Abstract
Objectives
Vitamin D may prolong cancer survival by inhibiting tumor progression and metastasis, however, there are limited epidemiologic studies regarding the association between circulating 25-hydroxyvitamin D (25(OH)D) and lung cancer survival. The aim of this study was to examine the relationship between serum 25(OH)D and lung cancer specific survival and to evaluate whether vitamin D binding protein (DBP) concentration modified this association.
Materials and Methods
25(OH)D and DBP were measured in fasting serum samples from 500 male lung cancer cases in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CI) for lung cancer related death according to quartiles of season-specific 25(OH)D, DBP, and the molar ratio of 25(OH)D:DBP, a proxy for free circulating 25(OH)D.
Results
Comparing highest to lowest quartiles, serum 25(OH)D (HR=1.18; 95% CI: 0.89–1.56) and DBP (HR=0.95; 95% CI: 0.71–1.26) were not associated with lung cancer survival and DBP concentration did not modify the association with 25(OH)D (p for interaction=0.56). There was suggestion of an association between higher serum 25(OH)D and better survival from adenocarcinoma (HR=0.64; 95% CI: 0.17–2.45) and small cell carcinoma (HR=0.55; 95% CI: 0.21–1.45), but these estimates were based on a relatively small number of cases.
Conclusion
Serum 25(OH)D was not associated with overall lung cancer survival regardless of DBP concentration, however, these findings should be examined in other studies that include women and subjects with higher 25(OH)D levels.
Keywords: serum vitamin D, vitamin D binding protein, lung cancer, survival, cohort
INTRODUCTION
Lung cancer is the second most common cancer and the leading cause of cancer related death in men and women in the United States (1). Ecologic studies suggest that lung cancer mortality is lowest in patients diagnosed during the summer and autumn months, the time of year when vitamin D levels tend to be highest (2, 3). Vitamin D may influence cancer survival and inhibit tumor progression by suppressing metastasis, proliferation, and angiogenesis, or by promoting apoptosis in cancer cells (4–6). 25-hydroxyvitamin D (25(OH)D) is the primary form of circulating vitamin D and the accepted measure of vitamin D status, integrating vitamin D obtained from sunlight exposure and dietary intake (7).
Evidence suggests that higher serum concentrations of 25(OH)D are associated with better prognosis for cancer patients (8), however, there are limited epidemiologic studies of serum 25(OH)D concentration in relation to survival among lung cancer cases. Two studies observed better survival among lung cancer cases with higher circulating 25(OH)D measured shortly after diagnosis (9, 10), but other studies observed that higher 25(OH)D concentration at diagnosis was not association with survival (11) or associated with worse survival (12). To our knowledge no other studies have examined pre-diagnostic serum 25(OH)D in relation to survival among lung cancer cases.
The vitamin D binding protein (DBP) is the primary transport protein of 25(OH)D (13). In addition to being a carrier for vitamin D metabolites, DBP may also influence survival through its other functions including actin scavengering, macrophage activation, and neutrophil chemotaxis (14–16). Previous studies have suggested that serum DBP modifies 25(OH)D risk associations with pancreatic (17), prostate (18), and bladder cancers (19), and was itself inversely related to pancreatic (17) and renal cell carcinoma (20), findings that support the need to measure DBP when evaluating associations between 25(OH)D and disease outcomes. To our knowledge, no previous study has assessed whether DBP concentration modifies the association between 25(OH)D and lung cancer survival.
We examined whether circulating 25(OH)D prior to diagnosis was associated with survival in 500 men diagnosed with lung cancer from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study and whether DBP concentrations modified this association.
METHODS
Study population
The ATBC Study, which has been described previously (21), was a randomized, double-blind, placebo controlled, chemoprevention trial with daily supplementation with either α-tocopherol (50 mg/day), β-carotene (20 mg/day), both supplements, or placebo. The 29,133 men who enrolled between 1985 and 1988 were residents of southwestern Finland, aged 50–69 years, and smoked ≥5 cigarettes per day. Study supplementation continued for 5–8 years (median, 6.1 years) until death or trial closure on April 30, 1993. The study was approved by the institutional review boards of the National Cancer Institute and the National Public Health Institute of Finland and written informed consent was obtained from each participant. Participants completed a food frequency questionnaire (22) and a questionnaire to obtain information about general risk factors.
The current investigation was based on a nested case-control study of serum 25(OH)D that included 500 lung cancer cases (International Classification of Diseases 9, code 162) diagnosed through April 30, 2005 (23). Cases were identified by the Finnish Cancer Registry, which provides nearly 100% case ascertainment for ATBC study subjects (24). For cases diagnosed before May 1999, one or two study physicians reviewed medical records and histopathologic information to confirm the diagnosis and staging. For all subsequent cases, the date of diagnosis and histology were based solely on information from the Finnish Cancer Registry. Tumor stage information was available for cases diagnosed before May 1999. Histology data were available for all but 87 cases; the main histology subtypes were squamous cell carcinoma (n=179), adenocarcinoma (n=72), and small cell carcinoma (n=100), as defined by International Classifications of Diseases for Oncology, 2nd edition, codes, 80702–80708, 81403–82508, and 80413–80493, respectively. Lung cancer related deaths were identified from the Finnish National Register of Causes of Death.
Laboratory measurements
Overnight fasting serum samples were collected at the pre-randomization baseline visit and stored at −70°C. Median time from blood collection until diagnosis was 10 years (range: 0–20 years). Concentrations of 25(OH)D were measured in matched case-control sets (23) by Heartland Assays, Inc. (Ames, IA), using the DiaSorin Liaison 25(OH)D TOTAL assay (25). Blinded quality control samples were included in each batch and comprised 5% of the samples. Nested components-of-variance analysis (26) was used to calculate the interbatch and intrabatch coefficients of variation that ranged between 12.3%–13.6% and 9.3%–11.0%, respectively.
DBP was measured using the Quantikine Human Vitamin D Binding Protein Immunoassay kit (Catalog number DVDBP0, R&D Systems, Inc., Minneapolis, MN) at the Clinical Support Laboratory, SAIC-Frederick, Inc., Frederick National Laboratory for Cancer Research (Frederick, MD). Blinded quality control samples were included in each batch and comprised approximately 10% of the total samples. Interbatch and intrabatch coefficients of variation were 10.8% and 15.2%, respectively. DBP concentration measurement was available for 480 of the 500 cases.
Statistical analysis
Survival time was calculated from the date of lung cancer diagnosis until the date of death from lung cancer, death from another cause, or December 31, 2012, whichever came first. Men that did not die from lung cancer were censored at their date of death or at the end of the follow-up period if still alive. Survival time by 25(OH)D categories was estimated using the Kaplan–Meier method. Cox proportional hazards regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for lung cancer survival by quartiles of 25(OH)D, DBP, and the 25(OH)D:DBP molar ratio, a proxy for free circulating vitamin D (27, 28). Quartile cut-points were based on the distribution of 25(OH)D and DBP in all cases. To account for seasonal variation, season-specific 25(OH)D quartile cut-points were created for each season of blood collection (darker months: November-April or sunnier months: May-October) and then merged into one variable. Season-standardized 25(OH)D was created using residuals from a model regressing log-transformed 25(OH)D against calendar week of blood collection (29). Associations with survival were further evaluated by clinically pre-defined cut-points of serum 25(OH)D (<25, 25 - <37.5, 37.5 - <50, and ≥50 nmol/L) (29). Schoenfeld residuals were used to test the proportional hazards assumption, which was fulfilled for 25(OH)D (p = 0.96), DBP (p = 0.41), and the 25(OH)D:DBP molar ratio (p = 0.45).
To assess for potential confounding, factors from Table 1 were entered into univariate models to determine if the effect estimate for 25(OH)D or DBP changed more than 10%. Family history of lung cancer and daily intake of energy, calcium, and fat were the only factors to alter the effect estimates by more than 10%. The final multivariable model adjusted for age at randomization (continuous), date of blood collection (continuous), family history of lung cancer (yes, no, missing), total daily intake of energy (quartiles, missing category), calcium (quartiles, missing category), and fat (quartiles, missing category), and factors commonly associated with lung cancer risk or prognosis including cigarettes smoked per day (continuous), total years smoked (continuous), BMI (continuous), stage at diagnosis (stage I/2, stage 3/4, unknown/missing), age at diagnosis (continuous), total serum cholesterol (continuous), daily alcohol intake (no alcohol, quartiles, missing), and trial supplementation (α-tocopherol: yes/no, β-carotene: yes/no). To test for linear trend, a term with the median values of each quartile for the main effect was entered into the model as a continuous ordinal variable.
Table 1.
Select baseline characteristics for 500 male lung cancer cases in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study.
| Characteristic | Fatal Lung Cancers (n = 428) |
Lung Cancer Survivors (n = 72) |
p-valuea |
|---|---|---|---|
| Age at randomization, y | 58.6 ± 4.9 | 59.1 ± 5.5 | 0.34 |
| Age at diagnosis, y | 68.5 ± 6.3 | 68.3 ± 6.6 | 0.62 |
| Survival time, y | 1.1 ± 2.1 | 5.0 ± 6.6 | 0.0005 |
| Body mass index, (kg/m2) | 25.5 ± 3.7 | 26.7 ± 4.3 | 0.04 |
| Education, % >elementary | 15.9 | 16.7 | 0.87 |
| Married, % | 79.4 | 87.5 | 0.11 |
| Cigarettes smoked/day | 23.2 ± 9.5 | 21.6 ± 9.4 | 0.22 |
| Total years smoked | 39.4 ± 6.4 | 39.9 ± 6.8 | 0.38 |
| Family history of lung cancer, %b | 14.4 | 11.4 | 0.59 |
| Stage at diagnosis | |||
| Stage 1 or 2, % | 15.9 | 33.3 | 0.002 |
| Stage 3 or 4, % | 46.5 | 34.7 | |
| Missing stage at diagnosis, % | 37.6 | 31.9 | |
| Histology | |||
| Squamous cell carcinoma | 34.1 | 45.8 | 0.007 |
| Adenocarcinoma | 13.1 | 22.2 | |
| Small cell carcinoma | 21.7 | 9.7 | |
| Other types and unknown | 31.1 | 22.2 | |
| Leisure physical activity ≥3 times/week, % | 20.8 | 25.0 | 0.46 |
| Energy intake, kcal/day | 2688 ± 736 | 2562 ± 849 | 0.06 |
| Vitamin D supplement use, % yes | 8.2 | 5.6 | 0.44 |
| Calcium supplement use, % yes | 11.9 | 11.1 | 0.84 |
| Dietary vitamin D intake, µg/day | 5.4 ± 3.0 | 5.3 ± 3.1 | 0.62 |
| Dietary calcium intake, mg/day | 1416 ± 605 | 1348 ± 618 | 0.26 |
| Total fat intake, g/day | 125 ± 42 | 117 ± 47 | 0.06 |
| Alcohol, g of ethanol/day | 17.6 ± 20.6 | 16.5 ± 21.0 | 0.31 |
| Season at blood draw, % May-October | 40.2 | 38.9 | 0.84 |
| Alpha-tocopherol intervention, % | 51.9 | 47.2 | 0.47 |
| Beta-carotene intervention, % | 52.8 | 58.3 | 0.38 |
| Serum 25(OH)D, nmol/L | 36.8 ± 21.3 | 39.4 ± 24.3 | 0.50 |
| Serum DBP, nmol/L | 5988 ± 1814 | 5670 ± 1643 | 0.21 |
| Serum 25(OH)D:DBP molar ratio (× 103) | 6.8 ± 4.8 | 7.9 ± 6.4 | 0.26 |
| Serum α-tocopherol, mg/L | 11.8 ± 3.4 | 11.5 ± 3.4 | 0.43 |
| Serum β-carotene, µg/L | 199 ± 138 | 207 ± 171 | 0.83 |
| Serum retinol, µg/L | 570 ± 137 | 573 ± 137 | 0.64 |
| Serum total cholesterol, mmol/L | 6.2 ± 1.2 | 6.1 ± 1.3 | 0.96 |
| Serum high-density lipoprotein cholesterol, mmol/L | 1.22 ± 0.33 | 1.10 ± 0.27 | 0.006 |
DBP = vitamin D binding protein, 25(OH)D = 25-hydroxyvitamin D.
Data are presented as means ± the standard deviation or proportions.
Wilcoxon rank sum test for continuous variables and the chi-squared test for categorical variables.
Family history data available for 55% of cases.
To explore potential effect modifiers, multivariable models were stratified at the median split for age, cigarettes per day, years smoked, serum levels of α-tocopherol, β-carotene, retinol, total cholesterol, HDL, and dietary fat and calcium intake. Models were also stratified by categories of leisure time physical activity (<3 times/week, ≥3 times/week), BMI (<25, ≥25 kg/m2), trial supplementation (α-tocopherol: yes/no, β-carotene: yes/no), tumor stage (stage1/2, stage 3/4), time from blood collection to diagnosis (<10 years, ≥10 years), and season at blood draw (November-April, May-October). To test whether interactions were statistically significant, a cross-product interaction term of quartiles of 25(OH)D and the characteristic coded as a two-level variable was included in a multivariable model.
Statistical analyses were conducted using SAS software version 9.2 (SAS Institute, Cary, NC) and all p-values were two sided. Kaplan–Meier curves were generated using R version 3.0.2.
RESULTS
Of the 500 lung cancer cases included in the analysis, 428 died from their disease. Lung cancer survivors were more likely to have a higher BMI and lower serum HDL cholesterol at baseline, and be diagnosed with earlier stage disease and non-small cell lung cancer (Table 1). Mean baseline concentrations of serum 25(OH)D and DBP did not significantly differ by survival status. Men with a higher serum concentration of 25(OH)D had significantly lower daily intake of calories, calcium, and fat, had higher dietary vitamin D intake, higher baseline serum β-carotene, were more likely to take vitamin D and calcium supplements, and less likely to have a family history of lung cancer (data not shown). Prognostic factors including tumor stage, BMI, age at diagnosis, smoking, and alcohol intake were not correlated with serum concentration of 25(OH)D, however, men in the highest quartile of serum 25(OH)D were significantly more likely to have small cell lung cancer than men with low concentrations of serum 25(OH)D (data not shown).
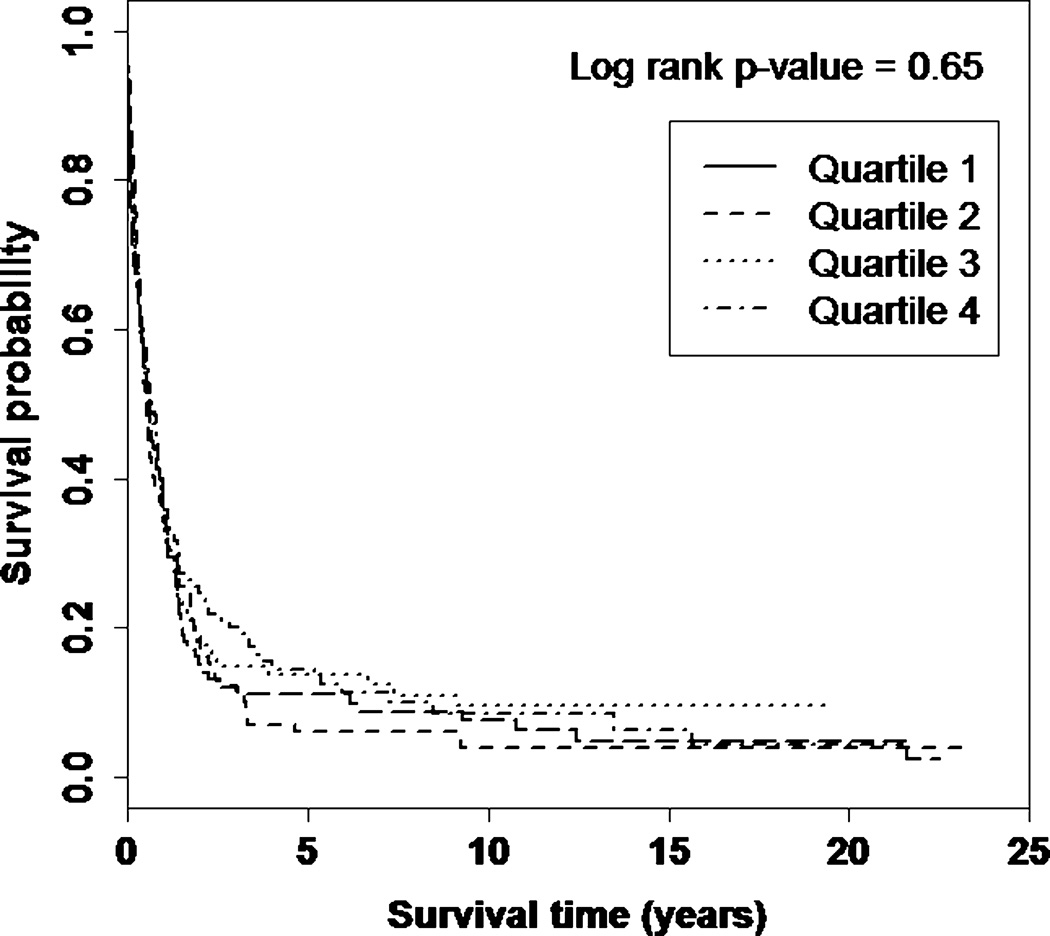
Lung cancer specific survival was similar across quartiles of serum 25(OH)D (log-rank p-value = 0.65) (Figure 1). Comparing highest to lowest quartiles, there was no statistically significant association between serum 25(OH)D and lung cancer survival (HR = 1.18; 95% CI: 0.89–1.56) (Table 2) and further adjustment for DBP concentration did not alter the findings (data not shown). Similar associations were observed for season-standardized 25(OH)D and clinically pre-defined 25(OH)D cut-points (data not shown). The association between serum 25(OH)D and lung cancer survival remained null when comparing men with sufficient (≥50 nmol/L, n = 125) versus insufficient (<50 nmol/L, n = 375) 25(OH)D concentration (multivariable HR = 1.15; 95% CI:0.91–1.45). Lung cancer survival was not associated with DBP concentration (HR = 0.95; 95% CI: 0.71–1.26), or the 25(OH)D:DBP molar ratio (HR = 0.90; 95% CI: 0.67–1.22), a proxy for free circulating vitamin D, although the second quartile of the 25(OH)D:DBP molar ratio was associated with longer survival (HR = 0.71; 95% CI: 0.52–0.94). Results for 25(OH)D, DBP, and the 25(OH)D:DBP molar ratio were unchanged after excluding cases diagnosed less than 2 years after blood collection (data not shown).
Figure 1. Kaplan–Meier curves showing the proportion surviving after lung cancer diagnosis by quartile of serum 25(OH)D.
Table 2.
Association between serum 25(OH)D, DBP, and the 25(OH)D:DBP molar ratio and lung cancer survival in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study.
| Quartile 1 HR (95% CI) |
Quartile 2 HR (95% CI) |
Quartile 3 HR (95% CI) |
Quartile 4 HR (95% CI) |
p-trend | |
|---|---|---|---|---|---|
| Season-specific 25(OH)Da | |||||
| Deaths/Total cases | 110/125 | 104/125 | 109/125 | 105/125 | |
| Model 1b | 1.00 (Reference) | 0.89 (0.68, 1.16) | 0.88 (0.68, 1.15) | 1.00 (0.76, 1.31) | 0.82 |
| Model 2c | 1.00 (Reference) | 1.08 (0.81, 1.43) | 0.97 (0.72, 1.29) | 1.18 (0.89, 1.56) | 0.31 |
| DBP, nmol/L | <4724 | 4724 - <5890 | 5890 - <7187 | ≥7187 | |
| Deaths/Total cases | 103/120 | 99/120 | 109/120 | 104/120 | |
| Model 1b | 1.00 (Reference) | 0.83 (0.63, 1.10) | 1.17 (0.90, 1.54) | 1.01 (0.77, 1.33) | 0.39 |
| Model 2c | 1.00 (Reference) | 0.79 (0.59, 1.06) | 1.02 (0.76, 1.35) | 0.95 (0.71, 1.26) | 0.78 |
| 25(OH)D:DBP molar ratio (×103) | <3.4 | 3.4 - <5.8 | 5.8 - <9.0 | ≥9.0 | |
| Deaths/Total cases | 107/120 | 103/120 | 108/120 | 97/120 | |
| Model 1b | 1.00 (Reference) | 0.74 (0.57, 0.98) | 0.86 (0.66, 1.13) | 0.83 (0.63, 1.10) | 0.49 |
| Model 2c | 1.00 (Reference) | 0.71 (0.52, 0.94) | 0.85 (0.64, 1.15) | 0.90 (0.67, 1.22) | 0.92 |
HR = hazards ratio; CI = confidence interval; 25(OH)D = 25-hydroxyvitamin D; DBP = vitamin D binding protein.
Cut-points for season specific quartiles (nmol/L): Winter (November - April) = Q1: <17.8, Q2: 17.8 - <25.3, Q3: 25.3 - <40.8, Q4: ≥40.8; Summer (May - October) = Q1: <29.5, Q2: 29.5 - <45.1, Q3: 45.1 - <60.8, Q4: ≥60.8.
Adjusted for age at randomization and date of blood collection.
Adjusted for age at randomization, date of blood collection, cigarettes per day, total years smoked, BMI, total serum cholesterol, calcium intake, energy intake, alcohol intake, fat intake, trial supplementation (α-tocopherol: yes/no; β-carotene: yes/no), family history of lung cancer, stage at diagnosis, and age at diagnosis.
Associations with serum 25(OH)D were not significantly different for men with serum DBP concentrations below (HR = 0.80; 95% CI: 0.51–1.27) or above the median (HR = 1.16; 95% CI: 0.76–1.76) (p for interaction = 0.56) (Table 3). Similarly, associations with serum DBP and the molar ratio of 25(OH)D:DBP did not vary by concentration of 25(OH)D and DBP, respectively.
Table 3.
Association between serum 25(OH)D, DBP, and the 25(OH)D:DBP molar ratio and lung cancer survival in stratified models, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study.
| Quartile 1 HR (95% CI) |
Quartile 2 HR (95% CI) |
Quartile 3 HR (95% CI) |
Quartile 4 HR (95% CI) |
p-trend | p-interaction | |
|---|---|---|---|---|---|---|
| Season-specific 25(OH)Da | ||||||
| DBP <median | ||||||
| Deaths/Total cases | 58/62 | 49/61 | 54/62 | 41/55 | 0.56 | |
| Model 1b | 1.00 (Reference) | 0.66 (0.45, 0.97) | 0.78 (0.54, 1.14) | 0.85 (0.56, 1.27) | 0.81 | |
| Model 2c | 1.00 (Reference) | 0.72 (0.47, 1.10) | 0.79 (0.51, 1.21) | 0.80 (0.51, 1.27) | 0.58 | |
| DBP ≥median | ||||||
| Deaths/Total cases | 46/54 | 53/59 | 53/61 | 61/66 | ||
| Model 1b | 1.00 (Reference) | 1.18 (0.79, 1.75) | 0.88 (0.59, 1.32) | 1.00 (0.68, 1.47) | 0.70 | |
| Model 2c | 1.00 (Reference) | 1.49 (0.96, 2.33) | 0.92 (0.59, 1.45) | 1.16 (0.76, 1.76) | 0.89 | |
| DBP | ||||||
| 25(OH)D <median | ||||||
| Deaths/Total cases | 56/64 | 51/59 | 49/57 | 50/56 | 0.20 | |
| Model 1b | 1.00 (Reference) | 0.92 (0.63, 1.35) | 1.13 (0.77, 1.66) | 1.35 (0.92, 1.98) | 0.08 | |
| Model 2c | 1.00 (Reference) | 0.85 (0.56, 1.29) | 0.91 (0.59, 1.41) | 1.36 (0.88, 2.09) | 0.17 | |
| 25(OH)D ≥median | ||||||
| Deaths/Total cases | 47/56 | 48/61 | 60/63 | 54/64 | ||
| Model 1b | 1.00 (Reference) | 0.74 (0.49, 1.11) | 1.22 (0.83, 1.79) | 0.79 (0.53, 1.17) | 0.73 | |
| Model 2c | 1.00 (Reference) | 0.85 (0.54, 1.34) | 1.39 (0.88, 2.17) | 0.76 (0.49, 1.16) | 0.43 | |
|
25(OH)D:DBP molar ratio DBP <median |
||||||
| Deaths/Total cases | 35/38 | 42/50 | 56/64 | 69/88 | 0.42 | |
| Model 1b | 1.00 (Reference) | 0.68 (0.43, 1.07) | 0.86 (0.56, 1.32) | 0.85 (0.56, 1.29) | 0.93 | |
| Model 2c | 1.00 (Reference) | 0.55 (0.33, 0.91) | 0.87 (0.53, 1.41) | 0.76 (0.48, 1.22) | 0.95 | |
| DBP ≥median | ||||||
| Deaths/Total cases | 72/82 | 61/70 | 52/56 | 28/32 | ||
| Model 1b | 1.00 (Reference) | 0.79 (0.56, 1.12) | 0.89 (0.62, 1.27) | 0.81 (0.52, 1.27) | 0.46 | |
| Model 2c | 1.00 (Reference) | 0.78 (0.54, 1.13) | 0.82 (0.55, 1.24) | 0.82 (0.50, 1.34) | 0.43 |
HR = hazard ratio; CI = confidence interval; 25(OH)D = 25-hydroxyvitamin D; DBP = vitamin D binding protein.
Cutpoints for season specific quartiles (nmol/L): Winter (November - April) = Q1: <17.8, Q2: 17.8 - <25.3, Q3: 25.3 - <40.8, Q4: ≥40.8; Summer (May - October) = Q1: <29.5, Q2: 29.5 - <45.1, Q3: 45.1 - <60.8, Q4: ≥60.8.
Adjusted for age at randomization and date of blood collection.
Adjusted for age at randomization, date of blood collection cigarettes per day, total years smoked, BMI, total serum cholesterol, calcium intake, energy intake, alcohol intake, fat intake, trial supplementation (α-tocopherol: yes/no; β-carotene: yes/no), family history of lung cancer, stage at diagnosis, and age at diagnosis.
In histology-specific analyses (Table 4), there was suggestion that higher 25(OH)D concentration was associated with reduced survival in cases of squamous cell carcinoma (HR = 1.34; 95% CI: 0.76–2.39), but increased survival for adenocarcinoma (HR = 0.64; 95% CI: 0.17–2.45) and small cell carcinoma (HR = 0.55; 95% CI: 0.21–1.45), although none of these associations were statistically significant and the estimates were based on a relatively small number of cases. Similarly, DBP concentration was not significantly associated with any histology type (data not shown).
Table 4.
Association between season-specific 25(OH)D quartilesa and survival by lung cancer histology type, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study.
| Quartile 1 HR (95% CI) |
Quartile 2 HR (95% CI) |
Quartile 3 HR (95% CI) |
Quartile 4 HR (95% CI) |
p-trend | |
|---|---|---|---|---|---|
| Squamous cell carcinoma | |||||
| Deaths/Total cases | 38/46 | 40/50 | 38/50 | 30/33 | |
| Model 1b | 1.00 (Reference) | 0.80 (0.51, 1.26) | 0.86 (0.54, 1.37) | 1.05 (0.64, 1.73) | 0.61 |
| Model 2c | 1.00 (Reference) | 0.94 (0.57, 1.54) | 0.82 (0.48, 1.39) | 1.34 (0.76, 2.39) | 0.31 |
| Adenocarcinoma | |||||
| Deaths/Total cases | 13/16 | 17/22 | 12/13 | 14/21 | |
| Model 1b | 1.00 (Reference) | 1.51 (0.70, 3.27) | 1.07 (0.48, 2.42) | 0.64 (0.28, 1.46) | 0.09 |
| Model 2c | 1.00 (Reference) | 1.71 (0.57, 5.11) | 1.63 (0.43, 6.14) | 0.64 (0.17, 2.45) | 0.24 |
| Small cell carcinoma | |||||
| Deaths/Total cases | 19/19 | 24/24 | 24/26 | 26/31 | |
| Model 1b | 1.00 (Reference) | 0.65 (0.36, 1.20) | 0.59 (0.31, 1.11) | 0.85 (0.47, 1.54) | 0.96 |
| Model 2c | 1.00 (Reference) | 0.31 (0.12, 0.82) | 0.47 (0.21, 1.05) | 0.55 (0.21, 1.45) | 0.99 |
HR = hazard ratio; CI = confidence interval; 25(OH)D = 25-hydroxyvitamin D; DBP = vitamin D binding protein.
Cutpoints for season specific quartiles (nmol/L): Winter (November - April) = Q1: <17.8, Q2: 17.8 - <25.3, Q3: 25.3 - <40.8, Q4: ≥40.8; Summer (May - October) = Q1: <29.5, Q2: 29.5 - <45.1, Q3: 45.1 - <60.8, Q4: ≥60.8.
Adjusted for age at randomization and date of blood collection.
Adjusted for age at randomization, date of blood collection cigarettes per day, total years smoked, BMI, total serum cholesterol, calcium intake, energy intake, alcohol intake, fat intake, trial supplementation (α-tocopherol: yes/no; β-carotene: yes/no), family history of lung cancer, stage at diagnosis, and age at diagnosis.
In subgroup analyses of 25(OH)D, there was a significant interaction with frequency of leisure-time physical activity (p for interaction = 0.01), with the highest versus lowest quartile of 25(OH)D concentration being associated with decreased survival in cases who reported physical activity less than three times per week (HR = 1.46; 95% CI: 1.05–2.03) and suggestion of improved survival in men with ≥3 activity sessions per week (HR = 0.53; 95% CI: 0.24–1.16) (Table 5). No other factors modified the association between serum 25(OH)D and lung cancer survival (data not shown).
Table 5.
Association between serum 25(OH)D and lung cancer survival according to frequency of leisure time physical activity, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study.
| Quartile 1 HR (95% CI) |
Quartile 2 HR (95% CI) |
Quartile 3 HR (95% CI) |
Quartile 4 HR (95% CI) |
p-trend | p-interaction | |
|---|---|---|---|---|---|---|
| Season-specific 25(OH)Da | ||||||
|
Leisure time physical activity <3 times/week |
||||||
| Deaths/Total cases | 86/100 | 86/102 | 91/103 | 76/88 | 0.01 | |
| Model 1b | 1.00 (Reference) | 0.87 (0.64, 1.18) | 0.86 (0.64, 1.16) | 1.23 (0.90, 1.67) | 0.33 | |
| Model 2c | 1.00 (Reference) | 1.13 (0.82, 1.57) | 1.01 (0.73, 1.41) | 1.46 (1.05, 2.03) | 0.07 | |
|
Leisure time physical activity ≥3 times/week |
||||||
| Deaths/Total cases | 24/25 | 18/23 | 18/22 | 29/37 | ||
| Model 1b | 1.00 (Reference) | 0.91 (0.49, 1.70) | 1.00 (0.53, 1.88) | 0.55 (0.31, 0.98) | 0.04 | |
| Model 2c | 1.00 (Reference) | 0.81 (0.38, 1.75) | 0.91 (0.42, 1.97) | 0.53 (0.24, 1.16) | 0.14 |
HR = hazard ratio; CI = confidence interval; 25(OH)D = 25-hydroxyvitamin D.
Cutpoints for season specific quartiles (nmol/L): Winter (November - April) = Q1: <17.8, Q2: 17.8 - <25.3, Q3: 25.3 - <40.8, Q4: ≥40.8; Summer (May - October) = Q1: <29.5, Q2: 29.5 - <45.1, Q3: 45.1 - <60.8, Q4: ≥60.8.
Adjusted for age at randomization and date of blood collection.
Adjusted for age at randomization, date of blood collection cigarettes per day, total years smoked, BMI, total serum cholesterol, calcium intake, energy intake, alcohol intake, fat intake, trial supplementation (α-tocopherol: yes/no; β-carotene: yes/no), family history of lung cancer, stage at diagnosis, and age at diagnosis.
DISCUSSION
Circulating serum concentrations of 25(OH)D and DBP were not associated with lung cancer specific survival in this cohort of male smokers, and DBP concentration did not modify associations with 25(OH)D. In histology specific analyses, there was suggestion that higher 25(OH)D concentration may be associated with improved survival for adenocarcinoma and small cell carcinoma, however, these associations were not statistically significant.
Results from previous studies of serum 25(OH)D and lung cancer specific survival have been inconsistent. A Norwegian study of 210 lung cancer patients that collected serum samples shortly after diagnosis, observed that higher serum 25(OH)D was associated with a statistically significant longer survival time (9). Similarly, a US study of 447 early stage non-small cell lung cancer patients also observed longer survival for those with higher serum 25(OH)D at diagnosis, however the association was not statistically significant (10). In contrast, higher serum 25(OH)D at diagnosis was associated with shorter survival time in a Chinese study of 87 non-small cell lung cancer cases (12). No association between serum 25(OH)D and survival was observed for 294 patients with advanced stage non-small cell carcinoma (11), suggesting that vitamin D may be less protective for later stage, more aggressive lung tumors. To our knowledge, only one study examined DBP concentration and lung cancer survival (30); among 148 non-small cell lung cancer cases with blood samples collected shortly after diagnosis, no survival association was seen for serum 25(OH)D, but lower serum DBP concentration was associated with significantly shorter survival.
It is biologically plausible for vitamin D to influence lung cancer survival. Lung tissue expresses both 1α-hydroxylase, the enzyme that converts 25(OH)D to the metabolically active 1,25-hydroxyvitamin D (1,25(OH)2D) (31), and the vitamin D receptor (VDR) which mediates the anti-proliferative and other effects of 1,25(OH)2D (32). Furthermore, experimental studies have observed that higher VDR expression in lung tumors is associated with improved survival (33) and that vitamin D inhibits metastasis and angiogenesis in lung cancer cells (4, 5). Select characteristics of the ATBC cohort may have contributed to the weak findings observed in the current study as well as a previous analysis in ATBC of serum 25(OH)D concentration and risk of lung cancer (23). The ATBC cohort is comprised entirely of smokers, and smoking may promote the inactivation of 1,25(OH)2D (34), thereby attenuating potential anti-metastatic effects of vitamin D. Also, mean serum 25(OH)D concentration in ATBC is lower than mean 25(OH)D levels observed in other cohort populations (29) due to the high latitude of Finland, which limits UVB synthesis of vitamin D in the skin, the low levels of vitamin D supplementation reported by participants, and that only 10% of subjects had blood drawn from June-August, owing to study clinics being closed during July, the time of year when UVB rays are strong and serum 25(OH)D levels tend to be highest. Therefore, 25(OH)D concentrations in the ATBC population were potentially too low to observe a beneficial effect on survival.
We observed that physical activity modified the association between serum 25(OH)D and lung cancer survival, with higher serum 25(OH)D associated with shorter survival among men reporting infrequent physical activity and longer survival for men who were regularly active. Greater physical activity is associated with higher serum 25(OH)D concentration, even in studies controlling for sun exposure (35, 36), possibly as a result of reduced bioavailability of vitamin D in individuals that are not physically active D (37). Regular moderate physical activity is also associated with a significantly lower risk of cancer mortality (38), potentially through improved insulin sensitivity and immune function and reduced systemic inflammation (39). Taken together, the survival benefits from physical activity and higher serum 25(OH)D among regularly active men, may be reflecting a synergistic effect that improves lung cancer survival.
Strengths of our study include pre-diagnostic measurement of 25(OH)D and DBP to minimize the potential for reverse causality, a large number of pathology confirmed lung cancer cases, up to 20 years of follow-up, and comprehensive assessment of potential confounders. The ATBC cohort only includes male smokers, which limits the generalizability of our findings to women and non-smokers. The results are likely not confounded by smoking, however, since smoking intensity and duration were not associated with 25(OH)D concentration and inclusion of smoking factors in multivariable models did not change the effect estimates. Treatment information was not available (although baseline vitamin D status should not have differed by subsequent treatment protocols), and cancer stage and histology were not available for all cases; therefore, the histology specific findings that suggest higher 25(OH)D is associated with longer adenocarcinoma and small cell carcinoma survival must be interpreted cautiously given the relatively small numbers of cases. Although stage was strongly associated with survival, it was not correlated with serum 25(OH)D concentration, and the proportion of subjects missing stage information was similar across quartiles of serum 25(OH)D. Therefore, if missing stage data does bias the results, it would act to mask a potential association between serum vitamin D and lung cancer survival. Another potential limitation is that the coefficients of variation for the assays used to measure serum 25(OH)D and DBP are slightly high, which may result in non-differential measurement error that could bias the results towards the null. We did not measure serum albumin, which would have allowed us to estimate free circulating vitamin D using mass action equations. However, given that only about 12% of vitamin D is carried on albumin, and the remaining 88% is carried on DBP, the 25(OH)D:DBP molar ratio is a meaningful way to estimate free circulating vitamin D concentration. Since the molar ratio may be an imperfect estimate, there is the potential for non-differential measurement error that would underestimate the association between free circulating vitamin D and lung cancer survival. Lastly, it is not known if baseline concentration of 25(OH)D and DBP are representative of typical concentrations over time. Circulating DBP concentrations tend to remain fairly stable over time (40) with little seasonal variability (28), but serum 25(OH)D fluctuates by season. However, several cohort studies have shown that individual 25(OH)D concentrations remain fairly stable over time (40–42).
CONCLUSION
In summary, higher serum concentration of 25(OH)D and DBP did not materially influence lung cancer survival in this population of male smokers. This association should be evaluated in other prospective studies, particularly among populations that include women and have higher mean levels of 25(OH)D. Furthermore, the potential differences in the association by histologic subtype suggested by our study should be examined further.
Highlights.
We examined the association between serum 25(OH)D and lung cancer survival.
Pre-diagnostic serum 25(OH)D concentration was measured in 500 lung cancer cases.
Higher serum 25(OH)D concentration was not associated with overall survival.
Higher serum 25(OH)D concentration may prolong survival for some histology types.
Vitamin D binding protein concentration did not modify associations with 25(OH)D.
Acknowledgements
This work was supported in part by the Intramural Research Program of the US National Institutes of Health and the National Cancer Institute. Additionally, this research was supported by US Public Health Service contracts N01-CN-45165, N01-RC-45035, N01-RC-37004, and HHSN261201000006C from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to report.
REFERENCES
- 1.American Cancer Society. Cancer Facts and Figures. 2013 http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2013.
- 2.Porojnicu AC, Robsahm TE, Dahlback A, Berg JP, Christiani D, Bruland OS, et al. Seasonal and geographical variations in lung cancer prognosis in Norway. Does Vitamin D from the sun play a role? Lung Cancer. 2007;55(3):263–270. doi: 10.1016/j.lungcan.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Lim HS, Roychoudhuri R, Peto J, Schwartz G, Baade P, Moller H. Cancer survival is dependent on season of diagnosis and sunlight exposure. Int J Cancer. 2006;119(7):1530–1536. doi: 10.1002/ijc.22052. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa K, Kawaura A, Kato S, Takeda E, Okano T. 1 alpha,25-Dihydroxyvitamin D(3) is a preventive factor in the metastasis of lung cancer. Carcinogenesis. 2005;26(2):429–440. doi: 10.1093/carcin/bgh332. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa K, Sasaki Y, Kato S, Kubodera N, Okano T. 22-Oxa-1alpha,25-dihydroxyvitamin D3 inhibits metastasis and angiogenesis in lung cancer. Carcinogenesis. 2005;26(6):1044–1054. doi: 10.1093/carcin/bgi049. [DOI] [PubMed] [Google Scholar]
- 6.Jiang F, Bao J, Li P, Nicosia SV, Bai W. Induction of ovarian cancer cell apoptosis by 1,25-dihydroxyvitamin D3 through the down-regulation of telomerase. J Biol Chem. 2004;279(51):53213–53221. doi: 10.1074/jbc.M410395200. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toriola AT, Nguyen N, Scheitler-Ring K, Colditz GA. Circulating 25-hydroxyvitamin D levels and prognosis among cancer patients: a systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23(6):917–933. doi: 10.1158/1055-9965.EPI-14-0053. [DOI] [PubMed] [Google Scholar]
- 9.Tretli S, Schwartz GG, Torjesen PA, Robsahm TE. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: a population-based study. Cancer Causes Control. 2012;23(2):363–370. doi: 10.1007/s10552-011-9885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W, Heist RS, Liu G, Asomaning K, Neuberg DS, Hollis BW, et al. Circulating 25-hydroxyvitamin D levels predict survival in early-stage non-small-cell lung cancer patients. J Clin Oncol. 2007;25(5):479–485. doi: 10.1200/JCO.2006.07.5358. [DOI] [PubMed] [Google Scholar]
- 11.Heist RS, Zhou W, Wang Z, Liu G, Neuberg D, Su L, et al. Circulating 25-hydroxyvitamin D, VDR polymorphisms, and survival in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(34):5596–5602. doi: 10.1200/JCO.2008.18.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Chen W, Hu ZB, Xu L, Shu YQ, Pan SY, et al. Plasma Vitamin D Levels And Vitamin D Receptor Polymorphisms Are Associated with Survival of Non-small Cell Lung Cancer. Chin J Cancer Res. 2011;23(1):33–37. doi: 10.1007/s11670-011-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta. 2006;372(1–2):33–42. doi: 10.1016/j.cca.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Chun RF. New perspectives on the vitamin D binding protein. Cell Biochem Funct. 2012;30(6):445–456. doi: 10.1002/cbf.2835. [DOI] [PubMed] [Google Scholar]
- 15.Nagasawa H, Uto Y, Sasaki H, Okamura N, Murakami A, Kubo S, et al. Gc protein (vitamin D-binding protein): Gc genotyping and GcMAF precursor activity. Anticancer Res. 2005;25(6A):3689–3695. [PubMed] [Google Scholar]
- 16.Metcalf JP, Thompson AB, Gossman GL, Nelson KJ, Koyama S, Rennard SI, et al. Gcglobulin functions as a cochemotaxin in the lower respiratory tract. A potential mechanism for lung neutrophil recruitment in cigarette smokers. Am Rev Respir Dis. 1991;143(4 Pt 1):844–849. doi: 10.1164/ajrccm/143.4_Pt_1.844. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein SJ, Stolzenberg-Solomon RZ, Kopp W, Rager H, Virtamo J, Albanes D. Impact of circulating vitamin D binding protein levels on the association between 25-hydroxyvitamin D and pancreatic cancer risk: a nested case-control study. Cancer Res. 2012;72(5):1190–1198. doi: 10.1158/0008-5472.CAN-11-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinstein SJ, Mondul AM, Kopp W, Rager H, Virtamo J, Albanes D. Circulating 25-hydroxyvitamin D, vitamin D-binding protein and risk of prostate cancer. Int J Cancer. 2013;132(12):2940–2947. doi: 10.1002/ijc.27969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondul AM, Weinstein SJ, Virtamo J, Albanes D. Influence of vitamin D binding protein on the association between circulating vitamin D and risk of bladder cancer. Br J Cancer. 2012;107(9):1589–1594. doi: 10.1038/bjc.2012.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mondul AM, Weinstein SJ, Moy KA, Mannisto S, Albanes D. Vitamin D-binding protein, circulating vitamin D and risk of renal cell carcinoma. Int J Cancer. 2014;134(11):2699–2706. doi: 10.1002/ijc.28596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The ATBC Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330(15):1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 22.Pietinen P, Hartman AM, Haapa E, Rasanen L, Haapakoski J, Palmgren J, et al. Reproducibility and validity of dietary assessment instruments I A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol. 1988;128(3):655–666. doi: 10.1093/oxfordjournals.aje.a115013. [DOI] [PubMed] [Google Scholar]
- 23.Weinstein SJ, Yu K, Horst RL, Parisi D, Virtamo J, Albanes D. Serum 25-hydroxyvitamin D and risk of lung cancer in male smokers: a nested case-control study. PLoS One. 2011;6(6):e20796. doi: 10.1371/journal.pone.0020796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korhonen P, Malila N, Pukkala E, Teppo L, Albanes D, Virtamo J. The Finnish Cancer Registry as follow-up source of a large trial cohort--accuracy and delay. Acta Oncol. 2002;41(4):381–388. doi: 10.1080/028418602760169442. [DOI] [PubMed] [Google Scholar]
- 25.Ersfeld DL, Rao DS, Body JJ, Sackrison JL, Jr., Miller AB, Parikh N, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem. 2004;37(10):867–874. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Fears TR, Ziegler RG, Donaldson JL, Falk RT, Hoover RN, Stanczyk FZ, et al. Reproducibility studies and interlaboratory concordance for androgen assays in female plasma. Cancer Epidemiol Biomarkers Prev. 2000;9(4):403–412. [PubMed] [Google Scholar]
- 27.Al-oanzi ZH, Tuck SP, Raj N, Harrop JS, Summers GD, Cook DB, et al. Assessment of vitamin D status in male osteoporosis. Clin Chem. 2006;52(2):248–254. doi: 10.1373/clinchem.2005.059568. [DOI] [PubMed] [Google Scholar]
- 28.Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P. Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J Clin Invest. 1981;67(3):589–596. doi: 10.1172/JCI110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallicchio L, Helzlsouer KJ, Chow WH, Freedman DM, Hankinson SE, Hartge P, et al. Circulating 25-hydroxyvitamin D and the risk of rarer cancers: Design and methods of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):10–20. doi: 10.1093/aje/kwq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner AM, McGowan L, Millen A, Rajesh P, Webster C, Langman G, et al. Circulating DBP level and prognosis in operated lung cancer: an exploration of pathophysiology. Eur Respir J. 2013;41(2):410–416. doi: 10.1183/09031936.00002912. [DOI] [PubMed] [Google Scholar]
- 31.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181(10):7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menezes RJ, Cheney RT, Husain A, Tretiakova M, Loewen G, Johnson CS, et al. Vitamin D receptor expression in normal, premalignant, and malignant human lung tissue. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1104–1110. doi: 10.1158/1055-9965.EPI-07-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SH, Chen G, King AN, Jeon CK, Christensen PJ, Zhao L, et al. Characterization of vitamin D receptor (VDR) in lung adenocarcinoma. Lung Cancer. 2012;77(2):265–271. doi: 10.1016/j.lungcan.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsunawa M, Amano Y, Endo K, Uno S, Sakaki T, Yamada S, et al. The aryl hydrocarbon receptor activator benzo[a]pyrene enhances vitamin D3 catabolism in macrophages. Toxicol Sci. 2009;109(1):50–58. doi: 10.1093/toxsci/kfp044. [DOI] [PubMed] [Google Scholar]
- 35.Scragg R, Camargo CA., Jr Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2008;168(6):577–5786. doi: 10.1093/aje/kwn163. discussion 587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brock K, Cant R, Clemson L, Mason RS, Fraser DR. Effects of diet and exercise on plasma vitamin D (25(OH)D) levels in Vietnamese immigrant elderly in Sydney, Australia. J Steroid Biochem Mol Biol. 2007;103(3–5):786–792. doi: 10.1016/j.jsbmb.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 37.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 38.Arem H, Moore SC, Park Y, Ballard-Barbash R, Hollenbeck A, Leitzmann M, et al. Physical activity and cancer-specific mortality in the NIH-AARP Diet and Health Study cohort. Int J Cancer. 2014;135(2):423–431. doi: 10.1002/ijc.28659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8(3):205–211. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 40.Sonderman JS, Munro HM, Blot WJ, Signorello LB. Reproducibility of serum 25-hydroxyvitamin d and vitamin D-binding protein levels over time in a prospective cohort study of black and white adults. Am J Epidemiol. 2012;176(7):615–621. doi: 10.1093/aje/kws141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofmann JN, Yu K, Horst RL, Hayes RB, Purdue MP. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2010;19(4):927–931. doi: 10.1158/1055-9965.EPI-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171(8):903–908. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]