Abstract
Prenatal smoking cessation has been described as an empathic action “for the baby,” but this has not been empirically demonstrated. We capitalized on a genetically-characterized extant dataset with outstanding measurement of prenatal smoking patterns and maternal face processing data (as an indicator of empathy) to test this hypothesis, and explore how empathy and smoking patterns may be moderated by a genetic substrate of empathy, the oxytocin receptor gene (OXTR). Participants were 143 Caucasian women from the East Boston Family Study with repeated prospective reports of smoking level, adjusted based on repeated cotinine bioassays. Salivary DNA and face processing (Diagnostic Analysis of Nonverbal Accuracy-2) were assessed 14 years later at an adolescent follow-up of offspring. Two-thirds of participants reported smoking prior to pregnancy recognition. Of these, 21% quit during pregnancy; 56% reduced smoking, and 22% smoked persistently at the same level. A significant interaction between face processing and OXTR variants previously associated with increased sensitivity to social context, rs53576GG and rs2254298A, was found (β = -.181; p = .015); greater ability to identify distress in others was associated with lower levels of smoking during pregnancy for rs53576(GG)/rs2254298(A) individuals (p = .013), but not for other genotypes (p = .892). Testing this “empathy hypothesis of prenatal smoking cessation” in larger studies designed to examine this question can elucidate whether interventions to enhance empathy can improve prenatal smoking cessation rates.
Keywords: Pregnancy smoking, oxytocin receptor gene, social cognition, nonverbal accuracy, cognitive empathy, differential susceptibility
Introduction
Maternal smoking during pregnancy remains a common preventable risk factor for poor pregnancy and birth outcomes [1] and putatively, for externalizing problems across the lifespan [2] despite decades of attempts to reduce its prevalence [3]. While there is a great need to understand how to motivate pregnant smokers to quit and stay abstinent through delivery, most research has focused on psychosocial risks associated with continued smoking rather than modifiable psychological characteristics related to successful cessation [4].
Most women who do successfully quit smoking during pregnancy appear to merely suspend smoking during gestation, then resume after delivery [5] suggesting that motivation to change smoking behavior upon recognition of a pregnancy may be driven by the perceived harm of smoking to the baby, rather than perceived risks to personal health [6]. Moreover, pregnancy involves a change in identity as viewed by others [7]; pregnant women are visibly carrying, and face pressures to conform to societal expectations to assume healthy behaviors [8]. Thus, beyond variations in nicotine dependence [9], differences in prenatal smoking behavior may also reflect important psychological differences among smokers [10], in particular, processes related to concern for others, and differences in sensitivity to social context [11-13]. We aimed to provide an initial empirical foundation for the conceptualization of prenatal smoking behavior change as an empathic behavior, moderated by genetic indicators of sensitivity to social context, with the ultimate goal of identifying targets for future interventions [14].
Processing of negative facial emotions as a predictor of empathic behavior
One way to measure empathic capacity directly is to assess the ability to accurately identify distress in others through processing of facial expressions [15]. Face processing ability, particularly regarding distress in others, has been shown to be predictive of prosocial (helping) behaviors [16], and individuals lacking in concern for others show deficits in processing facial expressions of fear, sadness, and anger [17]. In this study, we draw on available face processing data as an indicator of empathy, and the tendency to act on behalf of others’ needs, in this case, the needs of the fetus.
Oxytocin receptor polymorphisms and sensitivity to social context
The nanopeptide neurohormone oxytocin has been extensively studied in relation to the tendency to exhibit concern for others, the development of early maternal behaviors [18], neurodevelopmental disorders characterized by social deficits [19,20], and more recently, individual responses to addictive substances [21]. Two single nucleotide polymorphisms (SNPs) in the third intron of the oxytocin receptor gene (OXTR), rs53576 (G/A) and rs2254298 (G/A), have been the subject of intense inquiry as they relate to individual differences in empathy and prosocial behaviors [22]. While the effect of these SNPs on gene functioning are unknown, and they are unlikely to confer main effects individually, there is recent evidence linking the combination of the rs53576GG and rs2254298A variants to face processing ability in children [23]. Moreover, as with the monoamine oxidase gene [24], the serotonin transporter gene [25], and dopamine-related genes [26], these OXTR variants may interact with environmental or other factors to influence psychological outcomes.
For example, relative to A carriers, individuals with the rs53576GG genotype show greater sympathetic reactivity to social stress [27] and increased vulnerability towards attachment problems if exposed to childhood maltreatment, yet seem to benefit more from the stress-buffering effects of social support [28]. Thompson et al. (2011) demonstrated that girls who were A carriers at rs2254298 had higher anxiety and depressive symptoms if exposed to early adversity, but had lower levels under favorable environmental conditions [29]. Hence, rather than conferring risk or protective main effects, rs53576GG and rs2254298A in combination may potentiate an individual’s sensitivity to social context in a ‘for better or for worse’ fashion [30].
Within the conceptualization of prenatal smoking cessation as an empathic behavior related to concern for fetal health, moderated by sensitivity to social context, we analyzed an extant genetically-characterized pregnancy cohort with ideal measurement of smoking patterns and available maternal face-processing data to provide an initial empirical foundation for this line of inquiry. We predicted that among women with the GG/A genotype at OXTR rs53576 and rs2254298, greater empathic capacity would be associated with lower levels of smoking during pregnancy. Limitations of utilizing this sample are noted; face processing was assessed in mothers many years later when offspring were adolescents and the sample is relatively small. However, as many larger studies do not have in-depth measurement of these constructs, the current investigation represents a first step towards larger prospective studies specifically designed to examine this question.
Material and Methods
Participants were from a prospective pregnancy cohort recruited from a neighborhood health clinic in East Boston before 20 weeks of gestation, enrolled in the Maternal Infant Smoking Study of East Boston (MISSEB) between 1986 and 1992 [31] who then participated in a follow-up study of their offspring, the East Boston Family Study (EBFS) [32]. Sample details and study procedures have been described previously [24]. For the current paper, only Caucasian mothers from whom saliva was obtained for genotyping and from whom face-processing data were obtained were included in analyses (Figure 1). Mothers with face-processing data did not differ from those without in terms of smoking during pregnancy or other variables, described below.
Figure 1.
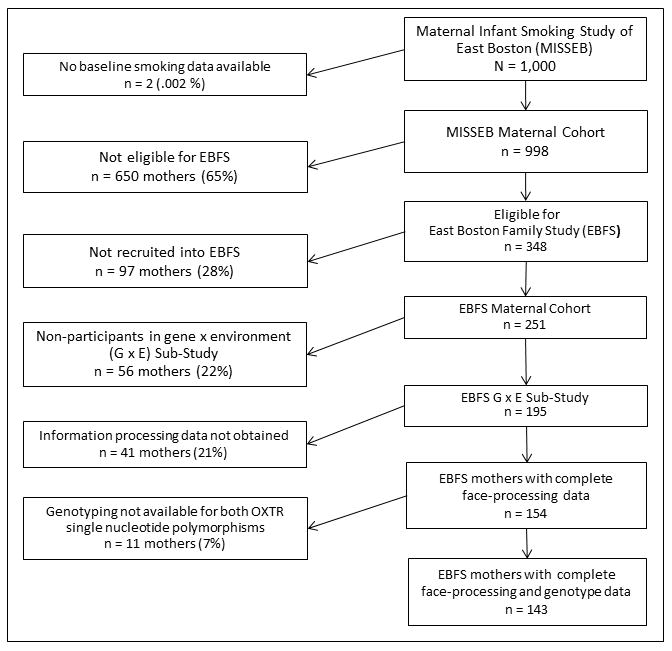
Study Flow Chart
Pregnancy Smoking
Smoking was assessed at each prenatal visit (mean of 6.4 ± 1.7 visits; range = 2 – 12) by self-report using timeline follow-back methodology [33] combined with blood and urine cotinine radioimmunoassays [34]. Smoking patterns were established via a ‘best-estimate’ approach such that non-disclosure and underreporting were corrected based on serum cotinine values, employing statistical methods previously described [35]. A continuous corrected mean serum cotinine measure of average cigarettes per day across pregnancy was used as the dependent variable.
OXTR Genotyping
Salivary DNA was collected using DNA Genotek Oragene Self-Collection Kits and quantitated with a fluorescent Quant-iT PicoGreen dsDNA Assay (Invitrogen, Carlsbad, CA, USA) after extraction, then normalized to 10 ng/μL. SNP markers were genotyped using TaqMan® SNP Genotyping Assays (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). TaqMan® PCR reactions were done with Universal Master Mix Amperase® UNG, 0.25 μL Taqman probe mix and 2.25 μL water for a 5 μL total volume. PCR conditions (Perkin Elmer 9700 Thermocycler (Applied Biosystems, Foster City, CA) were: one AmpErase® step at 50.0°C for 2 minutes, one enzyme activation step at 95.0°C for 10 minutes, 40 alternating cycles of denaturation at 92.0°C for 15 seconds, and reannealing and extension at 58.0°C for 1 minute. Fluorescence intensity of the final PCR product was measured using an LjL Analyst AD fluorescence microplate reader (LjL Biosystems, Sunnyvale, CA, http://www.moleculardevices.com) and LjL Criterion-Host Software. Genotyping was performed blind to all phenotypic data.
Empathic capacity measured by face processing accuracy
Maternal receptive knowledge of facial emotions was assessed using the computer-administered Diagnostic Analysis of Nonverbal Accuracy-2 (DANVA-2) [36]. Participants viewed 24 standardized child and adult faces displaying expressions of happiness, sadness, fear, and anger for two seconds, then identified the emotion (forced choice). Following signal detection theory we computed a D-prime variable to reflect accuracy in detecting each discrete emotion [37-38]. To assess ability to accurately identify distress in others, key to empathy, we used the average D-prime value for adult and child facial expressions of fear, sadness, and anger.
Control variables
To assesses how empathic capacity was related to changes in smoking due to pregnancy awareness, we controlled for the amount smoked prior to recognizing pregnancy, ascertained by self-report at the baseline prenatal visit (range 0 – 61 cigarettes/day; mean = 9.9; SD = 11.7). This also served as a proxy for nicotine dependence, a known barrier to prenatal smoking cessation [9]. Prenatal alcohol use was controlled using a dichotomous variable reflecting < 2 drinks versus ≥ 2 drinks on any occasion. Prenatal drug use was controlled using a dichotomous variable reflecting any reported illicit drug use. As the role of impulsivity in smoking maintenance is well-documented [39], we controlled for impulsivity using the eight-item impulsivity subscale of the Zuckerman-Kuhlman Personality Questionnaire [40]. Since depression is known to affect face processing accuracy [41], we controlled for depression using a dichotomous variable reflecting the presence of depressive symptoms from the Young Adult Diagnostic Interview Schedule [42] conducted at the time of DANVA-2 assessments.
Analysis
Distributions of rs53576 and rs2254298 genotypes were tested for Hardy-Weinberg equilibrium (HWE) using χ2. SNPs were tested for linkage disequilibrium using standard calculations based on allele frequencies [43]. As rs53576GG and rs2254298A may dominantly moderate the influence of social context on psychological outcomes [27-30], and relate to face processing ability in children [23], a dichotomous variable was created to reflect the presence or absence of this allele combination. Student’s t-test and chi square analyses were employed to examine differences in continuous and dichotomous variables, respectively, between smokers and non-smokers. Bivariate correlation analysis was conducted to rule out multilinear relationships among variables. The association between face processing and smoking during pregnancy was assessed using linear regression, with the OXTR allele combination as a moderator, and amount smoked prior to recognition of pregnancy, prenatal alcohol and drug use, impulsivity, depression, education, and income as covariates. Mean imputation was used for missing control variable data (smoking history 6.4%; prenatal alcohol use 3.5%; prenatal drug use 3.5%; impulsivity 7.2%; depression 5.7%; graduated high school 2.8%; family income 6.4%). Statistical analyses were performed using SPSS 22.0 (SPSS, Inc., Chicago, IL, USA).
Results
Participants were primarily high school graduates with annual household incomes of approximately $40,000 and a mean age of 27.0 years (SD = 5.4). Sixty-two percent (n = 89) reported smoking just prior to recognizing the pregnancy. Of these, 21% (n = 19) quit during pregnancy; 56% (n = 50) reduced their smoking or smoked intermittently during pregnancy, and 22% (n = 20) smoked persistently at the same level throughout pregnancy. Participants smoked an average of 10.7 cigarettes per day prior to recognition of pregnancy (SD = 12.1) and 6.6 cigarettes/day (SD = 8.9) across gestation. Rates of prenatal alcohol and drug use were 10.9% and 6.5%, respectively.
SNPs demonstrated HWE; χ2 for rs53576 and rs2254298 were 1.15 and 2.56, respectively (p > .05). SNPs were in linkage equilibrium (D = 0; r2 = 0). Genotype frequencies for the rs53576/rs2254298 allele combination were: GG/GG: 52; GG/AG: 19; GG/AA: 1; AG/GG: 34; AG/AG: 20; AG/AA: 0; AA/GG: 14; AA/AG: 3; AA/AA: 0. Prevalence of the OXTR rs53576(GG)/rs2254298(A) allele combination was 14.3%.
Regression coefficients testing the moderation of face processing and smoking during pregnancy by the OXTR allele combination are shown in Table 1. Neither SNP variant tested individually showed significant main effects or interaction effects on smoking during pregnancy. However, the interaction of the OXTR allele combination and face processing was significantly associated with smoking during pregnancy. Among rs53576(GG)/rs2254298(A) individuals, greater accuracy in detecting negative emotions in others was associated lower levels of smoking during pregnancy. Face processing was unrelated to prenatal smoking patterns for women without this genotype (Figure 2). Findings were specific to smoking behavior in relation to recognition of the pregnancy since smoking history and prenatal alcohol and drug use were controlled for in the model.
Table 1.
Regression coefficients testing the interaction of OXTR and face processing on smoking during pregnancya (n = 143)
| B | SE | β | t | p | |
|---|---|---|---|---|---|
| Face processing accuracyb | -.003 | .068 | -.003 | -.049 | .961 |
| OXTR allele combinationc | .180 | .188 | .063 | .955 | .341 |
| Face processing x OXTR allele combination | -.408 | .166 | -.181 | -2.461 | .015 |
| Smoking historyd | .669 | .064 | .670 | 10.441 | <.001 |
| Prenatal alcohol usee | .146 | .199 | .045 | .733 | .465 |
| Prenatal drug usef | .116 | .273 | .028 | .424 | .672 |
| Impulsivityg | -.062 | .065 | -.062 | -.954 | .342 |
| Depressionh | .113 | .164 | .043 | .689 | .492 |
| Graduated high school | .100 | .153 | .042 | .653 | .515 |
| Family incomei | -.024 | .068 | -.024 | -.350 | .727 |
Group-corrected mean serum cotinine during
Accuracy in detecting child and adult sad, fear, and angry faces, Diagnostic Analysis of Nonverbal Accuracy-2
OXTR rs53576(GG) and rs2254298(A)
Cigarettes /day prior to recognition of pregnancy
≥ 2 drinks/sitting at any time during pregnancy
Any illicit drug use during pregnancy
Zuckerman-Kuhlman Personality Questionnaire, Impulsivity Subscale
Past year major depressive disorder
1 = Less than $5,000; 2 = $5,000 - $7500; 3 = $7501 – $10,000; 4 = $10,001 – 15,000; 5= $15,001 – $20,000; 6 = $20,001 - $30,000; 7 = $30,001 - $40,000; 8 = $40,001 - $50,000; 9 = $50,000 - $75,000; 10 = $75,001 - $100,000; 11 = $100,001 - $200,000; 12 = Over $200,000
Figure 2.
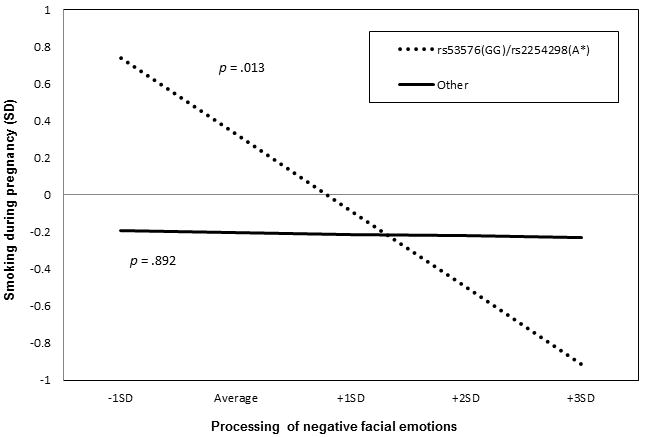
Moderation of face processing and smoking during pregnancy by oxytocin receptor gene (OXTR) allele combination
Smoking during pregnancy = continuous corrected mean serum cotinine during pregnancy; Processing of negative facial emotions = accuracy in detecting child and adult sad, fear, and angry faces, Diagnostic Analysis of Nonverbal Accuracy-2; SD = standard deviation
To rule out the important possibility that results were driven by genetic differences between smokers and non-smokers, we performed a post hoc analysis among only the 103 women who smoked prior to recognizing the pregnancy. The interaction of the OXTR allele combination and face processing was still associated with a lower level of smoking during pregnancy in this subsample (β= -.229, p = .023), supporting the validity of results found in the primary analysis.
Discussion
Consistent with a conceptualization of smoking during pregnancy is an early maternal behavior driven in part, by the capacity for empathy, and moderated by sensitivity to social pressures to assume health behaviors during pregnancy, we found that mothers with social-context-sensitive variants of OXTR and a greater capacity to detect negative emotions in others were more likely to have reduced or stopped smoking during pregnancy. While intermediate mechanisms remain to be elucidated, our results contribute to this promising field of understanding how genetic protective factors may interact with psychological substrates to predict behavior.
Previous work in the prenatal smoking cessation field has overwhelmingly focused on risks for continued smoking. Our results suggest that the capacity to suspend smoking during gestation may reflect not only the relative absence of risk, but the presence of adaptive emotional competencies, specifically, a greater ability to empathize with others, in this case, presumably, the developing fetus. To advance the field, we need to look beyond why smokers continue to smoke during pregnancy, to understanding how and why they may be motivated to quit in response to pregnancy.
Our results also point to complex biologic and psychological substrates of prenatal smoking that must be considered in transmission pathways regarding child psychopathology. In specific genetic contexts, greater smoking during pregnancy was associated with relative maternal deficits in the processing of negative facial expressions--deficits that may extend to early parenting behavior. Thus, some women who smoke more during pregnancy may not only be less able to suspend addictive behaviors, but also less likely to recognize distress cues in their children, thereby adversely affecting responsive interaction, a crucial foundation to children’s self-regulatory capacity [44] and attachment [45].
This investigation capitalized on a genotyped prenatal cohort with outstanding multi-method measurement of smoking patterns and a single performance-based assessment of face processing as an indicator of emotional competence. It is important to emphasize that the effect of variation at rs53576 and rs2254298 on OXTR functioning is unknown and bioinformatic analysis of these SNPs was not performed. Thus, findings should be considered a first step in a program of research designed to identify mutable targets for reducing smoking during pregnancy. Personality traits linked to health behavior are modifiable, and such changes have been associated with changes in health behavior [46]. The optimal follow-up study would utilize multiple measures of empathy in smokers prior to recognition of pregnancy, and follow mothers postpartum to examine whether differences in empathy are also evident in mother-infant interactions.
Conclusions
We add preliminary evidence to the growing body of literature on the diverse and complex role of the oxytocin system in human socio-affiliative behaviors and addictive behaviors.
Acknowledgments
We are deeply appreciative of the expertise provided by Vanja Dukic, PhD, and Kate Pickett, PhD, and editorial assistance by Stephanie Schuette, BA.
Role of funding sources
Work was supported by grants R01 DA015223, R01 DA023653-01A2, and R01 DA023653-03S1 from the National Institute on Drug Abuse (NIDA) (PI: Lauren Wakschlag), the Walden & Jean Young Shaw Foundation (PI: Lauren Wakschlag), and the Northwestern Memorial Foundation (PI: Suena Massey). Funding sources had no role in the design, collection, analysis or interpretation of data, writing the manuscript, or the decision to submit results for publication.
Footnotes
Conflict of Interest
All authors declare that they have no conflicts of interest.
Contributor Information
Suena H. Massey, Email: suena.massey@northwestern.edu.
Ryne Estabrook, Email: restabrook@northwestern.edu.
T. Caitlin O’Brien, Email: caitlin.obrien@mail.ic.edu.
Daniel S. Pine, Email: pined@mail.nih.gov.
James L. Burns, Email: james-l-burns@northwestern.edu.
Suma Jacob, Email: sjacob@umn.edu.
Edwin H. Cook, Email: ecook@psych.uic.edu.
Lauren S. Wakschlag, Email: lauriew@northwestern.edu.
References
- 1.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research. 2004;6:S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 2.Wakschlag LS, Leventhal BL, Pine DS, Pickett KE, Carter AS. Elucidating early mechanisms of developmental psychopathology: The case of prenatal smoking and disruptive behavior. Child Dev. 2006;77:893–906. doi: 10.1111/j.1467-8624.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- 3.Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database System Review. 2009;3 doi: 10.1002/14651858.CD001055.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massey SH, Compton MT. Psychological differences between smokers who spontaneously quit during pregnancy and those who do not: a review of observational studies and directions for future research. Nicotine & Tobacco Research. 2012:nts142. doi: 10.1093/ntr/nts142. [DOI] [PubMed] [Google Scholar]
- 5.Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy: Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000-2005. Department of Health and Human Services, Center for Disease Control and Prevention; 2009. [PubMed] [Google Scholar]
- 6.Stotts AL, Diclemente CC, Carbonari JP, Mullen PD. Pregnancy smoking cessation: a case of mistaken identity. Addict Behav. 1996;21:459–471. doi: 10.1016/0306-4603(95)00082-8. [DOI] [PubMed] [Google Scholar]
- 7.Ramona-Thieme M. Becoming a mother: research on maternal identity from Rubin to the present. 1995 Becoming a mother: Research on maternal identity from Rubin to the present. [Google Scholar]
- 8.Wigginton B, Lee C. Stigma and hostility towards pregnant smokers: Does individuating information reduce the effect? Psychology & Health. 2013;28:862–873. doi: 10.1080/08870446.2012.762101. [DOI] [PubMed] [Google Scholar]
- 9.Woodby LL, Windsor RA, Snyder SW, Kohler CL, Diclemente CC. Predictors of smoking cessation during pregnancy. Addiction. 1999;94:283–292. doi: 10.1046/j.1360-0443.1999.94228311.x. [DOI] [PubMed] [Google Scholar]
- 10.Massey SH, Lieberman DZ, Reiss D, Leve LD, Shaw DS, Neiderhiser JM. Association of clinical characteristics and cessation of tobacco, alcohol, and illicit drug use during pregnancy. Am J Addict. 2011;20:143–150. doi: 10.1111/j.1521-0391.2010.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakschlag LS, Pickett KE, Middlecamp MK, Walton LL, Tenzer P, Leventhal BL. Pregnant smokers who quit, pregnant smokers who don’t: Does history of problem behavior make a difference? Soc Sci Med. 2003;56:2449–2460. doi: 10.1016/s0277-9536(02)00248-4. [DOI] [PubMed] [Google Scholar]
- 12.Pickett KE, Wilkinson RG, Wakschlag LS. The psychosocial context of pregnancy smoking and quitting in the Millennium Cohort Study. J Epidemiol Community Health. 2009;63:474–480. doi: 10.1136/jech.2008.082594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massey SH, Neiderhiser JM, Shaw DS, Leve LD, Ganiban JM, Reiss D. Maternal self concept as a provider and cessation of substance use during pregnancy. Addict Behav. 2012;37:956–961. doi: 10.1016/j.addbeh.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeager DS, Miu AS, Powers J, Dweck CS. Implicit Theories of Personality and Attributions of Hostile Intent: A Meta-Analysis, an Experiment, and a Longitudinal Intervention. Child Dev. 2013;84:1651–1667. doi: 10.1111/cdev.12062. [DOI] [PubMed] [Google Scholar]
- 15.Lamm C, Batson C, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J Cogn Neurosci. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- 16.Marsh AA, Kozak MN, Ambady N. Accurate identification of fear facial expressions predicts prosocial behavior. Emotion. 2007;7:239. doi: 10.1037/1528-3542.7.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh AA, Blair R. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neurosci Biobehav Rev. 2008;32:454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal–fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Francis S, Sagar A, Levin-Decanini T, Liu W, Carter C, Jacob S. Oxytocin and vasopressin systems in genetic syndromes and neurodevelopmental disorders. Brain Res. 2014 doi: 10.1016/j.brainres.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob S, Brune CW, Carter C, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buisman-Pijlman FT, Sumracki NM, Gordon JJ, Hull PR, Carter CS, Tops M. Individual differences underlying susceptibility to addiction: Role for the endogenous oxytocin system. Pharmacology Biochemistry and Behavior. 2014;119:22–38. doi: 10.1016/j.pbb.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Bakermans-Kranenburg MJ, van IJzendoorn MH. A sociability gene? Meta-analysis of oxytocin receptor genotype effects in humans. Psychiatr Genet. 2014;24:45–51. doi: 10.1097/YPG.0b013e3283643684. [DOI] [PubMed] [Google Scholar]
- 23.Slane MM, Lusk LG, Boomer K, Hare AE, King MK, Evans DW. Social cognition, face processing, and oxytocin receptor single nucleotide polymorphisms in typically developing children. Developmental Cognitive Neuroscience. 2014;9:160–171. doi: 10.1016/j.dcn.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakschlag LS, Kistner EO, Pine DS, Biesecker G, Pickett KE, Skol A, Dukic V, Blair R, Leventhal BL, Cox N. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Mol Psychiatry. 2009;15:928–937. doi: 10.1038/mp.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Risch N, Herrell R, Lehner T, Liang K-Y, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to rearing environment depending on dopamine-related genes: New evidence and a meta-analysis. Dev Psychopathol. 2011;23:39–52. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- 27.Norman GJ, Hawkley L, Luhmann M, Ball AB, Cole SW, Berntson GG, Cacioppo JT. Variation in the oxytocin receptor gene influences neurocardiac reactivity to social stress and HPA function: a population based study. Horm Behav. 2012;61:134–139. doi: 10.1016/j.yhbeh.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc Natl Acad Sci. 2011;108:19937–19942. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. 2011;36:144–147. doi: 10.1016/j.psyneuen.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brüne M. Does the oxytocin receptor polymorphism (rs2254298) confer ‘vulnerability’ for psychopathology or ‘differential susceptibility’? insights from evolution. BMC Medicine. 2012;10:38. doi: 10.1186/1741-7015-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RG, Van Vunakis H, Weiss ST, Speizer FE. The effect of maternal smoking during pregnancy on early infant lung function. American Review of Respiratory Disease. 1992;145:1129–1135. doi: 10.1164/ajrccm/145.5.1129. [DOI] [PubMed] [Google Scholar]
- 32.Tager IB, Ngo L, Hanrahan JP. Maternal smoking during pregnancy. Effects on lung function during the first 18 months of life. Am J of Respir Crit Care Med. 1995;152:977–983. doi: 10.1164/ajrccm.152.3.7663813. [DOI] [PubMed] [Google Scholar]
- 33.Sobell LC, Sobell MB. Timeline follow back: A calendar method for assessing alcohol and drug use (Users Guide) Toronto: Addiction Research Foundation; 1996. [Google Scholar]
- 34.Wang X, Tager IB, Van Vunakis H, Speizer FE, Hanrahan JP. Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes. A prospective cohort study. Int J Epidemiol. 1997;26:978–988. doi: 10.1093/ije/26.5.978. [DOI] [PubMed] [Google Scholar]
- 35.Dukic VM, Niessner M, Benowitz N, Hans S, Wakschlag L. Modeling the relationship of cotinine and self-reported measures of maternal smoking during pregnancy: a deterministic approach. Nicotine & Tobacco Research. 2007;9:453–465. doi: 10.1080/14622200701239530. [DOI] [PubMed] [Google Scholar]
- 36.Nowicki S, Jr, Carton J. The measurement of emotional intensity from facial expressions. J Soc Psychol. 1993;133:749–750. doi: 10.1080/00224545.1993.9713934. [DOI] [PubMed] [Google Scholar]
- 37.Macmillan NA, Kaplan HL. Detection theory analysis of group data: estimating sensitivity from average hit and false-alarm rates. Psychol Bull. 1985;98:185. [PubMed] [Google Scholar]
- 38.Stanislaw H, Todorov N. Calculation of signal detection theory measures, Behavior Research Methods. Instruments, & Computers. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- 39.VanderVeen JW, Cohen LM, Cukrowicz KC, Trotter DR. The role of impulsivity on smoking maintenance. Nicotine & Tobacco Research. 2008;10:1397–1404. doi: 10.1080/14622200802239330. [DOI] [PubMed] [Google Scholar]
- 40.Zuckerman M. Zuckerman-Kuhlman Personality Questionnaire (ZKPQ): an alternative five-factorial model. Big Five Assessment. 2002:377–396. [Google Scholar]
- 41.Jacobs RH, Pine DS, Schoeny ME, Henry DB, Gollan JK, Moy G, Cook EH, Wakschlag LS. Maternal depressive history, teen 5HTTLPR genotype, and the processing of emotional faces: Exploring mechanisms of risk. Behaviour Research & Therapy. 2011;49:80–84. doi: 10.1016/j.brat.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Devlin B, Risch N. A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics. 1995;29:311–322. doi: 10.1006/geno.1995.9003. [DOI] [PubMed] [Google Scholar]
- 44.Wakschlag LS, Hans SL. Maternal smoking during pregnancy and conduct problems in high-risk youth: A developmental framework. Dev Psychopathol. 2002;14:351–369. doi: 10.1017/s0954579402002092. [DOI] [PubMed] [Google Scholar]
- 45.Schachner DA, Shaver PR, Mikulincer M. Patterns of nonverbal behavior and sensivity in the context of attachment relations. Journal of Nonverbal Behavior. 2005;29:141–169. [Google Scholar]
- 46.Conrod PJ, O’Leary-Barrett M, Newton N, Topper L, Castellanos-Ryan N, Mackie C, Girard A. Effectiveness of a selective, personality-targeted prevention program for adolescent alcohol use and misuse: a cluster randomized controlled trial. JAMA Psychiatry. 2013;70:334–342. doi: 10.1001/jamapsychiatry.2013.651. [DOI] [PubMed] [Google Scholar]


