Abstract
This review examines current understanding of how the lymphatic vessel network can optimize lymph flow in response to various mechanical forces. Lymphatics are organized as a vascular tree, with blind-ended initial lymphatics, precollectors, prenodal collecting lymphatics, lymph nodes, postnodal collecting lymphatics and the larger trunks (thoracic duct and right lymph duct) that connect to the subclavian veins. The formation of lymph from interstitial fluid depends heavily on oscillating pressure gradients to drive fluid into initial lymphatics. Collecting lymphatics are segmented vessels with unidirectional valves, with each segment, called a lymphangion, possessing an intrinsic pumping mechanism. The lymphangions propel lymph forward against a hydrostatic pressure gradient. Fluid is returned to the central circulation both at lymph nodes and via the larger lymphatic trunks. Several recent developments are discussed, including: evidence for the active role of endothelial cells in lymph formation; recent developments on how inflow pressure, outflow pressure, and shear stress affect pump function of the lymphangion; lymphatic valve gating mechanisms; collecting lymphatic permeability; and current interpretations of the molecular mechanisms within lymphatic endothelial cells and smooth muscle. Improved understanding of the physiological mechanisms by lymphatic vessels sense mechanical stimuli, integrate the information, and generate the appropriate response is key for determining the pathogenesis of lymphatic insufficiency and developing treatments for lymphedema.
Keywords: Lymphatic endothelium, lymphatic muscle, lymphatic myogenic response, lymphatic contractile cycle, lymphedema
Introduction
The mammalian lymphatic system has an important role in overall health, collectively through its contributions to extracellular fluid and protein homeostasis, lipid transport, and immunity. Lymphatic insufficiency causes lymphedema, which in its worst form is a deforming and debilitating disease with severe swelling throughout the body. Mild forms of lymphedema are more prevalent, and certain populations, like cancer survivors, are at-risk for development of lymphedema (Beesley et al., 2007; Petrek et al., 2001). As with all living tissues, organs, and organ systems, a key characteristic of the lymphatic system is its ability to detect and respond to a variety of physical and chemical cues, in order to optimize function under varying conditions. Lymph flow can vary widely, with 10–15 fold changes recorded, when standing up from a supine position, and as high as 63-fold by foot warming in standing individuals (Olszewski et al., 1977). Others have reviewed the impact of mechanical forces on lymphatic development and lymphangiogenesis in detail (Planas-Paz and Lammert, 2013; Wiig and Swartz, 2012). This review will highlight recent advances to our understanding of how lymphatic vessels optimize lymph flow in response to mechanical forces.
Organization of the lymphatic system
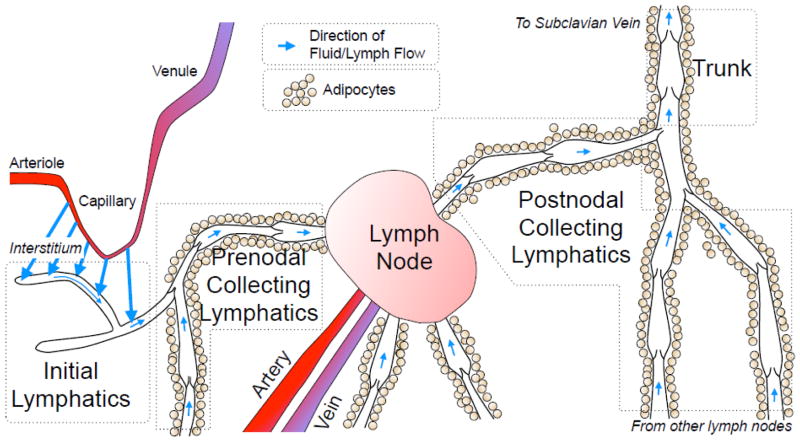
Lymphatics are organized as a vessel tree (Fig. 1), with the most distal, blind-ended vessels serving as the site where interstitial fluid enters the system to form lymph. Terms to describe these blind-ended vessels include lymphatic capillaries (based on fluid exchange function), terminal or peripheral lymphatics (both based on location at the end of the vascular tree), and initial lymphatics (based on being the site of lymph formation). The initial lymphatics are thin-walled vessels composed of a single layer of endothelial cells, with an incomplete basement membrane. They typically form interconnected networks with features that are tissue specific, like the blind-ended lacteals inside intestinal villi (a comprehensive review of tissue-specific initial lymphatic networks is featured in (Schmid-Schonbein, 1990)). The diameters of initial lymphatics vary widely depending on tissue and species. The smallest initial lymphatics reported are the lacteals within rat intestinal villi, as small as 5–15 μm in specimens fixed and viewed by electron microscopy, or 15–30 μm when filled with fluid and viewed by intravital microscopy (Lee, 1979; Ushiki, 1990). Much larger initial lymphatic diameters, as high as 450 μm, have been observed in the bat wing (which are also exceptional in that they possess a smooth muscle layer) (Webb and Nicoll, 1944). Rat mesenteric and human skin initial lymphatics typically fall within ranges of 25–50 and 35–70 μm, respectively (Fischer et al., 1996; Zweifach and Prather, 1975).
Fig. 1.
Schematic view of lymph formation and transport. The interstitial fluid formed by capillary filtration moves toward initial lymphatics (large blue arrows). This fluid then moves along the initial lymphatic network into contractile prenodal collecting lymphatics (small blue arrows). The collecting lymphatics frequently are surrounded by adipocytes, and bring lymph to one or more lymph nodes, where some of the lymph fluid is lost to the blood circulation. A single postnodal collecting lymphatic exits a lymph node, eventually coalescing with other collecting lymphatics into a larger lymph trunk. The two largest, main trunks of this vascular tree (thoracic duct, right lymph duct) empty into the left and right subclavian veins, respectively.
The endothelial cells of initial lymphatics feature specialized intercellular junctions, described as “buttons” that give each cell an “oak leaf” shape (Baluk et al., 2007; Murfee et al., 2007). These button junctions display an alternating pattern of the adhesion proteins VE-cadherin and PECAM-1, forming overlapping leaflets of cell membrane between adjacent cells (Baluk et al., 2007), and are thought to act as microscopic one-way valves, termed primary valves, that favor entry of fluids, macromolecules, and lymphocytes into the lymphatic lumen (Murfee et al., 2007; Schmid-Schonbein, 1990; Trzewik et al., 2001). In addition to this paracellular route of fluid entry, the initial lymphatic endothelial cells have significant expression of the water channel aquaporin-1, suggesting transcellular water transport across the initial lymphatic may contribute to lymph formation (Gannon and Carati, 2003).
The initial lymphatic vessel segments coalesce into larger, collecting lymphatics. The collecting lymphatics are distinguished by having both endothelial and smooth muscle layers, and an adventitia that can contain fibroblasts, connective tissue, MHC-II+ antigen presenting cells, nerves that innervate the vessel, and vasa vasorum (Bridenbaugh et al., 2013; Schmid-Schonbein, 1990; Zawieja et al., 2008). While the lymphatic smooth muscle cell organization varies between species, in general the amount of smooth muscle increases when moving centrally along the lymphatic tree. The functional importance of the smooth muscle layer is that it establishes both vessel tone and a phasic contractile cycle that drives lymph flow (von der Weid, 2001; Zawieja et al., 2008). Another important feature of collecting lymphatics is their organization into segments, termed lymphangions, which have unidirectional valves at each end. These valves, which are bicuspid flaps composed of endothelial cells and connective tissue, prevent backflow of lymph. This is of particular importance in humans standing upright, in which lymph must flow against a significant hydrostatic gradient. These luminal unidirectional valves are referred to as “secondary valves” to distinguish them from the microscopic leaflets between endothelial cells of initial lymphatics (Schmid-Schonbein, 1990; Zawieja et al., 2008). The transition from initial lymphatic to collecting lymphatic is easily distinguishable in some tissues, like mesentery. However in the skin, an intermediate network of lymphatics known as precollectors lies between the initial and collecting lymphatics. Notable features of precollectors is the appearance of secondary valves, yet there is no smooth muscle layer or phasic contractions (Schmid-Schonbein, 1990). Also, the precollector endothelial cells express lower levels of the membrane protein podoplanin than the endothelium of initial lymphatics, suggesting functional differences (Wick et al., 2008).
The anatomical pattern of collecting lymphatics, organized as a chain of lymphangions in series, combined with smooth muscle-generated contractions, enables collecting lymphatics to work as pumps. Each lymphangion is individually capable of propelling lymph forward to the next in series, in a rhythmic manner analogous to the pumping of the heart chambers, with systolic and diastolic phases (Benoit et al., 1989; Davis et al., 2012; Scallan et al., 2012). This active, intrinsic lymphatic pump plays a critical role in normal lymph flow from the extremities in humans (Olszewski and Engeset, 1980), and structural defects in the secondary valves that impair the ability of the pump to propel lymph forward results in lymphedema (Kriederman et al., 2003).
The collecting lymphatics deliver the lymph to one or more lymph nodes. These organs are meeting centers for lymphocytes and antigen-bearing cells, where the profile of antigens arriving from the feeding lymphatics can be sampled (Kogan and von Andrian, 2008). As a result, the cellular content of lymph changes dramatically at the lymph node, with the significant numbers of monocytes, macrophages, and dendritic cells found in prenodal (afferent) lymph essentially absent in postnodal (efferent) lymph (Hay and Andrade, 1998). In addition, lymphocytes exit the lymph nodes primarily through postnodal lymphatics, causing lymphocyte counts to be higher in postnodal lymph than in prenodal lymph (Kogan and von Andrian, 2008). The lymph nodes also alter the protein concentration of lymph. Protein-free fluid can cross the blood-lymph barrier when there is disequilibrium in Starling forces (hydrostatic and colloid oncotic pressures). Prenodal lymph with a relatively low colloid osmotic pressure becomes more concentrated when passing through the lymph node, due to loss of protein-free fluid into the blood. (Adair and Guyton, 1983; Adair et al., 1982). The combined modification of protein concentration and cellular composition thus make lymph in postnodal collecting lymphatics different from that seen in prenodal collecting lymphatics.
The postnodal collecting lymphatics coalesce into larger lymphatic trunks. The right lymphatic duct drains the right arm, right side of the head, and right thoracic cavity, and empties into the right subclavian vein. The thoracic duct drains lymph from all other parts of the body, emptying into the left subclavian vein (Schmid-Schonbein, 1990). Like the smaller collecting lymphatics, these vessels possess a smooth muscle layer with phasic and tonic contractile activity (Gashev et al., 2004).
Influence of mechanical forces on lymph formation
Tissue fluid volume is determined upon fluid delivery minus removal. While arteries are responsible for fluid delivery, removal by veins is incomplete due to the filtration of plasma in the capillaries and postcapillary venules. Lymphatics remove the remainder, with interstitial fluid accumulation serving as a driving force for lymph formation. When the rate of fluid entering the interstitium increases, the limited space available facilitates the hydraulic conductivity of additional fluid through the interstitium toward the lymphatics (Bert and Reed, 1995; Guyton et al., 1966). This is readily apparent in the demonstration that continuous intravenous infusion of saline, which expands plasma volume, produces a significant increase in lymph flow (Benoit et al., 1989; Rahbar et al., 2014). Interstitial fluid flow and lymph formation serve as compensatory mechanisms that establish a “margin of safety” against edema formation (Granger, 1979). Without these mechanisms, uncompensated expansion of interstitial fluid volume will lead to tissue edema. Recent advances and current understanding of the lymph formation mechanisms will be outlined here. For a more in-depth analysis, a recent review by Wiig and Swartz is an excellent resource (Wiig and Swartz, 2012).
Initial lymphatics typically lie a short distance (hundreds of μm) from the capillaries and postcapillary venules (Schmid-Schonbein, 1990). In order to produce lymph, a gradient must be established for fluid movement from these sites of blood-tissue exchange, across the interstitium, and into the lymphatic lumen. The movement of fluid and various solutes across the microvascular barrier is determined by the Starling forces (hydrostatic and osmotic pressure gradients) and the permeability of the endothelium, favoring net filtration into the interstitium (Durán et al., 2008). Pressure gradients favoring movement of interstitial fluid toward lymphatics have also been reported (Hogan, 1981b; Zhang et al., 2000). However, the mechanism for passage of fluid across the interstitium and into the lymphatics has been less straightforward.
It is generally accepted that average interstitial fluid pressure is slightly lower than atmospheric pressure, as originally observed by Guyton using subcutaneously implanted perforated capsules (Guyton, 1963). Early measurements of intraluminal pressures of mesenteric initial lymphatics were performed by Zweifach and colleagues and were on average slightly higher than atmospheric pressure (Hargens and Zweifach, 1977; Zweifach, 1973; Zweifach and Prather, 1975). Later measurements in human skin lymphatics produced comparable findings, with a range including both subatmospheric (negative) and positive values (Spiegel et al., 1992). These measurements presented the problem of an apparent uphill pressure gradient for lymph formation. An osmotic pressure gradient hypothesis was proposed, however accumulated experimental evidence has not supported this (Casley-Smith, 1975; Schmid-Schonbein, 1990; Wiig and Swartz, 2012; Zawieja et al., 2008).
An alternative explanation takes into consideration that the interstitial and lymphatic fluid pressures are not static, but fluctuate with tissue movements. The hydrostatic hypothesis postulates that oscillating movements like contraction of the heart or tidal movements of the lungs transmit force to the tissues, which ripples through the interstitium toward lymphatics. These oscillating forces transiently change the interstitial-intraluminal fluid pressure gradient, producing periodic movement into initial lymphatics. This hypothesis originated from the demonstration that arterial pulsations were required for local lymphatic removal of subcutaneously injected tracer molecules in a rabbit ear model; steady perfusion of the artery at the same mean arterial pressure stopped lymph flow (Cressman and Blalock, 1939). Transient changes in pressure gradients favoring lymph formation were subsequently shown in the bat wing, which has atypical contractile initial lymphatics (Hogan, 1981a; Hogan, 1981b). More recent evidence has come from study of rodent diaphragmatic and intercostal lymphatics, which receive cyclic pressure waves from cardiac contractions and the pulmonary tidal movements (Grimaldi et al., 2006; Moriondo et al., 2005; Negrini and Del Fabbro, 1999; Negrini and Moriondo, 2013; Negrini et al., 2004). In some of these studies, simultaneous measurement of interstitial and intralymphatic pressure revealed an apparent pressure gradient favoring lymph formation (Moriondo et al., 2005; Negrini et al., 2004). Intralymphatic pressure decreased and increased significantly during spontaneous inspiration and expiration, respectively, but not during paralysis and mechanical ventilation of the lung (Moriondo et al., 2005). Tissue deformation in other organs, due to activities such as walking, passive limb movements, or gentle skin massage also promotes lymph formation (Jacobsson and Kjellmer, 1964; McGeown et al., 1988b; Olszewski et al., 1977; Olszewski and Engeset, 1980). In heart, skeletal muscle, lung, and intestine, non-contracting initial lymphatic networks predominate, with collecting lymphatics appearing near the point where lymphatic vessels exit from the tissues (Schmid-Schonbein, 1990). Thus it is conceivable, but remains to be proven, that the lymph formation mechanism involving oscillating pressures and gradients is universal. At least one study has suggested that in the sheep hindlimb, the arterial pulsation-driven pressure gradient mechanism does not drive lymph formation (McGeown et al., 1988a), suggesting other tissue-specific mechanisms.
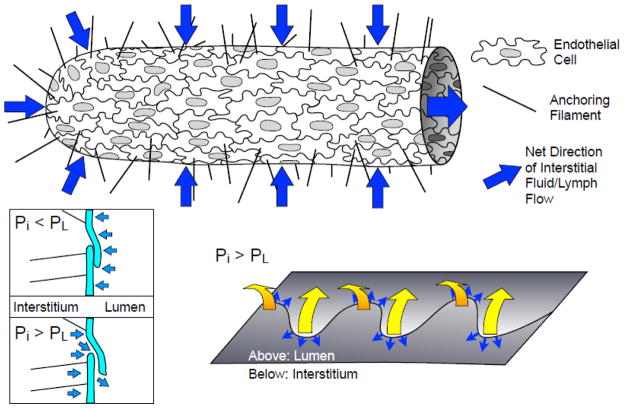
A key aspect of initial lymphatic structure that can enable lymph formation is the presence of the microscopic primary valves (Fig. 2) between endothelial cells (Lynch et al., 2007; Mendoza and Schmid-Schonbein, 2003; Schmid-Schonbein, 2003; Trzewik et al., 2001). The alternating pattern of VE-cadherin and PECAM-1 labeling at the junctions of the oak leaf-shaped initial lymphatic endothelial cells defines the locations of the primary valves (Baluk et al., 2007; Murfee et al., 2007). In the case of hydrostatic oscillations in interstitial fluid pressure, these valves would prevent back-and-forth flow of fluid between the interstitium and lymphatic lumen. While anchoring filaments help to maintain the patency of the initial lymphatic lumen, these one-way leaflet structures are thought to allow fluid to enter lymphatics when fluid pressure gradients favor lymph formation, and close when intralymphatic fluid pressure exceeds the interstitial fluid pressure (Lynch et al., 2007; Trzewik et al., 2001).
Fig. 2.
Initial lymphatics and lymph formation. The initial lymphatics are composed of a single layer of oak-leaf shaped endothelial cells with discontinuous basement membrane and anchoring filaments that project into the interstitium, and are the site of interstitial fluid entry (blue arrows). Fluid can pass through leaflets of cell membrane at adjacent endothelial cells when the interstitial pressure (Pi) exceeds luminal pressure (PL). The leaflets are closed when PL > Pi (bottom left; light blue arrows represent net pressure gradient), and open when PL < Pi. The sites where these leaflets are thought to open are the along the far edges of the “oak leaf” junction lobes (bottom right). The yellow arrows show the direction of the leaflet movement when PL < Pi and the small blue arrows show where fluid is thought to pass across these specialized lymphatic endothelial junctions.
Recent evidence also suggests active involvement of lymphatic endothelial cells in lymph formation. On one hand, transport vesicles have long been postulated to serve as a transendothelial transport mechanism (Dobbins, 1971; Dobbins and Rollins, 1970; Leak, 1971). A recent study using a bioengineered intestinal lymph lacteal model, in which an abundance of lipid-containing vesicles appeared to shuttle across endothelial cells, and basal-to-apical transport of chylomicrons was favored, strongly suggests such mechanisms contribute to lymph formation (Dixon et al., 2009). On the other hand, various chemical and physical stimuli cause lymphatic endothelial cells to alter their shape and barrier function (Breslin, 2011; Breslin and Kurtz, 2009; Breslin et al., 2007b; Cromer et al., 2013; Miteva et al., 2010). One of these studies showed that increased transmural flow across lymphatic endothelium upregulates aquaporin-2, an adaptation which may increase permeability in order to optimize drainage (Miteva et al., 2010). Future studies aimed at elucidating how lymphatic endothelial cells adapt to local mechanical forces and chemical cues will hopefully illuminate how these processes may optimize lymph flow, immune cell entry, and lipid transport.
Lymph flow in initial lymphatic networks
Initial lymphatics may connect directly to a collecting lymphatic or form hexagonal networks in which the components of lymph can move freely. The direction of net lymph flow will be determined again by fluid pressure gradients. In studies where a fluorescent tracer is subcutaneously injected in the distal tail of the mouse, a gradual uptake of this tracer into the cutaneous lymphatic network was observed, followed by central movement along the network toward the body. This gradual movement of tracer is thought to reflect the lymph flow occurring in deeper collecting lymphatics, due to the frequent connections with the initial lymphatic network (Hagendoorn et al., 2004; Swartz et al., 1996).
Impact of mechanical forces on collecting lymphatic contractions
Once lymph enters the collecting lymphatics, it must be propelled against a hydrostatic gradient. While passive compression of lymphatics due to extrinsic events like skeletal muscle movements can aid in this process, the primary mechanism for lymph flow is the intrinsic pumping of lymphangions (Olszewski and Engeset, 1980; von der Weid and Zawieja, 2004; Zawieja et al., 2008). The collecting lymphatic smooth muscle layer provides the driving force to move lymph, through its generation of a phasic contractile cycle (Fig. 3). These phasic contractions compress the lumen, producing fluid movement, and the unidirectional, secondary valve system forces the fluid movement downstream towards the central circulation (Schmid-Schonbein, 1990). The uphill gradient to be overcome may be substantial, as in the case of the human legs while standing. However, the organization of lymphatic vessels as a series of lymphangions, separated by secondary valves, prevents the large buildup of pressure due to gravity (Olszewski et al., 1977; Olszewski and Engeset, 1980). With this pattern, each lymphangion only needs to overcome the outflow pressure imposed by the subsequent, downstream lymphangion to open the downstream valve and propel its contents forward (Hargens and Zweifach, 1977). Lymphangions in series may phasically contract in a peristaltic or segmented manner; both can achieve forward of lymph (Armenio et al., 1981; Zawieja et al., 2008). Zweifach and colleagues measured pressures of mesenteric lymphatic networks and found that gradually increasing pressures in each downstream segment (Hargens and Zweifach, 1976; Hargens and Zweifach, 1977; Zweifach and Prather, 1975). In addition, they also observed that elevated luminal pressure caused an increase in phasic contraction frequency (Hargens and Zweifach, 1977). A later study by Benoit and colleagues showed that edemagenic stress caused by intravenous infusion of excess fluids also increased contraction frequency of mesenteric lymphatics (Benoit et al., 1989). As lymph formation can rise and fall, the ability of lymphangions to respond appropriately to optimize lymph flow represents an important mechanism for energy efficiency of the system (Gasheva et al., 2006).
Fig. 3.
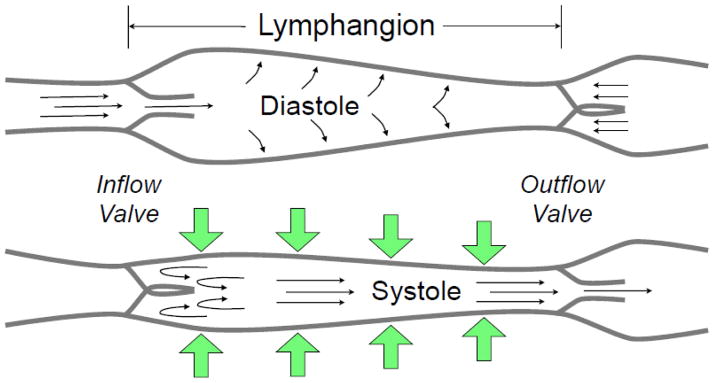
Collecting lymphatic phasic contractions. Like the heart, collecting lymphatics have a period of brisk contraction (systole) and a period of relaxation (diastole) between each phasic contraction. Each lymphangion, defined as the segment between two unidirectional valves, can typically exhibit systole and diastole. When a lymphangion is relaxed, the inflow (or upstream) valve will open (given sufficient inflow pressure) for filling of the lymphangion to occur. During systole, the phasic contraction pushes the lymph both upstream and downstream, but the inflow valve closes, so that lymph is forced forward through the outflow valve. The exact valve gating and phasic contraction frequency and force depend heavily on the outflow pressure (afterload), inflow pressure (preload), and the flow of lymph (shear stress), as discussed in the text.
The collecting lymphatic contractile cycle can be described as having periodic, phasic contractions superimposed over vessel tone, as the vessel does not relax completely between phasic contractions. As in arterioles, changes in tone can be elicited by vasodilators or vasoconstrictors, increased luminal pressure produces a myogenic constriction, and elevations in flow elicit an endothelium-dependent relaxation (Zawieja, 2009). The mechanism governing tone has some similarities with that seen in other types of smooth muscle, in that extracellular Ca2+ is a requirement and Ca2+-dependent activation of myosin light chain (MLC) kinase (MLCK) drives the development of actin-myosin mediated tonic contraction (Wang et al., 2009). Rho Kinase (ROCK), which phosphorylates the MLC phosphatase (MLCP) targeting subunit MYPT-1, leading to inactivation of MLCP, also promotes tonic constriction of lymphatics (Hosaka et al., 2003; Kurtz et al., 2014b; Souza-Smith et al., 2013). There is also evidence that PKC increases Ca2+-sensitivity by activating CPI-17-mediated inhibition of MLCP to increase tone (Dougherty et al., 2008; Dougherty et al., 2014). New evidence also suggests that lymphatic tone can be sustained by slow-cycling latch bridges (Dougherty et al., 2014), as seen in vascular smooth muscle (Dillon et al., 1981).
The mechanisms controlling phasic contractions are less clear. Action potentials and transient increases in intracellular free Ca2+ ([Ca2+]i) have been shown to precede phasic contractions of lymphatic vessels (Imtiaz et al., 2007; Shirasawa and Benoit, 2003; Souza-Smith et al., 2011; von der Weid et al., 2008). The pacemaker may involve Ca2+-activated Cl− currents or hyperpolarization-activated inward current (McCloskey et al., 1999; Toland et al., 2000; von der Weid et al., 2008). In addition, recent work shows that inhibition of ROCK can prevent phasic contractions while allowing the Ca2+ transients to persist (Kurtz et al., 2014b). The shortening velocity of lymphatic smooth muscle is much greater than observed in other smooth muscle types and much closer to that of striated muscle (Benoit et al., 1989; Zhang et al., 2013). This appears to be due to the presence of cardiac muscle contractile proteins in addition to those typically found in smooth muscle. For example, fast isoforms of myosin, such as the smooth muscle B myosin heavy chain (MHC) and the fetal cardiac/skeletal slow-twitch β-MHC, have been identified within lymphatic smooth muscle (Muthuchamy et al., 2003). Cardiac Troponin-C (cTN-C) and cTN-T may also play a role in the phasic contractile nature of lymphatic vessels (Muthuchamy and Zawieja, 2008). The brisk contraction of lymphatic smooth muscle fibers, oriented circumferentially on the vessel, reduces luminal diameter, but can also cause some axial shortening of the lymphangion. Phasic contractions may be propagated to an adjacent lymphangion (upstream or downstream) through gap junctions (Zawieja et al., 1993). Recent work also shows that basal endothelial-derived NO, under conditions in which lymphatics are not stimulated by various mechanical forces or with agents like acetylcholine, reduces phasic contraction amplitude (Scallan and Davis, 2013).
Luminal Pressure and Collecting Lymphatic Contractions
Studies of the lymphatic pressure-diameter relationship show that like in blood vessels, at any given diameter the total pressure is the sum of the passive pressure due to the composition of the lymphatic wall and active pressure generated by the smooth muscle layer (Ohhashi et al., 1980; Zhang et al., 2006). Passive tension is dependent upon the connective tissue in the lymphatic wall, which contains an abundance of collagen and elastin fibers (Rahbar et al., 2012). Recent studies of the passive properties of rat mesenteric lymphatics showed that these vessels are compliant at the low intraluminal at which they typically operate (1–5 cm H2O). However, a sharp stiffness transition occurs in lymphatics at pressures above 5 cm H2O (Rahbar et al., 2012). The lymphatic wall is impacted by three main stresses. First, there is the wall tension, which is the circumferential stress (hoop stress) across the entire lymphatic wall. Second, stretching along the length of the lymphangion causes axial stress. Both of these are strongly influenced by the transmural pressure (luminal pressure minus outside pressure). The third stress on the wall is the shear stress caused by the flow of lymph parallel to and in contact with the wall (Dixon, 2010). Under normal conditions, lymphangions sense when luminal pressure and flow change, integrate the information, and readjust their pump function to handle the fluid load. In the in vivo setting, these parameters are influenced by the rate of delivery of new lymph into the lymphangion, and the load against which the lymphangion must work to propel lymph forward.
Studies utilizing isolated, cannulated collecting lymphatics have significantly advanced our understanding of how lymphangions respond to changes in lymph pressure and flow. These procedures allow for the tight control and independent manipulation of the imposed pressure and flow, chemical composition of the bathing solution, and temperature. The studies have revealed separate mechanisms for how lymphangions react to changes in 1) luminal pressure (and by extension, transmural pressure when the outside pressure is held constant), and 2) shear stress.
The response of most collecting lymphatics to changes in transmural pressure involves intrinsic readjustment of both tonic and phasic contractions. Generally, an elevation in transmural pressure increases both the frequency and force of phasic contractions, until a maximum is reached at which phasic contractions become weaker against the increasing load (Davis et al., 2009b; Eisenhoffer et al., 1994; McHale and Roddie, 1976; Ohhashi et al., 1980; Reddy and Staub, 1981; Zhang et al., 2007). The development of more sophisticated protocols to impose step increases in luminal pressure revealed a myogenic constriction characterized by a reduction of the end-diastolic diameter and indicative of a stretch-induced increase in lymphatic tone (Davis et al., 2009a; Souza-Smith et al., 2010). A recent series of elegant experiments by Davis and colleagues has examined the contributions of both inflow pressure (preload) and outflow pressure (afterload) on isolated collecting lymphatics with two secondary valves. The protocols utilized step or ramp changes in either of these pressures, while also monitoring the luminal pressure of the lymphangion between the two valves with a smaller servo-null pipette. The pressure data were recorded together with video that was used to obtain the intraluminal diameters in multiple segments, and open/closed state of the valves (Davis et al., 2012; Scallan et al., 2013b; Scallan et al., 2012).
First considering afterload, some isolated rat mesenteric lymphangions possess the ability to propel lymph forward when outflow pressures are as high as 12 cm H2O when an inflow pressure of 1 cm H2O is imposed. In these lymphangions, when the inflow pressure was clamped at 1 cm H2O while ramping the outflow pressure from 1 to 12 cm H2O, the output segment distended while the preceding lymphangion showed a slight myogenic constriction. Both segments displayed increased contraction frequency and decreased phasic contraction amplitude (due to a progressive rise in end systolic diameter) (Davis et al., 2012; Scallan et al., 2013b). The decrease in amplitude is due to the greater amount of stroke work needed to compress the lumen against higher pressures (Zhang et al., 2007). As the luminal pressure increased, with each phasic contraction, the central lymphangion was able to produce a slightly higher pressure than the outflow pressure, opening its outflow valve. (Davis et al., 2012). The pressure-volume loops constructed from the data in this study resembled those typically observed for the heart. Quantification of end-systolic pressure-volume relationship (ESPVR) showed that step increases in outflow pressure caused a leftward shift, while the rate of rise in luminal pressure during systole was also increased, suggesting that lymphatic smooth muscle contractility increases in response to elevated afterload, or homeometric autoregulation (Davis et al., 2012).
Considering preload, several studies have shown that increased inflow pressure enhances pump function of chains of lymphangions by the Frank-Starling mechanism (heterometric autoregulation) (Eisenhoffer et al., 1995; Elias et al., 1990; Li et al., 1998; McHale and Roddie, 1976). Recent studies by Scallan and colleagues tested how single lymphangions respond to increases in preload (Scallan et al., 2012). Step increases in inflow pressure increased the luminal pressure, phasic contraction frequency, and end diastolic diameter, and initially decreased phasic contraction amplitude and stroke volume, which both recovered after a few minutes. The pressure-volume loop analysis revealed a leftward shift in ESPVR, suggesting an increase in lymphatic contractility (Scallan et al., 2012). In addition, in some lymphatics in which afterload was progressively increased by a ramp protocol, a critical pressure could be reached at which they failed to eject their contents, resulting in pump failure. The pressure-volume loops for these lymphangions changed drastically as they reached a limiting outflow that resulted in negative stroke work, i.e. retrograde filling (Davis et al., 2012). This limiting afterload for successful ejection is dependent upon preload. In addition, when preload is very small, an afterload-induced increase in contractility may be insufficient to cause ejection due to low luminal pressure. In this case, a lymphangion could accommodate, by regulating diastolic filling, to increase contraction strength to overcome the afterload (Scallan et al., 2012). These studies show that the response of single lymphangions to preload conditions is complex and involves multiple mechanisms, including both the Frank-Starling mechanism and true changes in contractility.
The cellular mechanism for the myogenic response of collecting lymphatics must involve the ability of the smooth muscle cells to sense stretch, yet a specific mechanism for this in lymphatics has not yet been identified. However, downstream signals have been identified, such as changes in [Ca2+]i oscillations after step increases in luminal pressure (Shirasawa and Benoit, 2003). Recent studies show an increased frequency of calcium transients coupled to the elevated contraction frequency, and a gradual increase in the diastolic [Ca2+]i (between transients) that correlated with the myogenic constriction (Souza-Smith et al., 2011; Souza-Smith et al., 2013). Downstream, both MLCK and RhoA/ROCK have been implicated by studies utilizing pharmacologic agents (Hosaka et al., 2003; Souza-Smith et al., 2013; Wang et al., 2009). An additional recent observation of particular interest is that pressure increases in a downstream lymphangion can cause myogenic constriction and increased phasic contraction frequency and force in the preceding lymphangion (Scallan et al., 2013b). This response can occur even if the valve separating the two lymphangions is closed, indicating the signal must be transmitted via the lymphatic wall. Normal function of the downstream lymphangion’s smooth muscle layer was critical, because if it is selectively impaired by a kinase inhibitor or by pharmacologic hyperpolarization, the upstream response was also inhabited (Scallan et al., 2013b). The cellular and molecular mechanisms that mediate lymphatic myogenic constriction and relaxation represent an important future area of focus for the field.
Fluid Shear Stress from Lymph Flow and Collecting Lymphatic Contractions
Like small arteries and arterioles, collecting lymphatics possess a flow-mediated vasodilator response. Studies using lymphatics from different tissues of the rat, in which flow changes were imposed, while luminal pressure was held constant, revealed a flow-induced inhibition of the lymphatic pump (Gashev et al., 2004; Gashev et al., 2002; Gasheva et al., 2006). This response has been considered as an energy-conserving mechanism for periods when lymph formation is high and pressure gradients favor forward flow. If the lymphatics were to continue to pump phasically under such conditions, this activity would be counterproductive, as the periodic constrictions in diameter would actually increase resistance to forward to flow (Gashev et al., 2004; Gashev et al., 2002). Several studies have identified that the mechanism for flow-mediated modulation of the lymphatic contractile cycle requires endothelial production of NO (Gashev et al., 2002; Mizuno et al., 1998; Shirasawa et al., 2000; von der Weid et al., 1996; von der Weid et al., 2001). Presumably the action of NO is activation of soluble guanylate cyclase, accelerated production of cGMP, and activation protein kinase G (Gasheva et al., 2013; Kurtz et al., 2014a). Interestingly, in aged rats (22 months old), shear stress-induced relaxation of lymphatic smooth muscle is only partially inhibited by blockade of NO synthase, unlike the 9-month old controls (Nagai et al., 2011). In the aged rats, histamine appears to also serve as an endothelium-derived lymphatic relaxing factor (Nizamutdinova et al., 2014).
The flow of lymph through collecting lymphatics is influenced by both extrinsic and intrinsic forces. The intrinsic forces generated by phasic contractile activity of lymphangions is characterized by brief periods of rapid forward movement of fluid and an interphase of either no flow or very slow flow in the forward or reverse direction (Dixon, 2010; Dixon et al., 2007; Dixon et al., 2006; Dixon et al., 2005). Experiments in which an electrode sensitive for NO was placed adjacent to a pumping lymphatic in the rat mesentery in vivo showed that with each phasic contraction, the brief increase in luminal fluid shear stress caused a local increase in NO synthesis (Bohlen et al., 2009). This mechanism may assist in timing the local relaxation of lymphatic smooth muscle during the contractile cycle. Extrinsic forces, such as very high rates of lymph formation, may cause periods of forward flow (observed by movement of leukocytes contained within the lymph) in the absence of but lymphangion pumping. Inhibition of NO, or deletion of eNOS, has been shown to attenuate the forward movement of lymph in such cases (Hagendoorn et al., 2004). A recent study of collecting lymphatic vessels isolated from eNOS−/− mice suggests that basal NO (unstimulated conditions) only impacts phasic contraction amplitude, while elevated NO (acetylcholine stimulation) affects all other aspects of lymphatic contractility (Scallan and Davis, 2013), suggesting that the action of NO is context-specific.
Collecting Lymphatic Valve Gating
The requirement of the secondary valve system for forward flow of lymph is well established, but only recently have secondary valves become on object of intense study. This interest has been driven in part by the discovery that certain genetic mutations, such as those of the FOXC2 gene that cause lymphedema distichiasis, produce malformation of the secondary valves (Brice et al., 2005; Mellor et al., 2011; Petrova et al., 2004). FoxC2+/− mice recapitulate the symptoms of the human disease (Kriederman et al., 2003).
The secondary valves are thought to act as passive gates to retrograde fluid flow largely through passive mechanisms determined by their structure, low Reynolds number and highly viscous flow, and the trans-valve pressure gradient (Schmid-Schonbein, 1990). The valve leaflets are composed to a layer of endothelial cells, folded so that the basal sides of the cells face each other, with a matrix composed in large part of elastin fibers in between. At the base of each valve, collagen fibers support the interface between the leaflets and lymphatic wall (Mazzoni et al., 1987; Rahbar et al., 2012). Recent studies have also provided new insights about the valve gating mechanisms. When no trans-valve pressure gradient is present, both the inflow and outflow valves of a lymphangion typically remain open for much of diastole. In contrast, when the outflow pressure is higher than the inflow, the gating pattern of the valves is more like the ventricular valves in the cardiac cycle (Davis et al., 2011). In the same study, it was also observed that a certain minimal pressure gradient (inflow pressure < outflow pressure) is required for valve closure, and that this gradient is dependent upon the degree to which the vessel is distended. In addition, the valves could be opened in the presence of a negative trans-valve pressure gradient (inflow pressure < outflow pressure) that was smaller than the pressure gradient for closing but was also dependent to a lesser degree on vessel distension (Davis et al., 2011). Combined, these data indicate that the valves display hysteresis, have a bias to remain open, and the trans-valve pressure gradients for valve closure are dependent upon transmural pressure. Additional data also indicates that lymphatic vessel tone can impact valve gating (Scallan et al., 2013a). These characteristics imply a complex gating pattern that is susceptible to failure at high outflow pressures (Bertram et al., 2014; Bertram et al., 2011; Bertram et al., 2013). Indeed, a “valve lock” mechanism can occur, in which an output valve remains open when phasic contractions at high outflow pressures become insufficient to drive lymph forward (Scallan et al., 2013b). With the valve locked open, the outflow pressure would then be transmitted through the lymphangion to the inflow valve. If this series of events were to repeat multiple times in upstream lymphangions, the chain of lymphangions that normally prevents against large pressure gradient formation would be lost, causing catastrophic failure of the pumping mechanism (Scallan et al., 2013b).
Permeability of Collecting Lymphatics
In recent years the rat mesenteric collecting lymphatic wall was also shown to also be permeable to macromolecules (Scallan and Huxley, 2010). Of particular interest, the collecting lymphatic permeability could be altered by atrial and brain natriuretic peptides, indicating a potential physiological function for this lymphatic-tissue exchange (Scallan et al., 2013a). Likewise, the impacts of inflammatory agonists or pharmacological agents on the permeability/barrier function of cultured lymphatic endothelial cells, grown as monolayers or in tubes, have also been reported (Breslin, 2011; Breslin et al., 2007b; Cromer et al., 2013; Price et al., 2008). The permeability coefficients for albumin determined from single-perfused rat mesenteric lymphatic vessels lie within the same range as those for postcapillary venules (Scallan and Huxley, 2010). Like postcapillary venules, collecting lymphatics have continuous VE-cadherin labeling at their endothelial intercellular junctions (Baluk et al., 2007; Kurtz et al., 2014a; Wong et al., 1999). This pattern is also seen in cultured lymphatic endothelial cells, grown either as monolayers or in tubes (Breslin et al., 2007a; Price et al., 2008). Enhancing the continuous pattern of VE-cadherin at junctions by experimentally elevating cAMP caused a concomitant decrease in permeability (Price et al., 2008). Thus, VE-cadherin crucial role in limiting paracellular transport across the lymphatic vessel wall as it does in the central circulation (Corada et al., 2001).
The determination of permeability to albumin in single-perfused rat mesenteric lymphatics was performed over a range of luminal hydrostatic pressures, corresponding to the native pressure measured prior to each experiment (Scallan and Huxley, 2010). Plotting the permeability data against luminal hydrostatic pressure revealed a positive correlation that would be expected if fluid and solute move together through common pathways (Huxley and Scallan, 2011; Scallan and Huxley, 2010). In addition, the repeated increases and decreases in luminal hydrostatic pressure, when balanced with the other Starling forces, suggest that the direction of albumin flux across the lymphatic wall could go in either direction. The existing data suggest that albumin flux is outwards except when the luminal pressure falls below ~2 cm H2O (Huxley and Scallan, 2011; Scallan and Huxley, 2010).
Another mechanical force that may influence permeability of collecting lymphatic vessels is shear stress on the endothelium. While studies utilizing intact lymphatic vessels remain to be performed, a study of cultured lymphatic endothelial cells grown in flow chambers suggests that shear stress influences barrier function (Breslin and Kurtz, 2009). In that study, step increases in shear stress enhanced barrier function, while stepping shear stress back down to baseline caused a reduction in barrier integrity. While the mechanism is largely unknown, inhibition of the small GTPase Rac1, which directs cytoskeletal reorganization at intercellular junctions, attenuated the shear-induced barrier enhancement (Breslin and Kurtz, 2009). While adaptations of the initial lymphatic endothelial barrier can optimize lymph formation, it is less clear at this point what physiological role permeability of the collecting lymphatic wall may have in signaling to the surrounding tissues. In both cases, much remains to be discovered about what cellular and molecular mechanisms control lymphatic permeability.
Summary and Perspective
Significant advances have been made in the last decade to our understanding of lymphatic vessel function. Most of these advances have uncovered previously unseen phenomena by utilizing innovative techniques to visualize lymphatic function in vivo or perform technically challenging measurements on isolated lymphatic vessels. Recent innovations to study pump function of lymphatic vessels isolated from mice opens the door to a wide variety of transgenic and knockout models to expand our knowledge (Liao et al., 2011; Scallan and Davis, 2013). New techniques to non-invasively study lymphatic function in both animals and humans are also emerging and will aid with understanding human lymphedema (Aldrich et al., 2012; Maus et al., 2012; Nelson et al., 2014; Rasmussen et al., 2010; Tan et al., 2011; Weiler and Dixon, 2013; Weiler et al., 2012). These new approaches will help with the continued need to investigate the cellular and molecular mechanisms by which lymphatic vessels respond to mechanical cues to optimize lymph flow.
Highlights.
The impact of mechanical forces within the body on lymph flow is discussed.
Lymph formation involves pressure gradients and endothelial cell activities.
Preload, afterload, and shear stress affect contractility in collecting lymphatics.
Lympahtic tone and trans-valve pressures affect valve gating.
Peptide hormaones can change collecting lymphatic permeabilty to macromolecules.
Acknowledgments
The author is supported by the National Heart, Lung, and Blood Institute, and the National Institute on Alcohol Abuse and Alcoholism under the National Institutes of Health, under award numbers R01HL098215 and R21AA020049.
Footnotes
The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adair TH, Guyton AC. Modification of lymph by lymph nodes. II. Effect of increased lymph node venous blood pressure. Am J Physiol. 1983;245:H616–22. doi: 10.1152/ajpheart.1983.245.4.H616. [DOI] [PubMed] [Google Scholar]
- Adair TH, et al. Quantitation of changes in lymph protein concentration during lymph node transit. Am J Physiol. 1982;243:H351–9. doi: 10.1152/ajpheart.1982.243.3.H351. [DOI] [PubMed] [Google Scholar]
- Aldrich MB, et al. Lymphatic abnormalities in the normal contralateral arms of subjects with breast cancer-related lymphedema as assessed by near-infrared fluorescent imaging. Biomed Opt Express. 2012;3:1256–65. doi: 10.1364/BOE.3.001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenio S, et al. Spontaneous contractility in the human lymph vessels. Lymphology. 1981;14:173–8. [PubMed] [Google Scholar]
- Baluk P, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–62. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley V, et al. Lymphedema after gynecological cancer treatment: prevalence, correlates, and supportive care needs. Cancer. 2007;109:2607–14. doi: 10.1002/cncr.22684. [DOI] [PubMed] [Google Scholar]
- Benoit JN, et al. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol. 1989;257:H2059–69. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- Bert JL, Reed RK. Flow conductivity of rat dermis is determined by hydration. Biorheology. 1995;32:17–27. doi: 10.3233/bir-1995-32102. [DOI] [PubMed] [Google Scholar]
- Bertram CD, et al. Development of a model of a multi-lymphangion lymphatic vessel incorporating realistic and measured parameter values. Biomech Model Mechanobiol. 2014;13:401–16. doi: 10.1007/s10237-013-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram CD, et al. Simulation of a chain of collapsible contracting lymphangions with progressive valve closure. J Biomech Eng. 2011;133:011008. doi: 10.1115/1.4002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram CD, et al. Incorporating measured valve properties into a numerical model of a lymphatic vessel. Comput Methods Biomech Biomed Engin. 2013 doi: 10.1080/10255842.2012.753066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen HG, et al. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol. 2009;297:H1319–28. doi: 10.1152/ajpheart.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin JW. ROCK and cAMP promote lymphatic endothelial cell barrier integrity and modulate histamine and thrombin-induced barrier dysfunction. Lymphat Res Biol. 2011;9:3–11. doi: 10.1089/lrb.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin JW, et al. Vascular endothelial growth factor-C stimulates the lymphatic pump by a VEGF receptor-3-dependent mechanism. Am J Physiol Heart Circ Physiol. 2007a;293:H709–18. doi: 10.1152/ajpheart.00102.2007. [DOI] [PubMed] [Google Scholar]
- Breslin JW, Kurtz KM. Lymphatic endothelial cells adapt their barrier function in response to changes in shear stress. Lymphat Res Biol. 2009;7:229–37. doi: 10.1089/lrb.2009.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin JW, et al. VEGF-C alters barrier function of cultured lymphatic endothelial cells through a VEGFR-3-dependent mechanism. Lymphat Res Biol. 2007b;5:105–13. doi: 10.1089/lrb.2007.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice G, et al. Milroy disease and the VEGFR-3 mutation phenotype. J Med Genet. 2005;42:98–102. doi: 10.1136/jmg.2004.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridenbaugh EA, et al. An immunological fingerprint differentiates muscular lymphatics from arteries and veins. Lymphat Res Biol. 2013;11:155–71. doi: 10.1089/lrb.2013.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casley-Smith JR. A theoretical support for the transport of macromolecules by osmotic flow across a leaky membrane against a concentration gradient. Microvasc Res. 1975;9:43–8. doi: 10.1016/0026-2862(75)90050-3. [DOI] [PubMed] [Google Scholar]
- Corada M, et al. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–84. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- Cressman RD, Blalock A. The effect of the pulse upon the flow of lymph. Proc Soc Exp Biol Med. 1939;41:140–144. [Google Scholar]
- Cromer WE, et al. The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis. 2013 doi: 10.1007/s10456-013-9393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, et al. Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol. 2009a;296:H293–302. doi: 10.1152/ajpheart.01040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, et al. Rate-sensitive contractile responses of lymphatic vessels to circumferential stretch. J Physiol. 2009b;587:165–82. doi: 10.1113/jphysiol.2008.162438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, et al. Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol Heart Circ Physiol. 2011;301:H48–60. doi: 10.1152/ajpheart.00133.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, et al. Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am J Physiol Heart Circ Physiol. 2012;303:H795–808. doi: 10.1152/ajpheart.01097.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon PF, et al. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science. 1981;211:495–7. doi: 10.1126/science.6893872. [DOI] [PubMed] [Google Scholar]
- Dixon JB. Lymphatic lipid transport: sewer or subway? Trends Endocrinol Metab. 2010;21:480–7. doi: 10.1016/j.tem.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB, et al. Image correlation algorithm for measuring lymphocyte velocity and diameter changes in contracting microlymphatics. Ann Biomed Eng. 2007;35:387–96. doi: 10.1007/s10439-006-9225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB, et al. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- Dixon JB, et al. A tissue-engineered model of the intestinal lacteal for evaluating lipid transport by lymphatics. Biotechnol Bioeng. 2009;103:1224–35. doi: 10.1002/bit.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB, et al. Measuring microlymphatic flow using fast video microscopy. J Biomed Opt. 2005;10:064016. doi: 10.1117/1.2135791. [DOI] [PubMed] [Google Scholar]
- Dobbins WO., 3rd Intestinal mucosal lacteal in transport of macromolecules and chylomicrons. Am J Clin Nutr. 1971;24:77–90. doi: 10.1093/ajcn/24.1.77. [DOI] [PubMed] [Google Scholar]
- Dobbins WO, 3rd, Rollins EL. Intestinal mucosal lymphatic permeability: an electron microscopic study of endothelial vesicles and cell junctions. J Ultrastruct Res. 1970;33:29–59. doi: 10.1016/s0022-5320(70)90117-6. [DOI] [PubMed] [Google Scholar]
- Dougherty PJ, et al. Calcium sensitivity and cooperativity of permeabilized rat mesenteric lymphatics. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1524–32. doi: 10.1152/ajpregu.00888.2007. [DOI] [PubMed] [Google Scholar]
- Dougherty PJ, et al. PKC activation increases calcium sensitivity of permeabilized lymphatic muscle via myosin light chain 20 phosphorylation dependent and independent mechanisms. Am J Physiol Heart Circ Physiol. 2014 doi: 10.1152/ajpheart.00732.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán WN, et al. Microcirculatory Exchange Function. In: Tuma RF, et al., editors. Handbook of Physiology: Microcirculation. Academic Press - Elsevier; San Diego, CA: 2008. pp. 81–124. [Google Scholar]
- Eisenhoffer J, et al. Importance of valves and lymphangion contractions in determining pressure gradients in isolated lymphatics exposed to elevations in outflow pressure. Microvasc Res. 1995;49:97–110. doi: 10.1006/mvre.1995.1008. [DOI] [PubMed] [Google Scholar]
- Eisenhoffer J, et al. Pressure-flow relationships in isolated sheep prenodal lymphatic vessels. Am J Physiol. 1994;267:H938–43. doi: 10.1152/ajpheart.1994.267.3.H938. [DOI] [PubMed] [Google Scholar]
- Elias RM, et al. Lymphatic pumping in response to changes in transmural pressure is modulated by erythrolysate/hemoglobin. Circ Res. 1990;67:1097–106. doi: 10.1161/01.res.67.5.1097. [DOI] [PubMed] [Google Scholar]
- Fischer M, et al. Flow velocity of single lymphatic capillaries in human skin. Am J Physiol. 1996;270:H358–63. doi: 10.1152/ajpheart.1996.270.1.H358. [DOI] [PubMed] [Google Scholar]
- Gannon BJ, Carati CJ. Endothelial distribution of the membrane water channel molecule aquaporin-1: implications for tissue and lymph fluid physiology? Lymphat Res Biol. 2003;1:55–66. doi: 10.1089/15396850360495709. [DOI] [PubMed] [Google Scholar]
- Gashev AA, et al. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation. 2004;11:477–92. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- Gashev AA, et al. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol. 2002;540:1023–37. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasheva OY, et al. Cyclic guanosine monophosphate and the dependent protein kinase regulate lymphatic contractility in rat thoracic duct. J Physiol. 2013;591:4549–65. doi: 10.1113/jphysiol.2013.258681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasheva OY, et al. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol. 2006;575:821–32. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger HJ. Role of the interstitial matrix and lymphatic pump in regulation of transcapillary fluid balance. Microvasc Res. 1979;18:209–16. doi: 10.1016/0026-2862(79)90029-3. [DOI] [PubMed] [Google Scholar]
- Grimaldi A, et al. Functional arrangement of rat diaphragmatic initial lymphatic network. Am J Physiol Heart Circ Physiol. 2006;291:H876–85. doi: 10.1152/ajpheart.01276.2005. [DOI] [PubMed] [Google Scholar]
- Guyton AC. A concept of negative interstitial pressure based on pressures in implanted perforated capsules. Circ Res. 1963;12:399–414. doi: 10.1161/01.res.12.4.399. [DOI] [PubMed] [Google Scholar]
- Guyton AC, et al. Interstitial fluid pressure. 3. Its effect on resistance to tissue fluid mobility. Circ Res. 1966;19:412–9. doi: 10.1161/01.res.19.2.412. [DOI] [PubMed] [Google Scholar]
- Hagendoorn J, et al. Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circ Res. 2004;95:204–9. doi: 10.1161/01.RES.0000135549.72828.24. [DOI] [PubMed] [Google Scholar]
- Hargens AR, Zweifach BW. Transport between blood and peripheral lymph in intestine. Microvasc Res. 1976;11:89–101. doi: 10.1016/0026-2862(76)90080-7. [DOI] [PubMed] [Google Scholar]
- Hargens AR, Zweifach BW. Contractile stimuli in collecting lymph vessels. Am J Physiol. 1977;233:H57–65. doi: 10.1152/ajpheart.1977.233.1.H57. [DOI] [PubMed] [Google Scholar]
- Hay JB, Andrade WN. Lymphocyte recirculation, exercise, and immune responses. Can J Physiol Pharmacol. 1998;76:490–6. doi: 10.1139/cjpp-76-5-490. [DOI] [PubMed] [Google Scholar]
- Hogan RD. The initial lymphatics and tissue fluid pressure. In: Hargens AR, editor. Tissue Fluid Pressure and Composition. Williams and Wilkins; Baltimore, MD: 1981a. pp. 155–163. [Google Scholar]
- Hogan RD. Lymph formation in the bat wing. In: Garlick D, editor. Progress in Microcirculation Research First Australasian Symposium on the Microcirculation Sydney 1980. University of New South Wales; Kensington: 1981b. pp. 261–282. [Google Scholar]
- Hosaka K, et al. Rho-Rho kinase pathway is involved in the regulation of myogenic tone and pump activity in isolated lymph vessels. Am J Physiol Heart Circ Physiol. 2003;284:H2015–25. doi: 10.1152/ajpheart.00763.2002. [DOI] [PubMed] [Google Scholar]
- Huxley VH, Scallan J. Lymphatic fluid: exchange mechanisms and regulation. J Physiol. 2011;589:2935–43. doi: 10.1113/jphysiol.2011.208298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz MS, et al. Pacemaking through Ca2+ stores interacting as coupled oscillators via membrane depolarization. Biophys J. 2007;92:3843–61. doi: 10.1529/biophysj.106.095687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson S, Kjellmer I. Flow and Protein Content of Lymph in Resting and Exercising Skeletal Muscle. Acta Physiol Scand. 1964;60:278–85. doi: 10.1111/j.1748-1716.1964.tb02889.x. [DOI] [PubMed] [Google Scholar]
- Kogan AN, von Andrian UH. Lymphocyte Trafficking. In: Tuma RF, et al., editors. Handbook of Physiology: Microcirculation. Academic Press - Elsevier; San Diego, CA: 2008. pp. 449–482. [Google Scholar]
- Kriederman BM, et al. FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema-distichiasis syndrome. Hum Mol Genet. 2003;12:1179–85. doi: 10.1093/hmg/ddg123. [DOI] [PubMed] [Google Scholar]
- Kurtz KH, et al. Involvement of H1 and H2 receptors and soluble guanylate cyclase in histamine-induced relaxation of rat mesenteric collecting lymphatics. Microcirculation. 2014a doi: 10.1111/micc.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz KH, et al. Rho kinase enhances contractions of rat mesenteric collecting lymphatics. PLoS One. 2014b;9:e94082. doi: 10.1371/journal.pone.0094082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak LV. Studies on the permeability of lymphatic capillaries. J Cell Biol. 1971;50:300–23. doi: 10.1083/jcb.50.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS. Lymph capillary pressure of rat intestinal villi during fluid absorption. Am J Physiol. 1979;237:E301–7. doi: 10.1152/ajpendo.1979.237.3.E301. [DOI] [PubMed] [Google Scholar]
- Li B, et al. Pressure-volume relationships in sheep mesenteric lymphatic vessels in situ: response to hypovolemia. Microvasc Res. 1998;56:127–38. doi: 10.1006/mvre.1998.2089. [DOI] [PubMed] [Google Scholar]
- Liao S, et al. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci U S A. 2011;108:18784–9. doi: 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch PM, et al. The primary valves in the initial lymphatics during inflammation. Lymphat Res Biol. 2007;5:3–10. doi: 10.1089/lrb.2007.5102. [DOI] [PubMed] [Google Scholar]
- Maus EA, et al. Near-infrared fluorescence imaging of lymphatics in head and neck lymphedema. Head Neck. 2012;34:448–53. doi: 10.1002/hed.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni MC, et al. Structure of lymphatic valves in the spinotrapezius muscle of the rat. Blood Vessels. 1987;24:304–12. doi: 10.1159/000158707. [DOI] [PubMed] [Google Scholar]
- McCloskey KD, et al. Hyperpolarisation-activated inward current in isolated sheep mesenteric lymphatic smooth muscle. J Physiol. 1999;521(Pt 1):201–11. doi: 10.1111/j.1469-7793.1999.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeown JG, et al. Arterial pulsation and lymph formation in an isolated sheep hindlimb preparation. J Physiol. 1988a;405:595–604. doi: 10.1113/jphysiol.1988.sp017350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeown JG, et al. Effects of varying patterns of external compression on lymph flow in the hindlimb of the anaesthetized sheep. J Physiol. 1988b;397:449–57. doi: 10.1113/jphysiol.1988.sp017011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol. 1976;261:255–69. doi: 10.1113/jphysiol.1976.sp011557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor RH, et al. Mutations in FOXC2 in humans (lymphoedema distichiasis syndrome) cause lymphatic dysfunction on dependency. J Vasc Res. 2011;48:397–407. doi: 10.1159/000323484. [DOI] [PubMed] [Google Scholar]
- Mendoza E, Schmid-Schonbein GW. A model for mechanics of primary lymphatic valves. J Biomech Eng. 2003;125:407–14. doi: 10.1115/1.1568128. [DOI] [PubMed] [Google Scholar]
- Miteva DO, et al. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res. 2010;106:920–31. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno R, et al. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol. 1998;274:R790–6. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- Moriondo A, et al. Transmural pressure in rat initial subpleural lymphatics during spontaneous or mechanical ventilation. Am J Physiol Heart Circ Physiol. 2005;289:H263–9. doi: 10.1152/ajpheart.00060.2005. [DOI] [PubMed] [Google Scholar]
- Murfee WL, et al. Discontinuous expression of endothelial cell adhesion molecules along initial lymphatic vessels in mesentery: the primary valve structure. Lymphat Res Biol. 2007;5:81–9. doi: 10.1089/lrb.2007.1005. [DOI] [PubMed] [Google Scholar]
- Muthuchamy M, et al. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J. 2003;17:920–2. doi: 10.1096/fj.02-0626fje. [DOI] [PubMed] [Google Scholar]
- Muthuchamy M, Zawieja D. Molecular regulation of lymphatic contractility. Ann N Y Acad Sci. 2008;1131:89–99. doi: 10.1196/annals.1413.008. [DOI] [PubMed] [Google Scholar]
- Nagai T, et al. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation. 2011;18:463–73. doi: 10.1111/j.1549-8719.2011.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini D, Del Fabbro M. Subatmospheric pressure in the rabbit pleural lymphatic network. J Physiol. 1999;520(Pt 3):761–9. doi: 10.1111/j.1469-7793.1999.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini D, Moriondo A. Pleural function and lymphatics. Acta Physiol (Oxf) 2013;207:244–59. doi: 10.1111/apha.12016. [DOI] [PubMed] [Google Scholar]
- Negrini D, et al. Transmural pressure during cardiogenic oscillations in rodent diaphragmatic lymphatic vessels. Lymphat Res Biol. 2004;2:69–81. doi: 10.1089/lrb.2004.2.69. [DOI] [PubMed] [Google Scholar]
- Nelson TS, et al. Minimally invasive method for determining the effective lymphatic pumping pressure in rats using near-infrared imaging. Am J Physiol Regul Integr Comp Physiol. 2014;306:R281–90. doi: 10.1152/ajpregu.00369.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizamutdinova IT, et al. Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels. Microcirculation. 2014 doi: 10.1111/micc.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohhashi T, et al. Active and passive mechanical characteristics of bovine mesenteric lymphatics. Am J Physiol. 1980;239:H88–95. doi: 10.1152/ajpheart.1980.239.1.H88. [DOI] [PubMed] [Google Scholar]
- Olszewski W, et al. Flow and composition of leg lymph in normal men during venous stasis, muscular activity and local hyperthermia. Acta Physiol Scand. 1977;99:149–55. doi: 10.1111/j.1748-1716.1977.tb10365.x. [DOI] [PubMed] [Google Scholar]
- Olszewski WL, Engeset A. Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am J Physiol. 1980;239:H775–83. doi: 10.1152/ajpheart.1980.239.6.H775. [DOI] [PubMed] [Google Scholar]
- Petrek JA, et al. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92:1368–77. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Petrova TV, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–81. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- Planas-Paz L, Lammert E. Mechanical forces in lymphatic vascular development and disease. Cell Mol Life Sci. 2013;70:4341–54. doi: 10.1007/s00018-013-1358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GM, et al. Effect of cyclic AMP on barrier function of human lymphatic microvascular tubes. Microvasc Res. 2008;76:46–51. doi: 10.1016/j.mvr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbar E, et al. Lymph transport in rat mesenteric lymphatics experiencing edemagenic stress. Microcirculation. 2014 doi: 10.1111/micc.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbar E, et al. Passive pressure-diameter relationship and structural composition of rat mesenteric lymphangions. Lymphat Res Biol. 2012;10:152–63. doi: 10.1089/lrb.2011.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JC, et al. Human Lymphatic Architecture and Dynamic Transport Imaged Using Near-infrared Fluorescence. Transl Oncol. 2010;3:362–72. doi: 10.1593/tlo.10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy NP, Staub NC. Intrinsic propulsive activity of thoracic duct perfused in anesthetized dogs. Microvasc Res. 1981;21:183–92. doi: 10.1016/0026-2862(81)90031-5. [DOI] [PubMed] [Google Scholar]
- Scallan JP, Davis MJ. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J Physiol. 2013;591:2139–56. doi: 10.1113/jphysiol.2012.250662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, et al. Permeability and contractile responses of collecting lymphatic vessels elicited by atrial and brain natriuretic peptides. J Physiol. 2013a;591:5071–81. doi: 10.1113/jphysiol.2013.260042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, Huxley VH. In vivo determination of collecting lymphatic vessel permeability to albumin: a role for lymphatics in exchange. J Physiol. 2010;588:243–54. doi: 10.1113/jphysiol.2009.179622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, et al. Constriction of isolated collecting lymphatic vessels in response to acute increases in downstream pressure. J Physiol. 2013b;591:443–59. doi: 10.1113/jphysiol.2012.237909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, et al. Independent and interactive effects of preload and afterload on the pump function of the isolated lymphangion. Am J Physiol Heart Circ Physiol. 2012;303:H809–24. doi: 10.1152/ajpheart.01098.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- Schmid-Schonbein GW. The second valve system in lymphatics. Lymphat Res Biol. 2003;1:25–9. doi: 10.1089/15396850360495664. discussion 29–31. [DOI] [PubMed] [Google Scholar]
- Shirasawa Y, Benoit JN. Stretch-induced calcium sensitization of rat lymphatic smooth muscle. Am J Physiol Heart Circ Physiol. 2003;285:H2573–7. doi: 10.1152/ajpheart.00002.2003. [DOI] [PubMed] [Google Scholar]
- Shirasawa Y, et al. Physiological roles of endogenous nitric oxide in lymphatic pump activity of rat mesentery in vivo. Am J Physiol Gastrointest Liver Physiol. 2000;278:G551–6. doi: 10.1152/ajpgi.2000.278.4.G551. [DOI] [PubMed] [Google Scholar]
- Souza-Smith FM, et al. Measurement of cytosolic Ca2+ in isolated contractile lymphatics. J Vis Exp. 2011;58:3438. doi: 10.3791/3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Smith FM, et al. Adaptation of mesenteric collecting lymphatic pump function following acute alcohol intoxication. Microcirculation. 2010;17:514–24. doi: 10.1111/j.1549-8719.2010.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Smith FM, et al. Reduced RhoA activity mediates acute alcohol intoxication-induced inhibition of lymphatic myogenic constriction despite increased cytosolic [Ca(2+)] Microcirculation. 2013;20:377–84. doi: 10.1111/micc.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel M, et al. Pressure of lymphatic capillaries in human skin. Am J Physiol. 1992;262:H1208–10. doi: 10.1152/ajpheart.1992.262.4.H1208. [DOI] [PubMed] [Google Scholar]
- Swartz MA, et al. Transport in lymphatic capillaries. I. Macroscopic measurements using residence time distribution theory. Am J Physiol. 1996;270:H324–9. doi: 10.1152/ajpheart.1996.270.1.H324. [DOI] [PubMed] [Google Scholar]
- Tan IC, et al. Assessment of lymphatic contractile function after manual lymphatic drainage using near-infrared fluorescence imaging. Arch Phys Med Rehabil. 2011;92:756–764 e1. doi: 10.1016/j.apmr.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toland HM, et al. Ca(2+)-activated Cl(−) current in sheep lymphatic smooth muscle. Am J Physiol Cell Physiol. 2000;279:C1327–35. doi: 10.1152/ajpcell.2000.279.5.C1327. [DOI] [PubMed] [Google Scholar]
- Trzewik J, et al. Evidence for a second valve system in lymphatics: endothelial microvalves. Faseb J. 2001;15:1711–7. doi: 10.1096/fj.01-0067com. [DOI] [PubMed] [Google Scholar]
- Ushiki T. The three-dimensional organization and ultrastructure of lymphatics in the rat intestinal mucosa as revealed by scanning electron microscopy after KOH-collagenase treatment. Arch Histol Cytol. 1990;53(Suppl):127–36. doi: 10.1679/aohc.53.suppl_127. [DOI] [PubMed] [Google Scholar]
- von der Weid PY. Review article: lymphatic vessel pumping and inflammation--the role of spontaneous constrictions and underlying electrical pacemaker potentials. Aliment Pharmacol Ther. 2001;15:1115–29. doi: 10.1046/j.1365-2036.2001.01037.x. [DOI] [PubMed] [Google Scholar]
- von der Weid PY, et al. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J Physiol. 1996;493:563–75. doi: 10.1113/jphysiol.1996.sp021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid PY, et al. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol. 2008;295:H1989–2000. doi: 10.1152/ajpheart.00007.2008. [DOI] [PubMed] [Google Scholar]
- von der Weid PY, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int J Biochem Cell Biol. 2004;36:1147–53. doi: 10.1016/j.biocel.2003.12.008. [DOI] [PubMed] [Google Scholar]
- von der Weid PY, et al. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol Heart Circ Physiol. 2001;280:H2707–16. doi: 10.1152/ajpheart.2001.280.6.H2707. [DOI] [PubMed] [Google Scholar]
- Wang W, et al. Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. Am J Physiol Heart Circ Physiol. 2009;297:H726–34. doi: 10.1152/ajpheart.00312.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb RL, Nicoll PA. Behavior of lymphatic vessels in the living bat. Anat Rec. 1944;88:351–367. [Google Scholar]
- Weiler M, Dixon JB. Differential transport function of lymphatic vessels in the rat tail model and the long-term effects of Indocyanine Green as assessed with near-infrared imaging. Front Physiol. 2013;4:215. doi: 10.3389/fphys.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler M, et al. Sensitivity analysis of near-infrared functional lymphatic imaging. J Biomed Opt. 2012;17:066019. doi: 10.1117/1.JBO.17.6.066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick N, et al. Lymphatic precollectors contain a novel, specialized subpopulation of podoplanin low, CCL27-expressing lymphatic endothelial cells. Am J Pathol. 2008;173:1202–9. doi: 10.2353/ajpath.2008.080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92:1005–60. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- Wong RK, et al. Cadherin-5 redistribution at sites of TNF-alpha and IFN-gamma-induced permeability in mesenteric venules. Am J Physiol. 1999;276:H736–48. doi: 10.1152/ajpheart.1999.276.2.H736. [DOI] [PubMed] [Google Scholar]
- Zawieja DC. Contractile physiology of lymphatics. Lymphat Res Biol. 2009;7:87–96. doi: 10.1089/lrb.2009.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawieja DC, et al. Distribution, propagation, and coordination of contractile activity in lymphatics. Am J Physiol. 1993;264:H1283–91. doi: 10.1152/ajpheart.1993.264.4.H1283. [DOI] [PubMed] [Google Scholar]
- Zawieja DC, et al. Microlymphatic Biology. In: Tuma RF, et al., editors. Handbook of Physiology: Microcirculation. Academic Press - Elsevier; San Diego, CA: 2008. pp. 125–158. [Google Scholar]
- Zhang R, et al. Length-tension relationships of small arteries, veins, and lymphatics from the rat mesenteric microcirculation. Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpheart.01000.2005. [DOI] [PubMed] [Google Scholar]
- Zhang R, et al. Length-dependence of lymphatic phasic contractile activity under isometric and isobaric conditions. Microcirculation. 2007;14:613–25. doi: 10.1080/10739680701436160. [DOI] [PubMed] [Google Scholar]
- Zhang R, et al. Maximum shortening velocity of lymphatic muscle approaches that of striated muscle. Am J Physiol Heart Circ Physiol. 2013;305:H1494–507. doi: 10.1152/ajpheart.00898.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WB, et al. Distribution of interstitial fluid pressure and fluid volumes in hind-limb skin of rats: relation to meridians? Clin Physiol. 2000;20:242–9. doi: 10.1046/j.1365-2281.2000.00254.x. [DOI] [PubMed] [Google Scholar]
- Zweifach BW. Micropressure measurements in the terminal lymphatics. Bibl Anat. 1973;12:361–5. [PubMed] [Google Scholar]
- Zweifach BW, Prather JW. Micromanipulation of pressure in terminal lymphatics in the mesentery. Am J Physiol. 1975;228:1326–35. doi: 10.1152/ajplegacy.1975.228.5.1326. [DOI] [PubMed] [Google Scholar]