Abstract
The cloning of leptin in 1994 was an important milestone in obesity research. In those days obesity was stigmatized as a condition caused by lack of character and self-control. Mutations in either leptin or its receptor were the first single gene mutations found to cause morbid obesity, and it is now appreciated that obesity is caused by a dysregulation of central neuronal circuits. From the first discovery of the leptin deficient obese mouse (ob/ob), to the cloning of leptin (ob aka lep) and leptin receptor (db aka lepr) genes, much has been learned about leptin and its action in the central nervous system. The initial high hopes that leptin would cure obesity were quickly dampened by the discovery that most obese humans have increased leptin levels and develop leptin resistance. Nevertheless, leptin target sites in the brain represent an excellent blueprint for distinct neuronal circuits that control energy homeostasis. A better understanding of the regulation and interconnection of these circuits will further guide and improve the development of safe and effective interventions to treat obesity. This review will highlight our current knowledge about the hormone leptin, its signaling pathways and its central actions to mediate distinct physiological functions.
Keywords: Leptin receptor, Neuronal circuits, Feeding, Energy expenditure, Glucose homeostasis, Reward, Leptin transport
1. Introduction
Even before leptin was cloned in 1994 [1], its presence was predicted based on ob/ob (leptin deficient) and db/db (leptin receptor deficient) mice. Douglas Coleman and colleagues performed parabiosis studies, where they joined the circulation of ob/ob and db/db mice. They concluded from these studies that ob/ob mice were missing a circulating factor that was plentiful in db/db mice. This circulating factor would cure obesity in ob/ob mice, while db/db mice were unresponsive to it [2].
It took over 40 years for the discovery of the gene that was thought to be responsible for the observed effect in ob/ob and db/db parabiosis studies; at a time when positional cloning was still in its infancy. The discovery of the hormone leptin by cloning was initially hailed as a cure for human obesity, and the production of recombinant leptin followed quickly after that [3]. As a proof of concept, daily injections of recombinant leptin fully corrected obesity and other associated neuroendocrine abnormalities in rare cases of leptin deficient humans and rodents [4,5]. However, for most obese patients leptin levels were high and correlated positively with their adiposity [6]. Also, leptin injections were ineffective to reduce body weight and food intake in obese mice compared to lean controls [7]; a condition now termed leptin resistance (for more detailed reading on this topic please see [8]). Thus, the vast majority of overweight and obese patients are unresponsive to leptin. Yet, despite its clinical ineffectiveness to treat general obesity, the importance of leptin signaling for the maintenance of normal energy homeostasis is undebated, and patients with leptin deficiency, chronically low leptin levels (lipodystrophy or anorexia) or insulin deficiency may benefit from leptin treatment [9].
The past decade of research progress has continually expanded and refined the sites and mechanisms through which leptin acts to regulate energy homeostasis. Initial work demonstrated that leptin acts predominantly via the long form leptin receptor (LepRb) in the central nervous system, as deletion of LepRb from peripheral tissues does not affect energy homeostasis [10]. Attempts to understand leptin’s effect on central feeding circuits initially highlighted the hypothalamic arcuate nucleus (ARC) [11], yet more recent work demonstrates that non-ARC LepRb populations importantly contribute to distinct physiologic aspects of leptin function [12]. This ever growing literature provides an improved, but still incomplete, picture of leptin function within the complex neural systems that control food intake and energy expenditure [13].
2. The leptin gene and peptide
Leptin (from the Greek word leptos, meaning “thin”) is derived from the lep gene, located on chromosome 7, which transcribes a 167 amino acid peptide with a molecular weight of 16kD. The lep gene sequence is highly preserved across mammals, and leptin orthologs exist in amphibians, reptiles and fish with considerable divergence in primary amino acid sequences. The function of leptin is highly conserved in all mammalian and non-mammalian leptin due to the preservation of key second and tertiary structures allowing the formation of disulfide bridges [14]. Leptin belongs to the family of long-chain helical cytokines, which includes leukemia inhibitory factor, ciliary neurotrophic factor (CNTF) and human growth hormone, based on its crystal structure [15].
3. Leptin production
Leptin is produced and secreted predominantly from adipose tissue into the circulation. Circulating leptin levels positively reflect adipose tissue size, and communicate energy storage status to the brain [6,7]. Leptin expression and circulating levels show circadian fluctuations, and also change with nutritional state [16]. Fasting decreases circulating leptin levels, while feeding or obesity increases leptin levels [17]. Preventing the fasting induced fall of leptin reverses common physiological adaptations to fasting [17,18], highlighting the importance of leptin levels for energy homeostasis.
Leptin expression and secretion are regulated by many factors, e.g. inflammatory cytokines, glucocorticoids and insulin [19]. Also, sympathetic norepinephrine release and β-adrenergic receptor activation in adipose tissue are critical to decrease leptin gene expression in response to leptin injections [20] and are required for the reduction in circulating leptin levels during fasting [21]. Circulating leptin levels also reflect the physiological potency of leptin, such that ob/ob mice show pronounced responses to injected leptin, while hyperleptinemia results in diminished leptin response [22]. Indeed, hyperleptinemia is sufficient and necessary to induce leptin resistance, even though weight gain and hyperphagia are unaffected by the presence or absence of hyperleptinemia [23]. Overall there is compelling evidence that hyperleptinemia induces leptin resistance, but the importance of leptin resis-tance for whole body energy homeostasis and obesity develop-ment remains to be conclusively resolved [8].
4. Central leptin access
Leptin is too large to passively cross the blood brain barrier (BBB) and is instead transported across the BBB by a regulated, saturable transport system. Even though the molecular identity of this leptin transporter system is still unclear, it acts independent of LepRb [24]. While it is often implied that the ARC is outside the BBB, the existence of a functional BBB in the ARC is well established and indicated by a lack of fenestrated capillaries. Fenestrated capillaries are found in select brain regions in close proximity to the ventricular space, collectively termed circumventricular organs (CVO’s). The median eminence (ME) is a CVO and as such clearly contains fenestrated capillaries. However, the border between the ME and ARC is lined by tanycytes, which are highly specialized glial cells connected via tight junctions. These tanycytes therefore shield the ARC from the circulation and the adjacent median eminence [25].
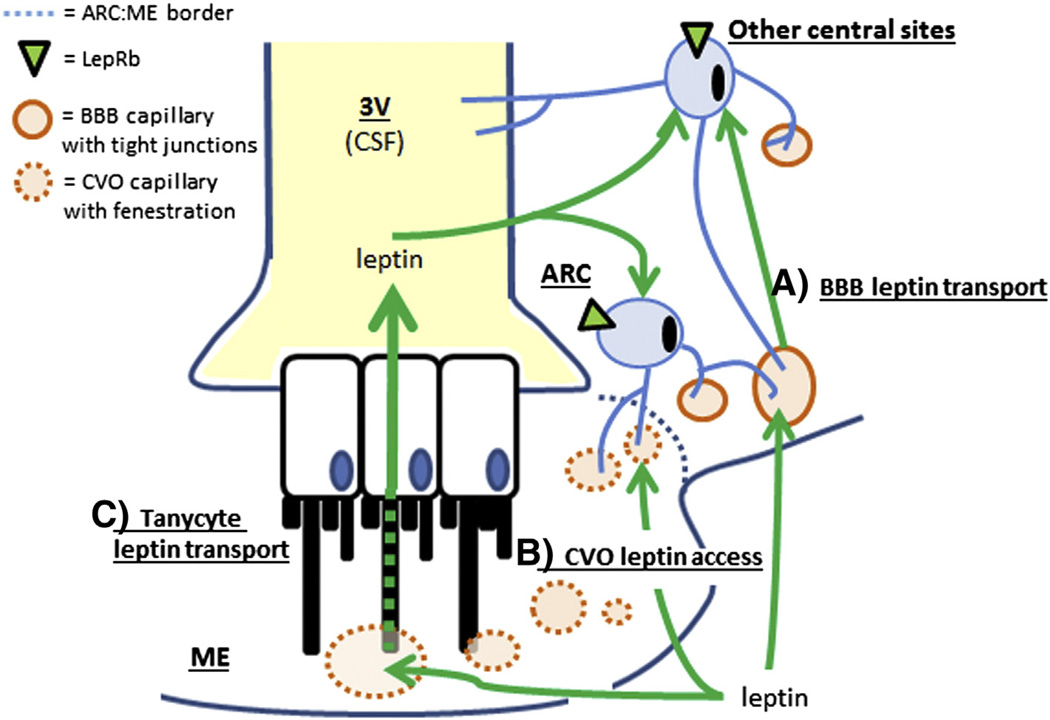
These and other data strongly indicate that the ARC is protected from the general circulation by the BBB and the ME/ ARC tanycyte barrier, and that circulating signals cannot reach ARC neurons via passive diffusion. However, several lines of interesting data indicate that ARC neurons, particularly those in close proximity to the ME, are uniquely positioned to respond to circulating signals such as leptin,. First, fasting causes fenestrated capillaries to extend from the ME to proximal parts of the ARC, possibly allowing the diffusion of leptin to neurons at the ARC–ME border [26]. Second, tanycytes can transport leptin into the cerebrospinal fluid (CSF) from where leptin reaches LepRb target cells [27]. Third, the proximal ARC also connects to the perivascular space of the median eminence (Virchow–Robin space), allowing blood-derived substances to reach proximal ARC neurons by perivascular routes [28]. Finally, many ARC LepRb neurons send projections across the tanycyte barrier and into the ME and thereby gain direct access to circulating leptin levels [29]. While leptin likely reaches most central LepRb neurons via a saturable transport across the BBB or through the CSF, ARC LepRb neurons are uniquely positioned to detect changes in circulating leptin independent of a BBB and thereby respond to these changes with increased time- and dose-dependent sensitivity [29]. This unique anatomy may also explain why ARC LepRb neurons are prone to develop leptin resistance while other leptin target sites remain leptin sensitive [30]. In line with this hypothesis, tanycyte-mediated leptin transport is sensitive to leptin resistance, and select improvement of tanycyte transport reverses DIO and reinstates leptin sensitivity [27]. The different routes of leptin access to the brain in ARC and non-ARC LepRb neurons that have been proposed in the literature are summarized in Fig. 1.
Fig. 1.
Mechanisms of central leptin access. Schematic drawing depicting the border at the level of the median eminence (ME) and arcuate nucleus (ARC) as an example to show different mechanisms of central leptin access. A. Saturable transport of leptin across the blood brain barrier (BBB). B. Direct access of leptin receptor neurons to the circulation via projections close to fenestrated capillaries (perivascular space) in circumventricular organs (CVO’s, e.g. median eminence, area postrema, organum vasculosum). C. Leptin transport via tanycytes into cerebrospinal fluid (CSF) in the ventricular space (e.g. the third ventricle, 3V).
5. Leptin receptors and signaling
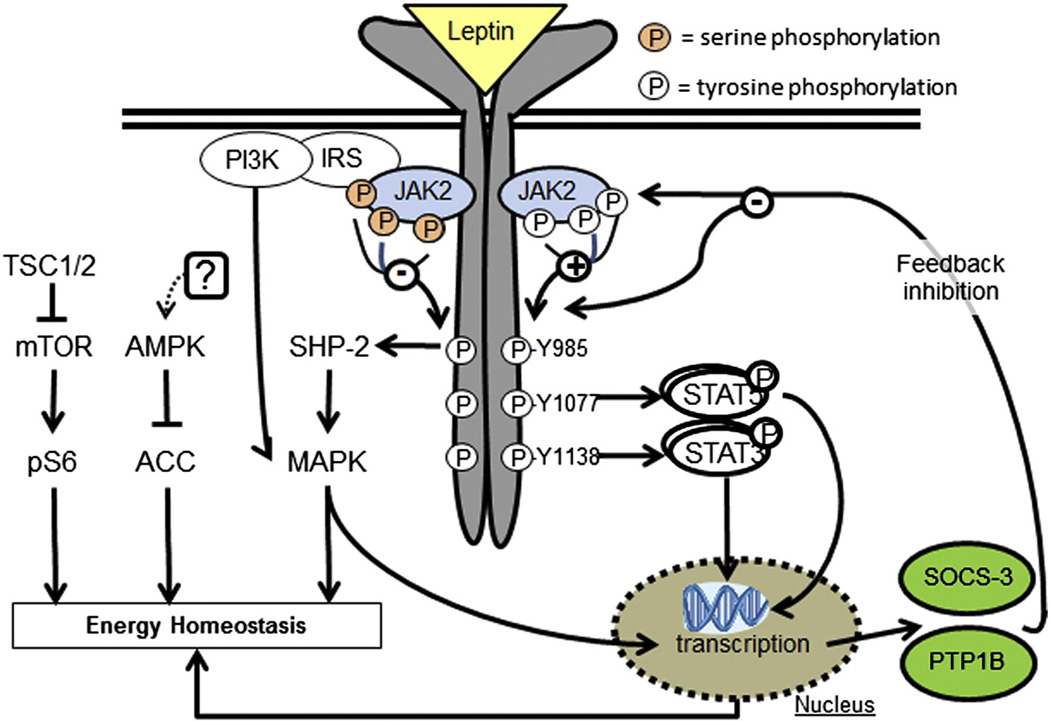
Six leptin receptor isoforms (LepRa-f) are generated by alternative splicing of the lepr gene (aka db gene). These isoforms share a common leptin binding domain but differ in their intracellular domains. LepRa, b, c, d and f are trans-membrane receptors that all possess the box 1 motif required for binding of janus kinase 2 (JAK2). LepRe uniquely lacks a transmembrane domain and is a soluble LepR isoform, allowing LepRe to bind circulating leptin and inhibit central leptin transport. LepRb features an extended intracellular signaling domain that is phosphorylated at three distinct tyrosine residues by activated JAK2, and this long form of the leptin receptor is responsible for the main effects of leptin on energy homeostasis and other neuroendocrine functions [8].
LepRb is a typical class I cytokine receptor without intrinsic kinase activity; instead leptin binding to LepRb allows the recruitment and activation of JAK2, which propagate phosphorylation of JAK2 itself and three tyrosine residues on LepRb (Y985, Y1077and Y1138). Each of these phosphorylation sites induces a specific signaling pathway with distinct physiological leptin functions. Y985 activates src-homology-2 domain protein (SHP-2) and mitogen-activated-protein-kinase (MAPK) signaling and mediates negative feedback signaling of the leptin signaling pathway. Y1077 activates signal-transducer-and-activator-of-transcription-5 (STAT5) signaling and mediates reproductive effects of leptin. Finally, Y1138 activates STAT3 signaling and mediates the main effects of leptin on energy homeostasis and neuroendocrine functions, but has little effect on reproduction [8]. Leptin-induced pSTAT3 has also been used to indicate relative changes in leptin sensitivity and the physiological state of leptin resistance is nicely recapitulated by decreased leptin-activated STAT3 [30].
LepRb signaling is linked to two adaptor molecules, which serve as negative regulators of leptin signaling: suppressor-of-cytokine-signaling-3 (SOCS-3) and phosphotyrosine phospha-tase-1B (PTP1B). SOCS-3 gene expression is increased by leptin-induced pSTAT3 and SOCS-3 peptide binds to Y985 and JAK2 to block leptin signaling in a classic feedback inhibition pathway [31]. Selective mutation of Y985, to prevent leptin-induced Y985 phosphorylation, results in lean mice with enhanced leptin signaling and demonstrates the role of Y985 in negative feedback signaling [32]. Overexpression of SOCS-3 decreases leptin signaling; conversely, heterozygote SOCS-3 deletions (homozygous animals are embryonic lethal) enhance leptin sensitivity and decrease high-fat-diet (HFD) induced weight gain [33,34]. Similarly, PTP1B mRNA is upregulated by STAT3 signaling and PTP1B deficiency results in enhanced leptin action [35], even though the exact interaction of PTP1B with LepRb is not exactly known. Most importantly, leptin resistant mice have increased hypothalamic PTP1B and/or SOCS-3 mRNA expression, which contributes to their leptin resistance [35–37]. Other LepRb regulated transcripts include several hypothalam-ic neuropeptides like pro-opiomelanocortin (POMC), cocaine-and-amphetamine-regulated-transcript (CART), agouti-related-protein (AgRP), and neuropeptide Y (NPY) [38,39].
JAK2 can also be phosphorylated at several amino acid residues, which modulates JAK2 activity and LepRb signal transduction [40,41], e.g. phosphorylation of insulin-receptor-substrates (IRS) and activate phosphoinositol-3-kinase (PI3K) [42]. Leptin induced PI3K signaling is required for leptin’s effects on food intake and sympathetic nerve activity, and further involves activation of mammalian-target-of-rapamycin (mTOR) signaling [43,44]. Leptin also regulates 5’-adenosine monophosphate-activated protein kinase (AMPK) phosphoryla-tion, a metabolic master switch that regulates energy fluxes. In peripheral tissues leptin stimulates AMPK activity to promote catabolic pathways (fatty acid oxidation, glucose transport) [45]. Conversely, in the brain leptin inhibits AMPK activity, which specifically regulates food intake via regulation of hypothalamic neuropeptides [46] (Fig. 2).
Fig. 2.
Leptin signaling pathways and cellular leptin resistance. Schematic drawing of signaling pathways induced via the long form leptin receptor (LepRb). PI3K = phosphatidylinositol-3-kinase; IRS = insulin receptor substrate; JAK2 = janus kinase-2; ER = endoplasmatic reticulum, STAT = signal-transducer-and-activator-of-transcription; SOCS-3 = suppressor-of-cytokine-signaling-3; PTP1B = phosphotyrosine phosphatase 1B; TSC1/2 = tuberous-sclerosis1/2; mTOR = mammalian-target-of-rapamycin; pS6 = phosphorylated ribosomal protein S6; AMPK = AMP-activated protein kinase; ACC = acetyl-CoA carboxylase; SHP-2 = src-homology-2 containing phosphotyrosine phosphatase 2; MAPK = mitogen-activated-protein-kinase.
6. Leptin and central control of energy homeostasis
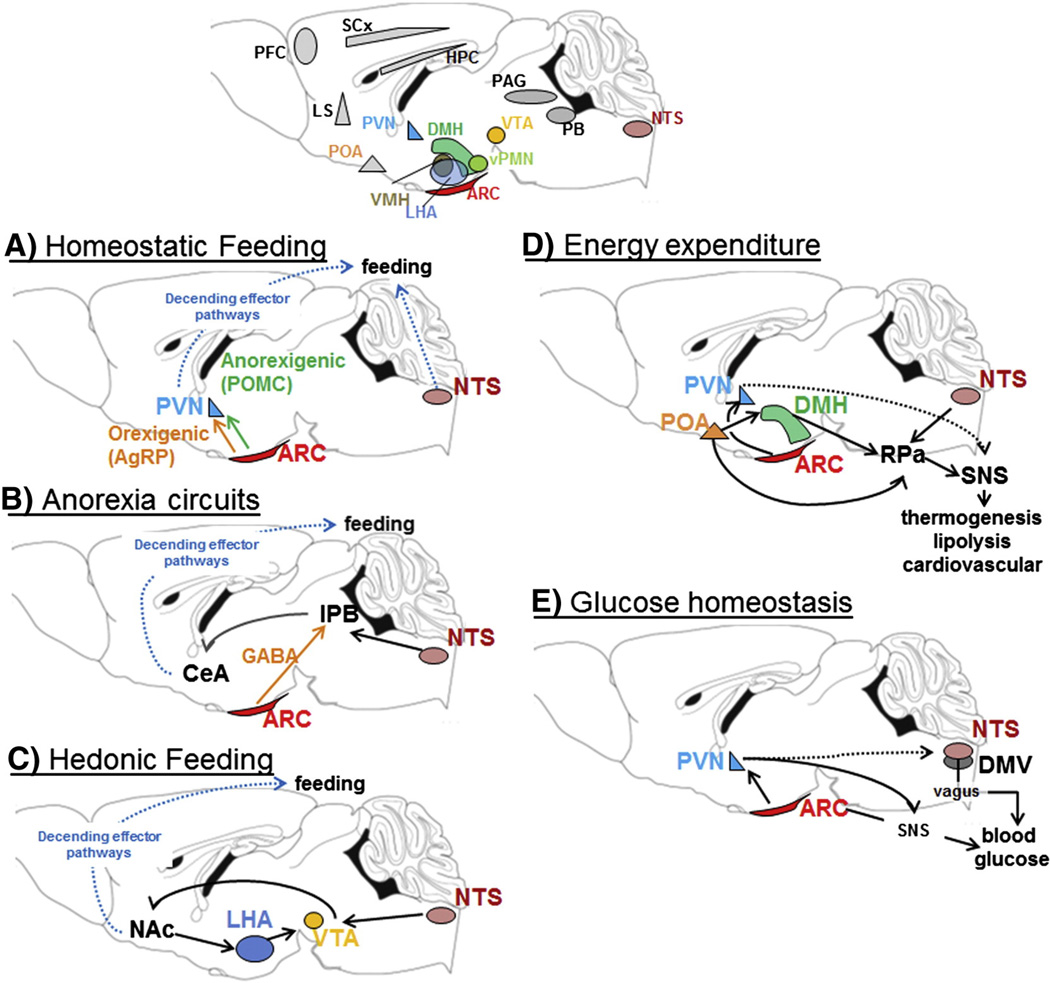
After the discovery that leptin acts in the brain to regulate energy homeostasis, the distribution of LepRb expressing neurons revealed the hypothalamus as main target for central leptin action. This observation was consistent with earlier lesion studies demonstrating that crude hypothalamic lesions, extending through several hypothalamic nuclei, generally resulted in severe obesity [47]. We now appreciate that diverse hypothalamic and extra-hypothalamic LepRb populations collectively contribute to leptin’s overall effect on energy homeostasis (Fig. 3A – E).
Fig. 3.
Central LepRb expression sites and related neuronal circuits. Leptin acts on diverse central circuits to regulate distinct aspects of energy homeostasis. A–E: Examples of select central circuits that have been studied in more detail for leptin function. Many other LepRb populations (gray areas in top panel) remain to be studied and integrated into a comprehensive picture of energy homeostasis. Specifically higher, cortical brain structures and descending effector pathway have not been well integrated into leptin regulated energy homeostasis. PVN = paraventricular nucleus; ARC = arcuate nucleus; NTS = nucleus of the solitary tract; AgRP = agouti-related-peptide; POMC = proopiomelanocoritin; CeA = amygdala; lPB = lateral parabrachial nucleus; GABA = γ-aminobutyric acid; Glu = glutamate; Nac = nucleus accumbens; LHA = lateral hypothalamic area; VTA = ventral tegmental area; POA = prooptic area; DMH = dorso-medial hypothalamus; RPa = raphe pallidus; SNS = sympathetic nervous system, DMV = dorso-motor complex of vagus; PAG = periaqueductal gray; SCs = sensory cortex; HPC = hippocampus.
6.1. Homeostatic feeding circuits
LepRb is strongly expressed in the ARC and LepRb expression co-localized with two neuronal populations that were well known for their opposing effectson food intake and differential regulation in response to changes in nutritional state: orexigenic NPY/AgRP neurons and anorexigenic POMC/CART neurons. Leptin decreases anorexigenic NPY and AgRP neuro-peptide gene expression and inhibits NPY/AgRP neurons, while fasting has the opposite effect. Conversely, leptin promotes POMC and CART gene expression and activates POMC/CART neurons, while fasting produces the opposite effect [11]. More recently, in vivo pharmacogenetic and optogenetic activation or inhibition of AgPR and POMC neurons confirmed the physiological importance of their neuronal activation states in the control of food intake and energy expenditure [48,49]; where AgRP neurons caused acute (within minutes) and POMC neurons long-term (24 h) changes in food intake [49]. Thus, anorexigenic leptin action differentially regulates orexigenic and anorexigenic neurons and the expression and release of their neuropeptides onto second order neurons. Here, homeostatic inputs from the ARC are conveyed via the melanocortin system within the paraventricular nucleus (PVN) or lateral hypothalamic area (LHA), where POMC-derived peptides (e.g.a-melanin-stimulating-hormone) activate melanocortin-4-recepotors (MC4R), while AgRP inhibits MC4R function [11]. This elegant and simple leptin signaling pathway is found in all newer physiology text books and is an important pathway for homeostatic regulation of feeding (Fig. 3A).
Even though this ARC-centric leptin signaling pathway remains valid, several excellent studies have refined and revised the role of ARC neurons in the response to leptin (for a recent excellent and detailed review on this topic see [50]). The availability of conditional gene manipulation systems (Cre/loxP and derivatives) has provided the opportunity to test the role of leptin signaling within specific tissue, cell type, or neuronal population. Contrary to general expectations, the targeted deletion of LepRb from ARC neurons did not recapitulate the severe obesity and hyperphagia observed in db/db mice, and instead only caused a mild obese phenotype [51]. These results indicated that additional LepRb populations were critical to mediate anorexic leptin action and body weight control. Subsequent studies targeted LepRb neurons in other hypotha-lamic and extra-hypothalamic sites [52–57]. Taken together, this work suggests that no single LepRb population explains the severe obesity phenotype of db/db mice, but that the sum of leptin action on LepRb neurons and their interaction with each other account for leptin’s effect on energy homeostasis. It is now also appreciated that distinct LepRb populations are responsible for different aspects of physiological leptin function (Fig. 3A–E) as further highlighted below.
6.2. Anorexia circuits
Central injection of AgRP or NPY robustly increased food intake, even though genetic ablation of AgRP or NPY surprisingly failed to decrease food intake or body weight [58]. Compensatory mechanisms during development were suspected, and indeed later studies demonstrated that ablation of AgRP neurons in adult mice caused severe hypophagia and even death from starvation, while neonatal ablation had no effect on energy intake [59]. NPY/AgRP neurons are inhibitory neurons and co-express the inhibitory neurotransmitter γ-amino-butyric-acid (GABA). Unexpectedly, the starvation behavior in ablated mice was independent of melanocortin signaling [60], however, it could be fully prevented by microinjections of the neurotransmitter GABA into the parabrachial nucleus (PB), while injections into other classic second order structures like the PVN and LHA had no effects [60,61]. The PB is activated by visceral malaise, e.g. induced by toxins, lithium chloride, but also by an excessive meal [62,63]. It was subsequently found that GABAergic AgRP inputs to the PB oppose excitatory brainstem inputs to the PB that induce severe nausea [64]. As noted above, leptin inhibits AgRP neurons and thus GABA release in the PB, which may enhance meal-induced satiety. Furthermore, LepRb is also directly expressed within the nucleus of the solitary tract (NTS) in the brainstem [65,66] and NTS leptin action promotes anorexia independent of hypothalamic inputs [54,67]. Many NTS acting and satiety inducing peptides (e.g. GLP-1) act via the PB and leptin action enhances their potency [68]. Thus, leptin-induced anorexia may further diminish GABA inputs from AgRP neurons, or induce excitatory NTS inputs to the PB to enhance meal induced satiety in addition to homeostatic feeding circuits though the PVN (Fig. 3B).
6.3. Hedonic feeding circuits
An important aspect of food intake is the motivation to seek out food despite dangers from predators or the environment. The organism’s nutritional state greatly influences the motivation to eat, and fasting robustly enhances motivated behavior [69]. The drop in leptin levels during fasting contributes to the increased motivation, while leptin administration reduces the rewarding value of food and thus the motivation to eat [70,71].
The master pathway involved in the regulation of motivated behavior is the mesolimbic pathway where midbrain dopamine (DA) expressing neurons in the ventral tegmental area (VTA) release DA into the nucleus accumbens (NAc). The release of DA into the NAc encodes motivated events, which may be either positive (rewarding) or negative (aversive) [72]. DA release thus modulates the motivation to work for reward, or how much the reward is “wanted”. This is nicely demonstrated in hyperdopaminergic mice, where increased DA release increases the motivation to work for a food treat [73].
It is well established that leptin impacts food reward, and that it does so at least in part via VTA DA neurons [70,55,74,75]. LepRb is expressed directly on some VTA DA neurons, with leptin inhibiting these VTA LepRb neurons [55] and preventing sucrose-induced DA release into the NAc [76]; thus providing a valid explanation how leptin could decrease food reward. In contrast, ob/ob mice show decreased NAc DA content and leptin stimulates expression and activation of the rate limiting enzyme for DA production (tyrosine hydroxylase) and increases DA content in the NAc [70,75]. Thus, dopamine deficiency in the NAc of ob/ob mice may diminish reinforcement, which could cause compensatory motivated behaviors, like food intake, in an attempt to enhance reinforcement [77,78]. The stimulatory effects of leptin on VTA function are mediated via LepRb neurons in the LHA, which innervate the VTA [75]. Finally, recent data demonstrated that the above mentioned homeo-static and anorexic aspects of feeding substantially interact with hedonic feeding paradigms [79,80], and complex behaviors like feeding should be understood as an integration of distinct feeding behaviors [81] (Fig. 3C).
6.4. Energy expenditure circuits
Energy expenditure is the counterpart of food intake, and body weight is stable when energy expenditure and intake are in balance. Energy expenditure is influenced by locomotor activity, ambient temperature as well as nutritional state. Acute leptin injections in lean normal mice do not influence energy expenditure per se, but lack of leptin in ob/ob mice or lipodystrophic mice causes low body temperature (including frequent torpor bouts) and cold-sensitivity. Leptin injections correct the reduced body temperature and cold sensitivity [5,82], supporting an important role of leptin in thermoregu-lation. Leptin-deficient mice are also characterized by brown adipose tissue (BAT) atrophy and decreased uncoupling protein-1 (UCP1) expression, a key protein for BAT-derived heat production that is fueled by lipolysis [83]. In addition, leptin also induces weight loss independent of food intake, via mechanisms that require UCP1 [84], suggesting that food-independent body weight control by leptin is mediated via BAT thermogenesis.
The dorsomedial hypothalamus and dorsal hypothalamic area (DMH/DHA) have been highlighted as brain areas that regulate BAT thermogenesis, considering that cold exposure activates DMH/DHA neurons [85]. The activity of DMH/DHA neurons also depends on inhibitory and excitatory inputs from other central sites, e.g. the preoptic area (POA), which acts as a temperature sensor and integrates local temperature with afferent information from peripheral and deep-body thermoreceptors [86]. The POA connects with the DMH/DHA to regulate further downstream effector neurons in the raphe pallidus (RPa) that control sympathetic BAT activity [86]. LepRb is expressed in BAT-related neurons in the DMH/DHA and POA [87] and DMH/DHA LepRb neurons are sufficient and necessary to regulate energy expenditure and body weight via mechanisms that are independent of food intake [88] but involve sympathetic activation of BAT thermogenesis [89]. The DMH also contains NPY expressing neurons that impact whole body energy expenditure by increasing the sympathetic nervous system, even though these effects are largely independent of leptin function [90].
Interestingly, the brainstem mediates thermoregulatory leptin functions independent of the hypothalamus, as observed in decerebrate rats [91] and other systems [92]. Leptin injection into the 4th ventricle of decerebrate rats mildly raises brown fat and body temperature, but strikingly, co-injection of leptin with thyroid releasing hormone (TRH) robustly enhanced the thermogenic capacities of TRH [92]. This sensitizing effect is mediated by direct leptin effects on NTS LepRb neurons [93], which may innervate and stimulate RPa neurons to induce BAT thermogenesis.
Changes in energy expenditure and thermogenesis always require the simultaneous adaptation of cardiovascular outputs and lipolysis. Both are similarly regulated by the sympathetic nervous system or in case of lipolysis also by the HPA axis [94], although whether identical or parallel neuronal circuits are engaged is unclear. Circulating leptin levels correlate with blood pressure [95] and leptin induces not only BAT, but also renal sympathetic outputs via the ARC and NTS [96,97]. However, whether these circuits cross-talk with DMH induced energy expenditure remains to be investigated. Furthermore, intracel-lular leptin signaling cascades are involved in distinct effects of leptin. While leptin-induced pSTAT3 is essential to maintain normal energy expenditure [98], leptin’s effect on blood pressure requires PI3K activation [99]. These circuits are summarized in Fig. 3D.
6.5. Glucose homeostasis circuits
Leptin action in the CNS also exerts a powerful effect on blood glucose homeostasis [100]. The earliest evidence that leptin contributes directly to glucose homeostasis came from exper-iments in leptin or leptin receptor deficient mice. Both ob/ob and db/db mice are markedly hyperglycemic, hyperinsulinemic, and glucose intolerant, and this hyperglycemia is independent of their excessive bodyweight [39]. However, the hyperglycemia of ob/ob mice can be readily normalized by infusion of leptin into the brain [101,102], demonstrating that leptin’s effects on blood glucose homeostasis are primarily mediated via the central nervous system.
Defining the specific brain areas and neuronal populations that mediate leptin’s effects on blood glucose remains an active area of investigation. Much as with energy balance, the preponderance of studies support a critical role for the hypothalamus, and most studies have focused particularly on the arcuate nucleus, in leptin-dependent regulation of glucose homeostasis. Emphasis on the arcuate nucleus extends from data demonstrating that restoration of leptin action within just the ARC is sufficient to normalize blood glucose levels in LepRb deficient mice or rats [44,103], with recent data strongly implicating arcuate AgRP neurons [104]. Additional work focused on signaling events downstream of LepRb collectively indicate that PI3K, but not STAT3 signaling, mediates the effects of leptin on glucose homeostasis. Mice lacking leptin-induced Stat3 signaling do not exhibit the marked hyperglycemia observed in db/db mice [98]. Conversely, pharmacological inhibition of PI3K signaling prevents leptin-enhanced insulin sensitivity and glucose tolerance [44], and POMC-specific ablation of PI3K signaling alters glucose homeostasis [105]. These data strongly implicate the ARC as a critical site for leptin-dependent regulation of blood glucose homeostasis.
Leptin’s effect on blood glucose homeostasis is further processed via downstream systems that regulate the autonomic nervous system. For instance, leptin acts in the hypothalamus to suppress hepatic glucose production, and vagal innervation of the liver is an important component of this effect [106]. Similarly, leptin alters glucose and lipid metabolism in peripheral tissues via sympathetic outflow and activation of AMPK signaling [45,46]. Thus, central leptin action is linked to autonomic pathways that control hepatic glucose production and peripheral tissue glucose disposal. Leptin also engages pathways which indirectly contribute to blood glucose levels. For instance, anorexic leptin action limits the introduction of carbohydrate to the system, while both leptin and insulin act within the CNS to alter peripheral lipid metabolism [107] (Fig. 3E).
Finally, the glucose lowering effect of brain leptin action involves both insulin-dependent and insulin-independent mechanisms. Considering insulin’s established role in the regulation of blood glucose homeostasis, several experiments have established that leptin acts to improve insulin sensitivity [44,106,108]. Yet in addition to this insulin sensitizing effect, recent studies indicate that leptin also lowers glucose levels independently of insulin. This insulin independent effect was convincingly shown in mice and rats with ablated pancreatic β-cells and insulin-deficiency using streptozotocin (STZ) treatment, where central leptin fully normalizes their severe hyperglycemia [109]. Leptin receptor expression solely within GABA and POMC neurons is sufficient to mediate this glucose lowering effect of leptin. AgRP neurons are GABAergic neurons, and thus these data could implicate AgRP and POMC neurons as key populations in leptin’s antidiabetic effects [110]. However, the majority of leptin receptor expressing neurons signal via GABA, so that it is not entirely clear if other non-ARC neurons contribute to the glucose lowering effect of leptin. Taken together, these data not only demonstrate the powerful effects of central leptin action to control blood glucose, they also indicate that this effect does not require insulin. This brain centered glucoregulatory system has therefore been postulated to contribute significantly to the regulation of blood glucose via mechanisms that are independent of, but synergize with, the classic insulin-dependent model of blood glucose homeostasis [100].
Leptin’s ability to regulate blood glucose independent of food intake and body weight provides strong evidence that leptin is not just an appetite or obesity hormone. Instead, leptin acts in the brain to coordinate a series of physiological responses, with blood glucose homeostasis being a critical and perhaps primary endpoint. Except for a relatively small number of leptin deficient humans, leptin has been largely ineffective in the clinical treatment of obesity. In contrast, leptin has shown positive effects in reducing the hyperglyce-mia associated with leptin-deficient lipodystrophy, and there is enthusiasm that leptin may also be an effective clinical treatment for insulin deficient, Type 1 diabetes [111].
7. Conclusion and perspective
Over the last 20 years much has been learned about the distinct physiological functions regulated by leptin and the central circuits that mediate these leptin-dependent effects. The result is a more comprehensive understanding of the complex network that underlies leptin regulated feeding behavior and other homeostatic functions. Several LepRb expression sites remain unstudied and their physiological function is unclear. Specifically, the role of higher brain structures, like sensory and prefrontal cortices, and their integration into homeostatic leptin action are not well understood. The development of drugs to treat obesity has been disappointing due to either safety issues or weight regain in the long term. It is hoped that a more comprehensive understanding of neuronal circuits that control energy homeostasis will help us to develop new strategies for the treatment of obesity.
Acknowledgment
This work was supported by NIH grants R01-DK092587, P20-RR02195, P30-DK072476 (HM) and R01-DK081563 (CM).
Abbreviations
- ob/ob mice
leptin deficient mice
- db/db mice
leptin receptor deficient mice
- LepRb
long form leptin receptor
- ARC
arcuate nucleus
- CNTF
ciliary neurotrophic factor
- BBB
blood brain barrier
- CVO
circumventricular organ
- ME
median eminence
- CSF
cerebrospinal fluid
- JAK2
janus-kinase-2
- Y985/1077/1138
tyrosine residues 985/1077/1138
- SHP-2
src homology-2 domain protein
- MAPK
mitogen-activated-protein-kinase
- STAT3/5
signal-transducer-and-activator-of transcription-3/5
- pSTAT3
phospho-STAT3
- SOCS-3
suppressor-of-cytokine-signalig-3
- PTP1B
phosphotyrosinephosphatase-1B
- HFD
high-fat-diet
- POMC
pro-opiomelanocortin
- CART
cocaine-and-amphetamine-regulated-transcript
- AgRP
agouti-related-protein
- NPY
neuropeptide Y
- IRS
insulin-receptor-substrates
- PI3K
phosphoinositol-3-kinase
- mTOR
mammalian-target-of-rapamycin (mTOR)
- AMPK
5’-adenosine monophosphate-activated protein kinase
- PVN
paraventricular nucleus
- LHA
lateral hypothalamic area
- MC4R
melanocortin-4-recepotors
- GABA
γ-amino-butyric-acid
- PB
parabrachial nucleus
- NTS
nucleus of the solitary tract
- GLP-1
glucagone-like-peptide-1
- DA
dopamine
- VTA
ventral tegmental area
- NAc
nucleus accumbens
- BAT
brown adipose tissue
- UCP1
uncoupling protein-1
- DMH/DHA
dorsomedial hypothalamus and dorsal hypothalamic area
- POA
preoptic area
- RPa
raphe pallidus
- TRH
thyroid releasing hormone
- STZ
streptozotocin.
Footnotes
All authors declare no conflict of interest.
REFERENCES
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Coleman DL. A historical perspective on leptin. Nat Med. 2010;16(10):1097–1099. doi: 10.1038/nm1010-1097. [DOI] [PubMed] [Google Scholar]
- 3.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 4.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 5.Harris RB, Zhou J, Redmann SM, Jr, Smagin GN, Smith SR, Rodgers E, et al. A leptin dose–response study in obese (ob/ob) and lean (+/?) mice. Endocrinology. 1998;139(1):8–19. doi: 10.1210/endo.139.1.5675. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2(5):589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 7.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1(12):1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 8.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Filho G, Mastronardi CA, Licinio J. Leptin treatment: facts and expectations. Metabolism. 2014 doi: 10.1016/j.metabol.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Guo K, McMinn JE, Ludwig T, Yu YH, Yang G, Chen L, et al. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology. 2007;148(8):3987–3997. doi: 10.1210/en.2007-0261. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 12.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9(2):117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berthoud HR, Zheng H, Shin AC. Food reward in the obese and after weight loss induced by calorie restriction and bariatric surgery. Ann N Y Acad Sci. 2012;1264(1):36–48. doi: 10.1111/j.1749-6632.2012.06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denver RJ, Bonett RM, Boorse GC. Evolution of leptin structure and function. Neuroendocrinology. 2011;94(1):21–38. doi: 10.1159/000328435. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, et al. Crystal structure of the obese protein leptin-E100. Nature. 1997;387(6629):206–209. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 16.Saladin R, De VP, Guerre-Millo M, Leturque A, Girard J, Staels B, et al. Transient increase in obese gene expression after food intake or insulin administration. Nature. 1995;377(6549):527–529. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- 17.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115(12):3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagogo-Jack S. Human leptin regulation and promise in pharmacotherapy. Curr Drug Targets. 2001;2(2):181–195. doi: 10.2174/1389450013348623. [DOI] [PubMed] [Google Scholar]
- 20.Mantzoros CS, Qu D, Frederich RC, Susulic VS, Lowell BB, Maratos-Flier E, et al. Activation of beta(3) adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice. Diabetes. 1996;45(7):909–914. doi: 10.2337/diab.45.7.909. [DOI] [PubMed] [Google Scholar]
- 21.Swoap SJ, Weinshenker D. Norepinephrine controls both torpor initiation and emergence via distinct mechanisms in the mouse. PLoS One. 2008;3(12):e4038. doi: 10.1371/journal.pone.0004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahu A. Resistance to the satiety action of leptin following chronic central leptin infusion is associated with the development of leptin resistance in neuropeptide Y neurones. J Neuroendocrinol. 2002;14(10):796–804. doi: 10.1046/j.1365-2826.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- 23.Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PLoS One. 2010;5(6):e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maness LM, Banks WA, Kastin AJ. Persistence of blood-to-brain transport of leptin in obese leptin-deficient and leptin receptor-deficient mice. Brain Res. 2000;873(1):165–167. doi: 10.1016/s0006-8993(00)02520-8. [DOI] [PubMed] [Google Scholar]
- 25.Bolborea M, Dale N. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 2013;36(2):91–100. doi: 10.1016/j.tins.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA, et al. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 2013;17(4):607–617. doi: 10.1016/j.cmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014;19(2):293–301. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaver SW, Pang JJ, Wainman DS, Wall KM, Gross PM. Morphology and function of capillary networks in subregions of the rat tuber cinereum. Cell Tissue Res. 1992;267(3):437–448. doi: 10.1007/BF00319366. [DOI] [PubMed] [Google Scholar]
- 29.Faouzi M, Leshan R, Bjornholm M, Hennessey T, Jones J, Munzberg H. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology. 2007;148(11):5414–5423. doi: 10.1210/en.2007-0655. [DOI] [PubMed] [Google Scholar]
- 30.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145(11):4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 31.Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, et al. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275(51):40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 32.Bjornholm M, Munzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, et al. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117(5):1354–1360. doi: 10.1172/JCI30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab. 2006;17(9):365–371. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, et al. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4(2):123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, et al. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2(4):489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 36.White CL, Whittington A, Barnes MJ, Wang Z, Bray GA, Morrison CD. HF diets increase hypothalamic PTP1B and induce leptin resistance through both leptin-dependent and -independent mechanisms. Am J Physiol Endocrinol Metab. 2009;296(2):E291–E299. doi: 10.1152/ajpendo.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004;10(7):734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, et al. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46(12):2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45(4):531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 40.Argetsinger LS, Stuckey JA, Robertson SA, Koleva RI, Cline JM, Marto JA, et al. Tyrosines 868, 966, and 972 in the kinase domain of JAK2 are autophosphorylated and required for maximal JAK2 kinase activity. Mol Endocrinol. 2010;24(5):1062–1076. doi: 10.1210/me.2009-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks AS, Myers MG., Jr Regulation of Jak kinases by intracellular leptin receptor sequences. J Biol Chem. 2002;277(44):41547–41555. doi: 10.1074/jbc.M205148200. [DOI] [PubMed] [Google Scholar]
- 42.Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, et al. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6(6):431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Harlan SM, Guo DF, Morgan DA, Fernandes-Santos C, Rahmouni K. Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab. 2013;17(4):599–606. doi: 10.1016/j.cmet.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2(6):411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 46.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428(6982):569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 47.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 48.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeltser LM, Seeley RJ, Tschop MH. Synaptic plasticity in neuronal circuits regulating energy balance. Nat Neurosci. 2012;15(10):1336–1342. doi: 10.1038/nn.3219. [DOI] [PubMed] [Google Scholar]
- 51.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149(4):1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 53.Leshan RL, Louis GW, Jo YH, Rhodes CJ, Munzberg H, Myers MG., Jr Direct innervation of GnRH neurons by metabolic-and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci. 2009;29(10):3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, Dileone RJ, Bence KK, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11(1):77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51(6):801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 56.Leinninger GM, Myers MG., Jr LRb signals act within a distributed network of leptin-responsive neurones to mediate leptin action. Acta Physiol (Oxf) 2008;192(1):49–59. doi: 10.1111/j.1748-1716.2007.01784.x. [DOI] [PubMed] [Google Scholar]
- 57.Ring LE, Zeltser LM. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J Clin Invest. 2010;120(8):2931–2941. doi: 10.1172/JCI41985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol. 2002;22(14):5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 60.Wu Q, Palmiter RD. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. Eur J Pharmacol. 2011;660(1):21–27. doi: 10.1016/j.ejphar.2010.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137(7):1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reilly S. The parabrachial nucleus and conditioned taste aversion. Brain Res Bull. 1999;48(3):239–254. doi: 10.1016/s0361-9230(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 63.Maniscalco JW, Kreisler AD, Rinaman L. Satiation and stress-induced hypophagia: examining the role of hindbrain neurons expressing prolactin-releasing Peptide or glucagon-like Peptide 1. Front Neurosci. 2012;6:199. doi: 10.3389/fnins.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Q, Zheng R, Srisai D, McKnight GS, Palmiter RD. NR2B subunit of the NMDA glutamate receptor regulates appetite in the parabrachial nucleus. Proc Natl Acad Sci U S A. 2013;110(36):14765–14770. doi: 10.1073/pnas.1314137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395(4):535–547. [PubMed] [Google Scholar]
- 66.Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144(5):2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- 67.Harris RB, Bartness TJ, Grill HJ. Leptin responsiveness in chronically decerebrate rats. Endocrinology. 2007;148(10):4623–4633. doi: 10.1210/en.2006-1565. [DOI] [PubMed] [Google Scholar]
- 68.Kanoski SE, Zhao S, Guarnieri DJ, DiLeone RJ, Yan J, De Jonghe BC, et al. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. Am J Physiol Endocrinol Metab. 2012;303(4):E496–E503. doi: 10.1152/ajpendo.00205.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr. 2009;139(3):629–632. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51(6):811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 71.Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, et al. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry. 2011;69(7):668–674. doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci. 2012;15(10):1330–1335. doi: 10.1038/nn.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher ”wanting” but not ”liking” for sweet rewards. J Neurosci. 2003;23(28):9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Domingos AI, Vaynshteyn J, Voss HU, Ren X, Gradinaru V, Zang F, et al. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14(12):1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10(2):89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Domingos AI, Sordillo A, Dietrich MO, Liu ZW, Tellez LA, Vaynshteyn J, et al. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. Elife. 2013;2:e01462. doi: 10.7554/eLife.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berthoud HR. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011;21(6):888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23(3):39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- 79.Lippert RN, Ellacott KL, Cone RD. Gender-specific roles for the melanocortin-3 receptor in the regulation of the mesolimbic dopamine system in mice. Endocrinology. 2014;155(5):1718–1727. doi: 10.1210/en.2013-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alhadeff AL, Grill HJ. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am J Physiol Regul Integr Comp Physiol. 2014 doi: 10.1152/ajpregu.00179.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc. 2012;71(4):478–487. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gavrilova O, Leon LR, Marcus-Samuels B, Mason MM, Castle AL, Refetoff S, et al. Torpor in mice is induced by both leptin-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 1999;96(25):14623–14628. doi: 10.1073/pnas.96.25.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Himms-Hagen J. Defective brown adipose tissue thermogenesis in obese mice. Int J Obes. 1985;9(Suppl 2):17–24. [PubMed] [Google Scholar]
- 84.Commins SP, Watson PM, Frampton IC, Gettys TW. Leptin selectively reduces white adipose tissue in mice via a UCP1-dependent mechanism in brown adipose tissue. Am J Physiol Endocrinol Metab. 2001;280(2):E372–E377. doi: 10.1152/ajpendo.2001.280.2.E372. [DOI] [PubMed] [Google Scholar]
- 85.Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R47–R63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- 86.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93(7):773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, et al. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31(5):1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rezai-Zadeh K, Yu S, Jiang Y, Laque A, Schwarzenburg C, Morrison CD, et al. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol Metab. 2014 doi: 10.1016/j.molmet.2014.07.008. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Enriori PJ, Sinnayah P, Simonds SE, Garcia RC, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31(34):12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011;13(5):573–583. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology. 2009;150(4):1705–1711. doi: 10.1210/en.2008-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hermann GE, Barnes MJ, Rogers RC. Leptin and thyrotropin-releasing hormone: cooperative action in the hindbrain to activate brown adipose thermogenesis. Brain Res. 2006;1117(1):118–124. doi: 10.1016/j.brainres.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 93.Rogers RC, McDougal DH, Hermann GE. Leptin amplifies the action of thyrotropin-releasing hormone in the solitary nucleus: an in vitro calcium imaging study. Brain Res. 2011;1385:47–55. doi: 10.1016/j.brainres.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perry RJ, Zhang XM, Zhang D, Kumashiro N, Camporez JP, Cline GW, et al. Leptin reverses diabetes by suppression of the hypothalamic–pituitary–adrenal axis. Nat Med. 2014;20(7):759–763. doi: 10.1038/nm.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eikelis N, Schlaich M, Aggarwal A, Kaye D, Esler M. Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003;41(5):1072–1079. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- 96.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, et al. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108(7):808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53(2):375–380. doi: 10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421(6925):856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 99.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41(3 Pt 2):763–767. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 100.Schwartz MW, Seeley RJ, Tschop MH, Woods SC, Morton GJ, Myers MG, et al. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature. 2013;503(7474):59–66. doi: 10.1038/nature12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389(6649):374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 102.Liu L, Karkanias GB, Morales JC, Hawkins M, Barzilai N, Wang J, et al. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J Biol Chem. 1998;273(47):31160–31167. doi: 10.1074/jbc.273.47.31160. [DOI] [PubMed] [Google Scholar]
- 103.Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, et al. The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1(1):63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 104.Goncalves GH, Li W, Garcia AV, Figueiredo MS, Bjorbaek C. Hypothalamic agouti-related peptide neurons and the central melanocortin system are crucial mediators of leptin’s antidiabetic actions. Cell Rep. 2014;7(4):1093–1103. doi: 10.1016/j.celrep.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hill JW, Xu Y, Preitner F, Fukuda M, Cho YR, Luo J, et al. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology. 2009;150(11):4874–4882. doi: 10.1210/en.2009-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, et al. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology. 2009;150(10):4502–4511. doi: 10.1210/en.2009-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scherer T, Buettner C. Yin and Yang of hypothalamic insulin and leptin signaling in regulating white adipose tissue metabolism. Rev Endocr Metab Disord. 2011;12(3):235–243. doi: 10.1007/s11154-011-9190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401(6748):73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 109.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci U S A. 2008;105(37):14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci USA. 2010;107(40):17391–17396. doi: 10.1073/pnas.1008025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coppari R, Bjorbaek C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11(9):692–708. doi: 10.1038/nrd3757. [DOI] [PMC free article] [PubMed] [Google Scholar]