Abstract
Background
The comprehensive geriatric assessment (CGA) has developed as an important prognostic tool to risk stratify older adults and has recently been applied to the surgical field. In this systematic review, we examined the utility of CGA components as predictors of adverse outcomes among geriatric patients undergoing major oncologic surgery.
Materials and Methods
MEDLINE, Embase, and the Cochrane Library were searched for prospective studies examining the association of components of the CGA with specific outcomes among geriatric patients undergoing elective oncologic surgery. Outcome parameters included 30-day post-operative complications, mortality, and discharge to a non-home institution.
Results
The initial search identified 178 potentially relevant articles, with six studies meeting inclusion criteria. Deficiencies in instrumental activities of daily living (IADLs), ADLs, fatigue, cognition, frailty, and cognitive impairment were associated with increased post-operative complications. No CGA predictors were identified for post-operative mortality while frailty, deficiencies in IADLs, and depression predicted discharge to a non-home institution.
Conclusions
Across a variety of surgical oncologic populations and cancer types, components of the CGA appear to be predictive of post-operative complications and discharge to a non-home institution. These results argue for inclusion of focused geriatric assessments as part of routine pre-operative care in the geriatric surgical oncology population.
Keywords: cancer surgery, geriatric assessment, treatment outcome, survival, complication
1. INTRODUCTION
The growth of the elderly population in the United States parallels the proportional increase in geriatric surgical patients over the past decade. Aging affects a patient’s functional, physiologic and social wellbeing, and these challenges have profound impacts on treatment and care. Geriatric patients typically have a greater burden of comorbid medical conditions; however, these may incompletely represent the spectrum of physiologic age and vulnerability. The challenge of balancing these comorbidities with the need for surgery is compounded with the addition of a cancer diagnosis. Currently, the median age of cancer diagnosis (for all sites) is 66 years [1]. By 2030, the American Cancer Society predicts that the population over age 64 will increase to 20%, representing 70% of all prevalent cancers [1]. This suggests a growing need for risk stratification assessments that address geriatric cancer care, but innovative approaches have yet to enter into standard clinical practice.
There are currently a number of assessments used by geriatricians and primary care providers to measure older patients’ physiologic fitness. In geriatric clinics, a Comprehensive Geriatric Assessment (CGA) is often used to assess components of a patient’s physical, mental, and social wellbeing, providing a complete picture of the patient’s physiological age and capabilities regardless of chronologic age. A full CGA can take several hours to complete, and it includes standard assessments such as Activities of Daily Living, Geriatric Depression Score, and a timed “Get-Up and Go” test [2]. While useful, a CGA may not be feasible due to time constraints, especially in a busy surgical clinic [3].
Shorter, more efficient geriatric assessments can address these time constraints while adequately assessing surgical eligibility among geriatric cancer patients [4]. By analyzing different components of the CGA, investigators have sought to categorize patients as frail, moderately frail, or fit. Intuitively, frail patients will experience worse outcomes than those who are fit. However, for the intermediate (or moderately) frail, additional assessments could improve the decision making process regarding treatment. Studies examining the predictive value of different screening tests have found relatively consistent benefits of using these assessments for general surgery as well as surgical oncology across different types of cancer [1,3,5–9].
Recognizing the need for synthesis of the burgeoning body of geriatric surgical oncology literature evaluating the potential utility of these assessments, we performed a systematic review of existing studies to assess which components of the CGA may be the most robust predictors of clinically relevant outcomes in this context. The results of this study will provide support and direction in developing new screening protocols and possibly identify actionable targets of the CGA for future pre-operative intervention studies to mitigate the risk of adverse outcomes.
2. MATERIALS AND METHODS
We aimed to identify prospective cohort studies including patients scheduled to undergo elective surgery for cancer treatment and investigated the association of components of the CGA and postoperative outcomes.
2.1. Eligibility Criteria
Types of participants
As variability exists in the age defining a geriatric patient, our review allowed inclusion of any study with patients 60 years and older undergoing elective surgery for cancer.
Types of observations
Any combination of CGA components were included, such as fitness assessment, mental/cognitive assessment, depression, nutrition, comorbidities, fatigue, and/or laboratory values.
Types of outcome measures
Primary outcomes −30-day post-surgical mortality, complications within 30 days, and discharge to an institution.
Secondary outcomes – Intermediate-term (90 day) all-cause mortality
2.2. Search Methods: Electronic searches
For this review, we identified relevant studies by conducting searches of MEDLINE via PubMed, Embase, and the Cochrane Library for articles published between 2000–2013. The following searches were performed on September 10, 2013. MEDLINE was searched using the following combination of search terms: “Aged”[MeSH Terms] AND (“Surgical Procedures, Operative”[majr] OR “Neoplasms/surgery”[majr] OR “Neoplasm metastasis”[MeSH]) AND (“Geriatric assessment”[MeSH] OR “geriatric assessment”[tw]) AND (“Prospective studies”[MeSH] OR “Prospective”[tw] OR “prospectively”[tw] OR “Logistic Models”[MeSH]). Embase was searched using the following combination of search terms: ‘cancer surgery’ OR ‘surgery’/exp OR surgery AND (‘geriatric assessment’/exp OR ‘geriatric assessment’) AND (‘prediction’/exp OR prediction OR ‘prognosis’/exp OR prognosis OR ‘treatment outcome’/exp OR ‘treatment outcome’ OR ‘survival’/exp OR survival OR ‘complication’/exp OR ‘complication’) AND (‘prospective’ OR ‘prospective study’ OR ‘prospectively’). The Cochrane Library was searched using the search term Neoplasms/Surgery. Additional relevant studies were identified through a manual search of the reference lists of relevant articles.
2.3. Selection of studies
As described briefly above, studies selected for inclusion in this review met the following criteria: prospective study design; a study population of geriatric patients who underwent elective surgical treatment for cancer; studies that used pre-surgical assessments containing components of the CGA as predictors of patient outcomes; measurements of at least one of the following outcomes: post-surgical complications, 30-day mortality, or discharge to an institution; published in English and a publication year between 2000 and 2013. Publications focusing on delirium as the primary post-surgical complication were excluded.
2.4. Data Collection and Quality Assessment
Titles and abstracts of all studies retrieved by the searches were assessed by MAF to determine which reports warranted further examination. MAF and DTM then screened the full text of all potentially relevant articles. Data regarding study design and results were independently extracted from the reports by both MAF, DTM. Data were then confirmed by ABS. Specific items extracted included type of study, study setting, study population, content of the CGA and assessment method, and outcomes in association with CGA components.
Methodologic quality of each study was independently assessed by MAF using a self-designed quality assessment evaluating six criteria: prospective study, multiple institutions, clearly defined inclusion/exclusion criteria, use of validated questionnaires, presence of short- (30-day) and intermediate-term (90-day) follow-up, scored separately. Each criterion was scored as 0 if unmet or 1 if met, with the total quality score ranging from 0–6. Disagreements between MAF and DTM were discussed, and if persistent disagreement was noted, the third reviewer (ABS) provided the final opinion.
2.5. Data Items
The different components of CGA used in each study were identified, and results were recorded. CGA components were organized into separate categories including: cognition, mood, ADLs, IADLs, nutritional status, social support, comorbidity, polypharmacy, mobility/falls, frailty assessment, and laboratory values. If available, the odds ratios were recorded.
The three main outcomes of interest were post-surgical complications, 30-day mortality, and discharge to an institution. Other outcomes also recorded included length of stay, operative time, and readmissions, when available.
3. RESULTS
3.1. Characteristics of Included Studies
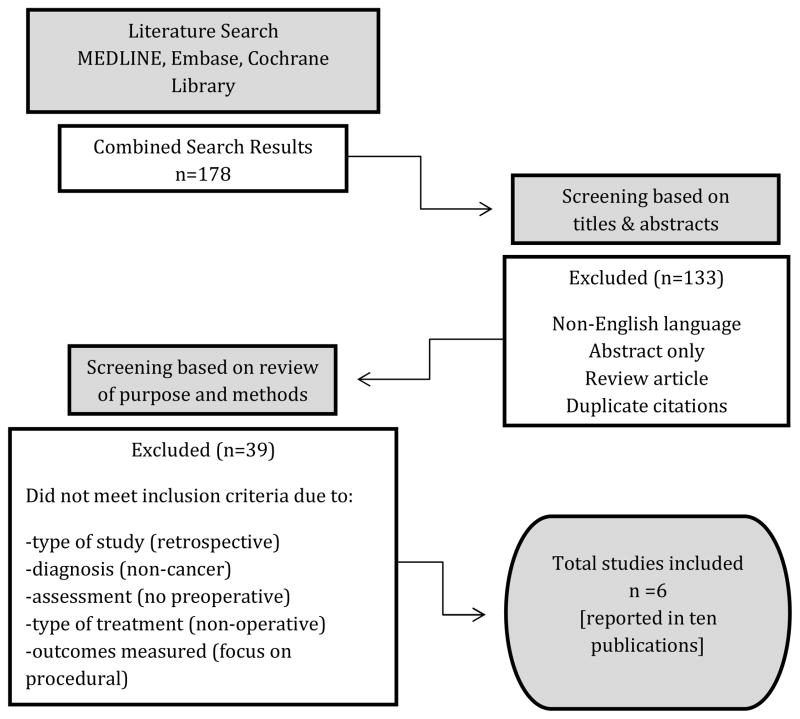
The literature search yielded 178 citations (84 from MEDLINE, 94 from Embase, and none from the Cochrane Library) (Figure 1). After screening abstracts, 45 potentially relevant articles were selected for further examination, excluding non-English articles, those with abstracts alone, review articles, and duplicate citations. The remaining articles were further reviewed for inclusion criteria by reading the full text. After exclusion of 39 further studies due to failure to meet inclusion criteria (i.e. retrospective studies, non-cancer diagnoses, absent CGA components, non-operative treatment, and absence of primary outcomes), a total of 10 publications from 6 studies were included in this review.
Figure 1.
Study Design
Characteristics of the six included studies demonstrated a variety of components of the CGA, summarized in Table 1. The association between CGA with outcomes following surgery was evaluated in two thoracic oncology cohorts, three colorectal cancer cohorts, and one large cohort for solid tumors (including breast, gastrointestinal, and genitourinary). The most often-incorporated components of the CGA included assessments of mood, ADLs, nutrition and comorbidities, while the least-included components were social support, mobility, frailty (as a composite measure), and polypharmacy.
Table 1.
Study Characteristics
| Study | Cancer surgery type |
Patients (n) |
Age (y, mean) |
Sex (% M) |
Outcomes assessed |
Components of Geriatric Assessment (CGA) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognition | Mood | ADL | IADL | Nutrition Status |
Social Support |
Comor- bidity |
Poly- pharmacy |
Mobility/ Falls |
Frailty | Labs | ||||||
| PACE Participants, et al.9 (2007) | Solid tumors-47% breast, 31% GI, 15% GU, 6% other | 460 | ≥70, 76.9 | 34% | 30d mortality, POC, LOS | X | X | X | X | |||||||
| Fukuse, et al.1 (2005) | Thoracic-82% lung/mediastinal | 120 | ≥60, 70.3 | 60% | POC, LOS, Operative time | X | X | X | X | X | X | |||||
| Kothari et al.3 (2011) | Thoracic- 65% lung, 30% esophageal, 5% pleural/tracheal | 60 | ≥70, median = 76 | 53% | 30d mortality, POC, LOS, Discharge disposition | X | X | X | X | |||||||
| Kristjansson et al.5 (2010) | Colorectal | 185 | ≥70, 79.6 | 43% | Mortality, 30-day POC, Readmission, 3m living situation | X | X | X | X | X | X | X | X | |||
| Tan et al.10 (2012) | Colorectal | 83 | >75, 81.5 | NR | Mortality, 30d major POC, Reoperation | X | X | X | X | |||||||
| Badgwell et al.11 (2013) | Abdominal-40% colorectal, 30% HBP, 14% gastric/duodenal | 111 | ≥65, median = 72 | 55% | 90d POC, LOS, Discharge to nursing facility, 30d readmissions | X | X | X | X | X | X | X | X | X | ||
NR = Not Recorded LOS = Length of stay POC = Post-operative complications HPB = hepatopancreatobiliary
3.2. Methodological Quality
The selected studies were assessed for quality by examining six factors as described in the Methods (Table 2). All fully reviewed studies had a prospective cohort design with clearly defined inclusion and exclusion criteria [1,3,5,9–11]. Furthermore, all studies included validated questionnaires and data were collected prior to surgery. General demographic data, including age, gender, history, and medical illnesses were recorded on hospital admission [1, 3,7]. Some studies used the Barthel index of ADL [1,5] while others used Katz index of ADL [3,7–9]. Cognitive status was most often measured using the Folstein mini mental status exam [1,3,5,6,9]. There was a range of differences in who conducted the preoperative interviews; some interviews were conducted by student doctors, research assistants, trained nurse practitioners, and medical doctors with or without training in geriatrics. [1,6,7,9]. Short-term follow-up (30 day post-operative complications and/or mortality) was assessed in all studies, with three additional studies including follow-up at 90 days [1,5,11]. Only three studies were conducted at multiple institutions [5,9,10].
Table 2.
Methodologic Quality of Included Studies
| Prospective study | Multiple institutions | Clearly defined inclusion/exclusion criteria | Validated questionnaire | Short term Follow-upa | Long term Follow-upb | Total | |
|---|---|---|---|---|---|---|---|
| Fukuse et al. [1] | 1 | 0 | 1 | 1 | 1 | 1 | 4 |
| Kothari et al. [3] | 1 | 0 | 1 | 1 | 1 | 0 | 4 |
| Kristjansson et al. [5] | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| PACE et al. [9] | 1 | 1 | 1 | 1 | 1 | 0 | 5 |
| Tan et al. [10] | 1 | 1 | 1 | 1 | 1 | 0 | 5 |
| Badgwell et al. [11] | 1 | 0 | 1 | 1 | 1 | 1 | 5 |
30-day follow-up
90-day follow-up
3.3. Complications
Complications were assessed in all studies, but recorded in various ways. All studies reported major complications, but only three studies defined these using the standard Clavien-Dindo classification system [5,10,11]. The remaining studies either did not specify the definition of a major complication [3,9] or defined these using a pre-specified list [1]. Only three studies specified the manner of complication ascertainment, two by the principal investigator [1,11] and one by a nurse data manager [3].
CGA predictors of overall complications included IADL dependency [9], moderate to severe Brief Fatigue Inventory score [9], and worse frailty scores [5,10] (Table 3). CGA predictors of major complications included higher ASA score [9], decreased mini-mental state exam score [1], dependency of ADLs [1] and IADLs [3], worse geriatric depression score [3], and worse frailty scores [5,10]. Only one study showed no predictors of 90-day overall or major complications [11]. Notably, no study revealed age as a predictor of either overall or major complications. Additionally, comorbidity index was not found to be a significant predictor of complications on univariable or multivariable analyses in those studies which included this in their preoperative assessment [1,5,9–11].
Table 3.
Geriatric Assessment Components and Association with Surgical Outcomes
| Outcomes | Post-operative Complications | Post-operative Mortality | Discharge to a non-home facility | Other Outcomes | |||
|---|---|---|---|---|---|---|---|
| Rates | Predictors | Rates | Predictors | Rates | Predictors | ||
| PACE et al.9 | 37.8% ≥1 overall 30d complication | IADL (OR 1.4; 95%CI 1.0–2.0) Moderate-Severe BFI (OR=1.5; 95% CI 1.2–2.1) | 3.5% overall 30d mortality | None | Longer LOS: Decreased ADL 2.0 (1.4–2.9) | ||
| 16% (n=75) ≥1 major 30d complication | ASA (OR=2.0; 95% CI: 1.1.–3.5) | ||||||
| Fukuse et al.1 | 16.7% | Decreased MMSE (p=0.03); Decreased ADLs) (p=0.04) No ORs reported |
|||||
| Kothari et al.3 | 13% major 30d complication | Dependency with IADL “shopping” (p=0.01); Geriatric depression scale “Have you dropped activities and interests?” (p=0.04) |
1.6% 30d mortality | None | 10% dc to non-home location | Dependency in IADLs (p=0.003); Geriatric Depression Score “Do you feel worthless the way you are now?” |
Longer LOS: Nutrition NSI NHC: illness that made patient change way they ate (p=0.04); unintentional ≥10 pound weight loss in 6 months (p=0.01); not always able to feed or shop for oneself (p=0.03) |
| Kristjannson et al.5 | 60% overall complications (78% severe) | Worse frailty score (OR=3.7; 95% CI: 1.7–7.9) | 2% 30d mortality | None | Readmissions higher for worse frailty score | ||
| Tan et al.10 | Surgical complications n=16 (19%) | Worse frailty score (OR = 3.5; 95% CI: 1.1–10.8) | |||||
| Medical complications; n=16 (19%) | Worse frailty score (OR = 3.5; 95% CI: 1.1–10.8) | ||||||
| Major complications (n=22); 27% | Worse frailty score (OR = 4.1; 95% CI: 1.4–11.6) | ||||||
| Badgwell et al.11 | 90-day overall complications (48%) n=53 | No predictors | 90-day mortality 3% (n=3) | None | DC status to SNF 10% | ECOG (<2): OR 4.5 (1.03–19.7); weight loss ≥10%: OR 6.5 (1.4–29.8); ASA>2: OR 5.1 (1.2–22.8) | 30-day readmissions 24% (no predictors); Increased LOS: weight loss ≥10% (OR=4.0; 95%CI 1.1–14.4); polypharmacy (OR=2.4; 95% CI 1.1–5.5) |
| 90-day major complications (21%) | No predictors | ||||||
3.4. Post-Operative Mortality
Post-operative mortality was assessed in four of six studies (Table 3) [3,5,9,11]. No CGA components were found to be predictors of 30-day or 90-day mortality.
3.5. Discharge to a Non-Home Institution
The ability of CGA components to predict discharge to an institutional setting was assessed in two of six studies (Table 3) [3,11]. Deficiencies in IADLs (specifically shopping) and feelings of worthlessness predicted discharge to a non-home institution [3]. Eastern Cooperative Oncology Group (ECOG) status >2 was also a strong predictor of discharge to a skilled nursing facility in one study [11].
3.6. Other Outcomes
Other outcomes assessed included length of stay, operative time, and post-operative readmission. Deficiency in ADLs [9], nutritional deficiency [3,11], inability to feed or shop for oneself [3], and polypharmacy [11] were associated with longer length of stay. Readmissions were examined by two studies [5,11], one of which revealed worse frailty score as an independent predictor of post-operative readmission [5].
4. DISCUSSION
With the aging population, the burden of cancer in the elderly has increasing public health significance. For older adults presenting with solid tumors, recommended surgical therapies are often potentially substantial physiologic stressors, and there is significant heterogeneity in patients’ ability to tolerate and recover from these treatments. Building on the well-established role of CGA as an adjunct to clinical care management from the gerontology literature, a number of investigators have explored the potential utility of abbreviated assessments incorporating elements of CGA as adjunctive pre-operative screening tools in the context of geriatric surgical oncology. Our systematic review seeks to synthesize this evidence, finding that specific components of the CGA, capturing elements outside the scope of standard clinical assessment, may predict post-surgical outcomes in the cancer setting. Importantly, our review also confirms that age alone was not an independent predictor of postoperative complications, highlighting the need for more granular functional assessment to optimize risk stratification in this patient population.
Despite heterogeneity in patient populations and surgical conditions, we found components of the CGA (such as IADLs, ADLs, cognition, and depression) to be consistently associated with adverse postoperative outcomes. These findings underscore the need for providers to consider the full spectrum of heterogeneity in the elderly population in order to determine the best course of treatment. The American College of Surgeons (ACS) recognizes the necessity for quality improvement in the surgical care of geriatric patients with the recent publication of guidelines for preoperative assessment in this population, including frailty assessment, and multiple components of the CGA (e.g. depression screening, nutritional status, cognitive ability, and functional status) [12]. Recommendations vary based on the patient’s age (predominantly >65 years), type of surgery (i.e. minor vs. major), and other risk factors.
The most robust predictors of post-operative complications among the surgical oncology population included deficiencies in IADLs, depression, decreased cognition, and decreased frailty composite scores. These items are consistent with the recently published ACS checklist for optimal preoperative assessment of the geriatric surgical patient [12]. This checklist includes 13 items, which may not be feasible in a busy surgical practice (Table 4). Therefore, attention to these above-listed components of the CGA may be warranted. Other items included on this checklist were assessed in several surgical oncology studies, including nutrition and polypharmacy. While the selected studies did not find an association between these items and post-surgical complications, they were predictive of longer length of stay, and therefore may be useful in counseling patients and family regarding anticipated hospital course [3,11].
Table 4.
Checklist for Optimal Preoperative Assessment of the Geriatric Surgical Patient (adapted from Chow et al, J Am Coll Surg, 2012 [12])
| Recommended Preoperative Assessments for Geriatric Surgical Patient |
|---|
| □ Cognitive Ability and Capacity to Understand Surgery |
| □ Depression Screen |
| □ Delirium Assessment |
| □ Alcohol or Substance Abuse Screen |
| □ Cardiac Evaluation |
| □ Pulmonary Risk Factor Assessment |
| □ Functional Status and Falls Assessment |
| □ Baseline Frailty Score |
| □ Nutritional Assessment & Intervention |
| □ Medication History & Evaluation for Polypharmacy |
| □ Patient Treatment Goals and Expectations |
| □ Family and Social Support Evaluation |
| □ Appropriate ordering of Diagnostic Tests |
The ACS also emphasized determination of a baseline frailty score through use of a frailty assessment evaluating shrinkage (unintentional weight loss), weakness (decreased grip strength), exhaustion (self-reported poor energy), low physical activity, and slowness (slow walking) [6]. Only two of six studies from our systematic review included this item in their evaluation [5,10], but frailty was a strong predictor of postoperative complications in both, and a predictor of readmissions in one [5]. This suggests that a frailty assessment should be routinely employed in the preoperative assessment, as it is a quick and efficient evaluation with potential for risk stratification prior to surgery.
Additional factors included on the ACS checklist but not identified in our systematic review included social support, falls, and alcohol use. These items were not examined in the studies we reviewed, and therefore, their utility in the surgical oncology population remains unknown. Further research will be necessary to evaluate their utility and justify their use in a busy practice. Other limitations highlighted by our systematic review include a paucity of studies evaluating the prospective use of the CGA in the surgical oncology patient population. Most studies were conducted at single institutions, with limited definitions of post-operative complications. Furthermore, only a small subset of malignancies was examined (predominantly thoracic and colorectal cancer).
Clearly, this systematic review supports that further research is necessary to expand our knowledge of the components of CGA with greatest utility for risk stratification among various cancer sites. A better understanding of the juxtaposition of CGA and post-operative outcomes could assist with patient and provider decision-making, especially in the context of a growing geriatric population, in which weighing the benefits and risks of surgery is increasingly less straightforward. Based on evidence from this systematic review, geriatric surgical oncology patients could benefit from an assessment of IADLs, ADLs, depression, frailty, and nutrition. Additional studies are needed to determine a specific assessment protocol to standardize pre-surgical evaluation of geriatric patients. However, the abbreviate geriatric assessment developed by Hurria et al. covers these essential domains, chosen for their reliability, validity, brevity, and prognostic ability to determine risk for morbidity and mortality in older patients. Furthermore, the assessment is brief, with 88% of patients in a multi-center trial of medical oncology patients able to complete the assessment within 22 minutes.[13] For patients with deficiencies in one or more of these components, pre-operative intervention in the form of preoperative physical therapy (i.e. “pre-hab”) or nutrition optimization to improve baseline deficiencies may be warranted. Geriatric patients should therefore be evaluated thoroughly, and physicians must understand and interpret the results of their assessments. If this cannot be accomplished in the surgical clinic, primary care physician or geriatrician referrals should be considered for further evaluation. As oncologic surgeons become increasingly aware of the utility of these assessments, patients may benefit greatly from their use through improved post-operative outcomes in this vulnerable population.
Acknowledgments
The project described was supported by the Medical Student Training in Aging Research (MSTAR) program in conjunction with the University Cancer Research Fund and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR001109 and UL1TR001111.
Footnotes
Author’s Contributions:
Megan A. Feng, MD – Data collection, analysis and interpretation, and writing the article.
Daniel T. McMillan, MD MPH – Data collection, analysis and interpretation, and writing the article.
Karen Crowell, MLIS – Data collection, conception and design of the project, analysis and interpretation.
Hyman Muss, MD – Conception and design of the project.
Matthew E. Nielsen, MD MS – Conception and design of the project, and critical revision of the article.
Angela B. Smith, MD – Data collection, conception and design of the project, writing the article, and critical revision of the article.
An earlier version of this review was presented at the American Geriatrics Society Annual Scientific Meeting in Seattle, WA on May 4-5, 2012.
Disclosure: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fukuse T, Satoda N, Hijiya K, Fujinaga T. Importance of a comprehensive geriatric assessment in prediction of complications following thoracic surgery in elderly patients. Chest. 2005;127:886–91. doi: 10.1378/chest.127.3.886. [DOI] [PubMed] [Google Scholar]
- 2.Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–52. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Kothari A, Phillips S, Bretl T, Block K, Weigel T. Components of geriatric assessments predict thoracic surgery outcomes. J Surg Res. 2011;166:5–13. doi: 10.1016/j.jss.2010.05.050. [DOI] [PubMed] [Google Scholar]
- 4.Overcash JA, Beckstead J, Extermann M, Cobb S. The abbreviated comprehensive geriatric assessment (aCGA): a retrospective analysis. Crit Rev Oncol Hematol. 2005;54:129–36. doi: 10.1016/j.critrevonc.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Kristjansson SR, Nesbakken A, Jordhoy MS, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol. 2010;76:208–17. doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–8. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 8.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and comorbidity. Ann Surg. 2009;250:449–55. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 9.Participants P, Audisio RA, Pope D, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol. 2008;65:156–63. doi: 10.1016/j.critrevonc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Tan KY, Kawamura YJ, Tokomitsu A, Tang T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg. 2012;204:139–43. doi: 10.1016/j.amjsurg.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Badgwell B, Stanley J, Chang GJ, et al. Comprehensive geriatric assessment of risk factors associated with adverse outcomes and resource utilization in cancer patients undergoing abdominal surgery. J Surg Oncol. 2013;108:182–6. doi: 10.1002/jso.23369. [DOI] [PubMed] [Google Scholar]
- 12.Chow WB, Rosenthal RA, Merkow RP, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg. 2012;215:453–66. doi: 10.1016/j.jamcollsurg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 29:1290–6. doi: 10.1200/JCO.2010.30.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]