Abstract
Motivation deficits are common in several disorders including schizophrenia, and are an important factor in both functioning and treatment adherence. Self-Determination Theory (SDT), a leading macro-theory of motivation, has contributed a number of insights into how motivation is impaired in schizophrenia. Nonetheless, self-report measures of motivation appropriate for people with severe mental illness (including those that emphasize SDT) are generally lacking in literature. To fill this gap, we adapted and abbreviated the well-validated General Causality Orientation Scale for use with people with schizophrenia and with other severe mental disorders (GCOS-clinical populations; GCOS-CP). In Study 1, we tested the similarity of our measure to the existing GCOS (using a college sample) and then validated this new measure in a schizophrenia and healthy control sample (Study 2). Results from Study 1 (N=360) indicated that the GCOS-CP was psychometrically similar to the original GCOS and provided good convergent and discriminant validity. In Study 2, the GCOS-CP was given to individuals with (N=44) and without schizophrenia (N=42). In line with both laboratory-based and observer-based research, people with schizophrenia showed lower motivational autonomy and higher impersonal/amotivated orientations. Additional applications of the GCOS-CP are discussed.
Keywords: Self-Determination Theory, Intrinsic motivation, Extrinsic Motivation, Amotivation
1. Introduction
Motivation dysregulation is common in several disorders including schizophrenia, bipolar disorder, and substance dependence (among others), and appears to be critical to functioning and quality of life (e.g., Gard et al., 2009; Johnson, 2005; Nakagami et al., 2008). Recently researchers have begun to investigate the specific mechanisms of motivational impairment in these disorders using basic science research as a guide. Self-Determination Theory (SDT), a leading macrotheory of motivation, helps identify environmental factors and personality tendencies that lead to adaptive or maladaptive motivated behavior (Deci and Ryan, 1985; 2000; Ryan and Deci, 2000), and is elucidating the specific deficits of motivation in schizophrenia, for example (e.g., Choi et al., 2010; Gard et al., 2014).
One component of SDT is Causality Orientation Theory, which describes motivation-based personality tendencies that orient individuals toward specific behavior in ambiguous situations (Deci and Ryan, 1985). Specifically, individuals can be more autonomous, control, or impersonal/amotivated in their motivation orientation. Autonomy oriented individuals tend to be motivated by engagement and inherent interest in activities, especially focusing on how they might be acting as their own agent, or how activities might deepen their experiences. Control oriented individuals are more often motivated by external praise and reward (especially monetary), and also away from punishment or criticism. Finally, individuals who lack opportunities for inherent engagement or reward may develop a more impersonal/amotivated orientation, and tend feel more disengagement with their actions; their behaviors feel as if they do not have a clear impact on the environment. Although there is an overlap with the autonomous orientation and what is often referred to as ‘intrinsic motivation’, as well as control orientation and ‘extrinsic motivation,’ the personality orientations described in SDT do not completely align with these forms of motivation. Rather the motivation orientations represent the tendency that an individual will interpret their behavior and the environment as more autonomous, control, or impersonal/amotivated. Naturally, individuals who tend to interpret ambiguous stimuli as potentially deepening their experience or self-expression are more likely to experience more intrinsic motivation, and individuals who tend to see ambiguous situations as involving control will be more extrinsically motivated by reward or away from punishment. Conversely, individuals that spend much of their time in environments that give them little opportunity to develop agency or self-expression, or that lack opportunities for reward are more likely to have lower levels of autonomy and a higher level of impersonal/amotivated orientation. In other words, both the nature of the environment as well as personality orientations are likely to have an effect on motivated behavior (e.g., Deci & Ryan, 2000).
1.1. Autonomy orientation
Although we are unaware of any research looking directly at self-reported autonomy motivation in schizophrenia, recent research has indicated that people with schizophrenia have lower levels of intrinsically motivated behavior, relative to healthy individuals (e.g., Choi et al., 2010; Medalia and Brekke, 2010). For example, in one study, people with schizophrenia showed significantly less intrinsic motivation to complete a cognitive task than healthy individuals, and this rating was connected to engagement in the task itself (Choi et al., 2010). In line with these findings we recently completed an Ecological Momentary Assessment (EMA) study which indicated that people with schizophrenia are also less likely than healthy individuals to set goals related to autonomy and competence in their daily lives (Gard et al., 2014). Finally, many studies have indicated that lower levels of intrinsic motivation are linked to key constructs such as neurocognition, social cognition, occupational functioning, and overall functioning in schizophrenia (e.g., Fervaha et al., 2014; Gard et al., 2009; Nakagami et al., 2008; Saperstein et al., 2011). Thus, intrinsic motivation appears to be a crucial area of impairment in schizophrenia.
In spite of this, there is a dearth of assessment measures of motivation for people with schizophrenia, or for individuals with severe mental illness. Some self-report measures of motivation, especially intrinsic motivation, have been utilized with mixed results. For example, Barch and colleagues (2008) found that people with schizophrenia did not differ from participants without schizophrenia in two intrinsic motivation domains, as measured by the Motivational Trait Questionnaire (MTQ, Heggestad and Kanfer, 2000). One possible reason for the lack of differences between individuals with schizophrenia and individuals without schizophrenia on intrinsic motivation may be because the MTQ is designed for use with relatively high functioning individuals (e.g., “It is important for me to outperform my co-workers.”). The lack of differences between individuals with schizophrenia and individuals without schizophrenia on intrinsic motivation may be because those with schizophrenia may not be relying on their own experiences when responding but, rather, without having experiential memories for a given item, may be responding how they believe they should answer (e.g., Robinson and Clore, 2002). The Intrinsic Motivation Inventory for Schizophrenia Research (IMI-SR), on the other hand, which measures the intrinsic motivation to complete a specific task (such as performing a cognitive battery), has indicated lower levels of intrinsic motivation in people with schizophrenia (Choi et al., 2010). This scale has clear utility in assessing something akin to the activation of an autonomous orientation in a specific task, but is designed for use with a specific task, and not on general motivated behavior. To summarize, lower levels of intrinsic motivation seen in research in schizophrenia indicates that people with schizophrenia would most likely report lower levels of trait autonomy motivation than individuals without schizophrenia, although this has not been directly tested to date.
1.2. Control orientation
The control orientation of SDT emphasizes approach towards rewards and approval, and avoidance of punishment or criticism (Deci and Ryan, 2000). In schizophrenia, there has been less emphasis on this research area (Silverstein, 2010), and thus far the evidence for or against impairment has been mixed. Indirect evidence, such as response to token economies and laboratory tasks, have shown that extrinsic motivators (i.e., monetary reinforcement) increase engagement in treatment and specific tasks for people with schizophrenia (e.g., Dickerson et al., 2005; Kern et al., 1995; Penn and Combs, 2000; Summerfelt et al., 1991). In contrast, research on ‘reward representation’ has shown that individuals with schizophrenia appear to have difficulties engaging in goal-directed behavior when a reward is not present (e.g., Gard et al., 2007; Heerey and Gold, 2007). Using EMA, in the study described above, we found that people with schizophrenia set goals that were motivated less by extrinsic reward than individuals without schizophrenia (Gard et al., 2014). However, there were no differences between people with and without schizophrenia on setting goals to avoid punishment or criticism. Beyond this EMA study, most of the work on punishment in schizophrenia has focused on monetary loss (e.g., Waltz et al., 2013), which differs from avoidance of criticism and punishment as defined by SDT (Deci and Ryan, 2000). Furthermore, to our knowledge there is no study focusing on the self-report of extrinsic motivation or specifically the control orientation in schizophrenia. Thus, it is currently unclear whether individuals with schizophrenia differ in terms of control orientation.
1.3. Impersonal/amotivated orientation
The impersonal/amotivated orientation in SDT is characterized by individuals who believe that they do not have agency in affecting outcomes and, therefore, want things to remain as they are; these individuals are likely to be amotivated and disengaged from goal-directed behavior. Deci and Ryan highlight that this orientation tends to develop when there are few opportunities in the environment for autonomy, or where there are few rewarding stimuli (e.g., Deci and Ryan, 2000). This orientation appears to be akin to the negative symptom amotivation/avolition (American Psychiatric Association, 2013). The literature has long characterized amotivation as one of the core negative symptoms of schizophrenia (e.g., Bleuler, 1950) and noted its relationship to functional outcome (e.g., Blanchard et al., 1998; Ho et al., 1998). Apathy, defined as “a lack of motivation that is not attributable to diminished level of consciousness, cognitive impairment, or emotional distress,” is one possible construct related to the impersonal/amotivated orientation (Marin, 1990; 1991). Research has indicated that apathy is higher in individuals with schizophrenia than in healthy comparison individuals and related to poorer functional outcomes in people with schizophrenia (Kiang et al., 2003). Given the centrality of amotivation in schizophrenia, as well as the research findings on apathy, it would seem likely that people with schizophrenia would report higher levels of the impersonal/amotivated orientation.
1.4. Assessing motivation orientations
One often-used scale to assess general motivation orientation in the general population is the General Causality Orientation Scale (GCOS; Deci and Ryan, 1985). The GCOS consists of 17 different vignettes and asks participants to rate each of the three examples of how they might think in response to each vignette, one ‘thought’ for each motivation orientation, totaling 51 responses. These responses are averaged for each motivation orientation – autonomy, control, impersonal/amotivated. The GCOS shows utility in a variety of contexts including why a person engages in exercise (Rose et al., 2001; Vancampfort et al., 2013), the link between exercise and well-being in older adults (Solberg, Halvari, and Ommundsen, 2013), in understanding conflict in romantic relationships (Knee, Porter, and Rodriguez, 2014), in general problem solving (Keatley, Clarke, and Hagger, 2013), and in workplace compliance (Wall, Palvia, and Lowry, 2013), to name a few. To our knowledge, two studies have used the GCOS to assess motivation in clinical populations, one of men and women with anorexia-nervosa as compared to a healthy comparison group (Strauss and Ryan, 1987) and, more recently, with individuals experiencing their first psychotic episode (completed without a healthy comparison group) (Breitborde, Kleinlein, and Srihari, 2013). Thus far, however, no studies have used the GCOS in populations of individuals with severe, chronic mental illness, where community functioning may be more severely impaired, such as in chronic schizophrenia. This may be due to the fact that the GCOS, like many vignette-based measures, assumes that responders are functioning well (e.g., one vignette begins: “You are embarking on a new career…”), and therefore may not tap into areas of life and functioning that are applicable to individuals who have more functioning difficulties. In addition the 51-item GCOS measure can be labor intensive for participants.
Therefore, in Study 1, we altered the wording, removed redundant items of the original GCOS, and administered the new and original scale to a large sample of undergraduates along with convergent and discriminant measures. We then tested whether the new abbreviated and adapted measure was reliable, formed an expected three-factor structure, and was similarly related to existing measures. We hypothesized that this new scale, the General Causality Orientation Scale for Clinical Populations (GCOS-CP), would be psychometrically similar to the original GCOS. In Study 2, we administered the GCOS-CP to people with and without chronic schizophrenia, and in line with previous research we hypothesized that people with schizophrenia would report lower levels of the autonomy orientation and higher levels of the impersonal/amotivated orientation relative to people without schizophrenia.
2. Method of Study 1
2.1. Participants
A diverse group of undergraduate university students (N = 360) participated in this study for extra course credit. Participants were given the option to do an alternate assignment for extra credit if they did not want to participate in this study. See Table 1 for demographic information.
Table 1.
Study 1 participant demographics.
| Overall Sample (N = 360) |
|
|---|---|
| Demographics | |
| Male, n (%) | 83 (23.10) |
| Age (years), M (SD) | 21.76 (6.55) |
| Ethnicity, n (%) | |
| Caucasian | 116 (31.10) |
| Asian-American | 110 (27.10) |
| Latino | 52 (13.90) |
| African-American | 17 (4.60) |
| Multi-Racial/Other | 78 (20.90) |
2.2. Scale adaptation
Alterations to the scale included adaptations to wording within the vignettes to be more applicable to a schizophrenia patient population (e.g., “You are embarking on a new career. You are most likely to think:” changed to be more applicable to a patient population to: “You are beginning a new hobby. An important consideration will likely be:”).
After each vignette, there are three prompts, one for each motivation orientation, and the participant is to respond to a 7-point Likert scale indicating how likely they are to have a specific thought (e.g., continued from the above vignette: “If you will be any good at the hobby” (Impersonal), “How interested you are in the hobby” (Autonomy), “If people will criticize your work” (Control). Following administration, scores are summed, with higher scores indicating higher propensity to be oriented in a given area.
2.3. Motivational orientation
The General Causality Orientation Scale (GCOS; Deci and Ryan, 1985) is a widely used, well-validated measurement of motivation orientation. This 51-item scale is structured through 17 vignettes requesting 3 responses to each scenario. Responses to vignettes are given on a 7-point Likert scale for each motivation orientation: autonomy, control, impersonal/amotivated. The General Causality Orientation Scale for Clinical Populations as administered, maintained the original 17 vignettes of the GCOS but adapted as described above. See online supplement for the final GCOS-CP scale.
2.4. Additional scales administered – convergent and discriminant measures
The scales we chose for construct validity were based on their similarity to Causality Orientation Theory (Motivational Trait Questionnaire (MTQ), Heggestad and Kanfer, 2000; Intrinsic Motivation Inventory (IMI), Ryan, 1982). Typically, the IMI is administered with a specific task and is aimed at assessing intrinsic motivation with regard to that reference task. For our purposes, participants were asked to “think about an important task [they] recently completed.” And to “indicate how true each statement is for [them] with regard to that task.” Other scales were chosen to assess construct validity due to their nature of measuring how one behaves in response to exogenous influences (Behavioral Inhibition/Activation Scale (BIS/BAS), Carver and White, 1994; Regulatory Focus Questionnaire (RFQ), Higgins et al., 2001). For discriminant validity we chose a questionnaire assessing social desirability, (Marlow-Crowne-A (MC-A), Reynolds, 1982).
2.5. Procedures
Participants completed questionnaires online through an industry-standard survey website (qualtrics.com). All participants agreed to implied consent and were then given either the GCOS or the GCOS-CP, with the other measure completed after the additional convergent/discriminant measures. As with many survey studies, participants did not necessarily respond to every question presented over the course of the survey and, therefore, results include varying participant numbers reflecting those that responded to questions being analyzed for each respective scale.
3. Results for Study 1
3.1. Inter-correlations, alpha, and factor analysis
Initially, responses to the adapted GCOS-CP 17 vignettes were analyzed. Items with less than a .30 item-total scale correlation were removed (Cronbach, 1951), as were question redundancies (e.g., “You have a school-aged daughter. On parents’ night, the teacher tells you that your daughter has been doing poorly and doesn’t seem involved in the work.” and “Your friend’s younger sister is a freshman in college. Your friend says she has been doing badly and asks you what he (she) should do about it.”). In total, 9 vignettes were eliminated, leaving a total of 8 vignettes. Internal consistency, (Cronbach’s α), for the remaining 24 items (8 vignettes with 3 orientation items per vignette) of the new GCOS-CP was acceptable for each subscale (αAutonomy =.74, αControl =.65, and αImpersonal = .67), which were comparable to the original published 51-item GCOS (αAutonomy =.74, αControl =.69, αImpersonal =.74; Deci and Ryan, 1985).
We then conducted a principal-components factor analysis, using varimax rotation, on the original GCOS measure and the GCOS-CP with the current sample. For the original GCOS, three factors explained approximately 34% of the variance and had eigenvalues greater than 1.0. These loaded onto the intended factors at values greater than .20, though several items had higher cross-loadings on unintended factors.
For the new GCOS-CP scale, a principal-components factor analysis using varimax rotation was also conducted, which resulted in 3 factors, explaining approximately 37% of the variance and eigenvalues greater than 1.0. Although some items did have cross-loadings, each item loaded highest on its intended factor. The factor-loading matrix for the GCOS-CP is listed in Table 2.
Table 2.
Factor loadings based on principle components analysis with varimax rotation for the 8 items of the General Causality Orientation Scale for Clinical Populations (GCOS-CP) from Study 1.
| GCOS-CP Item | Autonomy | Impersonal | Control |
|---|---|---|---|
| Autonomy Factor | |||
| 4b. How interested you are in the hobby. | .678 | ||
| 3c. Seek participation: get inputs from others who want to make decisions before you make the final call. | .671 | ||
| 5b. Ask her what’s been going on, and offer to help resolve it. | .639 | ||
| 7a. Share your observations with him (her) and try to find out what is going on for him (her). | .615 | −.404 | |
| 8a. Find an opportunity to explain why it bothers you; your friend or roommate may not even realize how much it is bothering you. | .591 | −.391 | .284 |
| 2b. Each make suggestions and then decide together on something that you both feel like doing. | .576 | −.211 | |
| 1a. Simply assigning times that each can break to avoid any problems. | .516 | ||
| 6c. Try to understand why your friend or roommate does it and why it is so upsetting for you. | .424 | ||
| Internal consistency (α) = .734 | |||
| Impersonal Factor | |||
| 8b. Say nothing; if your friend or roommate cared about you she would understand how you felt. | −.406 | .622 | |
| 5c. It’s hard to know what to do. | .607 | ||
| 7b. Ignore it because there’s not much you can do about it anyway. | −.456 | .588 | |
| 6b. Avoid your friend or roommate when he does it. | .587 | ||
| 3b. Follow precedent: you’re not really up to the task so you’d do it the way it’s been done before. | .568 | ||
| 2a. Leave it up to your friend/roommate; he/she probably wouldn’t want to do what you’d suggest. | .459 | ||
| 4a. If you will be any good at the hobby. | .249 | .424 | .323 |
| 1b. Find out from someone in authority what to do, or do what has been done in the past. | .233 | ||
| Internal consistency (α) = .666 | |||
| Control Factor | |||
| 8c. Demand that your friend or roommate start being more considerate; otherwise you’ll respond similarly. | −.234 | .261 | .675 |
| 6b. Point it out each time you notice it so you can have a better friendship. | −.247 | .611 | |
| 7b. Tell him (her) that you’re willing to spend time together only if he (she) makes more of an effort. | .608 | ||
| 2a. Talk your friend into doing what you want to do because you’ll both have fun. | .566 | ||
| 5c. Tell her she has not been as helpful lately. | .548 | ||
| 3b. Take charge: you don’t have to deal with everyone’s opinions that way. | .447 | ||
| 4a. If people will criticize your work. | .422 | ||
| 1b. Find out from someone in authority what to do, or do what has been done in the past. | .321 | ||
| Internal consistency (α) = .651 |
To assess similarity in responses for the GCOS-CP (n=314) and GCOS (n=306), we computed a Pearson’s correlation coefficient for each of the subscales. Results show that the GCOS-CP and GCOS are significantly related in each of the subscales (rAutonomy(300)=.66, p<.001; rControl(300)=.60, p<.001; rImpersonal(300)=.66, p<.001), suggesting that how one responds on the original GCOS scale is in line with how one responds on the newly adapted GCOS-CP scale (see Table 3).
Table 3.
Summary of Correlations for GCOS-CP and GCOS with Construct Validity Scales from Study 1.
| GCOS: Autonomy |
GCOS: Control |
GCOS: Impersonal |
GCOS-CP: Autonomy |
GCOS-CP: Control |
GCOS-CP: Impersonal |
|
|---|---|---|---|---|---|---|
| GCOS: Autonomy | - | - | - | .67*** | −.01 | −.32*** |
| GCOS: Control | - | - | - | .13* | .62*** | .08 |
| GCOS: Impersonal | - | - | - | −.26*** | .27*** | .67*** |
| MTQ: Personal Mastery | .32** | .01 | −.38** | .36*** | −.08 | −.39*** |
| MTQ: Competitiveness | −.09 | .30** | .15** | −.08 | .31*** | .13* |
| MTQ: Motivation Anxiety | −.03 | .13* | .52** | −.15* | .16** | .43*** |
| Chapman Social Scale | −.36** | −.08 | .14* | −.42*** | −.04 | .32*** |
| TEPS: Anticipatory | .30** | .25** | −.01 | .38*** | .18** | −.08 |
| TEPS: Consummatory | .31** | .06 | −.10 | .35*** | .05 | −.15* |
| PANAS-X: Positive Affect | .24** | .13* | −.24** | .28*** | .10 | −.28*** |
| PANAS-X: Negative Affect | −.16** | .04 | .37*** | −.26*** | .09 | .37*** |
| IMI: Interest/ Enjoyment | .10 | .04 | −.13* | .20*** | .02 | .67*** |
| IMI: Perceived Competence | .30*** | .13* | −.15** | .30*** | .01 | −.24*** |
| IMI: Perceived Choice | .07 | −.04 | −.16** | .16** | −.06 | −.15** |
| IMI: Pressure/Tension | −.06 | −.01 | .19** | −.10 | .10 | .17** |
| RFQ: Promotion | .33** | .02 | −.41** | .38*** | −.05 | −.41*** |
| RFQ: Prevention | .09 | −.16** | −.12* | .17** | −.14* | −.12* |
| BIS/BAS: Inhibition | .04 | .09 | .13* | .07 | .12* | .17** |
| BIS/BAS: Reward Representation | .23** | .12* | −.02 | .23*** | .01 | .01 |
| BIS/BAS: Drive | .15** | .08 | −.15** | .18** | .01 | −.14* |
| BIS/BAS: Fun Seeking | .20** | .12* | .19** | .13* | .03 | .13* |
| Big 5: Openness | .18** | .25** | .098 | .15* | .15* | .04 |
| Big 5: Conscientiousness | −.01 | .12* | .35** | −.21*** | .02 | .27*** |
| Big 5: Extraversion | −.35** | .04 | .10 | −.35*** | .09 | .24*** |
| Big 5: Agreeableness | −.07 | .08 | .29** | −.13* | .11 | .35*** |
| Big 5: Neuroticism | .22** | −.03 | −.17** | .18** | −.13* | −.19** |
| MC-A | −.08 | −.11 | −.16 | .03 | −.07 | −.12 |
Note.
= p <.05,
= p<.01,
= p<.001;
GCOS-CP = General Causality Orientation Scale for Clinical Populations, GCOS = General Causality Orientation Scale, MTQ = Motivational Trait Questionnaire, Chapman Social Scale = The Revised Social Anhedonia Scale, TEPS = Temporal Experience of Pleasure Scale, PANAS-X = Positive and Negative Affect Scale, IMI = Intrinsic Motivation Inventory, RFQ = Regulatory Focus Questionnaire, BIS/BAS = Behavioral Inhibition and Behavioral Activation Scale, Big 5 = Big Five Personality Scale, MC-A = Marlow Crowne Scale – Short Form A.
3.2. Convergent and discriminant validity analyses
3.2.1. Convergent validity
Pearson’s bivariate correlations were performed to determine convergent validity; see Table 3 for complete results. All subscales of the GCOS-CP appear to be in line with similar measures in a college sample.
As predicted the GCOS-CP autonomy orientation showed positive correlations with subscales designed to specifically assess intrinsic motivation. For example, the MTQ-Personal Mastery subscale correlated with GCOS-CP autonomy, r(307)=.31, p<.001, as well as the following IMI subscales: IMI-Interest/Enjoyment, r(302)=.12, p=.02; IMI-Perceived Competence, r(302)=.30, p<.001; IMI-Perceived Choice, r(302)=.14, p=.01. The GCOS-CP autonomy orientation was positively related, as hypothesized, to constructs tapping aspects of intrinsic motivation: RFQ-Promotion, r(299)=.31, p<.001; BAS-Reward Representation, r(300)=.22, p<.001; BAS-Drive, r(300)=.19, p=.001; Big 5-Openness, r(290)=.22, p<.001; Big 5-Extraversion, r(290)=.27, p<.001. An inverse relationship between the GCOS-CP autonomy orientation and MTQ-Motivation Anxiety was found, r(307)=−.11, p=.05. This relationship was not hypothesized, but is understandable as this subscales measure aspects of amotivation that are often inversely related to intrinsic motivation.
For the GCOS-CP control orientation a positive correlation was found with the MTQ-Competitiveness, which focuses on seeking competition and measuring one’s abilities in reference to others, r(307)=.29, p<.001. Additionally, other scales were related to the GCOS-CP controlled orientation in the manner expected: RFQ-Prevention, r(299)=−.14, p<.01; Big 5-Extraversion, r(289)=.17, p<.01.
Finally, the GCOS-CP impersonal/amotivated scale was correlated positively with the MTQ-Motivation Anxiety, r(307)=.42, p<.001. Additionally, the following anticipated positive correlations were found: BIS-Inhibition, r(300)=.13, p=.03; IMI-Pressure/Tension, r(302)=.12, p=.04. Inversely related to GCOS-CP impersonal orientation were, not surprisingly, scales positively correlated with intrinsic motivation MTQ-Personal Mastery, r(307)=−.29, p<.001; BAS-Drive, r(300)=−.11, p=.05; IMI-Interest/Enjoyment, r(302)=−.11, p<.05; IMI-Perceived Competence, r(302)=−.17, p=.003; IMI-Perceived Choice, r(302)=−.18, p=.002; RFQ-Promotion, r(299)=−.30, p<.001.
3.2.2. Discriminant validity
Participants completed the MC Social Desirability Scale (Crowne & Marlow, 1964) to assess whether any of the GCOS-CP orientation scales were inadvertently measuring one’s desire to ‘look good’. As expected, the MC social desirability scale was unrelated to all subscales of the GCOS-CP, rAutonomy(272)=−.10, p=.10; rControl(272)=.02, p=.79; rImpersonal(272)=.08, p=.20.
4. Discussion of Study 1
In this study the (adapted) GCOS-CP was very similar to the original GCOS in measuring motivation orientation, and the GCOS-CP showed a three-factor solution that corresponded to the three motivation orientations. In addition, the GCOS-CP showed both convergent (MTQ and IMI) and discriminant (MC) validity with other validated self-report measures. The IMI is typically used with a consistent task as the reference, rather than having a participant think about a recent important task; nonetheless, we found that the IMI subscales correlated with the GCOS-CP autonomy subscale as expected, lending some evidence for this type of use with the IMI. The GCOS-CP and GCOS were also positively correlated as expected and showed similar levels of internal consistency, indicating that there are few psychometric differences with the adapted measure. In sum, the GCOS-CP appears to be psychometrically similar to the original scale and, given the altered content to be more in line with individuals who may have lower levels of functioning, now has the potential for use as a brief instrument that can assess motivation orientation in populations with severe mental illness.
5. Validation of the GCOS-CP in a sample of people with schizophrenia: Study 2
In a separate study we administered the adapted GCOS-CP to participants both with and without schizophrenia and, based on previous research, hypothesized that people with schizophrenia would report lower levels of autonomy orientation and higher levels of impersonal/amotivated orientation relative to people without schizophrenia. Given the mixed evidence for an extrinsic motivation deficit in schizophrenia (e.g., Dickerson et al., 2005; Gard et al., 2014; Kern et al., 1995; Penn and Combs, 2000; Summerfelt et al., 1991), we were uncertain if people with schizophrenia would differ in the control orientation. Finally, we investigated whether the GCOS-CP motivation orientations were related to symptoms or functioning in our patient sample. To assess this, we compared the GCOS-CP orientations to patient symptoms using the Positive and Negative Syndrome Scale (PANSS; Kay and Sevy, 1990; Poole et al., 2000) and the abbreviated Quality of Life Scale for functioning (QLS; Bilker et al., 2003).
6. Method for Study 2
6.1. Participants
Forty-four clinically stable outpatients with schizophrenia (n= 28) or schizoaffective disorder (n= 16) and forty-two healthy control individuals participated in the study. Schizophrenia participants were recruited from two larger studies on motivation or cognitive impairment in schizophrenia, and were run in the present study as part of the baseline assessment battery, prior to their engagement in larger studies. Diagnoses, or lack of, for both groups were confirmed using the DSM-IV-Clinician Version (SCID: First et al., 1997). Exclusion criteria for all participants included a history of head trauma/loss of consciousness, neurological disorders, and non-fluency in English. Patients were excluded if there were significant changes in medication or in dosage in the previous 30 days, or hospitalization in the previous three months. Healthy comparison participants were recruited through community postings and bulletin boards, and were excluded if they met criteria for any Axis I disorder on the SCID. See Table 4 for participant characteristics and demographics.
Table 4.
Study 2 demographics for participants with schizophrenia and participants without schizophrenia. There were no significant differences between groups on demographic measures.
| People with Schizophrenia (n = 44) |
Healthy Controls (n = 42) |
|
|---|---|---|
| Demographics | ||
| Male, n (%) | 32 (74.4) | 27 (62.80) |
| Age (years), M (SD) | 38.62 (14.13) | 36.83 (15.07) |
| Education, M (SD) | 13.91 (2.66) | 14.17 (1.85) |
| Parental Education, M (SD) | 13.76 (2.91) | 14.25 (3.11) |
| Race, n (%) | ||
| Caucasian | 16 (36.36) | 22 (51.22) |
| Asian-American | 9 (20.45) | 8 (19.51) |
| Mexican-American / Other Latino | 7 (15.91) | 5 (12.2) |
| Multi-racial | 6 (13.64) | 2 (4.70) |
| African-American | 6 (13.64) | 5 (11.6) |
| Other | - | 1 (2.30) |
| Diagnosis, n (%) | ||
| Schizophrenia | 28 (63.64) | - |
| Schizoaffective Disorder | 16 (36.36) | - |
| Chlorpromazine Equiv. (M, SD) | 448.54 (606.28) | - |
| PANSS Positive, M (SD) | 14.91 (4.52) | - |
| PANSS Negative, M (SD) | 16.7 (5.3) | - |
6.2. Procedures
Participants were administered the GCOS-CP, and in a separate appointment, individuals with schizophrenia were given the PANSS (Kay and Sevy, 1990; Poole et al., 2000) and the abbreviated QLS (Bilker et al., 2003).
6.3. Data analyses
Paired samples t-tests were conducted to assess within-group comparisons. Analysis of variance models (ANOVAs) were conducted for between-group analyses, and Cohen’s d were computed to report effect size. Finally, Pearson’s bivariate correlations were conducted to assess relationships between GCOS-CP orientations and clinical outcome measures.
7. Results for Study 2
7.1. Within- and between-group differences
We first assessed within-group comparisons of motivation orientation in order to test whether one motivation orientation predominated for each participant group. People with schizophrenia were significantly more oriented toward autonomy than the control orientation, t(43)=9.21, p<.001, and were more oriented toward the autonomy orientation than the impersonal/amotivated orientation, t(43)=8.01, p<.001. This same pattern existed in people without schizophrenia; with higher levels of the autonomy orientation than the control orientation, t(41)=10.98, p<.001, and more autonomy than the impersonal/amotivated orientation, t(41)=12.82, p<.001. For the comparison of control versus impersonal orientations, people with schizophrenia were similarly oriented toward the control and impersonal orientations, t(43)=0.89, p= 38, whereas people without schizophrenia were significantly more oriented toward the control than impersonal, t(41)=3.16, p=.003), Thus, both groups endorsed the autonomous orientation over other motivation orientations, but there were group differences in whether control or impersonal orientation was relatively higher.
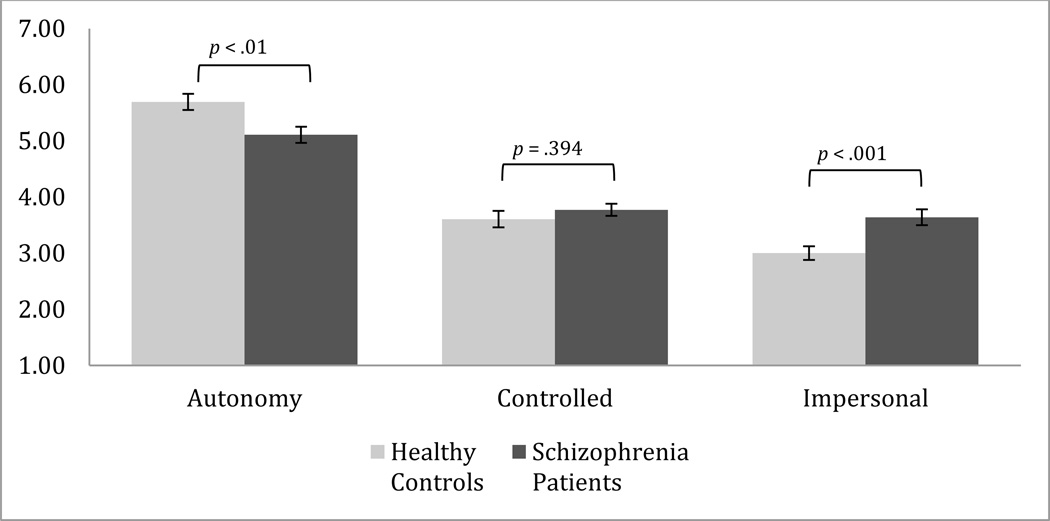
In terms of between group differences, and in line with previous research, people with schizophrenia reported a lower autonomy orientation, F(1,84)=7.89, p=.006, d=.63, and higher impersonal/amotivated orientation, F(1,84)=11.25, p<.001, d=.77, relative to people without schizophrenia. There were no differences between the groups on the control orientation, F(1,84)=0.73, p=.39 (see Figure 1).
Figure 1.
People with schizophrenia (n=44) report lower levels of autonomy and higher levels of impersonal motivation relative to individuals without schizophrenia (n=42).
7.2. Clinical sample inter-correlations and internal consistency
Internal consistency (Cronbach’s α) of the GCOS-CP was acceptable for each subscale (αAutonomy =.77, αControl =.52, and αImpersonal = .57). For schizophrenia patients, Pearson’s correlations were conducted comparing GCOS-CP subscales to one another. No significant correlations are found when comparing the autonomy and controlled orientations, r(44)=.267, p=.08), when comparing the autonomy and impersonal/amotivated orientations, r(44)=.101, p=.52), or when comparing the controlled and impersonal orientation, r(44)=.274, p=.07.
7.3. Schizophrenia participant correlations with motivation orientation
PANSS positive and negative symptom averages were not related to any GCOS-CP subscales. The QLS functioning scale was related to GCOS-CP autonomy, r(39)=.36, p=.03, but unrelated to the other GCOS-CP subscales, rControl(39)= −0.12, p=.94; rImpersonal(39)= −.16, p=.35.
To better understand the relationship of patient motivation orientations to symptoms and functioning we looked at the patient data in two ways: 1) we assessed patients who scored above versus below the autonomy scale median for control participants, and 2) we assessed patients who scored above versus below the Impersonal scale median for control participants.
When considering the GCOS-CP autonomy scale, as expected, we find 33 patients who were less autonomously oriented than the median and 11 patients who reported that they were more autonomously oriented than the median. For those who below the median, lower autonomy orientation was found to be significantly correlated with functioning as measured by the Quality of Life Scale, r(28)=.486, p=.009. Those individuals above the median, higher autonomy orientation was found to be negatively correlated at trend level to PANSS positive symptoms, r(9)=−.620, p=.08. For those who are generally autonomously motivated, higher levels of concurrent amotivation were related at trend level to PANSS negative symptoms, r(9)=.602, p=.09. These data suggest that individuals with schizophrenia who are particularly autonomously motivated may experience fewer positive psychotic symptoms and function better than those who are less autonomously motivated, but when they are concurrently amotivated, they may have increased negative symptoms.
When considering the GCOS-CP impersonal scale, we find 10 patients who were less impersonally oriented than the median and 34 patients who were more impersonally oriented than the median, as expected. Those who were below the median showed a trend level negative correlation between GCOS-CP amotivation and functioning on the Quality of Life Scale, r(8)=−.630, p=.09. Individuals who were more impersonally oriented than the control median showed a trend level negative correlation between GCOS-CP autonomy and PANSS positive symptoms, r(29)=−.326, p=.09, and a trend level positive correlation between GCOS-CP autonomy and functioning on the Quality of Life Scale, r(29)=.327, p=.08. Although the data are preliminary (and need replication in a larger sample) they appear to indicate that even for patients who have less amotivation, their level of amotivation may be related to their level of functioning.
8. Discussion for Study 2
This is the first study to our knowledge of motivation orientations with a sample of individuals with severe mental illness and a healthy comparison group. In this study we found that people with schizophrenia, similarly to people without schizophrenia, report being more autonomy oriented than control or impersonal/amotivated oriented. That is, both those with and without schizophrenia, report that the inherent value in an activity drives their motivation over other factors such as an external reward or punishment, or perceiving events as being uncontrollable. This finding is important, in that it highlights that individuals with schizophrenia do not, as a group, have across the board difficulties with agency, or completely lack a drive towards inherent enjoyment in motivated behavior. Instead people with schizophrenia appear to have relative deficits in autonomy when compared to individuals without schizophrenia. Thus, psychosocial treatment providers may wish to leverage existing experiences of autonomy to deepen and broaden connections to activities and behaviors that they currently have.
Our findings of decreased autonomy motivation in schizophrenia relative to healthy individuals are in line with results showing decreased intrinsic motivation in patients (Choi et al., 2010; Gard et al., in press). The results of the present study expand those findings by highlighting that people with schizophrenia appear to have some difficulty orienting their behavior towards self-expression and agency when faced with ambiguous situations. The findings here are also in line with research indicating that people with a first-episode of psychosis demonstrate lower autonomy orientation and higher impersonal/amotivated orientation than the general population (Breitborde, Kleinlein, and Srihari, 2013). In that study and the present study the level of autonomy orientation was significantly correlated with functioning, further indicating that agentic behavior may be an important psychosocial treatment target and gauge of treatment impact. Indeed, previous research has shown that an individual’s intrinsic motivation is an important component of successful rehabilitation (Medalia and Brekke, 2010; Nakagami, 2010), and people with schizophrenia demonstrate a relationship between low intrinsic motivation levels and lower functioning (Gard et al., 2009; Nakagami et al., 2008). Thus, the use of the GCOS-CP may offer important clinical information for treatment providers.
In addition to lower levels of autonomy, people with schizophrenia reported higher levels of impersonal/amotivated orientation. Surprisingly the impersonal/amotivation orientation was unrelated to overall patient functioning. This lack of a correlation between amotivation/impersonal orientation and functioning is in contrast to other studies that have looked at patient goals, and the symptom of apathy (e.g., Gard et al., 2014; Kiang et al., 2003). One possible explanation may be due to measurement differences. For example, in our previous EMA study we found a relationship between goals that were ‘amotivated,’ defined as setting disengaged goals or goals set primarily to ‘pass the time.’ This coding of activities and goals may differ from how people with schizophrenia self-report to ambiguous behavioral vignettes. Other research involving ‘apathy’ has indicated a relationship with functioning, although apathy tends to differ from impersonal/amotivation orientation in that it focuses on disinterest (Kiang et al., 2003), rather than a lack of agency in affecting outcomes. It is possible that patients’ self-report of amotivation due to agency is less related to functioning than the self-report of amotivation/apathy due to disinterest. Further research may wish to test whether the expression of disinterest rather than an impersonal/amotivation orientation is indeed more connected to functioning deficits. In an effort to understand motivation and patient functioning, we split the patient group to assess those who were more versus less amotivation and those who were more versus less autonomy oriented. Overall, the data indicate that those individuals with schizophrenia who are more amotivated may also have poorer functioning and those who are more autonomous orientation may have better functioning and may experience fewer positive psychotic symptoms. These finding suggest that the GCOS-CP may be a helpful tool to identify subgroups of amotivated patients.
Interestingly, there were no differences between individuals with or without schizophrenia on the control orientation. This is in contrast to recent work showing that people with schizophrenia tend to set goals that are less extrinsically rewarding (Gard et al., 2014). In that study, however, there were no differences between groups on goals that were set to avoid extrinsically negative (i.e., punishing) outcomes. Unfortunately, the GCOS-CP does not separate the tendency to approach an extrinsic reward from a tendency to avoid punishment. Thus, it may be that the lack of differences seen in the control orientation here is due to the conflation of these items in the GCOS/GCOS-CP.
It is also somewhat surprising that the GCOS-CP orientations were unrelated to negative symptoms in schizophrenia. However, a lack of a relationship between self-reported motivation and negative symptoms is more common than not. In fact three studies using self-report measures similar to this study also do not find a relationship between motivation and negative symptoms in schizophrenia (Barch et al., 2008; Breitborde, Kleinlein, and Srihari, 2013; Choi et al., 2010). One possibility is that a lack of a significant relationship could be due to patient self-report of trait behavior as it correlates to observer ratings of negative symptoms. In other words, it may be that patient report of impersonal/amotivated orientation is less predictive of patient outcomes than the autonomy orientation (which was related to functioning).
This study has a number of limitations. For one, the cross-sectional design provides only a snapshot of motivational orientation at a given moment, thus limiting our ability to see any fluctuations of motivation orientation change, or whether external influences (such as symptom change or stressors) influence motivation orientation. In addition, self-report measures of motivation orientation (or other constructs) are limited to some degree by the participant’s insight into their own behavior. While the GCOS/GCOS-CP avoids broad statements about personality traits about oneself, and instead focuses on reactions to vignettes, patients still must accurately report the thoughts that they might have in these situations. While our relationship between autonomy orientation and functioning provides some indication of the usefulness of this measure, future research may wish to add a behavioral measure, such as a motivated behavior task, in order to further confirm the findings. Additionally, it will be important for future studies in patients with schizophrenia to include additional, more detailed measures of negative symptoms and functioning, such as informant report, to more completely understand the relationship between negative symptoms and motivation. Finally, while this study focused on a measure of motivation personality orientation, SDT has consistently shown that the environment is a major influence on motivated behavior (e.g., Deci and Ryan, 2012; Philippe and Vallerand, 2008), and we did not measure environmental influences on motivation. Thus, the findings here do not speak to the reasons for lower autonomy or higher impersonal/amotivation in schizophrenia. It may be that patient environments are less facilitating of autonomy or lack opportunities that lead people with schizophrenia to develop these motivation orientations.
Although the current study included only people with schizophrenia, the design of the GCOS-CP may be applicable to other populations with motivational impairment. For example, the GCOS-CP may be useful for assessing motivation orientation in individuals with depression, bipolar disorder, dementia, and substance use disorders. The autonomy orientation, for example, could be predictive of problems in dysthymia and unipolar depression. Specifically, people with unipolar depression who demonstrate higher levels of autonomous motivation have increased symptom reduction and remission of depression than those who have lower levels of autonomous motivation (Zuroff et al., 2007). In bipolar disorder, individuals often exhibit extreme, atypical behavior directed at obtaining external rewards (Johnson et al., 2011a; Johnson et al., 2011b). Similarly, individuals with a substance use disorder often show heightened sensitivity to rewarding outcomes, such as drug-seeking behaviors (Franken, 2002). Thus, the GCOS-CP control orientation may be especially applicable these disorders, In terms of the impersonal/amotivation aspect of the GCOS-CP, individuals with dementia often show problems with amotivation and apathy, which negatively impact rehabilitation as well as interpersonal relationships with caregivers (e.g., William, 2005). Thus, the GCOS-CP may serve as a helpful addition to research with a variety of populations.
9. Conclusion
In conclusion, the current study provides a much-needed assessment of motivation orientations that is applicable to clinical populations where functioning is impaired. This brief questionnaire provided evidence for problems of autonomy and impersonal/amotivation in schizophrenia, and indicated that patient self-report of autonomy orientation was related to functioning. Thus, this brief measure may be a helpful addition to patient assessment for research and psychosocial treatment.
Supplementary Material
Acknowledgements
This research was supported by Award Number R21MH086801 to DEG from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Contributors:
SC designed the studies, collected and analyzed data, and wrote the initial draft of the report. LML collected and analyzed data and contributed to the editing of the report. DEG designed the studies, oversaw all aspects of the studies, and contributed to the writing and editing of the report.
Conflicts of Interest:
None.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: Author; 2013. [Google Scholar]
- Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: How much and how little we know. Schizophrenia Bulletin. 2005;31:875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophrenia Bulletin. 2010;36:919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Yodkovik N, Sypher-Locke H, Hanewinkel M. Intrinsic motivation in schizophrenia: Relationships to cognitive function, depression, anxeity, and personality. Journal of Abnormal Psychology. 2008;117:776–787. doi: 10.1037/a0013944. [DOI] [PubMed] [Google Scholar]
- Bilker WB, Brensinger C, Kurtz M, Kohler C, Gur RC, Siegel SJ, Gur RE. Development of an abbreviated schizophrenia Quality of Life Scale using a new method. Neuropsychopharmacology. 2003;28:773–777. doi: 10.1038/sj.npp.1300093. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Mueser KT, Bellak AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophrenia Bulletin. 1998;24:413–424. doi: 10.1093/oxfordjournals.schbul.a033336. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Translated by Joseph Zinkin. New York: International Universities Press; 1950. Dementia praecox; or, the group of schizophrenias. [Google Scholar]
- Bowie CR, Depp CA, McGrath JA. Prediction of real world functional impairments in community-dwelling schizophrenia and bipolar disorder patients. American Journal of Psychiatry. 2010;167:1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brietborde NJK, Kleinlein P, Srihari VH. Self-determination and first-episode psychosis: Associations with symptomatology, social and vocational functioning, and quality of life. Schizophrenia Research. 2012;137:132–136. doi: 10.1016/j.schres.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitborde NJK, Kleinlein P, Srihari VH. Causality orientations among individuals with first-episode psychosis. Psychosis, (ahead-of-print) 2013:1–4. doi: 10.1080/17522439.2012.762801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Choi J, Mogami T, Medalia A. Intrinsic Motivation Inventory: An adapted measure for schizophrenia research. Schizophrenia Bulletin. 2010;36:966–976. doi: 10.1093/schbul/sbp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PT-R) and NEO Five-Factor Inventory (NEO-FFI) Lutz, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- Crowne DP, Marlow D. A new scale of social desirability independent of psychopathology. Journal of Consulting Psychology. 1960;24:348–354. doi: 10.1037/h0047358. [DOI] [PubMed] [Google Scholar]
- Deci EL, Ryan RM. The general causality orientations scale: Self-determination in personality. Journal of Research in Personality. 1985;19:109–134. [Google Scholar]
- Deci EL, Ryan RM. The “what” and “why” of goal pursuits: Human needs and the self-determination of behavior. Psychological Inquiry. 2000;11:227–268. [Google Scholar]
- Deci EL, Ryan RM. Overview of Self-Determination Theory. The Oxford Handbook of Human Motivation; 2012. [Google Scholar]
- Dickerson FB, Tenhula WN, Green-Paden LD. The token economy for schizophrenia: review of the literature and recommendations for future research. Schizophrenia Research. 2005;75:405–416. doi: 10.1016/j.schres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- DiClemente CC. Addiction and change: How addictions develop and addicted people recover. New York: Guilford Press; 2003. [Google Scholar]
- Fernandez-Artamendi S, Fernandez-Hermida JR, Secades-Villa R, Garcia-Portilla P. Cannabis and Mental Health. Actas Esp Psiquiat. 2011;39:180–190. [PubMed] [Google Scholar]
- Fervaha G, Agid O, Foussias G, Remington G. Effect of intrinsic motivation on cognitive performance in schizophrenia: A pilot study. Schizophrenia Research. 2014;152:317–318. doi: 10.1016/j.schres.2013.11.037. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Foussias G, Remington G. Negative symptoms in schizophrenia: Avolition and Occam's razor. Schizophrenia Bulletin. 2010;36:359–369. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IHA. Behavioral approach system (BAS) sensitivity predicts alcohol craving. Personality and Individual Differences. 2002;32:349–355. [Google Scholar]
- Gard DE, Cooper S, Fisher M, Genevsky A, Mikels JA, Vinogradov S. Evidence for an emotion maintenance deficit in schizophrenia. Psychiatry Research. 2011;187:24–29. doi: 10.1016/j.psychres.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Fisher M, Garrett C, Genevsky A, Vinogradov S. Motivation and its relationship to neurocognition, social cognition, and functional outcome in schizophrenia. Schizophrenia Research. 2009;115:74–81. doi: 10.1016/j.schres.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Germans Gard M, Horan WP, Green MF. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Sanchez AH, Starr J, Cooper S, Fisher M, Rowand A, Vinogradov S. Using Self Determination Theory to understand motivation deficits in schizophrenia: The ‘Why’ of Motivated Behavior. Schizophrenia Research. 2014;156:217–222. doi: 10.1016/j.schres.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Matveeva TM, Kasanova Z, Strauss GP, Herbener ES, Collins AGE, Frank MJ. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Archives of General Psychiatry. 2012;69:129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: A deficit in the representation of value. Schizophrenia Bulletin. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits in functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophrenia Bulletin. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. Journal of Abnormal Psychology. 2007;116:268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Heggestad ED, Kanfer R. Individual differences in trait motivation: development of the Motivational Trait Questionnaire. International Journal of Educational Research. 2000;33:751–776. [Google Scholar]
- Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Higgins ET. Beyond pleasure and pain. American Psychologist. 1997;52:1280–1300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- Higgins ET, Friedman RS, Harlow RE, Chen Idson L, Ayduk ON, Taylor A. Achievement orientations from subjective histories of success: promotion pride versus prevention pride. European Journal of Social Psychology. 2001;31:3–23. [Google Scholar]
- Ho B-c, Nopoulos P, Flaum M, Arndt S, Andreasen NC. Two-year outcome in first-episode schizophrenia: Predictive value of symptoms for quality of life. American Journal of Psychiatry. 1998;155:1196–1201. doi: 10.1176/ajp.155.9.1196. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Mania and dysregulation in goal pursuit: A review. Clinical psychology review. 2005;25:241–262. doi: 10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Carver CS, Gotlib IH. Elevated ambitions for fame among persons diagnosed with bipolar I disorder. Journal of Abnormal Psychology. 2011b;121:602–609. doi: 10.1037/a0026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Edge MD, Holmes MK, Carter CS. The behavioral activation system and mania. Annual Review of Clinical Psychology. 2011a;8:243–267. doi: 10.1146/annurev-clinpsy-032511-143148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Sevy S. Pyramidical model of schizophrenia. Schizophrenia Bulletin. 1990;16:537–545. doi: 10.1093/schbul/16.3.537. [DOI] [PubMed] [Google Scholar]
- Keatley D, Clarke D, Hagger MS. Investigating the predictive validity of implicit and explicit measures of motivation in problem-solving behavioural tasks. British Journal of Social Psychology. 2013;52:510–524. doi: 10.1111/j.2044-8309.2012.02107.x. [DOI] [PubMed] [Google Scholar]
- Kern RS, Green MF, Goldstein MJ. Modification of performance on the span of apprehension, a putative marker of vulnerability to schizophrenia. Journal of Abnormal Psychology. 1995;104:385–389. doi: 10.1037//0021-843x.104.2.385. [DOI] [PubMed] [Google Scholar]
- Kiang M, Christensen BK, Remington G, Kapur S. Apathy in schizophrenia: clinical correlates and association with functional outcome. Schizophrenia Research. 2003;63:79–88. doi: 10.1016/s0920-9964(02)00433-4. [DOI] [PubMed] [Google Scholar]
- Knee CR, Porter B, Rodriguez LM. Self-determination and regulation of conflict in romantic relationships. Human Motivation and Interpersonal Relationships. 2014;139 [Google Scholar]
- Marin RS. Differential diagnosis and classification of apathy. American Journal of Psychiatry. 1990;147:22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- Marin RS. Apathy: a neuropsychiatric syndrome. Journal of Neuropsychiatry: Clinical Neuroscience. 1991;3:243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- Medalia A, Brekke J, editors. Theme: social contextual and physiological determinants of motivation in schizophrenia [Special theme] Schizophrenia Bulletin. 2010;36 [Google Scholar]
- Miller RR. Motivation for treatment: A review with special emphasis on alcoholism. Psychological Bulletin. 1985;98:84–107. doi: 10.1037/0033-2909.98.1.84. [DOI] [PubMed] [Google Scholar]
- Nakagami E, Hoe M, Brekke JS. The prospective relationships among intrinsic motivation, neurocognition, and psychosocial functioning in schizophrenia. Schizophrenia Bulletin. 2008;36:935–948. doi: 10.1093/schbul/sbq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesenn MH, Thomsen DK, Schneiber A, Tonnesvang J. Distinguishing general causality orientations from personality traits. Personality and Individual Differences. 2010;48:538–543. [Google Scholar]
- Penn DL, Combs D. Modification of affect perception deficits in schizophrenia. Schizophrenia Research. 2000;46:217–229. doi: 10.1016/s0920-9964(00)00005-0. [DOI] [PubMed] [Google Scholar]
- Philippe FL, Vallerand RJ. Actual environments do affect motivation and psychological adjustment: A test of Self-Determination Theory in a natural setting. Motivation and Emotion. 2008;32:81–89. [Google Scholar]
- Poole JH, Tobias FC, Vinogradov S. The functional relevance of affect recognition errors in schizophrenia. Journal of the International Neuropsychological Society. 2000;6:649–658. doi: 10.1017/s135561770066602x. [DOI] [PubMed] [Google Scholar]
- Reynolds WM. Development of reliable and valid short forms of the Marlowe-Crowne Social Desirability Scale. Journal of Clinical Psychology. 1982;38:119–125. [Google Scholar]
- Robinson MD, Clore GL. Belief and feeling: evidence for an accessibility model of emotional self-report. Psychological Bulletin. 2002;128:934. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- Rose EA, Markland D, Parfitt G. The development and initial validation of the Exercise Causality Orientations Scale. Journal of Sports Sciences. 2001;19:445–462. doi: 10.1080/026404101300149393. [DOI] [PubMed] [Google Scholar]
- Ryan RM. Control and information in the intrapersonal sphere: an extension of cognitive evaluation theory. Journal of Personality and Social Psychology. 1982;43:450–461. [Google Scholar]
- Ryan RM, Deci EL. Intrinsic and extrinsic motivations: Classic definitions and new directions. Contemporary Educational Psychology. 2000;25:54–67. doi: 10.1006/ceps.1999.1020. [DOI] [PubMed] [Google Scholar]
- Sayers SL, Curran PJ, Mueser KT. Factor structure and construct validity of the Scale for the Assessment of Negative Symptoms. Psychological Assessment. 1996;8:269–280. [Google Scholar]
- Scholten MRM, van Honk J, Aleman A, Kahn RS. Behavioral inhibition system (BIS), behavioral activation system (BAS) and schizophrenia: relationship with psychopathology and physiology. Journal of Psychiatry Research. 2006;40:638–645. doi: 10.1016/j.jpsychires.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Silverstein SM. Bridging the gap between extrinsic and intrinsic motivation in the cognitive remediation of schizophrenia. Schizophrenia Bulletin. 2010;36:949–956. doi: 10.1093/schbul/sbp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg PA, Halvari H, Ommundsen Y. Linking exercise and causality orientations to change in well-being among older adults: does change in motivational variables play a role? Journal of Applied Social Psychology. 2013;43:1259–1272. [Google Scholar]
- Summerfelt AT, Alphs LD, Wagman AM, Funderburk FR, Hierholzer RM, Strauss ME. Reduction of perseverative errors in patients with schizophrenia using monetary feedback. Journal of Abnormal Psychology. 1991;100:613–616. doi: 10.1037//0021-843x.100.4.613. [DOI] [PubMed] [Google Scholar]
- Strauss J, Ryan RM. Autonomy disturbances in subtypes of anorexia nervosa. Journal of Abnormal Psychology. 1987;96:254–258. doi: 10.1037//0021-843x.96.3.254. [DOI] [PubMed] [Google Scholar]
- Vallerand RJ, Blais MR, Lacouture Y, Deci EL. L’echelle des orientations generales a la causalite: Validation canadienne francaise du General Causality Orientations Scale. Canadian Journal of Behavioral Science. 1987;19:1–15. [Google Scholar]
- Vancampfort D, De Hert M, Vansteenkiste M, De Herdt A, Scheewe TW, Soundy A, Stubbs B, Probst M. The importance of self-determined motivation towards physical activity in patients with schizophrenia. Psychiatry Research. 2013;210:812–818. doi: 10.1016/j.psychres.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Wall JD, Palvia P, Lowry PB. Control-related motivations and information security policy compliance: The role of autonomy and efficacy. Journal of Information Privacy & Security. 2013;9:52–79. [Google Scholar]
- Williams AK. Motivation and Dementia. Topics in Geriatric Rehabilition. 2005;21:123–126. [Google Scholar]
- Wingo AP, Harvey PD, Baldessarini RJ. Neurocognitive impairment in bipolar disorder patients: functional implications. Bipolar Disorders. 2009;11:113–125. doi: 10.1111/j.1399-5618.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- Zuroff DC, Koestner R, Moskowitz DS, McBride C, Marshall M, Bagby RM. Autonomous motivation for therapy: A new common factor in brief treatments for depression. Psychotherapy Research. 2007;17:137–147. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.