Abstract
Leptin is secreted by adipose tissue and regulates energy homeostasis, neuroendocrine function, metabolism, immune function and other systems through its effects on the central nervous system and peripheral tissues. Leptin administration has been shown to restore metabolic and neuroendocrine abnormalities in individuals with leptin-deficient states, including hypothalamic amenorrhea and lipoatrophy. In contrast, obese individuals are resistant to leptin. Recombinant leptin is beneficial in patients with congenital leptin deficiency or generalized lipodystrophy. However, further research on molecular mediators of leptin resistance is needed for the development of targeted leptin sensitizing therapies for obesity and related metabolic diseases.
Keywords: energy homeostasis, hypothalamus, leptin, metabolism, obesity
Introduction
The discovery of leptin changed the knowledge of energy homeostasis and our view of adipose tissue from a simple energy depot to an active endocrine organ [1]. Leptin is mainly produced in adipose tissue and circulating leptin levels correlate well with the amount of body fat, reflecting energy status. Leptin plays an important role in regulating energy homeostasis, neuroendocrine and immune functions, and glucose, lipid and bone metabolism [2, 3]. While leptin administration reverses neuroendocrine and metabolic abnormalities in individuals with congenital leptin deficiency, common forms of obesity are typically associated with elevated leptin and resistance to leptin's effects on energy homeostasis [4]. Here, we review the biology of leptin, the current understanding of its physiologic and pathologic roles, and potential clinical applications.
Biology of leptin
Leptin is a 167-amino-acid peptide that is mainly expressed in white adipose tissue (WAT), but is also found in a variety of tissues including placenta, mammary gland, ovary, skeletal muscle, stomach, pituitary gland, and lymphoid tissue [5]. Circulating leptin levels are directly in proportion to the amount of body fat, thereby reflecting the status of long-term energy stores. In addition, leptin levels fluctuate according to changes in calorie intake with a marked decrease during starvation [6, 7]. Leptin is secreted in a pulsatile manner, displaying a circadian rhythm with lowest levels at mid-afternoon and highest levels at midnight. The pulsatile pattern of leptin secretion is similar in obese and lean subjects, but the pulse amplitude is higher in obese subjects [8]. Leptin levels exhibit sexual dimorphism. Although leptin levels decline significantly after the menopause, women tend to have higher levels than men even after controlling for body fat mass, suggesting a role of sex steroids [9]. Subcutaneous fat produces more leptin than visceral fat, and this may, in part, contribute to higher leptin levels in women compared to men [10]. Besides sex steroids, leptin levels are also regulated by other factors including insulin, glucocorticoids, catecholamine, and cytokines [11] (Table 1).
Table 1.
Factors regulating circulating leptin levels
| Factors increasing leptin | Factors reducing leptin |
|---|---|
| Excess energy stored as fat (obesity) | Low energy states with decreased fat stores (leanness; lipoatrophy) |
| Overfeeding | Fasting |
| Glucose | Cold exposure, and adrenergic agonists |
| Insulin | Thyroid hormone |
| Estrogen | Testosterone |
| Proinflammatory cytokines (TNF-α, IL-6) |
Adapted from reference [3]. IL, interleukin; TNF, tumor necrosis factor.
Leptin exerts its effects through binding to specific leptin receptors (LepRs) located throughout the central nervous system (CNS). Four alternatively spliced isoforms of LepR have been identified in humans. The long isoform of leptin receptor (LepRb) is highly expressed in the hypothalamus and other brain regions, where it regulates energy homeostasis and neuroendocrine function, and considered as the main leptin receptor [12]. While LepRb is primarily responsible for suppression of food intake and stimulation of energy expenditure, the short isoforms of LepR are thought to mediate the transport of leptin across the blood-brain barrier [13]. Evidence suggests that leptin transport into the hypothalamus is mediated by tanycytes through LepR activation [14]. The binding of leptin to LepRb activates several signaling pathways, including Janus kinase 2 (JAK2)-signal transducer and activator of transcription 3 (STAT3), insulin receptor substrate (IRS)-phosphatidylinositol 3-kinase (PI3K), SH2-containing protein tyrosine phosphatase 2 (SHP2)-mitogen-activated protein kinase (MAPK), and 5' adenosine monophosphate-activated protein kinase (AMPK)/acetyl-CoA carboxylase (ACC), and other pathways. The leptin signaling cascade is terminated by induction of suppressor of cytokine signaling 3 (SOCS3), which inhibits the JAK2-STAT3 pathway through a negative-feedback loop. Protein tyrosine phosphatase 1B (PTP1B) is also implicated in the inhibition of leptin signaling [2, 3, 15]. Activation JAK2-STAT3 signaling plays a crucial role in leptin's ability to regulate energy homeostasis [16, 17].
Obese individuals exhibit high levels of leptin expression in adipose tissue and have elevated circulating leptin levels, and these high leptin levels fail to reduce excess adiposity, indicating leptin resistance. Moreover, exogenous leptin administration has little effect on body fat in obese subjects [18]. Mechanisms underlying leptin resistance may include disruption of leptin signaling in hypothalamic and other CNS neurons, impaired leptin transport across blood-brain barrier, hypothalamic inflammation, endoplasmic reticulum stress, and autophagy [19, 20].
Leptin and energy homeostasis
Leptin acts on LepRb-expressing neurons in the brain. In the arcuate nucleus (ARC), leptin interacts with a complex neural circuit to control food intake, activating anorexigenic neurons that synthesize pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART), and inhibiting orexigenic neurons that synthesize agouti-related peptide (AgRP) and neuropeptide Y (NPY). During fasting, circulating leptin levels decline rapidly. The fall in leptin stimulates the expression of AgRP and NPY and suppresses POMC and CART, thereby increasing food intake and decreasing energy expenditure [21, 22]. The lateral hypothalamus contains neurons that express melanin-concentrating hormone (MCH) and orexins, which are decreased by leptin resulting in suppression of feeding [2, 23, 24]. A distinct population of LepRb-expressing neurons in the lateral hypothalamus which do not express MCH or orexins has been identified. These neurons innervate the ventral tegmental area (VTA), linking leptin to the hedonic control of feeding mediated by the mesolimbic dopamine system [25]. Leptin also acts on VMH neurons that express the transcription factor steroidogenic factor 1 (SF-1) [26]. Mice with SF-1 deletion in the VMH are susceptible to diet-induced obesity, associated with impaired thermogenesis [27]. More recently, brain-derived neurotrophic factor in the VMH has been linked to leptin's effects on feeding and energy balance [28].
LepRb-expressing neurons have also been found in the nucleus of the solitary tract (NTS) of the hindbrain, including subpopulations that express glucagon-like peptide 1 (GLP-1) and cholecystokinin (CCK). Leptin appears to act synergistically with GLP-1 and CCK in the NTS to promote satiety [29]. Peptide YY (PYY) is released from L cells of the ileum and colon in response to feeding, crosses blood-brain barrier and acts on Y2 receptor on NPY neurons in the ARC to inhibit the release of NPY, thereby reducing food intake in mice and humans [30, 31]. Peripheral administration of PYY in mice also activates neurons in the NTS, which may partly influence food intake. However, compared to wild-type mice, nearly identical reductions in food intake were observed in both leptin-deficient ob/ob and LepR-deficient db/db mice treated with PYY, indicating that leptin signaling is not essential for the anorectic action of PYY [32]. Clinical studies have shown that PYY levels are reduced in obese people [33–35], and increased after weight loss [36]. Although PYY transgenic mice crossed with ob/ob mice exhibit reduced body weight and adiposity, the physiological significance of this finding is unclear [37, 38].
Leptin interacts with the mesolimbic dopamine system and modulates the hedonic drive to feed [17, 39]. Neurons in the ventral tegmental area (VTA) expressing LepRb respond directly to leptin, resulting in suppression of feeding. Moreover, leptin modulates the mesolimbic dopamine system indirectly through the lateral hypothalamus [25, 40].
In addition to regulating food intake, leptin increases energy expenditure through sympathetic nerve activity. In rodents, leptin stimulates brown adipose tissue (BAT) thermogenesis by increasing the expression of uncoupling protein (UCP)-1 [41, 42]. The thermogenic effect of leptin is mediated partly by suppression of MCH and transcription factor Forkhead box O1 (FoxO1) [43, 44]. Mice lacking both leptin and MCH (double null) have less body fat than leptin-deficient ob/ob mice. While the mutant mice are similarly hyperphagic, the double null mice have greater energy expenditure and locomotor activity compared to ob/ob mice [45]. Mice lacking FoxO1 in SF-1 neurons of the VMH have increased energy expenditure with up-regulation of UCP-1 in BAT due to enhanced sympathetic activity.
Leptin has a neurotrophic effect on hypothalamic neurons implicated in feeding and energy homeostasis, as well as cortical and hippocampal neurons [46, 47]. Leptin-deficient ob/ob and LepR-deficient db/db mice have reduced brain weight, decreased cortical volume, and structural neuronal abnormalities, suggesting that leptin plays a role in brain growth and neuronal development [48, 49]. Neural projections from the ARC to the paraventricular nucleus are reduced in ob/ob mice, and these deficits are restored by leptin treatment during early life [46]. In addition to its role in early neuronal development, leptin modulates synaptic plasticity in adults. Leptin administration in ob/ob mice rapidly normalizes synaptic inputs to POMC and AgRP neurons to levels seen in wild-type mice [47]. Leptin's actions in neurodevelopment have been demonstrated in murine cerebral cortex and hippocampus [50]. Brain imaging studies have also revealed structural and functional deficits reversible by leptin treatment in humans with congenital leptin deficiency [51, 52].
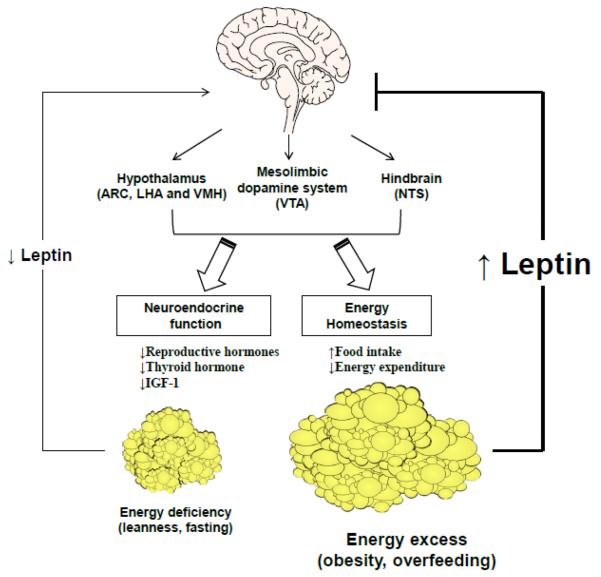
The importance of leptin in energy homeostasis is most evident in leptin deficiency. Thus, ob/ob mice with total leptin deficiency develop severe hyperphagia, low metabolic rate and rapid onset obesity, associated with high expression of NPY and MCH, and low expression of POMC in the hypothalamus (Fig. 1). These features are reversed by leptin treatment [11, 53]. As in rodents, humans with congenital leptin deficiency are hyperphagic and obese. Leptin treatment in these individuals results in a marked reduction in energy intake and body weight and fat [54, 55]. In contrast, the vast majority of obese people are insensitive to endogenous hyperleptinemia or leptin treatment, indicating the existence of “leptin resistance” in common forms of obesity arising from overnutrition and sedentary lifestyle [56–58] (Fig. 1).
Figure 1. The effects of leptin in states of energy excess and energy deficiency.
In states of energy deficiency such as fasting, circulating leptin levels decrease. As a result, food intake increases due to increased expression of orexigenic neuropeptides and decreased expression of anorexigenic neuropeptides. In addition, the decline of leptin modulates mesolimbic dopamine system and hindbrain circuits to increase food intake, and also has effects on neuroendocrine function and sympathetic nervous system, to decrease energy expenditure. In states of energy excess such as obesity and overfeeding, leptin levels increase; however, leptin's effects in the CNS are blunted due to leptin resistance. Recombinant leptin administration results in improvement in neuroendocrine and metabolic abnormalities in leptin-deficient states such as congenital leptin deficiency, hypothalamic amenorrhea, and congenital or acquired lipoatrophy. On the other hand, in states of leptin excess such as common forms of obesity and type 2 diabetes, recombinant leptin confers minimal benefits, indicating leptin resistance. The latter may be overcome with co-administration of amylin, leptin sensitizers or exercise. Leptin enhances weight loss maintenance in obesity by suppressing food intake and increasing energy expenditure. Abbreviations: ARC, arcuate nucleus; CNS, central nervous system; IGF, insulin-like growth factor; LHA, lateral hypothalamic area; NTS, nucleus of the solitary tract; VMH, ventromedial hypothalamus; VTA, ventral tegmental area.
Leptin and neuroendocrine function
Leptin levels in adipose tissue and plasma fall rapidly during fasting. Circulating leptin levels decrease in overweight men after negative energy balance is achieved with exercise and calorie restriction, indicating that leptin levels reflect energy status [59]. Low leptin levels during fasting trigger metabolic and hormonal responses in mice and humans [11, 60], consisting of hyperphagia, hypogonadotropic hypogonadism, and suppression of thyroid and growth hormone (GH) levels, which are prevented by physiologic doses of leptin [7, 61, 62] (Fig. 1). Leptin administration restores thyroid hormone, testosterone and luteinizing hormone (LH) levels in fasted mice [61], and LH pulsatility and testosterone levels in starved human volunteers [7, 61, 63]. These hormone changes are similar to congenital leptin deficiency which results in hypogonadism and failure of pubertal development. Leptin replacement facilitates pubertal development in ob/ob mice and leptin-deficient humans, establishing a crucial role of leptin in reproduction [54, 64, 65]. Leptin also prevents the pubertal delay associated with starvation, and exerts a permissive effect on the onset of puberty in normal mice [66–68]. Low leptin levels are linked to impaired leptin pulsatility and hypogonadism in hypothalamic amenorrhea and generalized lipoatrophy [69, 70]. Leptin treatment increases mean LH levels, LH pulse frequency, estradiol levels, and corrects abnormal thyroid and cortisol levels in hypothalamic amenorrhea [71, 72]. Similarly, in individuals with generalized lipodystrophy who have low leptin levels and insulin resistance, leptin replacement normalizes LH and sex steroid levels [73]. These findings suggest that leptin is an important signal linking energy stores to the neuroendocrine axis.
Gonadotropin-releasing hormone (GnRH) neurons lack LepR, suggesting an indirect action of leptin in the hypothalamus via NPY, POMC, and kisspeptin [74, 75]. Kisspeptin, a product of Kiss1 gene, stimulates GnRH secretion and increases LH levels in mice [76, 77]. Mutations in kisspeptin or kisspeptin receptor result in the lack of pubertal maturation and hypogonadotropic hypogonadism [78]. In comparison with wild-type mice, Kiss 1 mRNA levels are reduced in ob/ob mice and increase with leptin treatment [68, 77, 79]. However, mice with selective deletion of LepR from hypothalamic Kiss1 neurons show normal pubertal development and fertility, indicating that leptin action in Kiss1 neurons is not essential for puberty and reproduction [80]. A small population of Kiss1 neurons in the ARC express LepR [81, 82], but leptin signaling in these neurons occurs after completion of sexual maturation and is not crucial for leptin action during puberty [83, 84].
Leptin alters thyroid hormone regulation via multiple pathways. Leptin increases thyrotropin-releasing hormone (TRH) levels through up-regulation of proTRH gene expression, and by enhancing the processing of proTRH into mature TRH [85, 86]. Leptin-deficient ob/ob mice have reduced levels of thyroxine (T4) from birth [17]. Adult wild-type mice develop thyroid axis suppression during fasting [61]. Healthy humans have circadian and pulsatile levels of leptin and TSH, while congenital leptin deficiency results in a highly disorganized secretion of TSH [87]. After leptin replacement, leptin-deficient individuals exhibit an increase in thyroid hormone levels but no change in TSH [54]. Leptin administration prevents the fasting-induced suppression of TSH pulses but does not reverse the fall in tri-iodothyronine (T3) levels [7]. In women with hypothalamic amenorrhea, leptin administration increases free T3 and T4 levels but not TSH levels [71]. Leptin administration to weight-reduced individuals reverses the declines in T3 and T4 levels and in energy expenditure [88, 89].
In contrast to ob/ob mice which have reduced linear growth, suppression of the GH axis, and markedly elevated ACTH and corticosterone levels, humans with congenital leptin deficiency have normal linear growth and adrenal function [54, 55]. The mechanisms underlying the species differences in leptin regulation of growth hormone and hypothalamic-pituitary-adrenal axis are unclear.
Leptin and metabolism
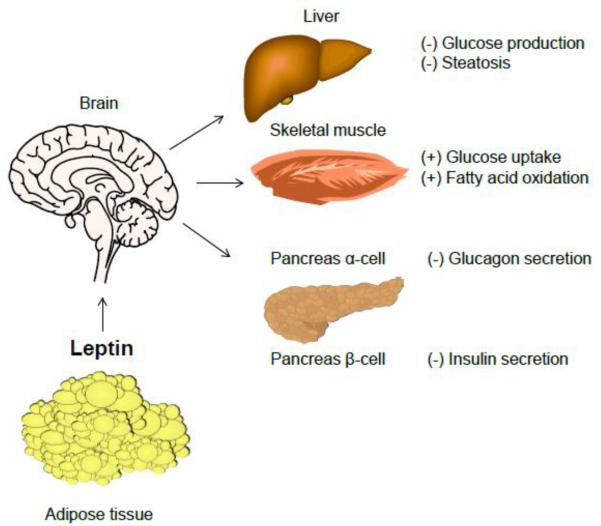
Total leptin deficiency in ob/ob mice and individuals with congenital leptin deficiency results in insulin resistance, diabetes, steatosis and other features of metabolic syndrome. In ob/ob mice, leptin treatment rapidly decreases glucose, insulin and lipids before weight loss is achieved [53, 90] (Fig. 2). In morbidly obese individuals with congenital leptin deficiency, leptin replacement dramatically decreases insulin resistance, steatosis, dyslipidemia and glucose levels [54, 65]. Similarly, central or peripheral leptin administration decreases insulin resistance, steatosis and glucose in generalized lipoatrophy [91–93]. In lipodystrophic humans with severe leptin deficiency, leptin administration results in drastic improvements in glucose and lipid metabolism [73, 94, 95], accompanied by significant reductions in hepatic and muscle triglyceride accumulation [96]. In contrast, leptin does not reverse hyperglycemia in people with moderate leptin deficiency, but decreases plasma and hepatic triglyceride levels [97]. Leptin treatment causes a preferential decrease in visceral fat in rats, promoting fat redistribution [98]. Similarly, leptin replacement in patients with lipoatrophy results in a reduction of truncal fat mass, accompanied by a selective decrease in visceral adiposity [95, 99].
Figure 2. The effects of leptin in glucose and lipid metabolism.
Leptin can affect glucose homeostasis through a variety of mechanisms, including modulation of ANS, hepatic glucose production, muscle glucose uptake, and glucagon secretion from pancreatic α-cells. Leptin inhibits insulin secretion, and stimulates fatty acid oxidation. Abbreviations: ANS, autonomic nervous system.
How does leptin regulate glucose and lipid metabolism? In non-obese diabetic mice, peripheral leptin administration normalized glycemia by suppressing glucagon levels and various hepatic intermediary metabolites [100]. Central administration of leptin also decreased glucose and glucagon levels through insulin-independent mechanisms [101]. It has been proposed that leptin replacement may serve as an adjunct therapy to insulin in patients with type 1 diabetes, by improving glucose and lipid metabolism [102, 103]. Studies suggest that leptin affects peripheral insulin sensitivity via CNS mechanisms independent of its effects on food intake and weight [104] (Fig. 2). Leptin potently suppresses hepatic glucose production partly by ameliorating hyperglucagonemia and increases peripheral glucose uptake, via multiple mechanisms including POMC- and AgRP-expressing neurons in the ARC [105–108]. Restoration of LepR expression in the ARC decreases insulin and glucose levels in LepR-null mice. Moreover, selective expression of LepRb in hypothalamic POMC neurons prevents diabetes in LepR-deficient db/db mice, independently of changes in food intake and weight [105, 106, 108]. Deletion of leptin targets, SOCS3 or PTP1B, in POMC neurons also improves glucose homeostasis [109, 110]. Furthermore, genetically mediated alteration of PI3K activity in POMC neurons affects hepatic insulin sensitivity [111]. Recent evidence has shown that selective re-expression of LepRb in AgRP neurons mediates leptin's anti-diabetic actions in db/db mice by suppressing glucagon [112]. Central leptin administration also suppresses hepatic glucose production and increases tissue glucose uptake through autonomic pathways [107]. Leptin increases AMPK phosphorylation and improves insulin sensitivity in skeletal muscle through activation of PI3K in the hypothalamus [113].
In addition to regulating insulin sensitivity, leptin alters glucose homeostasis through insulin. Leptin inhibits insulin gene expression and glucose-stimulated insulin secretion, and these actions adapt glucose levels to body fat stores [114, 115]. In turn, insulin stimulates both leptin synthesis and secretion, thus establishing an adipose-islet axis [116]. Leptin also protects pancreatic β-cells from lipotoxicity in various animal models [100, 117, 118].
Leptin regulates lipid metabolism independently of food intake. Central leptin administration inhibits de novo lipogenesis and stimulates lipolysis in adipose tissue and liver via activation of the sympathetic nervous system [119, 120]. Leptin stimulates fatty acid oxidation by up-regulating peroxisome proliferator-activated receptor γ-coactivator-1α, and decreasing triglyceride stores within white adipocytes and liver [121]. Leptin also stimulates fatty acid oxidation by activating AMPK in skeletal muscle, and preventing the accumulation of lipid metabolites associated with lipotoxocity [122]. Disruption of LepR in peripheral organs has no significant impact on metabolism, suggesting that leptin acts mainly in the brain to influence glucose and lipid metabolism [123].
Leptin appears to modulate bone metabolism both centrally and peripherally. In rodents, leptin regulates cortical bone formation via β-adrenergic stimulation or GH/ insulin-like growth factor (IGF)-1 effects on trabecular bone remodeling. Leptin may also influence cortical bone metabolism through neuropeptides in the hypothalamus, including NPY, an inhibitor of cortical bone formation [124, 125]. Peripherally, it has been shown that leptin acts on marrow stromal cells to enhance osteoblast differentiation and inhibit adipocyte differentiation. Leptin stimulates osteoblast proliferation and mineralization [126, 127]. Leptin's effect on bone biology is evident in leptin deficient states. For example, leptin treatment increases markers of bone formation, bone mineral density and content in the lumbar spine of patients with hypothalamic amenorrhea [71, 72, 128]. These effects of leptin may involve direct action on bone, increased IGF-1 levels, restoration of estradiol, and reduction of cortisol. Furthermore, leptin exerts anti-osteogenic effects via sympathetic nervous activation in the hypothalamus [129]. Sympathetic activation inhibits bone formation and increases bone resorption mediated by β2 adrenergic receptors in osteoblasts [130, 131].
Leptin and exercise
Successful long-term weight loss requires a reduction in food intake and an increase in physical activity. A negative energy balance achieved in 4 days by combining calorie restriction and exercise in obese subjects resulted in a rapid decline in leptin, indicating that changes in leptin levels reflect energy balance [59]. Human skeletal muscle expresses low levels of LepRb. As with plasma leptin levels, there is a sexual dimorphism in LepRb expression in human skeletal muscle, which could be partly explained by an inverse relationship between free testosterone level and LepRb in skeletal muscle [132]. Leptin is thought to directly increase glucose uptake and fatty acid oxidation in skeletal muscle [133, 134]. Peripheral leptin administration increases IGF binding protein-2 (IGFBP-2) in human skeletal myotubes. Central infusion of leptin in sheep also increased IGFBP-2 in skeletal muscle and improved glucose homeostasis [135, 136].
Leptin receptors and leptin signaling in skeletal muscle particularly in the leg muscles are reduced in obese subjects, suggesting a potential mechanism of leptin resistance in obesity [137]. Sprint exercise or intense intermittent exercise activates AMPK, which is also activated by leptin in human skeletal muscle [122, 138, 139]. Moreover, sprint exercise under fasting conditions enhances leptin signaling in human skeletal muscle [140]. In obese rodents, prolonged exercise suppresses PTP1B activity in the hypothalamus, and improves leptin signaling [141]. In healthy volunteers, one week of bed rest resulted in an increase in circulating leptin level without a concomitant increase in STAT3 phosphorylation in leg skeletal muscle, indicating an induction of leptin resistance in skeletal muscle. This was explained, at least partly, by an up-regulation of PTP1B expression in leg muscle [142]. Aging is also associated with dysregulation of leptin signaling and increased PTP1B expression in human skeletal muscle [143].
Leptin and immune function
Various studies have shown that leptin has important roles in modulating innate and adaptive immunity [144]. Leptin stimulates neutrophil chemotaxis and promotes macrophage phagocytosis, as well as production of pro-inflammatory cytokines such as interleukin (IL)-6, IL-12, tumor necrosis factor (TNF)-α [145, 146]. Recently, it has also been shown that leptin acts as a negative signal for the proliferation of regulatory T cells, while stimulating T helper 1 cells [144, 147]. Thus, leptin may contribute to the protection from infections and the development of autoimmunity [3, 144].
An in vivo study with ob/ob mice and short-term fasted normal mice showed that leptin treatment protected these mice from immune dysfunction associated with hypoleptinemia [148]. Compared to healthy subjects, individuals with congenital leptin deficiency have a higher incidence of infection, probably due to a reduction of circulating CD4+ T-cells and impaired T cell proliferation and cytokine release, all of which are normalized with leptin administration [54]. In women with hypothalamic amenorrhea who have chronic leptin deficiency, leptin replacement has been shown to increase soluble TNF-α receptor and restore both CD4+ T-cell counts and their in vitro proliferative responses, proving that leptin can facilitate immune reconstitution in subjects with chronic hypoleptinemia. In contrast, leptin administration has no major effects on immune cells or serum cytokines in individuals with acute leptin deficiency from short-term starvation [63, 149, 150].
Leptin can influence the development of autoimmune diseases. Leptin treatment potentiates experimental autoimmune encephalomyelitis, an animal model for multiple sclerosis in humans, in susceptible strains of mice, while leptin-deficient ob/ob mice are resistant to the induction and progression of the disease. Moreover, leptin treatment accelerated disease onset in susceptible mice [151, 152]. Patients with multiple sclerosis have increased leptin levels in blood and cerebrospinal fluid and reduced number of peripheral regulatory T cells compared to controls [153]. Taken together, these findings suggest potential roles of leptin in inflammatory and autoimmune diseases.
Clinical applications of leptin
As mentioned earlier, leptin treatment has robust effects in states of leptin deficiency [154] (Fig. 1). Leptin replacement dramatically reduces body weight and fat, and reverses neuroendocrine and metabolic abnormalities in individuals with congenital leptin deficiency [54, 65]. Leptin administration in women with hypothalamic amenorrhea restores normal menstrual cycles, corrects abnormalities in the gonadal, thyroid and adrenal axes, and improves bone mineral density and markers of bone formation [72, 128]. In subjects with congenital or acquired lipoatrophy, leptin treatment improves several metabolic parameters, including insulin sensitivity, dyslipidemia, and hepatic steatosis [94, 95, 155].
In contrast, common forms of obesity and type 2 diabetes are accompanied by leptin resistance (Fig. 1) [57, 58]. A combination therapy of leptin and leptin sensitizers has been suggested to overcome leptin resistance. Amylin acts synergistically with leptin to reduce body weight and adiposity in diet-induced obese rodents, while preventing the compensatory reduction in energy expenditure associated with weight loss [156, 157]. Clinical studies have shown that a combined treatment of leptin and amylin analog pramlintide results in more weight loss in obese subjects than either treatment alone. The effect of weight loss appeared to be additive rather than synergistic, implying that amylin does not improve the sensitivity to leptin [157, 158]. The additive actions of leptin and amylin involve overlapping intracellular signaling pathways in peripheral tissues in humans [159]. Unfortunately, a clinical trial of pramlintide/leptin therapy for obesity was discontinued due to induction of leptin antibodies [154]. In rodents, metformin, exendin-4, and fibroblast growth factor (FGF)-21 have been shown to enhance leptin sensitivity when co-administered with leptin [160, 161]. Co-administration of leptin with either exendin-4 or FGF21 resulted in restoration of leptin responsiveness in diet-induced obese mice after an initial body weight loss of 30% [161]. Exercise also acts as a leptin sensitizer and could be used to enhance leptin signaling in human skeletal muscle [140].
Recent work suggests that leptin plays a more important role in the maintenance of weight loss than weight loss per se. Reduced leptin levels during weight loss activate neuroendocrine mechanisms that may drive weight-reduced subjects to regain weight. In clinical studies of subjects with relative leptin deficiency due to weight loss, leptin treatment restored thyroid hormone levels, sympathetic nerve activity, and energy expenditure, and reversed declines in satiation in weight-reduced subjects, suggesting a role of leptin in weight loss maintenance [88, 89]. In addition, functional brain imaging has shown that leptin treatment prevents the changes in neural activity involved in the regulatory, emotional, and cognitive control of food intake following weight loss [162]. Studies are under way to determine whether leptin replacement can be an effective therapy for maintenance of weight loss [163].
Future potential areas of leptin therapy include neurodegenerative disorders such as Alzheimer's disease. A growing body of evidence suggests that leptin has a positive influence on neurogenesis, axon growth, synaptogenesis, and neuroprotection [50, 164]. Prospective studies have shown that high leptin levels, especially among non-obese individuals, are associated with a lower risk of dementia and Alzheimer's disease, implying a possible therapeutic role of leptin [165–167]. Furthermore, leptin may be targeted for diagnostic or therapeutic uses in the regulation of immunity and bone health.
Concluding remarks
Leptin is an adipocyte-secreted hormone that regulates food intake, energy homeostasis, neuroendocrine function, metabolism, and immune function. Studies have shown that leptin acts mainly on neuronal targets in the brain. Leptin replacement is an effective therapy in severe leptin deficiency, such as congenital leptin deficiency or generalized lipodystrophy. Further studies are needed to better understand the mechanisms underlying leptin resistance in common forms of obesity, and how these could be targeted specifically to treat obesity, diabetes and related metabolic diseases.
Acknowledgement
R.S.A is supported by National Institutes of Health grants P01-DK049210 and P30-DK19525.
Abbreviations
- ACC
acetyl-CoA carboxylase
- AgRP
agouti-related peptide
- AMPK
AMP-activated protein kinase
- ARC
arcuate nucleus
- BAT
brown adipose tissue
- CART
cocaine-and amphetamine-regulated transcript
- CCK
cholecystokinin
- CNS
central nervous system
- FGF
fibroblast growth factor
- FoxO1
Forkhead box O1
- GH
growth hormone
- GLP-1
glucagon-like peptide 1
- GnRH
gonadotropin-releasing hormone
- IGF
insulin-like growth factor
- IGFBP
IGF binding factor
- IL
interleukin
- IRS
insulin receptor substrate
- JAK2
Janus kinase 2
- LH
luteinizing hormone
- LHA
lateral hypothalamic area
- MAPK
mitogen-activated protein kinase
- MCH
melanin-concentrating hormone
- NPY
neuropeptide Y
- NTS
nucleus of the solitary tract
- LepR
leptin receptor
- LepRb
long isoform of leptin receptor
- PI3K
phosphatidylinositol 3-kinase
- POMC
pro-opiomelanocortin
- PTP1B
Protein tyrosine phosphatase 1B
- PYY
peptide YY
- SF-1
steroidogenic factor 1
- SHP2
SH2-containing protein tyrosine phosphatase 2
- SOCS3
suppressor of cytokine signaling 3
- STAT3
signal transducer and activator of transcription 3
- T3
tri-iodothyronine
- T4
thyroxine
- TNF
tumor necrosis factor
- TRH
thyrotropin-releasing hormone
- TSH
thyroid stimulating hormone
- UCP
uncoupling protein
- VMH
ventromedial hypothalamus
- VTA
ventral tegmental area
- WAT
white adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest There are no potential conflicts of interest relevant to this article.
Author contributions H.K.P and R.S.A co-wrote the review article.
References
- [1].Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- [2].Dalamaga M, Chou SH, Shields K, et al. Leptin at the intersection of neuroendocrinology and metabolism: Current evidence and therapeutic perspectives. Cell metabolism. 2013;18(1):29–42. doi: 10.1016/j.cmet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- [3].Moon HS, Dalamaga M, Kim SY, et al. Leptin's role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocrine reviews. 2013;34(3):377–412. doi: 10.1210/er.2012-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bluher S, Mantzoros CS. Leptin in humans: Lessons from translational research. The American journal of clinical nutrition. 2009;89(3):991S–7S. doi: 10.3945/ajcn.2008.26788E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Margetic S, Gazzola C, Pegg GG, et al. Leptin: A review of its peripheral actions and interactions. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26(11):1407–33. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- [6].Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. The New England journal of medicine. 1996;334(5):292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- [7].Chan JL, Heist K, DePaoli AM, et al. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. The Journal of clinical investigation. 2003;111(9):1409–21. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Licinio J, Mantzoros C, Negrao AB, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nature medicine. 1997;3(5):575–9. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- [9].Rosenbaum M, Leibel RL. Clinical review 107: Role of gonadal steroids in the sexual dimorphisms in body composition and circulating concentrations of leptin. The Journal of clinical endocrinology and metabolism. 1999;84(6):1784–9. doi: 10.1210/jcem.84.6.5787. [DOI] [PubMed] [Google Scholar]
- [10].Montague CT, Prins JB, Sanders L, et al. Depot- and sex-specific differences in human leptin mrna expression: Implications for the control of regional fat distribution. Diabetes. 1997;46(3):342–7. doi: 10.2337/diab.46.3.342. [DOI] [PubMed] [Google Scholar]
- [11].Ahima RS, Osei SY. Leptin signaling. Physiology & behavior. 2004;81(2):223–41. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- [12].Kelesidis T, Kelesidis I, Chou S, et al. Narrative review: The role of leptin in human physiology: Emerging clinical applications. Annals of internal medicine. 2010;152(2):93–100. doi: 10.1059/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bjorbaek C, Elmquist JK, Michl P, et al. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139(8):3485–91. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- [14].Balland E, Dam J, Langlet F, et al. Hypothalamic tanycytes are an erk-gated conduit for leptin into the brain. Cell metabolism. 2014;19(2):293–301. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. American journal of physiology Endocrinology and metabolism. 2009;297(6):E1247–59. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bates SH, Stearns WH, Dundon TA, et al. Stat3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421(6925):856–9. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- [17].Dardeno TA, Chou SH, Moon HS, et al. Leptin in human physiology and therapeutics. Frontiers in neuroendocrinology. 2010;31(3):377–93. doi: 10.1016/j.yfrne.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mantzoros CS, Flier JS. Editorial: Leptin as a therapeutic agent--trials and tribulations. The Journal of clinical endocrinology and metabolism. 2000;85(11):4000–2. doi: 10.1210/jcem.85.11.7062. [DOI] [PubMed] [Google Scholar]
- [19].Myers MG, Jr, Heymsfield SB, Haft C, et al. Challenges and opportunities of defining clinical leptin resistance. Cell metabolism. 2012;15(2):150–6. doi: 10.1016/j.cmet.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jung CH, Kim MS. Molecular mechanisms of central leptin resistance in obesity. Archives of pharmacal research. 2013;36(2):201–7. doi: 10.1007/s12272-013-0020-y. [DOI] [PubMed] [Google Scholar]
- [21].Ahima RS, Kelly J, Elmquist JK, et al. Distinct physiologic and neuronal responses to decreased leptin and mild hyperleptinemia. Endocrinology. 1999;140(11):4923–31. doi: 10.1210/endo.140.11.7105. [DOI] [PubMed] [Google Scholar]
- [22].Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic pomc neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–4. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- [23].Robertson SA, Leinninger GM, Myers MG., Jr Molecular and neural mediators of leptin action. Physiology & behavior. 2008;94(5):637–42. doi: 10.1016/j.physbeh.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Abizaid A, Gao Q, Horvath TL. Thoughts for food: Brain mechanisms and peripheral energy balance. Neuron. 2006;51(6):691–702. doi: 10.1016/j.neuron.2006.08.025. [DOI] [PubMed] [Google Scholar]
- [25].Leinninger GM, Jo YH, Leshan RL, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell metabolism. 2009;10(2):89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim KW, Sohn JW, Kohno D, et al. Sf-1 in the ventral medial hypothalamic nucleus: A key regulator of homeostasis. Molecular and cellular endocrinology. 2011;336(1–2):219–23. doi: 10.1016/j.mce.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim KW, Zhao L, Donato J, Jr, et al. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(26):10673–8. doi: 10.1073/pnas.1102364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liao GY, An JJ, Gharami K, et al. Dendritically targeted bdnf mrna is essential for energy balance and response to leptin. Nature medicine. 2012;18(4):564–71. doi: 10.1038/nm.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Garfield AS, Patterson C, Skora S, et al. Neurochemical characterization of body weight-regulating leptin receptor neurons in the nucleus of the solitary tract. Endocrinology. 2012;153(10):4600–7. doi: 10.1210/en.2012-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Batterham RL, Cowley MA, Small CJ, et al. Gut hormone pyy(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- [31].Riediger T. The receptive function of hypothalamic and brainstem centres to hormonal and nutrient signals affecting energy balance. The Proceedings of the Nutrition Society. 2012;71(4):463–77. doi: 10.1017/S0029665112000778. [DOI] [PubMed] [Google Scholar]
- [32].Halatchev IG, Cone RD. Peripheral administration of pyy(3-36) produces conditioned taste aversion in mice. Cell metabolism. 2005;1(3):159–68. doi: 10.1016/j.cmet.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [33].Alvarez Bartolome M, Borque M, Martinez-Sarmiento J, et al. Peptide yy secretion in morbidly obese patients before and after vertical banded gastroplasty. Obesity surgery. 2002;12(3):324–7. doi: 10.1381/096089202321088084. [DOI] [PubMed] [Google Scholar]
- [34].Guo Y, Ma L, Enriori PJ, et al. Physiological evidence for the involvement of peptide yy in the regulation of energy homeostasis in humans. Obesity. 2006;14(9):1562–70. doi: 10.1038/oby.2006.180. [DOI] [PubMed] [Google Scholar]
- [35].Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide yy in protein-mediated satiation and body-weight regulation. Cell metabolism. 2006;4(3):223–33. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- [36].Roth CL, Enriori PJ, Harz K, et al. Peptide yy is a regulator of energy homeostasis in obese children before and after weight loss. The Journal of clinical endocrinology and metabolism. 2005;90(12):6386–91. doi: 10.1210/jc.2005-1357. [DOI] [PubMed] [Google Scholar]
- [37].Boey D, Lin S, Enriquez RF, et al. Pyy transgenic mice are protected against diet-induced and genetic obesity. Neuropeptides. 2008;42(1):19–30. doi: 10.1016/j.npep.2007.11.003. [DOI] [PubMed] [Google Scholar]
- [38].Cooper JA. Factors affecting circulating levels of peptide yy in humans: A comprehensive review. Nutrition research reviews. 2014;27(1):186–97. doi: 10.1017/S0954422414000109. [DOI] [PubMed] [Google Scholar]
- [39].Gao Q, Horvath TL. Neuronal control of energy homeostasis. FEBS letters. 2008;582(1):132–41. doi: 10.1016/j.febslet.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hommel JD, Trinko R, Sears RM, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51(6):801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- [41].Haynes WG, Morgan DA, Walsh SA, et al. Receptor-mediated regional sympathetic nerve activation by leptin. The Journal of clinical investigation. 1997;100(2):270–8. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Scarpace PJ, Matheny M, Pollock BH, et al. Leptin increases uncoupling protein expression and energy expenditure. The American journal of physiology. 1997;273(1 Pt 1):E226–30. doi: 10.1152/ajpendo.1997.273.1.E226. [DOI] [PubMed] [Google Scholar]
- [43].Segal-Lieberman G, Bradley RL, Kokkotou E, et al. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(17):10085–90. doi: 10.1073/pnas.1633636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kim KW, Donato J, Jr, Berglund ED, et al. Foxo1 in the ventromedial hypothalamus regulates energy balance. The Journal of clinical investigation. 2012;122(7):2578–89. doi: 10.1172/JCI62848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Leinninger GM. Lateral thinking about leptin: A review of leptin action via the lateral hypothalamus. Physiology & behavior. 2011;104(4):572–81. doi: 10.1016/j.physbeh.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108–10. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- [47].Pinto S, Roseberry AG, Liu H, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304(5667):110–5. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- [48].Ahima RS, Bjorbaek C, Osei S, et al. Regulation of neuronal and glial proteins by leptin: Implications for brain development. Endocrinology. 1999;140(6):2755–62. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- [49].Steppan CM, Swick AG. A role for leptin in brain development. Biochemical and biophysical research communications. 1999;256(3):600–2. doi: 10.1006/bbrc.1999.0382. [DOI] [PubMed] [Google Scholar]
- [50].Bouret SG. Neurodevelopmental actions of leptin. Brain research. 2010;1350:2–9. doi: 10.1016/j.brainres.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Matochik JA, London ED, Yildiz BO, et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. The Journal of clinical endocrinology and metabolism. 2005;90(5):2851–4. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- [52].London ED, Berman SM, Chakrapani S, et al. Short-term plasticity of gray matter associated with leptin deficiency and replacement. The Journal of clinical endocrinology and metabolism. 2011;96(8):E1212–20. doi: 10.1210/jc.2011-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- [54].Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, t cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. The Journal of clinical investigation. 2002;110(8):1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. The New England journal of medicine. 1999;341(12):879–84. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- [56].Hukshorn CJ, van Dielen FM, Buurman WA, et al. The effect of pegylated recombinant human leptin (peg-ob) on weight loss and inflammatory status in obese subjects. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26(4):504–9. doi: 10.1038/sj.ijo.0801952. [DOI] [PubMed] [Google Scholar]
- [57].Moon HS, Matarese G, Brennan AM, et al. Efficacy of metreleptin in obese patients with type 2 diabetes: Cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60(6):1647–56. doi: 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mittendorfer B, Horowitz JF, DePaoli AM, et al. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes. 2011;60(5):1474–7. doi: 10.2337/db10-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Calbet JA, Ponce-Gonzalez JG, Perez-Suarez I, et al. A time-efficient reduction of fat mass in 4 days with exercise and caloric restriction. Scandinavian journal of medicine & science in sports. 2014 doi: 10.1111/sms.12194. [DOI] [PubMed] [Google Scholar]
- [60].Boden G, Chen X, Mozzoli M, et al. Effect of fasting on serum leptin in normal human subjects. The Journal of clinical endocrinology and metabolism. 1996;81(9):3419–23. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- [61].Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–2. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- [62].Chan JL, Williams CJ, Raciti P, et al. Leptin does not mediate short-term fasting-induced changes in growth hormone pulsatility but increases igf-i in leptin deficiency states. The Journal of clinical endocrinology and metabolism. 2008;93(7):2819–27. doi: 10.1210/jc.2008-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chan JL, Matarese G, Shetty GK, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(22):8481–6. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nature genetics. 1996;12(3):318–20. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- [65].Licinio J, Caglayan S, Ozata M, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4531–6. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gruaz NM, Lalaoui M, Pierroz DD, et al. Chronic administration of leptin into the lateral ventricle induces sexual maturation in severely food-restricted female rats. Journal of neuroendocrinology. 1998;10(8):627–33. doi: 10.1046/j.1365-2826.1998.00247.x. [DOI] [PubMed] [Google Scholar]
- [67].Cheung CC, Thornton JE, Nurani SD, et al. A reassessment of leptin's role in triggering the onset of puberty in the rat and mouse. Neuroendocrinology. 2001;74(1):12–21. doi: 10.1159/000054666. [DOI] [PubMed] [Google Scholar]
- [68].Elias CF, Purohit D. Leptin signaling and circuits in puberty and fertility. Cellular and molecular life sciences : CMLS. 2013;70(5):841–62. doi: 10.1007/s00018-012-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Miller KK, Parulekar MS, Schoenfeld E, et al. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: The effects of body composition and nutritional intake. The Journal of clinical endocrinology and metabolism. 1998;83(7):2309–12. doi: 10.1210/jcem.83.7.4975. [DOI] [PubMed] [Google Scholar]
- [70].Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: Normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet. 2005;366(9479):74–85. doi: 10.1016/S0140-6736(05)66830-4. [DOI] [PubMed] [Google Scholar]
- [71].Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. The New England journal of medicine. 2004;351(10):987–97. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- [72].Chou SH, Chamberland JP, Liu X, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(16):6585–90. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Musso C, Cochran E, Javor E, et al. The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism: clinical and experimental. 2005;54(2):255–63. doi: 10.1016/j.metabol.2004.08.021. [DOI] [PubMed] [Google Scholar]
- [74].Quennell JH, Mulligan AC, Tups A, et al. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology. 2009;150(6):2805–12. doi: 10.1210/en.2008-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hausman GJ, Barb CR, Lents CA. Leptin and reproductive function. Biochimie. 2012;94(10):2075–81. doi: 10.1016/j.biochi.2012.02.022. [DOI] [PubMed] [Google Scholar]
- [76].Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of kiss-1 mrna in the male rat. Neuroendocrinology. 2004;80(4):264–72. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- [77].Smith JT, Acohido BV, Clifton DK, et al. Kiss-1 neurones are direct targets for leptin in the ob/ob mouse. Journal of neuroendocrinology. 2006;18(4):298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- [78].Elias CF. Leptin action in pubertal development: Recent advances and unanswered questions. Trends in endocrinology and metabolism: TEM. 2012;23(1):9–15. doi: 10.1016/j.tem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Quennell JH, Howell CS, Roa J, et al. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152(4):1541–50. doi: 10.1210/en.2010-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Donato J, Jr, Cravo RM, Frazao R, et al. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in kiss1 neurons. The Journal of clinical investigation. 2011;121(1):355–68. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Louis GW, Greenwald-Yarnell M, Phillips R, et al. Molecular mapping of the neural pathways linking leptin to the neuroendocrine reproductive axis. Endocrinology. 2011;152(6):2302–10. doi: 10.1210/en.2011-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sanchez-Garrido MA, Tena-Sempere M. Metabolic control of puberty: Roles of leptin and kisspeptins. Hormones and behavior. 2013;64(2):187–94. doi: 10.1016/j.yhbeh.2013.01.014. [DOI] [PubMed] [Google Scholar]
- [83].Cravo RM, Frazao R, Perello M, et al. Leptin signaling in kiss1 neurons arises after pubertal development. PloS one. 2013;8(3):e58698. doi: 10.1371/journal.pone.0058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Elias CF. A critical view of the use of genetic tools to unveil neural circuits: The case of leptin action in reproduction. American journal of physiology Regulatory, integrative and comparative physiology. 2014;306(1):R1–9. doi: 10.1152/ajpregu.00444.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Legradi G, Emerson CH, Ahima RS, et al. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138(6):2569–76. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- [86].Sanchez VC, Goldstein J, Stuart RC, et al. Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone. The Journal of clinical investigation. 2004;114(3):357–69. doi: 10.1172/JCI21620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (tsh) and leptin concentrations in healthy adults and leptin-deficient subjects: Evidence for possible partial tsh regulation by leptin in humans. The Journal of clinical endocrinology and metabolism. 2001;86(7):3284–91. doi: 10.1210/jcem.86.7.7644. [DOI] [PubMed] [Google Scholar]
- [88].Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. The Journal of clinical investigation. 2005;115(12):3579–86. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kissileff HR, Thornton JC, Torres MI, et al. Leptin reverses declines in satiation in weight-reduced obese humans. The American journal of clinical nutrition. 2012;95(2):309–17. doi: 10.3945/ajcn.111.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Schwartz MW, Baskin DG, Bukowski TR, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide y gene expression in ob/ob mice. Diabetes. 1996;45(4):531–5. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- [91].Shimomura I, Hammer RE, Ikemoto S, et al. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401(6748):73–6. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- [92].Gavrilova O, Marcus-Samuels B, Graham D, et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. The Journal of clinical investigation. 2000;105(3):271–8. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Asilmaz E, Cohen P, Miyazaki M, et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. The Journal of clinical investigation. 2004;113(3):414–24. doi: 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. The New England journal of medicine. 2002;346(8):570–8. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- [95].Mulligan K, Khatami H, Schwarz JM, et al. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypoleptinemia. The Journal of clinical endocrinology and metabolism. 2009;94(4):1137–44. doi: 10.1210/jc.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. The Journal of clinical investigation. 2002;109(10):1345–50. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Simha V, Subramanyam L, Szczepaniak L, et al. Comparison of efficacy and safety of leptin replacement therapy in moderately and severely hypoleptinemic patients with familial partial lipodystrophy of the dunnigan variety. The Journal of clinical endocrinology and metabolism. 2012;97(3):785–92. doi: 10.1210/jc.2011-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Barzilai N, Wang J, Massilon D, et al. Leptin selectively decreases visceral adiposity and enhances insulin action. The Journal of clinical investigation. 1997;100(12):3105–10. doi: 10.1172/JCI119865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lee JH, Chan JL, Sourlas E, et al. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. The Journal of clinical endocrinology and metabolism. 2006;91(7):2605–11. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]
- [100].Wang MY, Chen L, Clark GO, et al. Leptin therapy in insulin-deficient type i diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(11):4813–9. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Fujikawa T, Chuang JC, Sakata I, et al. Leptin therapy improves insulin-deficient type 1 diabetes by cns-dependent mechanisms in mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(40):17391–6. doi: 10.1073/pnas.1008025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Toyoshima Y, Gavrilova O, Yakar S, et al. Leptin improves insulin resistance and hyperglycemia in a mouse model of type 2 diabetes. Endocrinology. 2005;146(9):4024–35. doi: 10.1210/en.2005-0087. [DOI] [PubMed] [Google Scholar]
- [103].Cummings BP, Bettaieb A, Graham JL, et al. Subcutaneous administration of leptin normalizes fasting plasma glucose in obese type 2 diabetic ucd-t2dm rats. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(35):14670–5. doi: 10.1073/pnas.1107163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Coppari R, Bjorbaek C. Leptin revisited: Its mechanism of action and potential for treating diabetes. Nature reviews Drug discovery. 2012;11(9):692–708. doi: 10.1038/nrd3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Coppari R, Ichinose M, Lee CE, et al. The hypothalamic arcuate nucleus: A key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell metabolism. 2005;1(1):63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- [106].Huo L, Gamber K, Greeley S, et al. Leptin-dependent control of glucose balance and locomotor activity by pomc neurons. Cell metabolism. 2009;9(6):537–47. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].German JP, Thaler JP, Wisse BE, et al. Leptin activates a novel cns mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology. 2011;152(2):394–404. doi: 10.1210/en.2010-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Berglund ED, Vianna CR, Donato J, Jr., et al. Direct leptin action on pomc neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. The Journal of clinical investigation. 2012;122(3):1000–9. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kievit P, Howard JK, Badman MK, et al. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in pomc-expressing cells. Cell metabolism. 2006;4(2):123–32. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- [110].Banno R, Zimmer D, De Jonghe BC, et al. Ptp1b and shp2 in pomc neurons reciprocally regulate energy balance in mice. The Journal of clinical investigation. 2010;120(3):720–34. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Hill JW, Xu Y, Preitner F, et al. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology. 2009;150(11):4874–82. doi: 10.1210/en.2009-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Goncalves GH, Li W, Garcia AV, et al. Hypothalamic agouti-related peptide neurons and the central melanocortin system are crucial mediators of leptin's antidiabetic actions. Cell reports. 2014;7(4):1093–103. doi: 10.1016/j.celrep.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Roman EA, Reis D, Romanatto T, et al. Central leptin action improves skeletal muscle akt, ampk, and pgc1 alpha activation by hypothalamic pi3k-dependent mechanism. Molecular and cellular endocrinology. 2010;314(1):62–9. doi: 10.1016/j.mce.2009.08.007. [DOI] [PubMed] [Google Scholar]
- [114].Seufert J, Kieffer TJ, Habener JF. Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptin-deficient ob/ob mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(2):674–9. doi: 10.1073/pnas.96.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Cases JA, Gabriely I, Ma XH, et al. Physiological increase in plasma leptin markedly inhibits insulin secretion in vivo. Diabetes. 2001;50(2):348–52. doi: 10.2337/diabetes.50.2.348. [DOI] [PubMed] [Google Scholar]
- [116].Seufert J. Leptin effects on pancreatic beta-cell gene expression and function. Diabetes. 2004;53(Suppl 1):S152–8. doi: 10.2337/diabetes.53.2007.s152. [DOI] [PubMed] [Google Scholar]
- [117].Lee Y, Ravazzola M, Park BH, et al. Metabolic mechanisms of failure of intraportally transplanted pancreatic beta-cells in rats: Role of lipotoxicity and prevention by leptin. Diabetes. 2007;56(9):2295–301. doi: 10.2337/db07-0460. [DOI] [PubMed] [Google Scholar]
- [118].Lee YH, Magkos F, Mantzoros CS, et al. Effects of leptin and adiponectin on pancreatic beta-cell function. Metabolism: clinical and experimental. 2011;60(12):1664–72. doi: 10.1016/j.metabol.2011.04.008. [DOI] [PubMed] [Google Scholar]
- [119].Gallardo N, Bonzon-Kulichenko E, Fernandez-Agullo T, et al. Tissue-specific effects of central leptin on the expression of genes involved in lipid metabolism in liver and white adipose tissue. Endocrinology. 2007;148(12):5604–10. doi: 10.1210/en.2007-0933. [DOI] [PubMed] [Google Scholar]
- [120].Buettner C, Muse ED, Cheng A, et al. Leptin controls adipose tissue lipogenesis via central, stat3-independent mechanisms. Nature medicine. 2008;14(6):667–75. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lee Y, Yu X, Gonzales F, et al. Ppar alpha is necessary for the lipopenic action of hyperleptinemia on white adipose and liver tissue. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(18):11848–53. doi: 10.1073/pnas.182420899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating amp-activated protein kinase. Nature. 2002;415(6869):339–43. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- [123].Guo K, McMinn JE, Ludwig T, et al. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology. 2007;148(8):3987–97. doi: 10.1210/en.2007-0261. [DOI] [PubMed] [Google Scholar]
- [124].Baldock PA, Allison S, McDonald MM, et al. Hypothalamic regulation of cortical bone mass: Opposing activity of y2 receptor and leptin pathways. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21(10):1600–7. doi: 10.1359/jbmr.060705. [DOI] [PubMed] [Google Scholar]
- [125].Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19(7):905–12. doi: 10.1007/s00198-007-0487-9. [DOI] [PubMed] [Google Scholar]
- [126].Thomas T, Gori F, Khosla S, et al. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140(4):1630–8. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- [127].Gordeladze JO, Drevon CA, Syversen U, et al. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. Journal of cellular biochemistry. 2002;85(4):825–36. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- [128].Sienkiewicz E, Magkos F, Aronis KN, et al. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism: clinical and experimental. 2011;60(9):1211–21. doi: 10.1016/j.metabol.2011.05.016. [DOI] [PubMed] [Google Scholar]
- [129].Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- [130].Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and cart. Nature. 2005;434(7032):514–20. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- [131].Takeda S, Karsenty G. Molecular bases of the sympathetic regulation of bone mass. Bone. 2008;42(5):837–40. doi: 10.1016/j.bone.2008.01.005. [DOI] [PubMed] [Google Scholar]
- [132].Guerra B, Fuentes T, Delgado-Guerra S, et al. Gender dimorphism in skeletal muscle leptin receptors, serum leptin and insulin sensitivity. PloS one. 2008;3(10):e3466. doi: 10.1371/journal.pone.0003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Ceddia RB, William WN, Jr., Curi R. Comparing effects of leptin and insulin on glucose metabolism in skeletal muscle: Evidence for an effect of leptin on glucose uptake and decarboxylation. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1999;23(1):75–82. doi: 10.1038/sj.ijo.0800762. [DOI] [PubMed] [Google Scholar]
- [134].Muoio DM, Dohm GL, Tapscott EB, et al. Leptin opposes insulin's effects on fatty acid partitioning in muscles isolated from obese ob/ob mice. The American journal of physiology. 1999;276(5 Pt 1):E913–21. doi: 10.1152/ajpendo.1999.276.5.E913. [DOI] [PubMed] [Google Scholar]
- [135].Hedbacker K, Birsoy K, Wysocki RW, et al. Antidiabetic effects of igfbp2, a leptin-regulated gene. Cell metabolism. 2010;11(1):11–22. doi: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- [136].Yau SW, Henry BA, Russo VC, et al. Leptin enhances insulin sensitivity by direct and sympathetic nervous system regulation of muscle igfbp-2 expression: Evidence from nonrodent models. Endocrinology. 2014;155(6):2133–43. doi: 10.1210/en.2013-2099. [DOI] [PubMed] [Google Scholar]
- [137].Fuentes T, Ara I, Guadalupe-Grau A, et al. Leptin receptor 170 kda (ob-r170) protein expression is reduced in obese human skeletal muscle: A potential mechanism of leptin resistance. Experimental physiology. 2010;95(1):160–71. doi: 10.1113/expphysiol.2009.049270. [DOI] [PubMed] [Google Scholar]
- [138].Gibala MJ, McGee SL, Garnham AP, et al. Brief intense interval exercise activates ampk and p38 mapk signaling and increases the expression of pgc-1alpha in human skeletal muscle. Journal of applied physiology. 2009;106(3):929–34. doi: 10.1152/japplphysiol.90880.2008. [DOI] [PubMed] [Google Scholar]
- [139].Guerra B, Guadalupe-Grau A, Fuentes T, et al. Sirt1, amp-activated protein kinase phosphorylation and downstream kinases in response to a single bout of sprint exercise: Influence of glucose ingestion. European journal of applied physiology. 2010;109(4):731–43. doi: 10.1007/s00421-010-1413-y. [DOI] [PubMed] [Google Scholar]
- [140].Guerra B, Olmedillas H, Guadalupe-Grau A, et al. Is sprint exercise a leptin signaling mimetic in human skeletal muscle? Journal of applied physiology. 2011;111(3):715–25. doi: 10.1152/japplphysiol.00805.2010. [DOI] [PubMed] [Google Scholar]
- [141].Chiarreotto-Ropelle EC, Pauli LS, Katashima CK, et al. Acute exercise suppresses hypothalamic ptp1b protein level and improves insulin and leptin signaling in obese rats. American journal of physiology Endocrinology and metabolism. 2013;305(5):E649–59. doi: 10.1152/ajpendo.00272.2013. [DOI] [PubMed] [Google Scholar]
- [142].Guerra B, Ponce-Gonzalez JG, Morales-Alamo D, et al. Leptin signaling in skeletal muscle after bed rest in healthy humans. European journal of applied physiology. 2014;114(2):345–57. doi: 10.1007/s00421-013-2779-4. [DOI] [PubMed] [Google Scholar]
- [143].Guadalupe-Grau A, Larsen S, Guerra B, et al. Influence of age on leptin induced skeletal muscle signalling. Acta physiologica. 2014;211(1):214–28. doi: 10.1111/apha.12273. [DOI] [PubMed] [Google Scholar]
- [144].Carbone F, La Rocca C, Matarese G. Immunological functions of leptin and adiponectin. Biochimie. 2012;94(10):2082–8. doi: 10.1016/j.biochi.2012.05.018. [DOI] [PubMed] [Google Scholar]
- [145].Lord GM, Matarese G, Howard JK, et al. Leptin modulates the t-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394(6696):897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- [146].Loffreda S, Yang SQ, Lin HZ, et al. Leptin regulates proinflammatory immune responses. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1998;12(1):57–65. [PubMed] [Google Scholar]
- [147].De Rosa V, Procaccini C, Cali G, et al. A key role of leptin in the control of regulatory t cell proliferation. Immunity. 2007;26(2):241–55. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- [148].Howard JK, Lord GM, Matarese G, et al. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. The Journal of clinical investigation. 1999;104(8):1051–9. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Chan JL, Moschos SJ, Bullen J, et al. Recombinant methionyl human leptin administration activates signal transducer and activator of transcription 3 signaling in peripheral blood mononuclear cells in vivo and regulates soluble tumor necrosis factor-alpha receptor levels in humans with relative leptin deficiency. The Journal of clinical endocrinology and metabolism. 2005;90(3):1625–31. doi: 10.1210/jc.2004-1823. [DOI] [PubMed] [Google Scholar]
- [150].Matarese G, La Rocca C, Moon HS, et al. Selective capacity of metreleptin administration to reconstitute cd4+ t-cell number in females with acquired hypoleptinemia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(9):E818–27. doi: 10.1073/pnas.1214554110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Matarese G, Sanna V, Di Giacomo A, et al. Leptin potentiates experimental autoimmune encephalomyelitis in sjl female mice and confers susceptibility to males. European journal of immunology. 2001;31(5):1324–32. doi: 10.1002/1521-4141(200105)31:5<1324::AID-IMMU1324>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- [152].Sanna V, Di Giacomo A, La Cava A, et al. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic t cell responses. The Journal of clinical investigation. 2003;111(2):241–50. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Matarese G, Carrieri PB, La Cava A, et al. Leptin increase in multiple sclerosis associates with reduced number of cd4(+)cd25+ regulatory t cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(14):5150–5. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Vatier C, Gautier JF, Vigouroux C. Therapeutic use of recombinant methionyl human leptin. Biochimie. 2012;94(10):2116–25. doi: 10.1016/j.biochi.2012.03.013. [DOI] [PubMed] [Google Scholar]
- [155].Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2011;17(6):922–32. doi: 10.4158/EP11229.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Trevaskis JL, Coffey T, Cole R, et al. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: Magnitude and mechanisms. Endocrinology. 2008;149(11):5679–87. doi: 10.1210/en.2008-0770. [DOI] [PubMed] [Google Scholar]
- [157].Roth JD, Roland BL, Cole RL, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: Evidence from nonclinical and clinical studies. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(20):7257–62. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Ravussin E, Smith SR, Mitchell JA, et al. Enhanced weight loss with pramlintide/metreleptin: An integrated neurohormonal approach to obesity pharmacotherapy. Obesity. 2009;17(9):1736–43. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Moon HS, Chamberland JP, Diakopoulos KN, et al. Leptin and amylin act in an additive manner to activate overlapping signaling pathways in peripheral tissues: In vitro and ex vivo studies in humans. Diabetes care. 2011;34(1):132–8. doi: 10.2337/dc10-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Kim YW, Kim JY, Park YH, et al. Metformin restores leptin sensitivity in high-fat-fed obese rats with leptin resistance. Diabetes. 2006;55(3):716–24. doi: 10.2337/diabetes.55.03.06.db05-0917. [DOI] [PubMed] [Google Scholar]
- [161].Muller TD, Sullivan LM, Habegger K, et al. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or fgf21. Journal of peptide science : an official publication of the European Peptide Society. 2012;18(6):383–93. doi: 10.1002/psc.2408. [DOI] [PubMed] [Google Scholar]
- [162].Rosenbaum M, Sy M, Pavlovich K, et al. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. The Journal of clinical investigation. 2008;118(7):2583–91. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Mantzoros CS, Magkos F, Brinkoetter M, et al. Leptin in human physiology and pathophysiology. American journal of physiology Endocrinology and metabolism. 2011;301(4):E567–84. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Paz-Filho G, Wong ML, Licinio J. The procognitive effects of leptin in the brain and their clinical implications. International journal of clinical practice. 2010;64(13):1808–12. doi: 10.1111/j.1742-1241.2010.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Holden KF, Lindquist K, Tylavsky FA, et al. Serum leptin level and cognition in the elderly: Findings from the health abc study. Neurobiology of aging. 2009;30(9):1483–9. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Lieb W, Beiser AS, Vasan RS, et al. Association of plasma leptin levels with incident alzheimer disease and mri measures of brain aging. JAMA : the journal of the American Medical Association. 2009;302(23):2565–72. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Davis C, Mudd J, Hawkins M. Neuroprotective effects of leptin in the context of obesity and metabolic disorders. Neurobiology of disease. 2014 doi: 10.1016/j.nbd.2014.04.012. [DOI] [PubMed] [Google Scholar]