plaas et al., 2011). Evaluation of the physiological significance of these effects with respect to joint health and OA will require additional in vivo studies.
Smad-Dependent TGFβ Signaling Functions in Cartilage
Smad2 and Smad3 are expressed throughout the growth plate. Smad2 is preferentially expressed in proliferative and prehypertrophic chondrocytes, whereas Smad3 is expressed at higher levels in prehypertrophic and hypertrophic chondrocytes. Smad4 is expressed in all zones (Billiar et al., 2004; Sakou et al., 1999). Smad2 and Smad3 have distinct roles in mediating TGFβ signaling. Smad3 binds DNA directly, whereas Smad2 regulates gene expression by interacting with Smad3 or other transcriptional factors (Massague et al., 2005). Smad2 is essential at early stages of embryonic development, while Smad3 may play a more important role in adult life (Song et al., 2009). Smad2−/− mice die at embryonic day 7.5–12.5 (Heyer et al., 1999; Nomura and Li, 1998; Waldrip et al., 1998; Weinstein et al., 1998). Smad3−/− mice are viable, but develop metastatic colorectal cancer, defects in the immune system, and present with OA-like symptoms (Datto et al., 1999; Li et al., 2006; Yang et al., 1999, 2001; Zhu et al., 1998). Smad3−/− mice develop degenerative joint disease resembling human OA, as characterized by increased chondrocyte hypertrophy and the presence of type X collagen-positive cells in articular cartilage, progressive loss of the joint surface, formation of osteophytes, and decreased production of proteoglycans in synovial joints (Yang et al., 2001).
As is the case for Tgfbr2 conditional knockout mice (Tgfbr2;Col2-cre) (Baffi et al., 2004), Smad3 conditional knockout mice (Smad3; Col2-Cre) do not exhibit profound cartilage defects at prenatal stages (Chen et al., 2012). Depletion of Smad3 in chondrocytes causes progressive articular cartilage degeneration, associated with increased expression of MMP13 and deficiency in key cartilage matrix constituents type II collagen and aggrecan (Chen et al., 2012).
The in vivo function of Smad2 in cartilage is still not clear. Overexpression of either Smad2 or Smad3 can block the spontaneous maturation observed in Smad3-deficient chondrocytes (Li et al., 2006). These data indicate that in spite of the fact that Smad2 and Smad3 regulate different sets of genes, Smad2 may partially compensate for Smad3 in preventing chondrocyte terminal differentiation. The extent to which TGFβ signaling utilizes Smad-dependent versus Smad-independent signaling in aspects of cartilage development and maintenance in vivo is as yet unclear, because direct comparisons of phenotypes using the same Cre drivers to ablate Smads versus TGFβ receptors have not been reported.
Noncanonical TGFβ Signaling in Cartilage
There exist numerous Smad-independent noncanonical pathways for transduction of TGFβ signals, including various MAPK, Rho-like GTPase, and phosphatidylinositol-3-kinase (PI3K)/AKT pathways (Moustakas and Heldin, 2005; Mu et al., 2012; Yeganeh et al., 2013; Zhang, 2009). The extent to which TGFβs mediate their effects through these pathways in cartilage in vivo is unknown, but there is solid evidence that these pathways are important for chondrogenesis.
TAK1 AND p38 IN CARTILAGE
The most extensively studied noncanonical pathways are those mediated by TGFβ activating kinase 1 (TAK1), a member of the MAPKKK family. TAK1 is activated by type I BMP and TGFβ receptors, and subsequently activates several MAP kinases (MAPKs), including p38, JNK, and ERK. Many reports have shown that MAPK activation converges with Smad signaling downstream of TGFβ to regulate cell apoptosis and epithelial–mesenchymal transition (EMT) (Holm et al., 2011; Lamouille and Derynck, 2007; Massague, 2012b; Mu et al., 2012; Wu and Hill, 2009). For example, the TAK1-JNK/p38 cascade functions in conjunction with the Smad-dependent pathway to regulate TGFβ-induced apoptosis. Moreover, siRNA knockdown of TRAF6, an upstream activator of TAK1, or treatment of cells with a chemical inhibitor of p38, efficiently blocked TGFβ-mediated apoptosis (Sorrentino et al., 2008; Yamashita et al., 2008; Yu et al., 2002). TGFβ promotes tumor growth by inducing EMT through a combination of Smad-dependent and Smad-independent effects mediated by p38 (Lee et al., 2006; Massague, 2012a; Thiery, 2003). Cooperation of Smad4 and p38 mediated signaling pathways is also required for normal tooth and palate formation (Xu et al., 2008). It is highly likely that TGFβ mediates its effects in chondrocytes through both canonical and noncanonical pathways.
In growth plate chondrocytes, TAK1 is critical for stimulating chondrocyte proliferation and differentiation. However, in developing cartilage, BMPs, rather than TGFβ, appear to be the major activators of TAK1. Whether this is the case in articular cartilage is unknown. Conditional deletion of TAK1 in cartilage (Tak1;Col2Cre) results in chondrodysplasia characterized by neonatal-onset runting, delayed formation of secondary ossification centers, and defects in formation of the elbow and tarsal joints (Greenblatt et al., 2010). Data from another research group showed that deletion of Tak1 in chondrocytes resulted in multiple developmental cartilage defects, including decreased chondrocyte proliferation and survival, delayed onset of hypertrophy, reduced MMP13 expression, and a failure to maintain interzone cells of the elbow joint (Gunnell et al., 2010). These defects resemble those seen in mice deficient for BMP receptors or ligands more than they do the phenotypes of mice deficient for components of TGFβ pathways. In accordance, chondrocytes from these mice show evidence of defective BMP signaling in vivo and in vitro. Somewhat unexpectedly, deletion of TAK1 seems to affect not only activation of the p38 MAPK signaling cascade, but also activation of canonical BMP Smads1/5/8. Deletion of Tak1 in limb mesenchyme (Tak1;Prx1Cre) resulted in widespread joint fusions, likely owing to the commitment of joint inter-zone cells to the chondrocyte lineage (Gunnell et al., 2010). Since TAK1 activates both p38/JNK/ERK MAPK and Smad1/5/8 pathways, the defects seen in Tak1;Col2Cre mice likely reflect reduced signaling through both canonical and noncanonical BMP pathways.
Although the above studies indicate that BMPs are major mediators of TAK1 pathways, TGFβ signaling also depends on TAK1 in several aspects of chondrogenesis. The most extensive data comes from studies of p38, a downstream effector of TAK1 signaling. Studies with genetically modified mice show that p38 pathways have various functions in cartilage, including inhibiting chondrocyte proliferation and differentiation, and maintaining cartilage integrity. p38 has 4 isoforms, α, β, γ, and δ, but only α, β, and γ are detectible in mouse cartilage (Li et al., 2010). Transgenic overexpression in chondrocytes of activated MKK6, a downstream mediator of TAK1 and an upstream activator of p38, resulted in dwarfism, inhibition of chondrocyte proliferation and differentiation, and a delay in primary and secondary ossification (Zhang et al., 2006). Inhibition of p38 in transgenic mice by cartilage-specific expression of a dominant-negative p38 (Col2a1-p38-DN) resulted in severely deficient endochondral bone formation and reduced limb length (Namdari et al., 2008). p38-DN heterozygotes developed osteoarthritis-like symptoms, indicating that chronic p38 deficiency is harmful to articular cartilage (Namdari et al., 2008). Whether this is due to early chondrogenesis defects that affect joint shape, or to a role for p38 in adult articular cartilage, is an important unknown.
p38 also has catabolic functions in cartilage. Increased p38 phosphorylation was found in human OA cartilage (Fan et al., 2007). Col10a1 is expressed in all hypertrophic chondrocytes, but MMP13 expression is restricted to the most terminally differentiated hypertrophic chondrocytes (Inada et al., 2004; MacLean et al., 2003). p38 inhibits Col10a1 expression (Li et al., 2010), but activates MMP13 expression (Chen et al., 2012). These results suggest that p38 prevents onset of chondrocyte hypertrophy, but stimulates their terminal differentiation, an effect also seen for TGFβ, as discussed above.
CROSSTALK BETWEEN SMAD3 AND p38 IN CARTILAGE
TGFβ can activate MAPK pathways through Smad-independent and Smad-dependent pathways. For example, a mutant TβRI receptor defective in Smad binding and activation, but retaining an intact kinase activity, is able to mediate TGFβ-induced activation of JNK and p38 through TAK1 (Itoh et al., 2003; Yu et al., 2002), indicating that Smads can be dispensable for TGFβ activation of JNK and p38. However, several studies have demonstrated that Smad2/3 and p38 act in concert to regulate aspects of chondrogenesis. For example, in vitro assays using chondrocytic ATDC5 cells showed that although TGFβ-dependent activation of p38 and ERK1/2 does not influence activation of R-Smads by TGFβ, inhibition of p38 or ERK1/2 inhibited TGFβ-induced transcriptional activity of both Smad2 and Smad4 (Watanabe et al., 2001). Hence, these studies suggest that TGFβ-induced activation of p38 or ERK1/2 is essential for transcriptional activation of Smad2 and Smad4, and for maximal activation of specific Smad-dependent transcriptional responses in ATDC5 cells (Watanabe et al., 2001).
Smad3 has been shown to modulate p38 activity. For example, Smad3 regulates p38 phosphorylation in chondrocytes. Assays using primary chondrocytes from Smad3−/− mice showed that loss of Smad3 promotes inactivation of p38, most likely by disrupting a pSmad3–p38 complex, thereby abrogating signaling through a TAK1/p38/ATF-2 pathway. Over-expression of ATF-2 or treatment with the p38 activator anisomycin inhibited expression of type X collagen, suggesting that Smad3 and p38 cooperate to repress the onset of hypertrophy (Li et al., 2010). p38 can also act independently of Smad3 to promote induction of MMP13 in growth plate chondrocytes (Chen et al., 2012). Smad3-mediated TGFβ signals transiently repress MMP13 expression. However, after 24 hr of TGFβ treatment, there is an increase of MMP13 expression; this induction is mediated by p38, but not Smad3 (Chen et al., 2012). Hence, a switch from Smad3-mediated signals to p38-mediated signals changes the outcome of TGFβ treatment from repression to activation of MMP13 expression. Whether this switch to a Smad3-independent effect correlates with a change in receptor utilization towards ALK1 is an interesting possibility.
In contrast to the above studies demonstrating cooperativity between Smad and p38 pathways, MAPK pathways negative regulate Smad signaling through phosphorylation of Smad linker sites (Fuentealba et al., 2007; Gao et al., 2009). The BMP and TGFβ type I receptors phosphorylate R-Smads at the C-terminal SXS site to initiate signal propagation. Subsequently, the R-Smad linker region is phosphorylated by MAPK, leading to a primed substrate for glycogen synthase kinase 3 (GSK3). GSK3 creates binding sites for the E3 ubiquitin protein ligases SMURF1 (SMAD-specific E3 ubiquitin protein ligase 1) or NEDD4L (neural precursor cell expressed developmentally downregulated protein 4-like), which target SMAD proteins for polyubiquitilation and proteasome-mediated degradation (Alarcon et al., 2009; Fuentealba et al., 2007; Gao et al., 2009). Besides MAPK pathways, PI3K/Akt pathway can also antagonize Smad-mediated effects. Akt can directly interact with Smad3 and inhibit Smad3-phosphorylation, nuclear localization, and Smad3-mediated transcription (Conery et al., 2004; Remy et al., 2004). The extent to which noncanonical pathways interact with Smad pathways in cartilage development remains poorly understood.
JNK, ERK, PI3K, AND RHO GTPASE PATHWAYS IN CARTILAGE
When compared with p38, there is less information on the functions of JNK and ERK pathways in cartilage. JNK1 and JNK2 are expressed in chondrocytes, but deletion of either isoform has not been associated with a skeletal phenotype (Beier and Loeser, 2010), suggesting that JNK1 and JNK2 may have overlapping functions in cartilage. High levels of activated JNK are seen in human OA cartilage (Clancy et al., 2001; Fan et al., 2007). In vitro experiments showed that inhibiting JNK blocks MMP13 expression in human chondrocytes (Im et al., 2007; Loeser et al., 2003), implicating JNK in the progression of OA.
Chondrocytes also express both ERK1 and ERK2. ERK1-null mice have no obvious skeletal or growth abnormalities (Pages et al., 1999), and ERK2 null mice die very early in embryogenesis as a result of defective trophoblast development (Saba-El-Leil et al., 2003). Constitutive activation of MEK1 in chondrocytes inhibits hypertrophic differentiation of growth plate chondrocytes, and negatively regulates bone growth without inhibiting chondrocyte proliferation, resulting in achondroplasia-like dwarfism (Murakami et al., 2004). Similar to p38 and JNK, ERK plays a role in stimulating MMP13 expression in human chondrocytes, and inhibiting ERK prevents MMP13 expression (Forsyth et al., 2002; Loeser et al., 2003). In vitro assay results showed that all three major MAP kinases (ERK1/2, p38a, and JNK1/2) must be activated at the same time to induce MMP expression, while inhibition of any one of the three is sufficient to inhibit MMP13 expression (Forsyth et al., 2002; Loeser et al., 2003).
The PI3K pathway has various stage-specific functions in cartilage development. Transgenic overexpression of an activated form of Akt, a downstream target of PI3K, in cartilage increased chondrocyte proliferation in the resting zone, and delayed hypertrophic differentiation in the growth plate, but promoted hypertrophic differentiation in craniobasal cartilaginous elements and vertebrae (Rokutanda et al., 2009). The differential effects on hypertrophy were shown to be a result of engagement of different pathways downstream of Akt. Organ culture experiments showed that Akt relays signals through mTOR, FoxO, and GSK3 pathways. The Akt–mTOR pathway was responsible for promoting chondrocyte proliferation, maturation, and cartilage matrix production. The Akt–FoxO pathway enhanced chondrocyte proliferation, but inhibited chondrocyte maturation and cartilage matrix production, while the Akt–GSK3 pathway negatively regulated three of the cellular processes in limb skeletons but not in vertebrae, as a result of less GSK3 expression in vertebrae (Rokutanda et al., 2009).
Cartilage-specific inactivation of PTEN, the main phosphatase counteracting PI3K activity, leads to profound defects in skeletal development in mice, including skeletal overgrowth, disorganization of growth plates with increasing resting cell proliferation, and fusion of the primary and secondary ossification centers (Ford-Hutchinson et al., 2007; Hsieh et al., 2009; Yang et al., 2008). As is the case for Akt, the function of PTEN varies depending on the time and location of skeletal development. The loss of PTEN delays chondrocyte hypertrophy in embryonic growth plates (Yang et al., 2008), whereas it accelerates hypertrophic chondrocyte maturation in adult mice (Ford-Hutchinson et al., 2007). Among the Akt isoforms (Akt1, Akt2, and Akt3), Akt1 is the most highly expressed in chondrocytes. Akt1−/− mice are small and have normal proliferative and hypertrophic zones, but exhibit decreased calcification in the growth plate. Akt1−/− mice formed fewer osteophytes in medial collateral ligament transection induced OA (Fukai et al., 2010).
The in vivo functions of Rho GTPases in chondrogenesis are not clear because in vivo models are lacking (e.g., conditional knockout mice). In vitro data indicate that RhoA, one of the main prototypes of Rho GTPas families, inhibits early chondrogenesis and hypertrophic chondrocyte differentiation by repressing Sox9 expression (Woods et al., 2005; Woods and Beier, 2006; Kumar and Lassar, 2009; reviewed in Beier and Loeser, 2010).
In summary, p38, JNK, ERK, PI-3 Kinase, and Rho GTPases pathways have different functions in regulating chondrocyte proliferation and differentiation according to the development stage and type of skeletal element (Table 1). p38, ERK, and PI3K pathways both promote and inhibit chondrocyte terminal differentiation. As a result, attempts at pharmacological intervention for OA using these pathways as targets must take these temporal and spatial differences in function into consideration. The extent to which these pathways are regulated by TGFβ in vivo is unknown, as is the extent to which TGFβ transduces its signals through these pathways in cartilage.
TABLE 1.
Genetic Modified Mouse Models on TGFβ Signaling on Cartilage Formation and Maintaining
| Gene | Models | Defects | References |
|---|---|---|---|
| Tgfβ1 | −/− | No defect in cartilage formation, 50% embryos die early | Shull et al. (1992), Dunker et al. (2001) |
| Tgfβ2 | −/− | Generalized chondrodysplasia | Sanford et al. (1997) |
| Tgfβ3 | −/− | No defects in chondrogenesis, perinatal lethality | Proetzel et al. (1995), Kaartinen et al. (1995) |
| Alk5 | Flox; Dermo1-Cre | Fused knee joints, short limbs and ectopic cartilaginous protrusions | Matsunobu et al. (2009) |
| Tgfbr2 | Flox; Prx1-Cre | Fused phalangeal joints, decreased chon. proliferation, delayed hypertrophic differentiation | Longobardi et al. (2012) |
| Flox; Wnt1-Cre | Craniofacial deformities | Iwata et al. (2012) | |
| Flox; Col2a1-Cre | No defect in appendicular elements, defective segmentation and formation of intervertebral discs | Baffi et al. (2004) | |
| Flox; Col2-CreER | osteoarthritis(OA)-like phenotype | Shen et al. (2013) | |
| DN-Tgfbr2a | Col11a2-promoter/enhancer; Flox; Prx1-Cre | Hypoplasia, absence of hypertrophic chondrocytes at 14.5 dpc | Hiramatsu et al. (2011) |
| Smad3 | −/− | Increased chondrocyte hypertrophy, osteoarthritis-like symptoms | Yang et al. (2001) |
| Flox; Col2a1-Cre | No cartilage defects at prenatal stages, articular cartilage degeneration | Chen et al. (2012) | |
| Smad4 | −/− | Early embryonic lethal | Chu et al. (2004) |
| Flox; Col2a1-Cre | Dwarfism, disorganized growth plate, delay in chondrocyte maturation | Zhang et al. (2005) | |
| Flox; Prx1-Cre | Halted limb bud development and carpal fusions | Benazet et al. (2012) | |
| Smad7 | −/− | Early postnatal lethality, retained proliferative chondrocytes, hypocellular cores, anterior/posterior transformation. | Estrada et al. (2013) |
| Tak1 | Flox; Prx1-Cre | Survive to the weaning stag, decreased chondrocyte proliferation, delays in both the onset and progression of chondrocyte maturation, joint fusions, | Gunnell et al. (2010) |
| Flox; Col2-Cre | Die before birth, decreased chondrocyte proliferation and survival, delayed onset of hypertrophy, elbow abnormalities | Gunnell et al. (2010) | |
| Flox; Col2a1-Cre | Survive postnatally for 2–3 weeks, neonatal-onset runting, decreased chondrocyte proliferation, delayed formation of secondary ossification centers, and defects in formation of the elbow and tarsal joints | Greenblatt et al. (2010) | |
| Flox; Col2-CreER | Display severe growth retardation and OA-like phenotype | Gao et al. (2013) | |
| CA-MKK6a | Col2a1 promoter | Dwarfism, inhibition of both chondrocyte proliferation and differentiation, and a delay in primary and secondary ossification | Zhang et al. (2006) |
| DN-P38a | Col2 promoter | Dwarfism, OA-like phenotype | Namdari et al. (2008) |
| CA-MEK1a | Col2a1 promoter | Dwarfism, inhibits hypertrophic differentiation, no defect of chondrocyte proliferation | Murakami et al. (2004) |
| Akt1 | −/− | Dwarfism, exhibit decreased calcification in the growth plate with fewer osteophyte formation in OA | Fukai et al. (2010) |
| Pten | Flox; Col2a1-Cre | Dyschondroplasia, defects in chondrocyte proliferation and maturation, and exhibit aberrant neoplastic cores | Yang et al. (2008) |
CA: Constitutive active, DN: Dominant negative.
CROSSTALK BETWEEN TGF-β SIGNALING AND BMP SIGNALING
BMP pathways control nearly every aspect of chondrogenesis (Song et al., 2009; Yoon and Lyons, 2004). Thus, understanding how BMP and TGFβ pathways intersect is fundamental to understand the mechanisms controlling cartilage formation and maintenance. TGFβ enhances BMP2-induced chondrogenesis in bovine synovial explants, improves the hyaline-like properties of neocartilage, and arrests differentiation at an early stage of hypertrophy (Shintani et al., 2013). In undifferentiated ATDC5 cells, which represent a proliferative stage, TGFβ enhanced BMP signaling, while BMP2 significantly reduced levels of TGFβ signaling (Keller et al., 2011). These results suggest that TGFβ promotes BMP signaling during early chondrogenesis and cell proliferation.
On the other hand, in vivo data demonstrated that Smad3 can repress Smad1/5/8 activation to prevent chondrocyte hypertrophy (Li et al., 2006). In accordance, there is an increase in the level of pSmad1/5/8 activity with the loss of Smad3. Smad3−/− chondrocytes were more responsive to BMP2, exhibiting increased type X collagen expression, pSmad1/5/8 levels, and BMP-responsive luciferase reporter activity (Li et al., 2006). In vitro assays using MDA-MB-231 breast cancer cell lines showed that TGFβ inhibits BMP responses by inducing the formation of pSmad3–pSmad1/5 complexes, which bind to BMP-responsive elements and mediate TGFβ-induced transcriptional repression (Gronroos et al., 2012). In ATDC5 cells, TGFβ suppresses BMP signaling and chondrocyte hypertrophy via SnoN, a transcriptional corepressor (Kawamura et al., 2012). SnoN is induced by TGFβ signaling in maturing chondrocytes and suppresses the BMP-Smad signaling pathway to inhibit hypertrophic maturation of chondrocytes (Kawamura et al., 2012).
Summary and Perspectives
There has been considerable progress toward understanding the physiological functions of TGFβ signaling network components and downstream pathways during cartilage development and maintenance. However, many questions remain regarding the relative importance of various pathways downstream of TGFβ, the role of TGFβ as opposed to other growth factors in activating these pathways, and the mechanisms by which TGFβ-regulated canonical and noncanonical pathways intersect. It is clear that the composition of TGFβ receptor complexes and crosstalk between Smad and non-Smad signaling pathways determines the final cellular response. Not every signaling component, however, is well understood with regard to in vivo functions at different developmental stages. For example, the functions of ALK5, ALK1, Smad2, and Smad4 in mediating TGFβ actions in cartilage development and maintenance are still not clear. Given that TGFβ can transduce both canonical TGFβ (Smad2/3) and BMP (Smad1/5/8) signals that have fundamentally different and usually opposing effects in cartilage, understanding the extent to which TGFβ utilizes BMP pathways in vivo is also an important goal.
References
- Akiyama H, Lefebvre V. Unraveling the transcriptional regulatory machinery in chondrogenesis. J Bone Miner Metab. 2011;29:390–395. doi: 10.1007/s00774-011-0273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon C, Zaromytidou AI, Xi Q, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139:757–769. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, Sohn P, Zeng X, et al. TGFβ2 mediates the effects of Hedgehog on hypertrophic differentiation and PTHrP expression. Development. 2002;129:1913–1924. doi: 10.1242/dev.129.8.1913. [DOI] [PubMed] [Google Scholar]
- Azhar M, Yin M, Bommireddy R, et al. Generation of mice with a conditional allele for transforming growth factor beta 1 gene. Genesis. 2009;47:423–431. doi: 10.1002/dvg.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffi MO, Slattery E, Sohn P, et al. Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol. 2004;276:124–142. doi: 10.1016/j.ydbio.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Ballock RT, Heydemann A, Wakefield LM, et al. TGF-beta 1 prevents hypertrophy of epiphyseal chondrocytes: Regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev Biol. 1993;158:414–429. doi: 10.1006/dbio.1993.1200. [DOI] [PubMed] [Google Scholar]
- Barry F, Boynton RE, Liu B, et al. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- Beier F, Loeser RF. Biology and pathology of Rho GTPase, PI-3 kinase-Akt, and MAP kinase signaling pathways in chondrocytes. J Cell Biochem. 2010;110:573–580. doi: 10.1002/jcb.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazet JD, Pignatti E, Nugent A, et al. Smad4 is required to induce digit ray primordia and to initiate the aggregation and differentiation of chondrogenic progenitors in mouse limb buds. Development. 2012;139:4250–4260. doi: 10.1242/dev.084822. [DOI] [PubMed] [Google Scholar]
- Bernabeu C, Lopez-Novoa JM, Quintanilla M. The emerging role of TGF-beta superfamily coreceptors in cancer. Biochim Biophys Acta. 2009;1792:954–973. doi: 10.1016/j.bbadis.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Billiar RB, St Clair JB, Zachos NC, et al. Localization and developmental expression of the activin signal transduction proteins Smads 2, 3, and 4 in the baboon fetal ovary. Biol Reprod. 2004;70:586–592. doi: 10.1095/biolreprod.103.018598. [DOI] [PubMed] [Google Scholar]
- Billinghurst RC, Dahlberg L, Ionescu M, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Investig. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson EN, Remst DF, et al. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J Immunol. 2009;182:7937–7945. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- Burks TN, Cohn RD. Role of TGF-beta signaling in inherited and acquired myopathies. Skelet Muscle. 2011;1:19. doi: 10.1186/2044-5040-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CG, Thuillier D, Chin EN, et al. Chondrocyte-intrinsic Smad3 represses Runx2-inducible matrix metalloproteinase 13 expression to maintain articular cartilage and prevent osteoarthritis. Arthritis Rheum. 2012;64:3278–3289. doi: 10.1002/art.34566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu GC, Dunn NR, Anderson DC, et al. Differential requirements for Smad4 in TGF beta-dependent patterning of the early mouse embryo. Development. 2004;131:3501–3512. doi: 10.1242/dev.01248. [DOI] [PubMed] [Google Scholar]
- Clancy R, Rediske J, Koehne C, et al. Activation of stress-activated protein kinase in osteoarthritic cartilage: Evidence for nitric oxide dependence. Osteoarthritis Cartilage. 2001;9:294–299. doi: 10.1053/joca.2000.0388. [DOI] [PubMed] [Google Scholar]
- Conery AR, Cao Y, Thompson EA, et al. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-beta induced apoptosis. Nat Cell Biol. 2004;6:366–372. doi: 10.1038/ncb1117. [DOI] [PubMed] [Google Scholar]
- Datto MB, Frederick JP, Pan L, et al. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker N, Schuster N, Krieglstein K. TGF-beta modulates programmed cell death in the retina of the developing chick embryo. Development. 2001;128:1933–1942. doi: 10.1242/dev.128.11.1933. [DOI] [PubMed] [Google Scholar]
- Estrada KD, Wang WG, Retting KN, et al. Smad7 regulates terminal maturation of chondrocytes in the growth plate. Dev Biol. 2013;382:375–384. doi: 10.1016/j.ydbio.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Soder S, Oehler S, et al. Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am J Pathol. 2007;171:938–946. doi: 10.2353/ajpath.2007.061083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford-Hutchinson AF, Ali Z, Lines SE, et al. Inactivation of Pten in osteochondroprogenitor cells leads to epiphyseal growth plate abnormalities and skeletal overgrowth. J Bone Miner Res. 2007;22:1245–1259. doi: 10.1359/jbmr.070420. [DOI] [PubMed] [Google Scholar]
- Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46:2368–2376. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- Fuentealba LC, Eivers E, Ikeda A, et al. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai A, Kawamura N, Saito T, et al. Akt1 in murine chondrocytes controls cartilage calcification during endochondral ossification under physiologic and pathologic conditions. Arthritis Rheum. 2010;62:826–836. doi: 10.1002/art.27296. [DOI] [PubMed] [Google Scholar]
- Gao L, Sheu TJ, Dong Y, et al. TAK1 regulates Sox9 expression in chondrocytes and is essential for postnatal development of the growth plate and articular cartilages. J Cell Sci. 2013;126:5704–5713. doi: 10.1242/jcs.135483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Alarcon C, Sapkota G, et al. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol Cell. 2009;36:457–468. doi: 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta. 2008;1782:197–228. doi: 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, et al. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt MB, Shim JH, Glimcher LH. TAK1 mediates BMP signaling in cartilage. Ann NY Acad Sci. 2010;1192:385–390. doi: 10.1111/j.1749-6632.2009.05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronroos E, Kingston IJ, Ramachandran A, et al. Transforming growth factor beta inhibits bone morphogenetic protein-induced transcription through novel phosphorylated Smad1/5–Smad3 complexes. Mol Cell Biol. 2012;32:2904–2916. doi: 10.1128/MCB.00231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnell LM, Jonason JH, Loiselle AE, et al. TAK1 regulates cartilage and joint development via the MAPK and BMP signaling pathways. J Bone Miner Res. 2010;25:1784–1797. doi: 10.1002/jbmr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer J, Escalante-Alcalde D, Lia M, et al. Postgastrulation Smad2-deficient embryos show defects in embryo turning and anterior morphogenesis. Proc Natl Acad Sci USA. 1999;96:12595–12600. doi: 10.1073/pnas.96.22.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck AP. Structural studies of the TGF-betas and their receptors: Insights into evolution of the TGF-beta superfamily. FEBS Lett. 2012;586:1860–1870. doi: 10.1016/j.febslet.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K, Iwai T, Yoshikawa H, et al. Expression of dominant negative TGF-beta receptors inhibits cartilage formation in conditional transgenic mice. J Bone Miner Metab. 2011;29:493–500. doi: 10.1007/s00774-010-0248-2. [DOI] [PubMed] [Google Scholar]
- Holm TM, Habashi JP, Doyle JJ, et al. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner A, Kemp P, Summers C, et al. Expression and distribution of transforming growth factor-beta isoforms and their signaling receptors in growing human bone. Bone. 1998;23:95–102. doi: 10.1016/s8756-3282(98)00080-5. [DOI] [PubMed] [Google Scholar]
- Hsieh SC, Chen NT, Lo SH. Conditional loss of PTEN leads to skeletal abnormalities and lipoma formation. Mol Carcinogen. 2009;48:545–552. doi: 10.1002/mc.20491. [DOI] [PubMed] [Google Scholar]
- Im HJ, Muddasani P, Natarajan V, et al. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J Biol Chemistry. 2007;282:11110–11121. doi: 10.1074/jbc.M609040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada M, Wang Y, Byrne MH, et al. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci USA. 2004;101:17192–17197. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Thorikay M, Kowanetz M, et al. Elucidation of Smad requirement in transforming growth factor-beta type I receptor-induced responses. J Biol Chem. 2003;278:3751–3761. doi: 10.1074/jbc.M208258200. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Ohta Y, Larmour C, et al. Toward regeneration of articular cartilage. Birth Defects Res. 2013;99:192–202. doi: 10.1002/bdrc.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata J, Hacia JG, Suzuki A, et al. Modulation of noncanonical TGF-beta signaling prevents cleft palate in Tgfbr2 mutant mice. J Clin Investig. 2012;122:873–885. doi: 10.1172/JCI61498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler CF, et al. Abnormal lung development and cleft palate in mice lacking TGF-b3 indicates defects of epithelial–mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Ann Rev Cell Dev Biol. 2009;25:629–648. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- Kawamura I, Maeda S, Imamura K, et al. SnoN suppresses maturation of chondrocytes by mediating signal cross-talk between transforming growth factor-beta and bone morphogenetic protein pathways. J Biol Chem. 2012;287:29101–29113. doi: 10.1074/jbc.M112.349415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller B, Yang T, Chen Y, et al. Interaction of TGFbeta and BMP signaling pathways during chondrogenesis. PloS one. 2011;6:e16421. doi: 10.1371/journal.pone.0016421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Karlsson S. Transforming growth factor-beta 1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol. 1993;143:3–9. [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Lassar AB. The transcriptional activity of Sox9 in chondrocytes is regulated by RhoA signaling and actin polymerization. Mol Cell Biol. 2009;29:4262–4273. doi: 10.1128/MCB.01779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Goumans MJ, Sjostrand LJ, et al. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Las Heras F, Gahunia HK, Pritzker KP. Articular cartilage development: a molecular perspective. Orthop Clin N Am. 2012;43:155–171. doi: 10.1016/j.ocl.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, et al. The epithelial–mesenchy-mal transition: New insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TF, Darowish M, Zuscik MJ, et al. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J Bone Miner Metab. 2006;21:4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TF, Gao L, Sheu TJ, et al. Aberrant hypertrophy in Smad3-deficient murine chondrocytes is rescued by restoring transforming growth factor beta-activated kinase 1/activating transcription factor 2 signaling: A potential clinical implication for osteoarthritis. Arthritis Rheum. 2010;62:2359–2369. doi: 10.1002/art.27537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TF, O’Keefe RJ, Chen D. TGF-beta signaling in chondrocytes. Front Biosci. 2005;10:681–688. doi: 10.2741/1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser RF, Forsyth CB, Samarel AM, et al. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J Biol Chem. 2003;278:24577–24585. doi: 10.1074/jbc.M304530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harbor Perspect Biol. 2013;5:a008334. doi: 10.1101/cshperspect.a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longobardi L, Li T, Myers TJ, et al. TGF-beta type II receptor/MCP-5 axis: At the crossroad between joint and growth plate development. Dev Cell. 2012;23:71–81. doi: 10.1016/j.devcel.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean HE, Kim JI, Glimcher MJ, et al. Absence of transcription factor c-maf causes abnormal terminal differentiation of hypertrophic chondrocytes during endochondral bone development. Dev Biol. 2003;262:51–63. doi: 10.1016/s0012-1606(03)00324-5. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signaling in development and disease. FEBS Lett. 2012a;586:1833. doi: 10.1016/j.febslet.2012.05.030. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012b;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Matsunobu T, Torigoe K, Ishikawa M, et al. Critical roles of the TGF-beta type I receptor ALK5 in perichondrial formation and function, cartilage integrity, and osteoblast differentiation during growth plate development. Dev Biol. 2009;332:325–338. doi: 10.1016/j.ydbio.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan FA, Denhez F, Kondaiah P, et al. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991;111:131–143. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Mu Y, Gudey SK, Landstrom M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347:11–20. doi: 10.1007/s00441-011-1201-y. [DOI] [PubMed] [Google Scholar]
- Mueller MB, Fischer M, Zellner J, et al. Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs. 2010;192:158–166. doi: 10.1159/000313399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377–1388. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Balmes G, McKinney S, et al. Constitutive activation of MEK1 in chondrocytes causes Stat1-independent achondroplasia-like dwarfism and rescues the Fgfr3-deficient mouse phenotype. Genes Dev. 2004;18:290–305. doi: 10.1101/gad.1179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namdari S, Wei L, Moore D, et al. Reduced limb length and worsened osteoarthritis in adult mice after genetic inhibition of p38 MAP kinase activity in cartilage. Arthritis Rheum. 2008;58:3520–3529. doi: 10.1002/art.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Li E. Smad2 role in mesoderm formation, left–right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- Oshima M, Oshima H, Taketo MM. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:KC 297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- Pages G, Guerin S, Grall D, et al. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- Pan Q, Yu Y, Chen Q, et al. Sox9, a key transcription factor of bone morphogenetic protein-2-induced chondrogenesis, is activated through BMP pathway and a CCAAT box in the proximal promoter. J Cell Physiol. 2008;217:228–241. doi: 10.1002/jcp.21496. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Dickinson ME, Moses HL, et al. In situ hybridization analysis of TGF beta 3 RNA expression during mouse development: comparative studies with TGF beta 1 and beta 2. Development. 1990;110:609–620. doi: 10.1242/dev.110.2.609. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Saxena B, Jones M, et al. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991;115:1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsillides AA, Beier F. Cartilage biology in osteoarthritis—Lessons from developmental biology. Nature reviews Rheumatology. 2011;7:654–663. doi: 10.1038/nrrheum.2011.129. [DOI] [PubMed] [Google Scholar]
- Plaas A, Velasco J, Gorski DJ, et al. The relationship between fibrogenic TGFbeta1 signaling in the joint and cartilage degradation in post-injury osteoarthritis. Osteoarthritis and cartilage/OARS. Osteoarthritis Res Soc. 2011;19:1081–1090. doi: 10.1016/j.joca.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Poole AR. Biochemical/immunochemical biomarkers of osteoarthritis: Utility for prediction of incident or progressive osteoarthritis. Rheum Dis Clin N Am. 2003;29:803–818. doi: 10.1016/s0889-857x(03)00056-5. [DOI] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, et al. Transforming growth factor beta 3 is required for secondary palate fusion. Nature. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy I, Montmarquette A, Michnick SW. PKB/Akt modulates TGF-beta signalling through a direct interaction with Smad3. Nat Cell Biol. 2004;6:358–365. doi: 10.1038/ncb1113. [DOI] [PubMed] [Google Scholar]
- Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- Rokutanda S, Fujita T, Kanatani N, et al. Akt regulates skeletal development through GSK3, mTOR, and FoxOs. Dev Biol. 2009;328:78–93. doi: 10.1016/j.ydbio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Saba-El-Leil MK, Vella FD, Vernay B, et al. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 2003;4:964–968. doi: 10.1038/sj.embor.embor939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakou T, Onishi T, Yamamoto T, et al. Localization of Smads, the TGF-beta family intracellular signaling components during endochondral ossification. J Bone Miner Metab. 1999;14:1145–1152. doi: 10.1359/jbmr.1999.14.7.1145. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Autio-Harmainen H, Vuorio E. Localization of the expression of types I, III, and IV collagen, TGF-beta 1 and c-fos genes in developing human calvarial bones. Dev Biol. 1988;130:324–334. doi: 10.1016/0012-1606(88)90438-1. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, et al. TGFβ2 knockout mice have multiple developmental defects that are nonoverlapping with other TGFβ knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Serra R. Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev Biol. 2007;310:304–316. doi: 10.1016/j.ydbio.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra R, Chang C. TGF-beta signaling in human skeletal and patterning disorders. Birth Defects Res Part C. 2003;69:333–351. doi: 10.1002/bdrc.10023. [DOI] [PubMed] [Google Scholar]
- Serra R, Johnson M, Filvaroff EH, et al. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro IM, Adams CS, Freeman T, et al. Fate of the hypertrophic chondrocyte: Microenvironmental perspectives on apoptosis and survival in the epiphyseal growth plate. Birth Defects Res Part C. 2005;75:330–339. doi: 10.1002/bdrc.20057. [DOI] [PubMed] [Google Scholar]
- Shen J, Li J, Wang B, et al. Deletion of the Type II TGF-beta receptor gene in articular chondrocytes leads to a progressive OA-like phenotype in mice. Arthritis Rheum. 2013;65:3107–3119. doi: 10.1002/art.38122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shintani N, Siebenrock KA, Hunziker EB. TGF-ss1 enhances the BMP-2-induced chondrogenesis of bovine synovial explants and arrests downstream differentiation at an early stage of hypertrophy. PloS one. 2013;8:e53086. doi: 10.1371/journal.pone.0053086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor beta-1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Estrada KD, Lyons KM. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 2009;20:379–388. doi: 10.1016/j.cytogfr.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Aswad R, Kanaan RA, et al. Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-beta1 to induce mesenchymal cell condensation. J Cell Physiol. 2007;210:398–410. doi: 10.1002/jcp.20850. [DOI] [PubMed] [Google Scholar]
- Sorrentino A, Thakur N, Grimsby S, et al. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- Spagnoli A, O’Rear L, Chandler RL, et al. TGF-beta signaling is essential for joint morphogenesis. J Cell Biol. 2007;177:1105–1117. doi: 10.1083/jcb.200611031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Dijke P, Yamashita H, Ichijo H, et al. Characterization of type I receptors for transforming growth factor-beta and activin. Science. 1994;264:101–104. doi: 10.1126/science.8140412. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial–mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Thorp BH, Anderson I, Jakowlew SB. Transforming growth factor-beta 1, -beta 2 and -beta 3 in cartilage and bone cells during endochondral ossification in the chick. Development. 1992;114:907–911. doi: 10.1242/dev.114.4.907. [DOI] [PubMed] [Google Scholar]
- Tuli R, Tuli S, Nandi S, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278:41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- van der Kraan PM, Blaney Davidson EN, Blom A, et al. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: Modulation and integration of signaling pathways through receptor-Smads. Osteoarthritis Cartilage. 2009;17:1539–1545. doi: 10.1016/j.joca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- van der Kraan PM, Goumans MJ, Blaney Davidson E, et al. Age-dependent alteration of TGF-beta signalling in osteoarthritis. Cell Tissue Res. 2012;347:257–265. doi: 10.1007/s00441-011-1194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrip WR, Bikoff EK, Hoodless PA, et al. Smad2 signaling in extraembryonic tissues determines anterior–posterior polarity of the early mouse embryo. Cell. 1998;92:797–808. doi: 10.1016/s0092-8674(00)81407-5. [DOI] [PubMed] [Google Scholar]
- Watanabe H, de Caestecker MP, Yamada Y. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic ATDC5 cells. J Biol Chem. 2001;276:14466–14473. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Yang X, Li C, et al. Failure of egg cylinder elongation and mesoderm induction in mouse embryos lacking the tumor suppressor smad2. Proc Natl Acad Sci USA. 1998;95:9378–9383. doi: 10.1073/pnas.95.16.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Attisano L. The TGFbeta superfamily signaling pathway. Dev Biol. 2013;2:47–63. doi: 10.1002/wdev.86. [DOI] [PubMed] [Google Scholar]
- Woods A, Beier F. RhoA/ROCK signaling regulates chondrogenesis in a context-dependent manner. J Biol Chem. 2006;281:13134–13140. doi: 10.1074/jbc.M509433200. [DOI] [PubMed] [Google Scholar]
- Woods A, Wang G, Beier F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem. 2005;280:11626–11634. doi: 10.1074/jbc.M409158200. [DOI] [PubMed] [Google Scholar]
- Wu MY, Hill CS. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev Cell. 2009;16:329–343. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Xu X, Han J, Ito Y, et al. Ectodermal Smad4 and p38 MAPK are functionally redundant in mediating TGF-beta/BMP signaling during tooth and palate development. Dev Cell. 2008;15:322–329. doi: 10.1016/j.devcel.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Fatyol K, Jin C, et al. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol Cell. 2008;31:918–924. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Sun Q, Teng Y, et al. PTEN deficiency causes dyschondroplasia in mice by enhanced hypoxia-inducible factor 1alpha signaling and endoplasmic reticulum stress. Development. 2008;135:3587–3597. doi: 10.1242/dev.028118. [DOI] [PubMed] [Google Scholar]
- Yang X, Chen L, Xu X, et al. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Letterio JJ, Lechleider RJ, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeganeh B, Mukherjee S, Moir LM, et al. Novel non-canonical TGF-beta signaling networks: emerging roles in airway smooth muscle phenotype and function. Pulm Pharmacol Therapeut. 2013;26:50–63. doi: 10.1016/j.pupt.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Yonekura A, Osaki M, Hirota Y, et al. Transforming growth factor-beta stimulates articular chondrocyte cell growth through p44/42 MAP kinase (ERK) activation. Endocrine J. 1999;46:545–553. doi: 10.1507/endocrj.46.545. [DOI] [PubMed] [Google Scholar]
- Yoon BS, Lyons KM. Multiple functions of BMPs in chondrogenesis. J Cell Biochem. 2004;93:93–103. doi: 10.1002/jcb.20211. [DOI] [PubMed] [Google Scholar]
- Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Tan XH, Li WL, et al. Smad4 is required for the normal organization of the cartilage growth plate. Dev Biol. 2005;284:311–322. doi: 10.1016/j.ydbio.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Zhang R, Murakami S, Coustry F, et al. Constitutive activation of MKK6 in chondrocytes of transgenic mice inhibits proliferation and delays endochondral bone formation. Proc Natl Acad Sci USA. 2006;103:365–370. doi: 10.1073/pnas.0507979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ziran N, Goater JJ, et al. Primary murine limb bud mesenchymal cells in long-term culture complete chondrocyte differentiation: TGF-beta delays hypertrophy and PGE2 inhibits terminal differentiation. Bone. 2004;34:809–817. doi: 10.1016/j.bone.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Richardson JA, Parada LF, et al. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–714. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]