Summary
The DNA damage response (DDR) occurs in the context of chromatin structure, and architectural features of chromatin contribute to DNA damage signaling and repair. While the role of chromatin decondensation in the DDR is established, we show here that chromatin condensation is integral to DDR signaling. We find that upon DNA damage, chromatin regions transiently expand before undergoing extensive compaction. Using a protein-chromatin tethering system to create defined chromatin domains, we show that interference with chromatin condensation results in failure to fully activate DDR. Conversely, forced induction of local chromatin condensation promotes ATM- and ATR-dependent activation of upstream DDR signaling in a break-independent manner. Finally, while persistent chromatin compaction enhanced upstream DDR signaling from irradiation-induced breaks, it reduced recovery and survival after damage. Our results demonstrate that chromatin condensation is sufficient for activation of DDR signaling and is an integral part of physiological DDR signaling.
Introduction
Upon sensing DNA damage, cells activate a complex signaling cascade termed the DNA damage response (DDR). The DDR triggers multiple cellular events including activation of DNA repair pathways, arrest of the cell cycle to allow time for repair, and in certain cases, initiation of senescence or apoptosis programs (Ciccia and Elledge, 2010). The DDR functions within the context of chromatin and alterations in the structure of chromatin, as well as chromatin modifications, have been implicated in the activation and transduction of the DDR (Lukas et al., 2011; Price and D'Andrea, 2013; Shi and Oberdoerffer, 2012). The most prominent histone modification in the DDR is phosphorylation of the histone variant H2AX by the PIKK family of kinases, including ATM, ATR and DNA-PK, which generate large chromatin domains of phosphorylated H2AX (γ-H2AX) around double-strand breaks (DSBs) (Lee and Paull, 2005; Rogakou et al., 1999; Stiff et al., 2004). The γ-H2AX mark acts as a platform for hierarchical recruitment and retention of key DDR factors, including the mediator protein MDC1, promoting amplification of the DDR by further ATM activation and consequent γ-H2AX spreading (Chapman and Jackson, 2008; Lou et al., 2006; Lukas et al., 2004; Stucki et al., 2005).
DDR activation leads to dynamic changes in chromatin structure, which contribute to the full-scale amplification and downstream functions of the DDR. Local chromatin decondensation, as well as histone reorganization and eviction have been observed after experimental induction of DSBs in mammalian cells (Berkovich et al., 2007; Kruhlak et al., 2006; Ziv et al., 2006) and expedite downstream aspects of the DDR, including signaling through the CHK1 and CHK2 effector kinases, and the engagement of repair pathways (Larsen et al., 2010; Murga et al., 2007; Murr et al., 2006; Polo et al., 2010; Smeenk et al., 2010).
A number of active chromatin processes to promote chromatin expansion for DNA repair have been proposed, including the phosphorylation and subsequent release of KAP-1, a binding partner of the structural heterochromatin protein HP1, as well as the relocalization of DNA breaks to the periphery of cytologically detectable heterochromatin domains (Chiolo et al., 2011; Goodarzi et al., 2008; Jakob et al., 2011; Ziv et al., 2006). HP1 variants themselves are also phosphorylated and released from heterochromatin regions after induction of DSBs (Ayoub et al., 2008; Dinant and Luijsterburg, 2009).
Somewhat paradoxically, proteins that promote chromatin compaction, such as HP1, KAP-1, SPOC1, su(var)3-9 methyltransferase variant 1 (SUV3-9), PDRM2 methyltransferase, macro H2A, and histone deacetylases (HDACs), have also been shown to be recruited to the sites of DSBs (Ayoub et al., 2009; Ayrapetov et al., 2014; Baldeyron et al., 2011; Khurana et al., 2014; Luijsterburg et al., 2009; Miller et al., 2010; Mund et al., 2012; Noon et al., 2010; Polo et al., 2010; Smeenk et al., 2010; Zarebski et al., 2009). Recent work suggests that a transient repressive chromatin domain enriched in the histone H3 lysine 9 di- and tri-methyl marks is established by PRDM2 and SUV3-9 methyltransferases being recruited to DNA damage sites (Ayrapetov et al., 2014; Khurana et al., 2014). H3K9me3 is known to stimulate binding and activation of the TIP60 acetyltransferase after DNA damage (Sun et al., 2009). TIP60 in turn acetylates ATM kinase, which promotes its activation (Sun et al., 2005). Interestingly, phosphorylation enhances the acetyltransferase activity of TIP60, and this modification can be induced by chromatin alterations, leading to ATM signaling independently of DNA breaks (Kaidi and Jackson, 2013). Here, we sought to directly test, in a controllable system, the role of chromatin condensation in the DDR signaling cascade and its impact on cell survival.
Results
Chromatin condensation is an integral part of the DDR
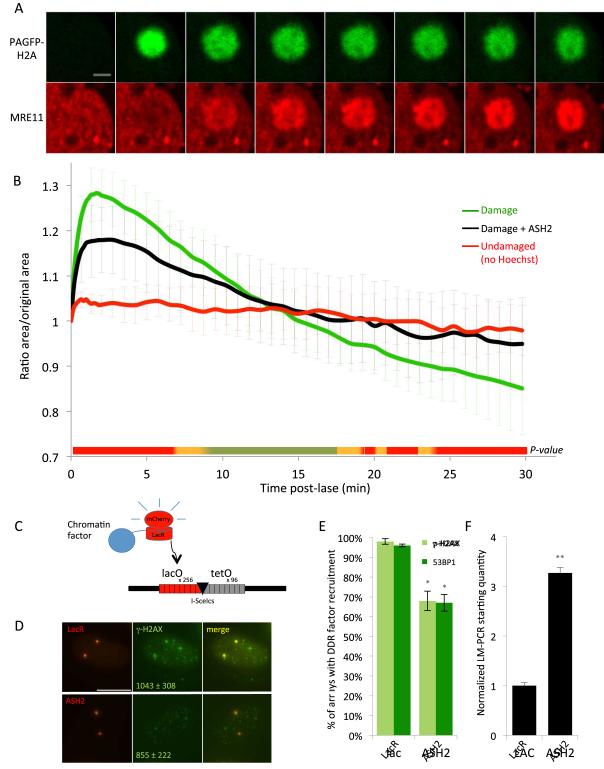
We sought to characterize changes in chromatin compaction in response to DNA damage. To this end, we used a previously characterized method based on a photo-activatable version of GFP (PAGFP) fused to the H2A core histone (Kruhlak et al., 2006). PAGFP can be activated simultaneously with laser microirradiation, allowing direct tracking of the chromatin dynamics of a damaged region (Kruhlak et al., 2006). In line with earlier observations (Kruhlak et al., 2006), upon local laser irradiation of a small spot of ~ 4.5 um in diameter, the damaged regions showed rapid expansion, reaching a maximum at about 1.5 min post-irradiation, with MRE11 recruitment detectable immediately (Fig. 1A, B; S1A). This expansion was followed by an extended linear re-compaction phase, reaching pre-damage levels by 15 min, followed by hypercondensation beyond the pre-damage baseline level by 20-30 min post-damage (Fig. 1B). No chromatin changes were observed in undamaged control cells (no Hoechst sensitization, 355 nm/405 nm laser irradiation; Fig. 1B). Damage-induced chromatin changes were dampened in both the expansion and compaction phases after overexpression of the Set1/Ash2 methyltransferase ASH2L (Fig. 1B), which globally increases the H3 lysine 4 methyl mark that is implicated in transcriptional activation and mediates chromatin expansion through recruitment of chromatin modifiers (Boyle et al., 2008; Chambeyron and Bickmore, 2004; Consortium et al., 2007; Ling et al., 2010; Luco et al., 2010; Santos-Rosa et al., 2002; Shimada et al., 2006). In agreement with recent findings (Khurana et al., 2014) we conclude that the DNA damage response involves initial expansion of chromatin followed by a phase of chromatin condensation.
Figure 1. Chromatin undergoes rapid expansion and compaction after DNA damage, and interference with these chromatin changes attenuates DDR signaling.
(A) Snapshots of a damaged chromatin region and recruitment of MRE11 at indicated timepoints. PAGFP-H2A, green; mCherry-tagged MRE11, red. Scale bar 3 μm. (B) Average area of damaged chromatin regions in vector control (green line) versus ASH2-overexpressing cells (black line) over time, an undamaged chromatin region is shown in red (no Hoechst sensitization).. p-values of ASH2 vs. control are shown in heat map below. Red, p< 0.01; orange, p<0.05; green, not significant. N >15 regions for each point. (C) Schematic of chromatin protein tethering system: 256 copies of the lac operator (lacO) and 96 copies of tet (tetO) flank an I-SceI cut site (I-SceIcs). Lac repressor fusions to either mCherry alone (LacR) or to chromatin proteins bind to the lac operator arrays after transient expression. (D) Maximum intensity projections of LacR or ASH2-tethered arrays (red) stained for γ-H2AX (green) after DSB induction by CFP-GR-I-SceI. Scale bar, 5 μm. Values show median integrated intensity of γ-H2AX at arrays ± median absolute deviation. (E) Percentage of LacR or ASH2-tethered arrays with γ-H2AX or 53BP1 enrichment 20 min post-DSB induction. Columns depict mean and error bars, SD. N ≥ 100 for each condition. *p < 0.05. (F) Ligation-mediated qPCR detecting the quantity of DSBs in I-SceI induced cells. Shown is the average ± SD of two independent experiments, each performed in triplicate. **p < 0.001. See also Supplemental Figure S1.
To directly probe the role of chromatin condensation in the DDR, we used a previously characterized Lac repressor/operator tethering system to create chromatin domains with defined properties (Soutoglou and Misteli, 2008). The system consists of ~10 kb tandem arrays of the Lac operator (lacO) sequence adjacent to an I-SceI endonuclease site stably integrated into human U2OS cells at two different chromosomal locations (Fig. 1C). Defined chromatin domains can be generated by virtue of tethering fusion proteins between chromatin modifiers and the Lac repressor (LacR) protein, which binds with high affinity to the lacO regions (Soutoglou and Misteli, 2008). Having observed an inhibitory effect of ASH2 on chromatin compaction after DNA damage (Fig. 1B), we sought to probe how interference with chromatin condensation affects DDR signaling. To this end, we tethered ASH2-LacR tagged with mCherry to the lacO arrays (Fig. 1D). As expected, tethering of ASH2 led to enrichment of the H3K4me3 mark at the array, but not at the cyclophilin A 3’ UTR site (Fig. S1B). As shown previously for the VP16 transactivator (Tumbar et al., 1999), tethering of ASH2 led to visible expansion of array sites, demonstrating local chromatin expansion (Fig. S1C). After establishment of the ASH2-expanded chromatin domain in cells, we transfected a glucocorticoid receptor-I-SceI endonuclease fusion (GR-I-SceI) into cells to synchronously and continuously induce DSB formation by addition of a synthetic GR ligand (dexamethasone), which stimulates translocation of the GR-I-SceI fusion into the nucleus (Soutoglou et al., 2007). Upon induction of DSBs in control cells expressing LacR-mCherry, γ-H2AX and 53BP1 accumulated at the I-SceI containing arrays in more than 90% of cells within 20 min (Fig. 1D, E). In contrast, the number of arrays which showed detectable accumulation of γ-H2AX and 53BP1 was reduced by ~35% in cells with expanded chromatin arrays due to tethering of ASH2 (Fig. 1D, E). In addition, the γ-H2AX signal at damaged arrays was slightly weaker in ASH2 expressing cells (median intensity: 1043 +/− 308) than in control cells (855 +/− 222, p < 0.05; Fig. 1D). Importantly, the reductions in γ-H2AX and 53BP1 were not due to lower levels of DSB induction in ASH2-expressing cells. On the contrary, ASH2-expressing cells showed an ~3-fold higher level of DSBs compared to LacR expressing control cells as determined by ligation-mediated PCR to directly quantify the total number of DSBs after 20 min of steady-state break induction (Fig. 1F; Soutoglou et al., 2007). Our finding of reduced γ-H2AX and 53BP1 recruitment to DSBs under conditions of persistent chromatin decondensation, despite higher levels of induced DSBs, points to a role of chromatin condensation in DDR signaling.
Chromatin condensation triggers DDR signaling
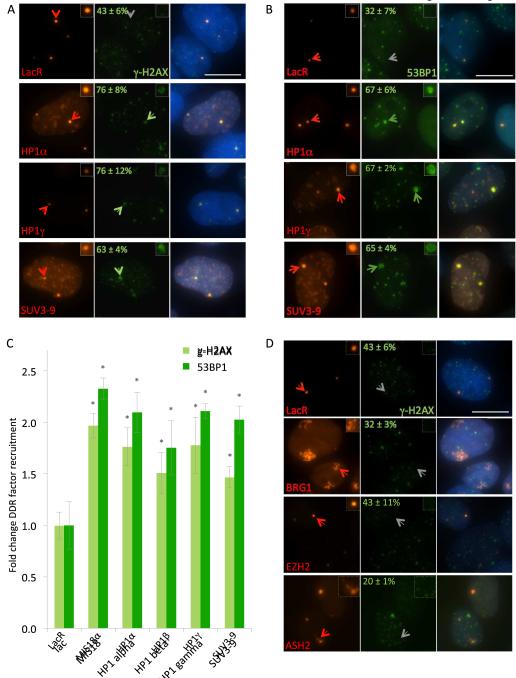
To directly assess the effect of chromatin condensation on the DDR, the heterochromatin structural protein HP1 or the su(var)3-9 methyltransferase variant 1 (SUV3-9) were tethered to the lacO repeats, since HP1 tethering to lacO has been previously shown to induce chromatin compaction and silencing as indicated by morphological and accessibility assays, as well as gene expression (Danzer and Wallrath, 2004; Li et al., 2003; Verschure et al., 2005). Consistent with condensation, tethering of these repressive chromatin proteins led to more compact arrays (Fig. S1D), while some arrays increased in size, likely due to spreading of heterochromatin by previously described cycles of nucleosome modification, structural heterochromatin protein binding, and further modification (Eissenberg and Reuter, 2009; Grewal and Jia, 2007). As expected, tethering of HP1α, HP1γ or SUV3-9 led to enrichment of the histone H3 lysine 9 tri-methyl mark (H3K9me3) at the array, as judged by chromatin immunoprecipitation (ChIP) and immunofluorescence against H3K9me3 (Fig. S2A,B). In line with chromatin compaction, tethering of heterochromatin factors also decreased accessibility to nuclease cutting (log2 SQ = −0.1 to −0.4), compared to the lac array alone, demonstrating chromatin compaction (Fig. S2C). Chromatin accessibility of distant silenced (HBB) or expressed (GAPDH) chromosomal loci was unaffected by expression and tethering of chromatin factors (Fig. S2D). Likewise, no changes in the overall levels or distribution of the H3K9me3 heterochromatin mark were detected by ChIP or immunostaining at unrelated loci (Fig. S2A,B), further confirming the site-specific, rather than global, effect of locally tethering chromatin compaction factors.
Tethering of chromatin compaction factors allowed us to directly assess the effect of chromatin condensation on DDR signaling. We found that tethering of HP1α, −β or −γ was sufficient to trigger DDR signaling, even in the absence of DNA damage (Fig. 2A). Upon tethering of HP1α, −β or −γ, ~75% of arrays showed accumulation of phospho-H2AX and ~65% showed recruitment of 53BP1 compared to 30-35% in LacR controls (Fig. 2A-C; p < 0.05). Similarly, tethering of SUV3-9 had a similar effect and led to a 2-fold increase in accumulation of γ-H2AX (63% of arrays) and recruitment of 53BP1 (65% of arrays) compared to LacR alone (Fig. 2A-C; p < 0.05). As a positive control for DDR activation, LacR-MIS18α was used, which creates DNA breaks when tethered to the array (Fig. 2C; see Fig. 3A, below). The mCherry-LacR-HP1 fusions and mCherry-LacR-SUV3-9 localized most intensely to arrays, but as expected, also appeared at sites of endogenous constitutive heterochromatin (Fig. 2A,B, S2B; Verschure et al., 2005). However, DDR factors were not co-recruited to constitutive heterochromatin, indicating specificity of the DDR trigger to the tethering array (Fig. 2A). This DDR activation was a specific response to chromatin condensation and did not reflect a response to arbitrary changes in chromatin structure since no DDR activation was observed upon chromatin decondensation in the absence of I-SceI cutting (Fig. 2D). Tethering of chromatin expansion factors, such as ASH2, VP16, or the SWI/SNF chromatin remodeler BRG1, visibly expanded the lac arrays but, in contrast to condensed arrays, did not autonomously recruit 53BP1 or induce γ-H2AX (Fig. 2D, and data not shown). Furthermore, tethering of EZH2, a polycomb family protein involved in facultative heterochromatin formation, did not recruit DDR factors (Fig. 2D), suggesting that activation of the DDR by chromatin condensation involves specific features of heterochromatin.
Figure 2. Compacted chromatin triggers DDR signaling.
(A) Maximum intensity projections of cells transfected with indicated mCherry-LacR constructs (red) stained with anti-γ-H2AX (green), and DAPI (blue). Green arrows indicate arrays enriched in γ-H2AX that are magnified 2X in the inset image. Gray arrows indicate arrays without significant γ-H2AX. Scale bar, 5 μm. Percentages ± SD of arrays staining positive for γ-H2AX are shown in the top center of each panel. (B) Images of cells as in (A), but stained with anti-53BP1 (green) (C) Quantification of γ-H2AX and 53BP1 colocalization frequency at the arrays. Values represent fold change in averages ± SD from 3 experiments (N ≥ 300 for each condition). * p < 0.05 compared to LacR alone. (D) Images of cells as in (A), but expressing indicated chromatin expansion factors or facultative heterochromatin proteins fused to the LacR protein. Scale bar, 5 μm. See also Supplemental Figure S2.
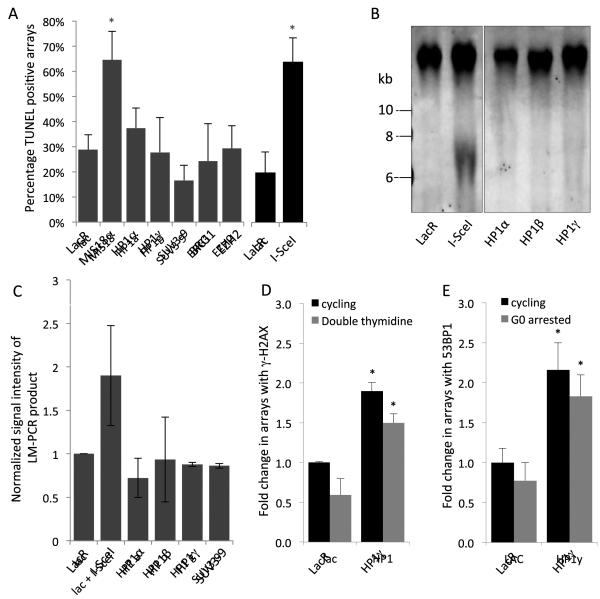
Figure 3. DDR signaling from arrays is not due to array breakage or replication defects.
(A) Quantification of arrays showing positive TUNEL signals. I-SceI transfected cells and MIS18α-arrays provide positive controls for DNA end detection. Values represent averages ± SD from at least 3 experiments. * p < 0.05 compared to lac alone, N > 200 for each. (B) Southern blot of genomic DNA isolated from indicated tethering conditions, using a probe to the lac array. I-SceI transfected cells provide positive control for breaks, unrelated lanes from the blot between I-SceI and HP1α omitted for simplicity. (C) Ligation-mediated PCR assay detecting damage within the lac array. I-SceI used as a positive control for breaks, this same DNA was used in the negative control reaction with no adaptor. Normalized signal intensity of PCR reactions are depicted by averages ± SD from 2 independent trials. (D) Cells pre-arrested in G1 by double thymidine block or (E) serum-starved prior to transfection with lac or HP1γ tethering constructs. Values represent averages ± SD of γ-H2AX or 53BP1 recruitment measured in cyclin A- or Ki67-negative cells, respectively, from 2-5 independent experiments. N > 150 per condition. *p < 0.05 compared to cycling cells. See also Supplemental Figure S3.
Several lines of evidence rule out that DDR signaling upon array-localized chromatin condensation is the result of DNA breaks induced by tethering. No labeling by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was detected (Fig. 3A), Southern blotting using probes to the Lac array did not reveal tethering-induced DSBs in the arrays (Fig. 3B) and no breaks were detected by ligation-mediated PCR (LM-PCR), which sensitively amplifies DSBs near the lacO integration site (Soutoglou and Misteli, 2008; Fig. 3C). Furthermore, limiting the expression of tethering constructs to 20 h after transient transfection prevented potential replication defects caused by extended tethering of fusion proteins to the Lac array (Beuzer et al., 2014; Jacome and Fernandez-Capetillo, 2011). In line with this, DDR activation by chromatin condensation was not a cell cycle effect or due to stalled replication, since it was similarly observed in cells arrested either at G1/S or in G0 (Fig. 3D, E). Taken together, these data suggest that condensed chromatin is sufficient to stimulate DDR signaling.
Condensed chromatin promotes activation of upstream parts of the DDR
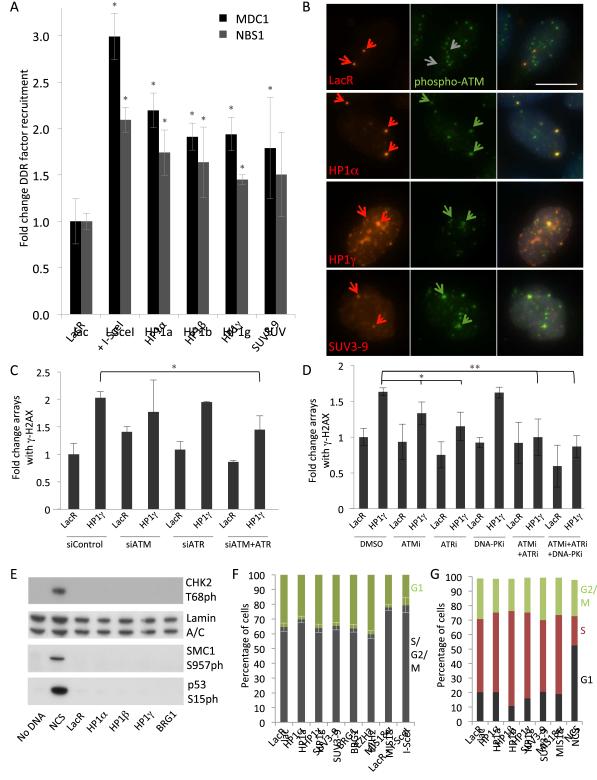
To assess whether chromatin condensation leads to recruitment of other factors, we measured the accumulation of DDR components at the condensed array (Fig. 4A). In addition to γ-H2AX and 53BP1, the MDC1 mediator protein and NBS1, a component of the MRE11-RAD50-NBS1 (MRN) complex were efficiently recruited to locally compacted chromatin domains (Fig. 2C, 4A). Their extent of recruitment was similar with typically 65-75% of arrays exhibiting recruitment, representing a 2-fold increase over control cells (Fig. 4A). Accumulation of these factors was not due to direct interaction with HP1 since their recruitment was inhibited under conditions where HP1 was tethered to a decondensed Lac array (Fig. S3A-D). This co-tethering experiment did not reflect out-competition of HP1 binding by the activators, since both fusions were able to bind the array without much competition as evident by visible decondensation with a robust HP1 signal. In addition, increasing amounts of lacR could not inhibit the HP1 effect, consistent with lac operators being incompletely occupied at any given time (Fig. S3 E,F). Although we cannot fully exclude the possibility that co-tethering of activators leads to enhanced clearance of DDR factors, we think this unlikely since we observe heightened levels of γ−H2AX at damaged arrays expanded by BRG1 (R.C.B. and T.M. data not shown). We take these data to suggest that HP1 does not act as a platform for DDR factor recruitment, but that condensed chromatin structures induce upstream DDR signaling.
Figure 4. Activation of upstream DDR signaling by condensed chromatin.
(A) Recruitment of MDC1 and NBS1 to condensed arrays, quantified as in Fig. 2C. Values represent average fold change ± SD from 3 independent experiments (N ≥ 200 for each condition). * p < 0.05 compared to LacR alone. (B) Maximum intensity projections of phospho-ATM (S1981) immunostaining at LacR, HP1- or SUV3-9 arrays. Green arrows, arrays enriched in phospho-ATM; gray arrows, no enrichment. Scale bar, 5 μm. (C) γ-H2AX formation in condensed chromatin following siRNA depletion of ATM and/or ATR, depicted as average fold change to control siRNA (siControl) transfected with mCherry-LacR ± SD from 2 experiments (N = 40-150), *p < 0.05. Knockdown is shown in Supplemental Figure S4. (D) DNA-PK, ATM and ATR were inhibited with KU55933 (ATMi), VE-821 (ATRi), or NU7441 (DNA-PKi), after tethering. Average ± SD of γ-H2AX recruitment compared to DMSO-treated cells transfected with LacR from 3 independent trials. N > 175 per condition, *p < 0.05, **p < 0.01. (E) Immunoblot analysis of activated DDR factors detected by phospho-specific antibodies. Loading control, Lamin A/C. (F) Cyclin A staining and (G) FACS cell cycle profiling of cell populations with tethered chromatin factors. Shown is average percentage of cells positive (S/G2, M phases), or negative (G1) for cyclin A staining, or as determined by DNA content.
The activation and amplification of the DDR involves several overlapping kinase activities, particularly, ATM, ATR and DNA-PK (Burma et al., 2001; Stiff et al., 2004; Ward and Chen, 2001). Active ATM monomers are exposed by their phosphorylation at serine 1981 (Bakkenist and Kastan, 2003), and tethering of SUV3-9, HP1α, −β or −γ resulted in robust accumulation of phosphorylated ATM at the array (Fig. 4B). To distinguish the contributions of ATM and ATR in chromatin-activated DDR signaling, we performed siRNA knockdowns of ATM or ATR as well as dual knockdown of both kinases simultaneously (Fig. S4A,B). DDR activation was only significantly inhibited when both ATM and ATR were depleted (Fig. 4C, p < 0.05). Experiments with specific kinase inhibitors confirmed these results. Treatment of cells containing LacR-HP1γ with specific inhibitors to both ATM and ATR, or to all three kinases led to a significant decrease in the frequency of γ-H2AX at the HP1-tethered arrays to near background levels (Fig. 4D, p < 0.01). Inhibitors of ATR or ATM alone led to more modest decreases in γ-H2AX signaling (p < 0.05), while an inhibitor specific to DNA-PK did not significantly curtail phosphorylation of H2AX (Fig. 4D). All together, these observations demonstrate that ATM and ATR are jointly involved in the chromatin-induced DDR signaling.
ATM signaling normally activates CHK2 kinase and downstream targets SMC1 and p53 (Hirao et al., 2000; Matsuoka et al., 2000; Yazdi et al., 2002). However, these factors were not activated by tethering of HP1α, −β, or −γ (Fig. 4E), suggesting that key effectors of the conventional DDR are not globally activated by condensed chromatin domains. Lack of activation of downstream cell cycle checkpoints by chromatin condensation was confirmed by cell-level analysis of Cyclin A immunostaining with I-SceI-expressing cells as a positive control for damage-activated checkpoints (Fig. 4F). We also found no difference in the cell cycle profiles of large populations using cell sorting, with NCS treatment as a positive control, since individual cell assessment for I-SceI expression was not possible by FACS (Fig. 4G). We conclude that chromatin condensation contributes to restricted activation of upstream components of the DDR, but not downstream effectors.
Condensed chromatin activates upstream components of DDR signaling in mitotic cells
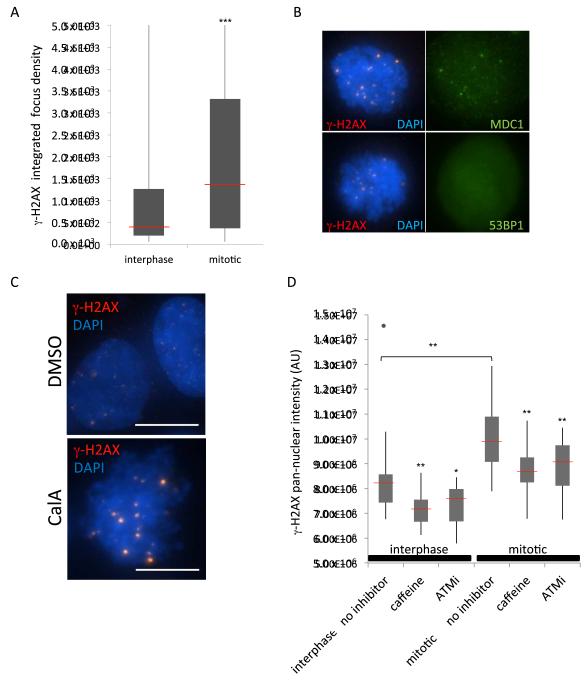
Mitotic chromosomes are an extreme case of naturally occurring condensed chromatin. If condensed chromatin contributes to DDR signaling and is sufficient to activate parts of the DDR, a prediction is that mitotic chromosomes should trigger upstream DDR signaling. Consistent with our findings on induced condensed chromatin domains, we detect accumulation of γ-H2AX on mitotic chromosomes, as previously observed by others (Ichijima et al., 2005; McManus and Hendzel, 2005). The intensity of γ-H2AX foci on mitotic chromosomes was increased about 3-fold compared to interphase levels as judged by integrated focus density measurements (Fig. 5A). In addition, mitotic chromosomes were decorated by MDC1 foci, which colocalized, albeit weakly in some cases, with γ-H2AX foci, while 53BP1 was excluded from mitotic γ-H2AX foci, as previously reported (Fig. 5B; Giunta et al., 2010; Nakamura et al., 2010; Oricchio et al., 2006). As expected, no free DNA ends were detected on mitotic chromosomes by TUNEL (Fig. S5), suggesting that the constitutive mitotic DDR signaling occurred in the absence of DNA damage. Similarly, an increase in γ-H2AX foci in the absence of detectable DNA breaks was observed upon premature chromosome condensation (PCC) induced by the phosphatase inhibitor Calyculin A (Coco-Martin and Begg, 1997; Huang et al., 2006; Fig. 5C, S5). Both ATM and ATR contributed to the mitotic chromatin-induced DDR, as treatment with caffeine diminished pan-nuclear γ-H2AX levels further than ATM inhibition alone (Fig. 5D). These findings are in line with the observation of mitotic activation of ATM and recruitment of the MRN complex and MDC1 to mitotic chromosomes in the absence of activation of downstream portions of the pathway (Giunta et al., 2010).
Figure 5. Mitotic chromosome condensation activates chromatin-induced DDR.
(A) Individual γ-H2AX foci quantified by integrated density measurements. Box: quartiles 1 - 3, whiskers: value range, red bar: median values, as follows: 487 (interphase), 1317 (mitosis). ***p < 0.001, N > 300 foci for each. (B) Mitotic cells were harvested by shake-off, attached to slides and stained for γ-H2AX (red), and either MDC1 or 53BP1 (green). Shown are maximum intensity projections with DAPI overlay (blue). (C) Increased γ-H2AX foci in cells treated with 50 nM CalA for 60 min, fixed and immunostained for γ-H2AX (red), and DAPI (blue). Scale bar, 10 μm. (D) Total nuclear intensity of γ-H2AX from cells in indicated treatments. Box and whiskers as in A. One single outlier (>1.5 times outside the interquartile range) is marked with a gray dot, and the red bars indicate median values. *p < 0.05, ** p < 0.01. See also Supplemental Figure S5.
Condensed chromatin boosts upstream DDR signaling, but is detrimental to downstream repair and recovery
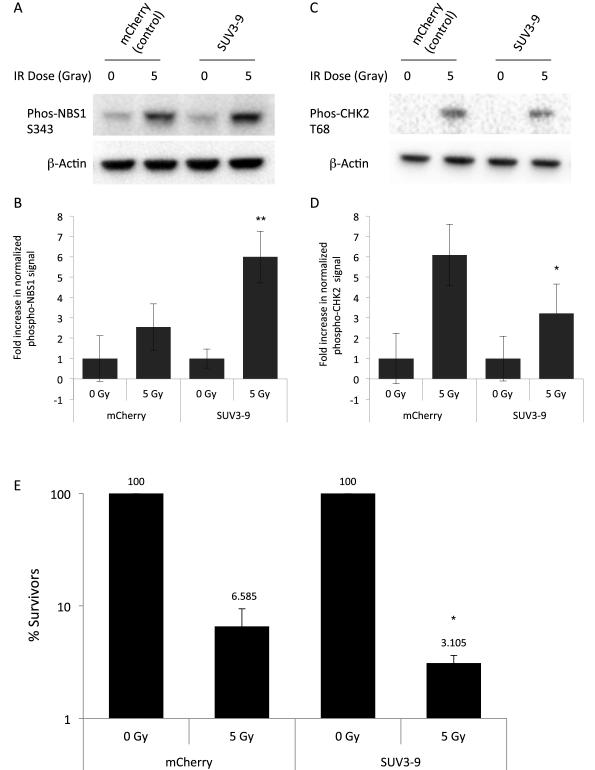
Given that condensed chromatin regions can generate local upstream DDR signaling, and the observation that damaged chromatin regions undergo compaction, we asked how these dynamics affect the signaling and repair of DSBs. To this end, we globally compacted chromatin by overexpressing the SUV3-9 methyltransferase, then produced DSBs throughout the genome with 5 Gy of γ-irradiation. Compared to controls, SUV3-9 overexpressing cells showed a greater than 2-fold increase in NBS1 phosphorylation, and increased γ-H2AX, suggesting enhanced early ATM signaling (Fig. 6A,B; S6A, p < 0.01). On the other hand, phosphorylation of CHK2 decreased by about 2-fold, representing a decreased ability of DSBs in compacted chromatin environments to signal to downstream effectors (Fig. 6C,D, p < 0.05). To finally ask whether persistent chromatin compaction affects the recovery and survival of cells from DNA damage, we performed clonogenic assays. Survival of SUV3-9 expressing cells was markedly decreased compared to control cells (Fig. 6E). From this, we conclude that, while chromatin compaction enhances upstream signaling, its interference with decondensation negatively impacts repair and recovery from DNA damage.
Figure 6. Persistent condensation of chromatin enhances upstream signaling from IR-induced breaks, but reduces cell survival after damage.
(A) Immunoblot of phospho-NBS1 in control or SUV3-9 overexpressing cells ±5 Gy γ-irradiation. β-actin shown as a loading control. (B) Quantification of phospho-NBS1 levels, normalized to β-actin. Values represent averages ± SD from 3 experiments. Unirradiated NBS1 levels are normalized to 1. **p < 0.01 compared to irradiated control. (C) Blot of phospho-CHK2 in conditions as in panel A and depicted identically. (D) Quantification of phospho-CHK2 as in B. *p < 0.05 compared to irradiated control. (E) Clonogenic survival assays of cells expressing SUV3-9. Surviving colonies were normalized to unirradiated controls. Values represent median ± median average deviation from 3 experiments. *p < 0.05 compared to irradiated control. See also Supplemental Figure S6.
Discussion
We provide evidence here that chromatin condensation contributes to DDR signaling. Based on our morphological observations, and in line with recent findings by others (Ayrapetov et al., 2014; Khurana et al., 2014), we propose that condensation of chromatin is an integral step in the damage response. Using chromatin tethering approaches to probe the functional effects of condensed chromatin during DDR, we find that condensed chromatin domains are sufficient to trigger ATM/ATR-dependent signaling and activate upstream, but not downstream, components of the DDR cascade. Conversely, interference with chromatin compaction at the site of DNA damage attenuates DDR signaling. These observations suggest that changes in chromatin structure are not just bystanders, but actively contribute to DDR activation. Fitting with a role of highly condensed chromatin as a trigger for DDR signaling, we find upstream parts of the DDR activated in naturally occurring highly condensed mitotic chromosomes. While condensation can augment upstream signaling, it renders damage refractory to repair and represses the recovery of cells from DNA damage, suggesting the need for a dynamic exchange in chromatin structure for an efficient DDR in physiological settings. Combined with recent observations by others (Ayrapetov et al., 2014; Khurana et al., 2014), these findings highlight the need for coordinated chromatin decondensation as well as condensation events for efficient activation of the DDR in a physiological setting.
A role of chromatin structure as a trigger of upstream DDR signaling is consistent with several previously reported circumstantial observations. Numerous studies have detected DDR activation under chromatin-altering conditions (Bakkenist and Kastan, 2003; Bencokova et al., 2009; Hunt et al., 2007). Hypotonic conditions, chromatin factor depletion, and treatments with trichostatin A or chloroquine that result in chromatin changes, but not DNA damage, have been reported to activate ATM (Bakkenist and Kastan, 2003; Kaidi and Jackson, 2013). In addition, damage-independent ATM activation has been reported in cells where senescence or replication stress has been induced, with concomitant formation of repressive chromatin (Olcina et al., 2013; Pospelova et al., 2009). Even in unperturbed cells, repressive chromatin domains such as sub-telomeric and centromeric regions show enrichment for γ-H2AX, as revealed by several genome-wide studies (Kitada et al., 2011; Lee et al., 2014; Seo et al., 2012; Szilard et al., 2010). DDR activation has also been suggested in condensed chromosomes progressing through mitosis. Mitotically-condensed and prematurely-condensed chromatin have previously been shown to activate ATM with a corresponding increase in γ-H2AX foci, and again, an absence of detectable DNA damage (Huang et al., 2006; Ichijima et al., 2005; McManus and Hendzel, 2005; Oricchio et al., 2006). Furthermore, in cells lacking the mitotic chromokinesin motor protein KIF4, abnormally highly condensed chromosomes are heavily decorated by γ-H2AX yet progress through mitosis, indicating the absence of extensive DNA lesions or checkpoint signaling (Mazumdar et al., 2006). Finally, recent work has raised the intriguing notion that ATR kinase activity is triggered by mechanical stimuli resulting from condensation in mitotic prophase and functions to modulate chromatin engagement with the nuclear envelope, preventing aberrant topological configurations (Kumar et al., 2014). The ability of condensed chromatin to activate ATR and ATM in all these distinct settings could reflect the evolutionary roots of this mechanism; future work will be needed to unveil commonalities, despite their divergent use for distinct biological processes.
This study and recently published work shows that chromatin compaction is an integral part of the generic DNA damage response (Ayrapetov et al., 2014; Khurana et al., 2014). We extend these findings by demonstrating that chromatin compaction is sufficient to trigger the upstream activation of the DDR independently of the DNA lesion. This conclusion is in line with earlier tethering experiments demonstrating that a DNA lesion is not an obligatory part of the DDR machinery and DDR signaling can occur in its absence, allowing for the possibility of DDR initiation by stimuli other than DNA damage, such as chromatin structure (Bonilla et al., 2008; Soutoglou and Misteli, 2008). We suggest that experimentally condensed chromatin provides the structural and molecular environment mimicking a DDR amplification step, leading to constrained ATM and ATR signaling without providing the full context of downstream DDR. A signaling function for chromatin may aid in amplification of the DDR; it has previously been noted that rapid activation of ATM kinase after a DSB occurs within seconds after irradiation and the majority of the relatively large cellular pool of ATM is activated by only a few strand breaks (Bakkenist and Kastan, 2003). This rapid and extensive activation of the DDR suggests the existence of cellular mechanisms that sense the damage signal with exquisite efficiency. Compaction of chromatin structure around the break site may represent a potent means to augment the signaling domain generated by a single DSB (Ayrapetov et al., 2014; Bakkenist and Kastan, 2003).
The establishment of a compact chromatin domain for enhancing DDR signaling is complementary to the observation that chromatin relaxation is required for amplification and activation of DDR effector pathways, and they likely occur in a dynamic exchange (Burgess et al., 2012; Soria et al., 2012). In a recent study, SUV3-9 was shown to be recruited to DSBs, establishing the repressive H3K9me3 histone mark at sites of damage, which captures HP1, KAP-1 and further SUV3-9, propagating the heterochromatin domain for tens of kb surrounding a DSB (Ayrapetov et al., 2014). Formation of a H3K9me3 repressive mark creates binding sites for the TIP60 acetyltransferase, which then contributes to the amplification of ATM activity (Ayrapetov et al., 2014; Sun et al., 2005; Sun et al., 2009). In agreement, we find that the establishment of a H3K9 methyl domain can promote signaling, while decompaction of chromatin by overexpression or tethering of the ASH2 H3K4 methyltransferase dampens the DDR and local dynamics of chromatin after damage. Later, ATM signaling leads to KAP-1 phosphorylation and release of the KAP-1/HP1/SUV3-9 and CHD3 complexes, promoting the relaxation of the chromatin that is essential to downstream signaling and repair (Ayrapetov et al., 2014; Goodarzi et al., 2011; Goodarzi et al., 2008). Impairing this relaxation by persistent compaction decreased the recovery of cells from damage in our clonogenic survival assays, consistent with a requirement for dynamic chromatin changes during DDR. Establishing the repressive chromatin domain is also important for the outcome of the DDR, as SUV3-9, PRDM2 or HP1-depletion impairs survival after DNA damage, and may shift repair pathway choice (Ayrapetov et al., 2014; Baldeyron et al., 2011; Khurana et al., 2014; Soria and Almouzni, 2013). This suggests that perturbing the dynamics of chromatin condensation and subsequent decondensation may be unfavorable to DDR. In addition, and not mutually exclusive, the temporal coordination of chromatin dynamics may be complemented by spatial separation of decondensed and condensed chromatin domains in the vicinity of a DSB, with relaxed and compact regions playing distinct roles in DDR signaling and repair. In fact, sub-compartments within DNA damage foci have been observed by super-resolution microscopy, and their chromatin environments proposed to be distinct (Chapman et al., 2012). Establishment of repressive chromatin may be beneficial to the damaged region to stabilize the damaged ends and concentrate DDR factors for more efficient signaling. In addition condensed domains may contribute to the transcriptional repression characteristic of damaged genome regions, and help keep the transcription machinery from interfering with downstream repair processes (Kruhlak et al., 2007; Pankotai et al., 2012; Price and D'Andrea, 2013; Shanbhag et al., 2010).
Taken together, these observations provide evidence that chromatin condensation is an integral, but transient step in the activation of DDR signaling, integrating observations of opposing dynamics of chromatin after damage. Our data allows for the possibility that, in addition to detecting bona fide DNA damage, the cellular surveillance machinery also senses changes in chromatin structure.
Experimental Procedures
Laser Microirradiation
Parental U2OS cells were transfected according to manufacturer’s protocol with Amaxa nucleofector V (Lonza) with a photoactivatable GFP-tagged H2A with or without DNA repair factors or chromatin modifiers, and plated on 2-chamber coverslip bottom slides (Lab-tek) for 20 h. Before imaging, cells were incubated with 0.1 μg/ml Hoechst 33324 for 1 h, , then switched FluoroBrite phenol red-free media containing 10% FBS, glutamine, and antibiotics without Hoechst and 5 mM HEPES. Imaging and laser damage/photoactivation was carried out as described previously on a Zeiss LSM510 with a 364 nm laser (Kruhlak et al., 2006), or on a Zeiss LSM780 with simultaneous 355 nm (10%) and 405 nm (5%) laser lines, with total UV laser output set to 20%, 10 iterations, and laser scan speed set to 7 (pixel dwell time 3.15 μs). The laser(s) were focused in a 30 pixel circle, and images taken every 10 sec for 2 min then every 45 s for 30 min, maintaining cells at 37°C and 5% CO2. Subsequent region area measurements were performed using ImageJ software.
Ligation-mediated PCR
Genomic DNA was purified from U2OS cell lines using the Qiagen Blood and Cell Culture DNA Mini Kit, and prepared for ligation-mediated PCR to detect random array breaks or I-SceI induced breaks as described previously (Soutoglou et al., 2007; Soutoglou and Misteli, 2008) respectively.
Chromatin factor tethering
Transient transfections were carried out using the Amaxa Nucleofector Kit V (Lonza), according to the manufacturer’s protocol. All tethering experiments were carried out using transient expression of the constructs for 20 h before harvesting or fixation, unless otherwise noted. For I-SceI expression, cells were transfected first with tethering constructs using the Amaxa Nucleofector kit V, and maintained in charcoal-stripped serum (Atlanta Biologicals). 12 h later, cells were transfected with CFP-GR-I-SceI construct per 1 million cells using the same protocol. After 12 h (24 h total), GR nuclear translocation was induced with dexamethasone (Sigma) at a concentration of 100 nM for 20 min.
Kinase inhibitor treatment
Kinase inhibitors were added to medium just after nucleofector transfection with the tethering constructs (see above), at the following final concentrations: KU55933, 10 μM; VE-821, 1 μM; NU7441, 1 μM. Cells were fixed and stained after 20 h of incubation, as described above.
siRNA knockdown
Dharmafect On-Target-plus SMART-pool siRNAs for ATM and ATR were used. 100 nM siRNAs were co-transfected with tethering constructs using Dharmafect1, according to the manufacturer’s instructions, and harvested for protein analysis and microscopy after 72 h.
Clonogenic survival assays
Assay was carried out essentially as described previously (Munshi et al., 2005), with the following changes: four million cells were transfected with overexpression constructs, plated in T75 flasks and incubated for 20 h post-transfection for full expression. Cells irradiated with 5 Gray of γ-irradiation using a 137Cs source, then trypsinized, cells counted, and plated at two cell densities, each in triplicate on 10 cm plates in standard medium. After 12-14 days of growth, colonies were fixed and stained with 0.25% crystal violet in ethanol before counting.
Supplementary Material
Acknowledgments
The authors thank Andrew Belmont, Daniel Foltz, Alexander Tarakhovsky, Evi Soutoglou, Maria Jasin, David Skalnik and Thomas Jenuwein for reagents. Members of the Misteli Lab, particularly Vassilis Roukos, provided technical support and comments. Imaging was performed in the NCI Fluorescence Imaging facility, and FACS was performed in the NCI Vaccine Branch FACS Core Facility. This work was supported by the Intramural Research Program of the National Institutes of Health (NIH), NCI, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests exist.
References
- Ayoub N, Jeyasekharan AD, Bernal JA, Venkitaraman AR. HP1-beta mobilization promotes chromatin changes that initiate the DNA damage response. Nature. 2008;453:682–686. doi: 10.1038/nature06875. [DOI] [PubMed] [Google Scholar]
- Ayoub N, Jeyasekharan AD, Venkitaraman AR. Mobilization and recruitment of HP1: a bimodal response to DNA breakage. Cell Cycle. 2009;8:2945–2950. [PubMed] [Google Scholar]
- Ayrapetov MK, Gursoy-Yuzugullu O, Xu C, Xu Y, Price BD. DNA double-strand breaks promote methylation of histone H3 on lysine 9 and transient formation of repressive chromatin. Proc Natl Acad Sci U S A. 2014;111:9169–9174. doi: 10.1073/pnas.1403565111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- Baldeyron C, Soria G, Roche D, Cook AJ, Almouzni G. HP1alpha recruitment to DNA damage by p150CAF-1 promotes homologous recombination repair. J Cell Biol. 2011;193:81–95. doi: 10.1083/jcb.201101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencokova Z, Kaufmann MR, Pires IM, Lecane PS, Giaccia AJ, Hammond EM. ATM activation and signaling under hypoxic conditions. Mol Cell Biol. 2009;29:526–537. doi: 10.1128/MCB.01301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovich E, Monnat RJ, Jr., Kastan MB. Roles of ATM and NBS1 in chromatin structure modulation and DNA double-strand break repair. Nat Cell Biol. 2007;9:683–690. doi: 10.1038/ncb1599. [DOI] [PubMed] [Google Scholar]
- Beuzer P, Quivy JP, Almouzni G. Establishment of a replication fork barrier following induction of DNA binding in mammalian cells. Cell Cycle. 2014;13:1607–1616. doi: 10.4161/cc.28627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla CY, Melo JA, Toczyski DP. Colocalization of sensors is sufficient to activate the DNA damage checkpoint in the absence of damage. Mol Cell. 2008;30:267–276. doi: 10.1016/j.molcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle AP, Davis S, Shulha HP, Meltzer P, Margulies EH, Weng Z, Furey TS, Crawford GE. High-resolution mapping and characterization of open chromatin across the genome. Cell. 2008;132:311–322. doi: 10.1016/j.cell.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess RC, Misteli T, Oberdoerffer P. DNA damage, chromatin, and transcription: the trinity of aging. Curr Opin Cell Biol. 2012 doi: 10.1016/j.ceb.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- Chambeyron S, Bickmore WA. Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Dev. 2004;18:1119–1130. doi: 10.1101/gad.292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JR, Jackson SP. Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage. EMBO Rep. 2008;9:795–801. doi: 10.1038/embor.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JR, Sossick AJ, Boulton SJ, Jackson SP. BRCA1-associated exclusion of 53BP1 from DNA damage sites underlies temporal control of DNA repair. J Cell Sci. 2012;125:3529–3534. doi: 10.1242/jcs.105353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiolo I, Minoda A, Colmenares SU, Polyzos A, Costes SV, Karpen GH. Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell. 2011;144:732–744. doi: 10.1016/j.cell.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco-Martin JM, Begg AC. Detection of radiation-induced chromosome aberrations using fluorescence in situ hybridization in drug-induced premature chromosome condensations of tumour cell lines with different radiosensitivities. Int J Radiat Biol. 1997;71:265–273. doi: 10.1080/095530097144148. [DOI] [PubMed] [Google Scholar]
- Consortium EP, Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer JR, Wallrath LL. Mechanisms of HP1-mediated gene silencing in Drosophila. Development. 2004;131:3571–3580. doi: 10.1242/dev.01223. [DOI] [PubMed] [Google Scholar]
- Dinant C, Luijsterburg MS. The emerging role of HP1 in the DNA damage response. Mol Cell Biol. 2009;29:6335–6340. doi: 10.1128/MCB.01048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg JC, Reuter G. Cellular mechanism for targeting heterochromatin formation in Drosophila. International review of cell and molecular biology. 2009;273:1–47. doi: 10.1016/S1937-6448(08)01801-7. [DOI] [PubMed] [Google Scholar]
- Giunta S, Belotserkovskaya R, Jackson SP. DNA damage signaling in response to double-strand breaks during mitosis. J Cell Biol. 2010;190:197–207. doi: 10.1083/jcb.200911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi AA, Kurka T, Jeggo PA. KAP-1 phosphorylation regulates CHD3 nucleosome remodeling during the DNA double-strand break response. Nat Struct Mol Biol. 2011;18:831–839. doi: 10.1038/nsmb.2077. [DOI] [PubMed] [Google Scholar]
- Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Lobrich M, Jeggo PA. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell. 2008;31:167–177. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Grewal SI, Jia S. Heterochromatin revisited. Nature reviews Genetics. 2007;8:35–46. doi: 10.1038/nrg2008. [DOI] [PubMed] [Google Scholar]
- Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- Huang X, Kurose A, Tanaka T, Traganos F, Dai W, Darzynkiewicz Z. Sequential phosphorylation of Ser-10 on histone H3 and ser-139 on histone H2AX and ATM activation during premature chromosome condensation: relationship to cell-cycle phase and apoptosis. Cytometry A. 2006;69:222–229. doi: 10.1002/cyto.a.20257. [DOI] [PubMed] [Google Scholar]
- Hunt CR, Pandita RK, Laszlo A, Higashikubo R, Agarwal M, Kitamura T, Gupta A, Rief N, Horikoshi N, Baskaran R, et al. Hyperthermia activates a subset of ataxia-telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res. 2007;67:3010–3017. doi: 10.1158/0008-5472.CAN-06-4328. [DOI] [PubMed] [Google Scholar]
- Ichijima Y, Sakasai R, Okita N, Asahina K, Mizutani S, Teraoka H. Phosphorylation of histone H2AX at M phase in human cells without DNA damage response. Biochem Biophys Res Commun. 2005;336:807–812. doi: 10.1016/j.bbrc.2005.08.164. [DOI] [PubMed] [Google Scholar]
- Jacome A, Fernandez-Capetillo O. Lac operator repeats generate a traceable fragile site in mammalian cells. EMBO Rep. 2011;12:1032–1038. doi: 10.1038/embor.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob B, Splinter J, Conrad S, Voss KO, Zink D, Durante M, Lobrich M, Taucher-Scholz G. DNA double-strand breaks in heterochromatin elicit fast repair protein recruitment, histone H2AX phosphorylation and relocation to euchromatin. Nucleic Acids Res. 2011;39:6489–6499. doi: 10.1093/nar/gkr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidi A, Jackson SP. KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling. Nature. 2013;498:70–74. doi: 10.1038/nature12201. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Khurana S, Kruhlak MJ, Kim J, Tran AD, Liu J, Nyswaner K, Shi L, Jailwala P, Sung MH, Hakim O, et al. A Macrohistone Variant Links Dynamic Chromatin Compaction to BRCA1-Dependent Genome Maintenance. Cell reports. 2014;8:1049–1062. doi: 10.1016/j.celrep.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Schleker T, Sperling AS, Xie W, Gasser SM, Grunstein M. gammaH2A is a component of yeast heterochromatin required for telomere elongation. Cell Cycle. 2011;10:293–300. doi: 10.4161/cc.10.2.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruhlak M, Crouch EE, Orlov M, Montano C, Gorski SA, Nussenzweig A, Misteli T, Phair RD, Casellas R. The ATM repair pathway inhibits RNA polymerase I transcription in response to chromosome breaks. Nature. 2007;447:730–734. doi: 10.1038/nature05842. [DOI] [PubMed] [Google Scholar]
- Kruhlak MJ, Celeste A, Dellaire G, Fernandez-Capetillo O, Muller WG, McNally JG, Bazett-Jones DP, Nussenzweig A. Changes in chromatin structure and mobility in living cells at sites of DNA double-strand breaks. J Cell Biol. 2006;172:823–834. doi: 10.1083/jcb.200510015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Mazzanti M, Mistrik M, Kosar M, Beznoussenko GV, Mironov AA, Garre M, Parazzoli D, Shivashankar GV, Scita G, et al. ATR Mediates a Checkpoint at the Nuclear Envelope in Response to Mechanical Stress. Cell. 2014;158:633–646. doi: 10.1016/j.cell.2014.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen DH, Poinsignon C, Gudjonsson T, Dinant C, Payne MR, Hari FJ, Rendtlew Danielsen JM, Menard P, Sand JC, Stucki M, et al. The chromatin-remodeling factor CHD4 coordinates signaling and repair after DNA damage. J Cell Biol. 2010;190:731–740. doi: 10.1083/jcb.200912135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Lee K, Legube G, Haber JE. Dynamics of yeast histone H2A and H2B phosphorylation in response to a double-strand break. Nat Struct Mol Biol. 2014;21:103–109. doi: 10.1038/nsmb.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- Li Y, Danzer JR, Alvarez P, Belmont AS, Wallrath LL. Effects of tethering HP1 to euchromatic regions of the Drosophila genome. Development. 2003;130:1817–1824. doi: 10.1242/dev.00405. [DOI] [PubMed] [Google Scholar]
- Ling G, Sugathan A, Mazor T, Fraenkel E, Waxman DJ. Unbiased, genome-wide in vivo mapping of transcriptional regulatory elements reveals sex differences in chromatin structure associated with sex-specific liver gene expression. Mol Cell Biol. 2010;30:5531–5544. doi: 10.1128/MCB.00601-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Z, Minter-Dykhouse K, Franco S, Gostissa M, Rivera MA, Celeste A, Manis JP, van Deursen J, Nussenzweig A, Paull TT, et al. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol Cell. 2006;21:187–200. doi: 10.1016/j.molcel.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Luco RF, Pan Q, Tominaga K, Blencowe BJ, Pereira-Smith OM, Misteli T. Regulation of alternative splicing by histone modifications. Science. 2010;327:996–1000. doi: 10.1126/science.1184208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijsterburg MS, Dinant C, Lans H, Stap J, Wiernasz E, Lagerwerf S, Warmerdam DO, Lindh M, Brink MC, Dobrucki JW, et al. Heterochromatin protein 1 is recruited to various types of DNA damage. J Cell Biol. 2009;185:577–586. doi: 10.1083/jcb.200810035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas C, Melander F, Stucki M, Falck J, Bekker-Jensen S, Goldberg M, Lerenthal Y, Jackson SP, Bartek J, Lukas J. Mdc1 couples DNA double-strand break recognition by Nbs1 with its H2AX-dependent chromatin retention. Embo J. 2004;23:2674–2683. doi: 10.1038/sj.emboj.7600269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J, Lukas C, Bartek J. More than just a focus: The chromatin response to DNA damage and its role in genome integrity maintenance. Nat Cell Biol. 2011;13:1161–1169. doi: 10.1038/ncb2344. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci U S A. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar M, Lee JH, Sengupta K, Ried T, Rane S, Misteli T. Tumor formation via loss of a molecular motor protein. Curr Biol. 2006;16:1559–1564. doi: 10.1016/j.cub.2006.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus KJ, Hendzel MJ. ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol Biol Cell. 2005;16:5013–5025. doi: 10.1091/mbc.E05-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaburn KJ. Fluorescence in situ hybridization on 3D cultures of tumor cells. Methods Mol Biol. 2010;659:323–336. doi: 10.1007/978-1-60761-789-1_25. [DOI] [PubMed] [Google Scholar]
- Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, Jackson SP. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol. 2010;17:1144–1151. doi: 10.1038/nsmb.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mund A, Schubert T, Staege H, Kinkley S, Reumann K, Kriegs M, Fritsch L, Battisti V, Ait-Si-Ali S, Hoffbeck AS, et al. SPOC1 modulates DNA repair by regulating key determinants of chromatin compaction and DNA damage response. Nucleic Acids Res. 2012;40:11363–11379. doi: 10.1093/nar/gks868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi A, Hobbs M, Meyn RE. Clonogenic cell survival assay. Methods in molecular medicine. 2005;110:21–28. doi: 10.1385/1-59259-869-2:021. [DOI] [PubMed] [Google Scholar]
- Murga M, Jaco I, Fan Y, Soria R, Martinez-Pastor B, Cuadrado M, Yang SM, Blasco MA, Skoultchi AI, Fernandez-Capetillo O. Global chromatin compaction limits the strength of the DNA damage response. J Cell Biol. 2007;178:1101–1108. doi: 10.1083/jcb.200704140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murr R, Loizou JI, Yang YG, Cuenin C, Li H, Wang ZQ, Herceg Z. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nat Cell Biol. 2006;8:91–99. doi: 10.1038/ncb1343. [DOI] [PubMed] [Google Scholar]
- Nakamura AJ, Rao VA, Pommier Y, Bonner WM. The complexity of phosphorylated H2AX foci formation and DNA repair assembly at DNA double-strand breaks. Cell Cycle. 2010;9:389–397. doi: 10.4161/cc.9.2.10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noon AT, Shibata A, Rief N, Lobrich M, Stewart GS, Jeggo PA, Goodarzi AA. 53BP1-dependent robust localized KAP-1 phosphorylation is essential for heterochromatic DNA double-strand break repair. Nat Cell Biol. 2010;12:177–184. doi: 10.1038/ncb2017. [DOI] [PubMed] [Google Scholar]
- Olcina MM, Foskolou IP, Anbalagan S, Senra JM, Pires IM, Jiang Y, Ryan AJ, Hammond EM. Replication stress and chromatin context link ATM activation to a role in DNA replication. Mol Cell. 2013;52:758–766. doi: 10.1016/j.molcel.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oricchio E, Saladino C, Iacovelli S, Soddu S, Cundari E. ATM is activated by default in mitosis, localizes at centrosomes and monitors mitotic spindle integrity. Cell Cycle. 2006;5:88–92. doi: 10.4161/cc.5.1.2269. [DOI] [PubMed] [Google Scholar]
- Pankotai T, Bonhomme C, Chen D, Soutoglou E. DNAPKcs-dependent arrest of RNA polymerase II transcription in the presence of DNA breaks. Nat Struct Mol Biol. 2012;19:276–282. doi: 10.1038/nsmb.2224. [DOI] [PubMed] [Google Scholar]
- Polo SE, Kaidi A, Baskcomb L, Galanty Y, Jackson SP. Regulation of DNA-damage responses and cell-cycle progression by the chromatin remodelling factor CHD4. Embo J. 2010;29:3130–3139. doi: 10.1038/emboj.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospelova TV, Demidenko ZN, Bukreeva EI, Pospelov VA, Gudkov AV, Blagosklonny MV. Pseudo-DNA damage response in senescent cells. Cell Cycle. 2009;8:4112–4118. doi: 10.4161/cc.8.24.10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price BD, D'Andrea AD. Chromatin remodeling at DNA double-strand breaks. Cell. 2013;152:1344–1354. doi: 10.1016/j.cell.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- Seo J, Kim SC, Lee HS, Kim JK, Shon HJ, Salleh NL, Desai KV, Lee JH, Kang ES, Kim JS, et al. Genome-wide profiles of H2AX and gamma-H2AX differentiate endogenous and exogenous DNA damage hotspots in human cells. Nucleic Acids Res. 2012;40:5965–5974. doi: 10.1093/nar/gks287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag NM, Rafalska-Metcalf IU, Balane-Bolivar C, Janicki SM, Greenberg RA. ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. Cell. 2010;141:970–981. doi: 10.1016/j.cell.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Oberdoerffer P. Chromatin dynamics in DNA double-strand break repair. Biochim Biophys Acta. 2012;1819:811–819. doi: 10.1016/j.bbagrm.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada N, Matsudo H, Osano K, Arakawa H, Buerstedde JM, Matsumoto Y, Chayahara K, Torihata A, Ono M. Activation of the chicken Ig-beta locus by the collaboration of scattered regulatory regions through changes in chromatin structure. Nucleic Acids Res. 2006;34:3794–3802. doi: 10.1093/nar/gkl469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeenk G, Wiegant WW, Vrolijk H, Solari AP, Pastink A, van Attikum H. The NuRD chromatin-remodeling complex regulates signaling and repair of DNA damage. J Cell Biol. 2010;190:741–749. doi: 10.1083/jcb.201001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Almouzni G. Differential contribution of HP1 proteins to DNA end resection and homology-directed repair. Cell Cycle. 2013;12:422–429. doi: 10.4161/cc.23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Polo SE, Almouzni G. Prime, repair, restore: the active role of chromatin in the DNA damage response. Mol Cell. 2012;46:722–734. doi: 10.1016/j.molcel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Soutoglou E, Dorn JF, Sengupta K, Jasin M, Nussenzweig A, Ried T, Danuser G, Misteli T. Positional stability of single double-strand breaks in mammalian cells. Nat Cell Biol. 2007;9:675–682. doi: 10.1038/ncb1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutoglou E, Misteli T. Activation of the cellular DNA damage response in the absence of DNA lesions. Science. 2008;320:1507–1510. doi: 10.1126/science.1159051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell. 2005;123:1213–1226. doi: 10.1016/j.cell.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci U S A. 2005;102:13182–13187. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jiang X, Xu Y, Ayrapetov MK, Moreau LA, Whetstine JR, Price BD. Histone H3 methylation links DNA damage detection to activation of the tumour suppressor Tip60. Nat Cell Biol. 2009;11:1376–1382. doi: 10.1038/ncb1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilard RK, Jacques PE, Laramee L, Cheng B, Galicia S, Bataille AR, Yeung M, Mendez M, Bergeron M, Robert F, et al. Systematic identification of fragile sites via genome-wide location analysis of gamma-H2AX. Nat Struct Mol Biol. 2010;17:299–305. doi: 10.1038/nsmb.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Sudlow G, Belmont AS. Large-scale chromatin unfolding and remodeling induced by VP16 acidic activation domain. J Cell Biol. 1999;145:1341–1354. doi: 10.1083/jcb.145.7.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschure PJ, van der Kraan I, de Leeuw W, van der Vlag J, Carpenter AE, Belmont AS, van Driel R. In vivo HP1 targeting causes large-scale chromatin condensation and enhanced histone lysine methylation. Mol Cell Biol. 2005;25:4552–4564. doi: 10.1128/MCB.25.11.4552-4564.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- Yazdi PT, Wang Y, Zhao S, Patel N, Lee EY, Qin J. SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint. Genes Dev. 2002;16:571–582. doi: 10.1101/gad.970702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarebski M, Wiernasz E, Dobrucki JW. Recruitment of heterochromatin protein 1 to DNA repair sites. Cytometry A. 2009;75:619–625. doi: 10.1002/cyto.a.20734. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Bielopolski D, Galanty Y, Lukas C, Taya Y, Schultz DC, Lukas J, Bekker-Jensen S, Bartek J, Shiloh Y. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat Cell Biol. 2006;8:870–876. doi: 10.1038/ncb1446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.