Abstract
We examined whether central melanocortin 3 and 4 receptor (MC3/4R) blockade attenuates the BP responses to chronic L-NAME or angiotensin II (Ang-II) infusion in Sprague Dawley rats implanted with telemetry transmitters, venous catheters and intracerebroventricular (ICV) cannula into the lateral ventricle. After 5 days of control measurements, L-NAME (10 μg/kg/day, i.v. – groups 1 and 2) or Ang II (10 ng/kg/min, i.v. – groups 3 and 4) were infused for 24 days and starting on day 7 of L-NAME or Ang II infusion the MC3/4R antagonist SHU-9119 (24 nmol/day, n=6/group – groups 1 and 3) or vehicle (saline 0.5 μl/hr, n=6/group – groups 2 and 4) was infused ICV for 10 days. A control normotensive group also received SHU-9119 for 10 days (n=5). L-NAME and Ang II increased BP by 40±3 and 56±5 mmHg, respectively; while heart rate (HR) was slightly reduced. MC3/4R blockade doubled food intake and reduced HR (~40 to ~50 bpm) in all groups. MC3/4R blockade caused only a small reduction in BP in normotensive group (4 mmHg) and no change in rats receiving Ang II, while markedly reducing BP by 21±4 mmHg in L-NAME treated rats. After SHU-9119 infusion was stopped, food intake, HR and BP gradually returned to values observed before SHU-9119 infusion was started. Ganglionic blockade performed at the end of L-NAME or Ang II infusion caused similar BP reduction in both groups. These results suggest that the brain MC3/4R contributes, at least in part, to the hypertension induced by chronic L-NAME infusion but not by Ang II.
Keywords: blood pressure, food intake, melanocortin system, CNS, heart rate
INTRODUCTION
One of the most important regulators of energy balance and body weight homeostasis is the central nervous system (CNS) melanocortin system. Activation of proopiomelanocortin (POMC) neurons leads to production and release of α-melanocyte stimulating hormone (α-MSH) which, in turn, activates melanocortin 3 and 4 receptors (MC3/4R) leading to suppressed appetite and increased energy expenditure, the latter promoted by increased sympathetic nerve activity (SNA) to thermogenic tissues such as brown adipose tissue1-3. Dysfunction of the melanocortin system in humans or rodents, caused either by mutations of the MC4R or POMC deficiency, is associated with marked hyperphagia, reduced energy expenditure, and severe early onset obesity that is accompanied by many characteristics of the metabolic syndrome, including hyperglycemia, insulin resistance and hyperleptinemia4-6. Some studies suggest that a defective melanocortin system may account for as much as 5-6% of early onset, morbid obesity in humans7-9.
In addition to its role in regulating appetite and energy balance, acute and chronic MC3/4R activation stimulate SNA to tissues that regulate cardiovascular function including the heart, blood vessels, and the kidneys, causing increased blood pressure (BP) and heart rate (HR)10-12. Studies in experimental animals as well as in humans suggest that a functional MC3/4R may be necessary for obesity to cause hypertension. For example, blood pressure of MC4R deficient mice is not elevated despite severe obesity, insulin resistance, hyperinsulinemia and other features of the metabolic syndrome 13,14. Likewise, humans with dysfunctional MC4R exhibit severe obesity and metabolic syndrome but are not hypertensive and actually have lower BP, reduced SNA and lower prevalence of hypertension than control obese subjects15. These observations support the concept that MC3/4R activation (in particular, MC4R activation) is required for excess weight gain to increase BP.
In addition to its importance in linking obesity with increased SNA and elevations in BP in HR in obesity, the brain melanocortin system may play a more fundamental role in regulation of blood pressure beyond obesity-induced hypertension. For instance, we showed that chronic MC3/4R blockade in lean spontaneous hypertensive rats (SHR), a model of hypertension associated with high sympathetic tone, markedly reduced their hypertension to a similar degree achieved by adrenergic receptor blockade16. This observation is consistent with the hypothesis that the brain MC3/4R is a key regulator of SNA and may be important in the development and maintenance of elevated BP in other commonly used experimental models of hypertension. Moreover, other factors including reduced nitric oxide (NO) availability appear to augment the impact of MC3/4R activation on cardiovascular function17.
Therefore, to test the hypothesis that MC3/4R is an important modulator of SNA and may play a fundamental role in BP control we examined the impact of chronic MC3/4R antagonism on two distinct and widely used models of hypertension caused by 1) reduced peripheral NO availability, a common feature in human obesity, by blocking oxide nitric synthase with L-NAME and 2) increased circulating angiotensin II (Ang II) levels by chronic infusion of Ang II. We found that chronic MC3/4R blockade doubled food intake and promoted weight gain while causing significant reductions in HR in both models of hypertension. However, despite a similar effect on appetite and HR, MC3/4R antagonism markedly attenuated the hypertension induced by chronic L-NAME infusion but failed to significantly buffer the increase in BP during chronic Ang II infusion.
METHODS
All experimental procedures conformed to the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Animal Surgery
Male Sprague-Dawley rats weighing between 300-350 g (Harlan Sprague-Dawley, Inc, Indianapolis, IN) were anesthetized with sodium pentobarbital (50 mg/kg) and atropine sulfate (0.37 mg/kg) was administered to prevent excessive airway secretion. A telemetry blood pressure transmitter (Model TA11PAC40, Data Sciences International, MN) was implanted in the abdominal aorta distal to the kidneys under sterile conditions as previously described16. A femoral vein catheter was implanted and the tip of the catheter was advanced into the inferior vena cava. The catheter was exteriorized through a stainless steel button implanted subcutaneously in the scapular region. A stainless steel cannula (26 gauge, 10 mm long) was also implanted into the brain right lateral ventricle using coordinates previously described18. Ten days after recovery from surgery, accuracy of the cannula was examined by determining the dipsogenic response to an acute injection of 100 ng of Ang II.
After recovery from surgery, the rats were housed individually in metabolic cages. The venous catheter was connected to a swivel and a continuous infusion of saline was maintained throughout the study. All rats received water and food ad libitum and total daily sodium intake was maintained constant at ~3.2 mEq/day by continuous infusion of 20 ml/day of 0.9% saline combined with a sodium deficient rat chow (0.006 mmol sodium/g of food, Harlan Teklad, Madison, WI).
Experimental Protocols
Experiment 1: Responses to chronic MC3/4R blockade during L-NAME induced hypertension
Two groups of rats were used in these experiments. After a 5-day control period, rats received intravenous (i.v.) L-NAME (10 μg/kg/min; Sigma, St. Louis, MO) infusion for 24 consecutive days. Beginning on day 7 of L-NAME treatment, the rats also received either an ICV infusion of the vehicle (0.9% saline, 0.5 μL/h, n=6) or the MC3/4R antagonist, SHU-9119 (24 nmol/day, n=6; Polypeptide Laboratories, Torrance, CA) for 10 days via an osmotic minipump (Alzet model 2002, Cupertino, CA) implanted subcutaneously in the scapular region and connected to the ICV cannula via tygon tubing. At the end of the 10-day SHU-9119 infusion, the tygon tubing was severed but the animals continued to be infused with L-NAME for additional 7 days. After this additional 7-day period of L-NAME infusion alone, L-NAME treatment was stopped and the animals were followed for a 5-day recovery period.
Experiment 2: Responses to chronic MC3/4R blockade during Ang II induced hypertension
Two groups of rats were also used in these experiments and the protocol was similar to experiment 1. After a 5-day control period, Ang II (10 ng/kg/min; Sigma, St. Louis, MO) was infused i.v. for 24 consecutive days. Beginning on day 7 of Ang II treatment, the rats also received either an ICV infusion of the saline vehicle or SHU-9119. At the end of the 10-day SHU-9119 infusion, the tygon tubing was severed but the animals continued to be infused with Ang II for additional 7 days. After this additional 7-day period of Ang II infusion alone, Ang II treatment was stopped and the animals followed a 5-day recovery period.
A control group of normotensive rats infused with SHU-9119 was also included in the protocol to account of the effects of MC3/4R antagonism in normotensive rats. This control groups was only infused with saline i.v. for the duration of the experiment and SHU-9119 was infused ICV for 10 days as described above.
Cardiac Sympathetic-Vagal Balance
To evaluate changes in sympathetic and parasympathetic tone to the heart L-NAME or Ang II hypertension and to chronic MC3/4R antagonism, we performed cardiac sympathetic-vagal balance measurements in separate groups of rats (n=4/group) under resting conditions by measuring the chronotropic effects of full-blocking doses of propranolol (4 mg/kg), a β-adrenergic receptor antagonist, and atropine (2 mg/kg), a muscarinic receptor blocker, at a volume per injection of 0.2 ml or less. Propranolol and atropine injections were performed at the end of the control period, 7 days after starting L-NAME or Ang II infusion, and again on day 10 of SHU-9119 treatment. After resting BP and HR were recorded for 30-60 min, propranolol was injected first and 15 minutes later, atropine was injected.
Acute Ganglionic Blockade
To determine the overall contribution of autonomic tone to the maintenance of BP in L-NAME- and Ang II-treated groups we injected a bolus dose of the ganglionic blocker hexamethonium (20 mg/kg, iv) at the end of the 3rd week of treatment. The volume per injection was 0.2 ml or less followed by 0.4 ml of saline to flush the catheter.
Statistical Methods
The results are expressed as means ± SEM. The data were analyzed by 1-way ANOVA with repeated measures followed by Dunnett’s post hoc test for comparisons between control and experimental values within each group when appropriate. Comparisons between different groups were made by 2- way ANOVA followed by Dunnett’s post hoc test when appropriate. Statistical significance was accepted at a level of P<0.05.
RESULTS
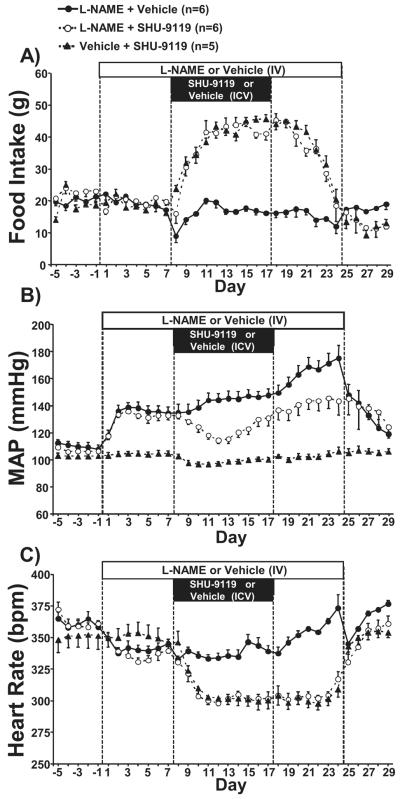
Chronic MC3/4R antagonism markedly increased food intake in L-NAME and vehicle treated rats
As shown in Figure 1A, chronic central MC3/4R blockade with SHU-9119 caused a significant increase in appetite leading to doubling of food intake during the last 5-6 days of SHU-9119 infusion in hypertensive L-NAME treated rats as well as in normotensive i.v. saline treated rats. After stopping SHU-9119 infusion, food intake remained elevated for an additional 3 to 4 days when it gradually fell toward control baseline values on day 7 post SHU-9119 infusion. L-NAME treatment had no effect on food intake.
Figure 1.
(A) Food intake, (B) mean arterial pressure, and (C) heart rate responses to L-NAME, the MC3/4R antagonist (SHU-9119), or both in Sprague-Dawley rats. * p<0.05 vs. control values within same group. # p<0.05 vs. L-NAME + Vehicle group.
Chronic MC3/4R antagonism attenuated hypertension induced by chronic L-NAME infusion and reduced HR in control and L-NAME treated groups
As expected chronic peripheral inhibition of NO formation by L-NAME raised mean arterial pressure (MAP) in ICV vehicle treated group by approximately 30 mmHg during the first week of treatment (Figure 1B). BP continued to increase during the course of the 24-day L-NAME treatment period reaching its peak (+60 mmHg) at the last 4 days of treatment (Figure 1B). After termination of L-NAME infusion MAP rapidly returned toward control values during the 5-day recovery period.
In the group treated with the MC3/4R antagonist MAP also increased to a similar degree during the first week of L-NAME treatment, however, when SHU-9119 began to be infused MAP dropped by ~27 mmHg during the first 5-6 days of ICV SHU-9119 infusion after which MAP gradually increased during the remaining days of L-NAME treatment but to a much lesser degree (varying from −15 to −25 mmHg) compared to ICV saline vehicle even after SHU-9119 infusion was terminated (Figure 1B). This suggests that MC3/4R antagonism not only attenuates hypertension induced by L-NAME but also exerts a long-lasting effect to buffer the increase in BP caused by reduced peripheral NO availability.
As we have previously demonstrated in normotensive SD rats18,19 SHU-9119 caused a small reduction in MAP (~5 mmHg), but this reduction in BP was only a fraction of the effect observed in L-NAME treated hypertensive rats (Figure 1B).
L-NAME treatment caused a small reduction in HR (~20 bpm) during the first 17 to 19 days which was caused mainly by an increase in cardiac parasympathetic tone whereas cardiac sympathetic tone remained unaffected (s 1), after which HR markedly increased in parallel with the increase in MAP during the last week of L-NAME treatment (Figure 1C). Chronic MC3/4R antagonism markedly reduced HR in both hypertensive and normotensive groups by 40 to 50 bpm (Figure 1C). This resulted in a difference in HR between the two L-NAME treated groups of 35 to 40 bpm (Figure 1C). The reduction in HR during MC3/4R antagonism was associated with increased parasympathetic tone to the heart in combination with a reduction in cardiac sympathetic tone (Table 1).
Table 1.
Changes in heart rate following an acute injection of propranolol or atropine in normotensive rats and rats made hypertensive by chronic infusion of L-NAME or Ang II.
| Groups/treatment | Propranolol (Δ bpm) |
Atropine (Δ bpm) |
|---|---|---|
| Vehicle + SHU-9119 | ||
| Control | −70±8 | 46±4 |
| SHU-9119 | −27±4* | 88±11* |
| L-NAME + SHU-9119 | ||
| Control | −55±12 | 34±8 |
| L-NAME | −59±14 | 53±4* |
| L-NAME + SHU-9119 | −33±13# | 55±8* |
| Ang II + SHU-9119 | ||
| Control | −61±8 | 51±4 |
| Ang II | −57±21 | 69±15* |
| Ang II + SHU-9119 | −15±8*† | 74±13* |
Values represent mean + SEM. Propranolol and atropine were injected on the last day of the control period, day 7 of L-NAME or Ang II infusion, and again on day 17 of L-NAME or Ang II infusion (e.g. day 10 of SHU-9119 treatment).
indicates p<0.05 vs. control values;
indicates p<0.05 vs. L-NAME alone;
indicates p<0.05 vs. Ang II alone.
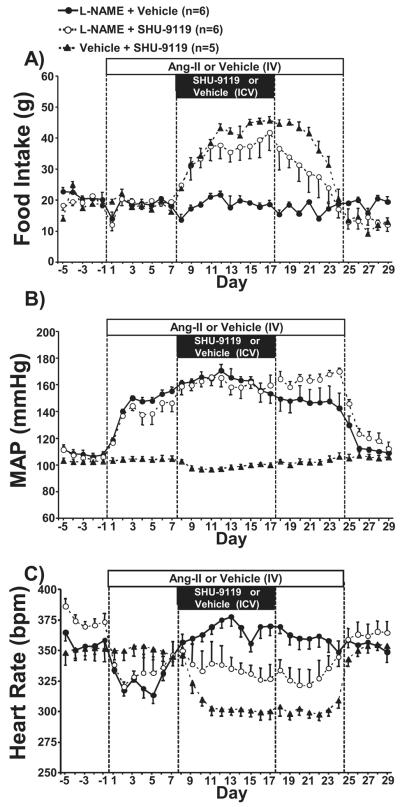
Chronic MC3/4R antagonism markedly increased food intake in Ang II treated rats
Chronic MC3/4R blockade also markedly increased food intake in rats chronically infused with Ang II, although the magnitude of the increase was slightly less pronounced when compared to control normotensive rats (Figure 2A). It is important to note that although the hyperphagia caused by SHU-9119 was attenuated by approximately 10% in the Ang II group, food intake still almost doubled compared to the days immediately before SHU-9119 infusion was initiated (from 20±1 to 39±2 g/day; Figure 2A). As observed in normotensive control and L-NAME treated groups, food intake also gradually returned to baseline levels after stopping SHU-9119 infusion in Ang II treated rats.
Figure 2.
(A) Food intake, (B) mean arterial pressure, and (C) heart rate responses to angiotensin II (Ang II), the MC3/4R antagonist (SHU-9119), or both in Sprague-Dawley rats. * p<0.05 vs. control values within same group. # p<0.05 vs. Ang II + Vehicle group. ‡ P<0.05 vs. Vehicle + SHU-9119 group.
Chronic MC3/4R antagonism failed to attenuate hypertension induced by chronic Ang II infusion but reduced HR in control and Ang II treated groups
Similar to L-NAME treatment, chronic i.v. Ang II infusion in SD rats raised MAP by approximately 40 mmHg (Figure 2B). Chronic central MC3/4R antagonism, however, did not significantly affect the hypertension induced by Ang II and BP levels in this group was similar to those observed in Ang II treated rats that received vehicle ICV infusion (Figure 2B). This suggests that, contrary to the hypertension induced by L-NAME, the elevation in BP caused by chronic Ang II is not modulated by the brain melanocortin system. After cessation of Ang II infusion, MAP returned to normotensive baseline levels (Figure 2B).
Chronic Ang II infusion in ICV vehicle treated rats was also associated with an initial phase of bradycardia lasting 6 to 7 days after which HR gradually increased past its initial baseline levels (Figure 2C). The effects of MC3/4R antagonism on HR during Ang II induced hypertension, however, were more complex when compared to the effects of MC3/4R on HR regulation in L-NAME treated rats described above. For example, SHU-9119 treatment did not cause a significant decrease in HR but prevented HR from increasing to levels observed in Ang II + vehicle group leading to a 30-35 bpm lower HR in Ang II + SHU9119 group (Figure 2C). Thus, despite the fact that SHU-9119 treatment did not lower HR to the same values observed in normotensive rats treated with SHU-9119 it still caused a comparable reduction in HR when compared to the HR values of the two groups infused with Ang II. HR returned to control values 7 to 8 days after cessation of SHU-9119 infusion. These observations indicate that Ang II does not appear to blunt the bradycardic action of chronic central MC3/4R antagonism.
Similar to what we observed in the L-NAME-treated group, the initial bradycardia caused by Ang II infusion was associated with an increase in parasympathetic tone to the heart with no change in cardiac sympathetic tone (Table 1). Furthermore, MC3/4R antagonism with SHU-9119 markedly reduced cardiac sympathetic tone (Table 1).
Acute ganglionic blockade reduced MAP in both groups
Ganglionic blockade with hexamethonium markedly reduced MAP in both groups of hypertensive rats, and this reduction was similar in L-NAME and Ang II hypertensive rats (Figure 3).
Figure 3.
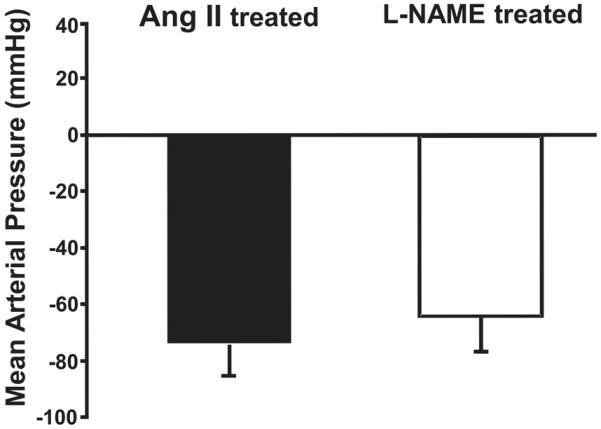
Change in mean arterial pressure in response to acute ganglionic blockade with hexamethonium in L-NAME- and angiotensin II (Ang II)-treated groups. Hexamethonium was injected on the last day of the 3rd week of L-NAME or Ang II treatment.
DISCUSSION
In the present study we demonstrated that chronic CNS MC3/4R antagonism results in comparable increases in appetite and reductions in HR in normotensive rats and in two distinct models of experimental hypertension caused by chronic i.v. L-NAME or Ang II infusion. However, the impact of central MC3/4R blockade on BP regulation markedly differed in these 3 groups. While MC3/4R antagonism significantly attenuated the hypertension induced by L-NAME and exerted a modest BP lowering effect in normotensive rats, it completely failed to alter BP levels in Ang II-induced hypertensive rats.
Our results together with previous studies highlight an important role of the brain melanocortin system in the development and/or maintenance of hypertension in distinct models of hypertension (e.g. L-NAME- and obesity-induced hypertension as well as in SHRs). Moreover, another novel aspect of the present study is that it shows that not all forms of hypertension (e.g. Ang II-induced hypertension) involve the brain MC3/4R receptors and it demonstrates that the exacerbated BP lowering effect of MC3/4R antagonism observed in L-NAME (present study) or in SHRs16 are not simply due to a higher baseline BP level where one may speculate that any antihypertensive therapy may exert a more profound reduction in BP than in normotensive controls.
Although the precise mechanisms by which MC3/4R influence BP regulation are not completely understood, previous studies suggest that MC3/4R modulate SNA. For instance, acute ICV injections of MC4R agonists raise SNA to several tissues, including the kidneys10-12 and combined α– and β–adrenergic receptors blockade completely prevented the hypertension caused by chronic central MC3/4R stimulation using the synthetic agonist MTII20. A role for modulation of SNA by CNS MC3/4R as the main mechanism by which the brain melanocortin system contributes to BP regulation is also supported by our finding that Ang II hypertensive rats are unresponsive to the BP lowering effects of SHU-9119. Using an elegant approach Yoshimoto et al.21 showed that chronic Ang II infusion in rats is associated with normal or even reduced renal SNA. Reduced renal SNA during chronic Ang II-induced hypertension has also been reported in rabbits and dogs on normal sodium diets using direct measurements of renal SNA or by quantifying renal norepinephrine spillover22-24. It is important to note that not all studies have observed reduced SNA during chronic Ang II infusion25 and that Ang II-induced changes in renal SNA may be time dependent as shown by Yoshimoto et al.21 Thus, although there is strong evidence to suggest that in forms of hypertension where SNA is reduced (especially renal SNA) the brain melanocortin system may not contribute to the elevated BP, it is possible that the brain melanocortin system may also modulate cardiovascular function via an alternative mechanism.
Contrary to what we observed in Ang II-induced hypertension, MC3/4R blockade markedly attenuated the hypertension caused by L-NAME infusion. Although we are not certain of the mechanisms leading to a greater fall in BP in this model caused by SHU-9119, a simple explanation would be that the hypertension induced by chronic L-NAME treatment may be associated with increased renal SNA. This explanation would be consistent with our previous observation that chronic MC3/4R antagonism in SHR, a model of high baseline SNA, marked lowered BP by an amount comparable to adrenergic receptor blockade16 and with our current data showing no change in blood pressure in Ang II treated rats during chronic SHU-9119 infusion. Previous studies in rabbits and rats, however, showed normal renal SNA in conscious animals made hypertensive by chronic L-NAME treatment26,27. Conversely, Young and colleagues28 showed that skin SNA, which is not under baroreflex control, was markedly elevated by short-term L-NAME infusion in healthy subjects. Thus, L-NAME may lead to increased SNA in organs/tissues not regulated by the baroreflex but not to organs under baroreflex control such as the kidneys. This possibility, however, may not explain the long-term reduction in BP caused by SHU-9119. Thus, an alternative explanation is that renal SNA, although not altered by L-NAME, is inappropriately high given the severity of the hypertension induced by L-NAME which would be expected to reduce renal SNA. But, independently of whether SNA to cardiovascular relevant tissues is inappropriately elevated or not during L-NAME-induced hypertension it is also possible that peripheral blockade of NO formation sensitizes the blood vessels and/or renal tubules to the pressor actions of adrenergic receptor stimulation, so that the sodium retaining effects of any given level of SNA would be augmented in the presence of L-NAME. For instance, Doodson et al.29 showed enhanced centrally induced sympathetic coronary vasoconstriction in cats pre-treated with L-NAME. We also found marked accentuated sympathetically-mediated hypertension induced by chronic MC3/4R activation in rats treated with L-NAME17. Whereas Hilzendeger et al.30 showed that blockade of brain AT1 receptors failed to alter the increase in renal SNA elicited by the MC3/4R agonist, MTII. This observation is in agreement with our findings that MC3/4R antagonism with SHU-9119 did not alter Ang II-induced hypertension while it reduced BP in L-NAME hypertension. However, it is possible that an adrenergic independent mechanism could explain the fall in BP during MC3/4R blockade in L-NAME hypertensive rats since acute ganglionic blockade lowered BP by a similar degree in L-NAME compared to Ang II hypertensive rats, suggesting an equal overall importance of SNA to the maintenance of BP in these models. Nevertheless, it is also important to recognize that acute ganglionic blockade may not recapitulate the effects of chronically blocking the SNS, especially if the chronic effects are mediated mainly by decreased renal SNA which, in turn, decreases renal tubular sodium reabsorption and leads to a slowly developing decreased in blood pressure over several days31. It is possible that renal SNA may be differentially affected by Ang II versus L-NAME hypertension. These differential effects on renal SNA could be particularly important if suppression of renal SNA mediates most of the chronic BP lowering effect of MC3/4R antagonism31. We, however, are not certain of the precise mechanism by which central MC3/4R blockade reduced BP in L-NAME but not Ang II-treated hypertensive rats. Although we hypothesize an involvement of renal SNA, our results may also suggest that a renal SNA independent mechanism contributes to the differential BP effect of chronic MC3/4R antagonism observed in these two distinct hypertensive models.
Although the impact of MC3/4R antagonism on BP regulation was remarkably different between L-NAME and Ang II induced hypertension, the effects of MC3/4R blockade on HR and food intake regulation were similar and resulted in significant reduction of HR concomitant with a doubling of food consumption. This finding is in accordance with our previous study showing that the bradycardic action of SHU-9119 was not different in SHRs compared to normotensive Wistar controls despite a marked reduction in BP in SHRs that was not seen in Wistar rats16. This supports the concept of differential control of appetite, HR and BP by the brain melanocortin system and highlights the importance of MC3/4R activation for increased weight gain to cause elevations in HR and BP. Our findings also suggest, albeit indirectly, that increased circulating levels of Ang II or reduced NO availability which often occur in obesity do not alter the importance of the brain MC3/4R in regulating appetite.
The reduction in HR in all groups receiving SHU-9119 was associated with increased parasympathetic and reduced sympathetic tone to the heart. Although the brain areas where MC3/4R exert their effects on the autonomic nervous system and BP regulation are still poorly understood, MC4R are abundant in the paraventricular nucleus of the hypothalamus (PVN) and in brainstem areas involved in autonomic regulation as well as in the intermediolateral medulla (IML)32,33. For example, acute stimulation of MC4R in the PVN or IML raises renal SNA and HR, respectively,32,33 while MC4R located on cholinergic preganglionic parasympathetic and sympathetic neurons appear to contribute, at least in part, to obesity hypertension34. However, additional studies are needed to determine the brain regions where the melanocortin system is most important for modulating cardiovascular function.
PERSPECTIVES
The CNS melanocortin system plays a key role linking obesity with sympathetic activation and hypertension. Our studies also support an important participation of brain MC3/4R in regulating BP in non-obese models of hypertension (e.g. SHR and L-NAME-induced hypertension). The fact that MC34R antagonism did not attenuate Ang II hypertension reinforces the notion that elevated baseline BP does not always predict an exacerbated BP lowering effect of MC3/4R blockade. The mechanisms responsible for the differential impact of chronic MC3/4R antagonism on BP regulation observed in L-NAME versus Ang II hypertension are still elusive and will require additional investigation. Overall, our results demonstrate a fundamental role of the CNS melanocortin system in the control of cardiovascular function that is both selective and differentially regulated. Unraveling the mechanisms responsible for this differential control of appetite, HR and BP by CNS melanocortin system will significantly improve our understanding of how the brain regulates metabolic and cardiovascular functions under physiological and pathological conditions.
NOVELTY AND SIGNIFICANCE.
1) What Is New?
Chronic MC3/4R antagonism significantly attenuates L-NAME-induced hypertension.
Chronic MC3/4R antagonism does not alter Ang II-induced hypertension.
MC3/4R blockade markedly increases appetite and reduces HR to a similar extent in Ang II- and L-NAME-treated rats.
Higher baseline BP does not predict the impact of chronic MC3/4R antagonism on BP regulation.
2) What Is Relevant?
The brain melanocortin system contributes to hypertension induced by chronic nitric oxide synthase inhibition but not to Ang II hypertension, suggesting that the brain melanocortin system may contribute to the elevated BP in specific models of hypertension.
Our results support the concept that the brain melanocortin system exerts a differential control of appetite, HR and BP regulation.
Summary
Despite exerting similar effects to increase appetite and to reduce HR in normotensive rats as well as in two distinct models of hypertension (e.g. chronic Ang II or L-NAME infusion), MC3/4R antagonism markedly attenuated L-NAME-induced hypertension whereas no attenuation of the increased BP caused by Ang II infusion was observed. Thus, while L-NAME hypertension appears to involve the brain melanocortin system Ang II-induced hypertension is independent of the brain melanocortin system.
Acknowledgments
SOURCES OF FUNDING This research was supported by grants from the National Heart, Lung and Blood Institute (PO1HL-51971), the National Institute of General Medical Sciences (P20 GM 104357), and by American Heart Association Scientist Development Grants to Alexandre A. da Silva and Jussara M. do Carmo.
Footnotes
CONFLICT OF INTEREST/DISCLOSURES None.
REFERENCES
- 1.Fan W, Boston BA, Keterson RA, Hruby VJ, Cone RD. Role of the melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 2.Hagen MM, Rushing PA, Schwartz MA, Yagaloff KA, Burn P, Woods SC, Seeley RJ. Role of the CNS melanocortin system in the response to overfeeding. J Neurosci. 1999;19:2362–2367. doi: 10.1523/JNEUROSCI.19-06-02362.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Keterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Target disruption of the melanocortin-4 receptor result in obesity. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 4.Balthasar N. Genetic dissection of neuronal pathways controlling energy homeostasis. Obesity. 2006;14:222S–227S. doi: 10.1038/oby.2006.313. [DOI] [PubMed] [Google Scholar]
- 5.Farooqi IS, O’Rahilly S. Genetic of obesity in humans. End Rev. 2006;27:710–718. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 6.Young EH, Wareham NJ, Faroqi S, Hinney A, Hebebrand J, Scherag A, O’Rahilly S, Barroso I, Sandher MS. The V103I polymorphism of the MC4R gene and obesity: population based studies and meta-analyses of 29 563 individuals. Int J Obes. 2007;31:1437–1441. doi: 10.1038/sj.ijo.0803609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;20:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 8.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with dominant form of obesity. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 9.Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutation are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106:253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosc. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interaction between the melanocortin system and leptin in the control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 12.Dunbar JC, Lu H. Proopiomelanocortin (POMC) products in the central regulation of sympathetic and cardiovascular dynamics: studies on melanocortin and opioid interactions. Peptides. 2000;21:211–217. doi: 10.1016/s0196-9781(99)00192-8. [DOI] [PubMed] [Google Scholar]
- 13.Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia and hyperleptinemia. Hypertension. 2005;46:326–332. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- 14.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. 2006;48:58–64. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]
- 15.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O’Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 16.da Silva AA, do Carmo JM, Kanyiscka B, Dubinion J, Hall JE. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension. 2008;51:884–890. doi: 10.1161/HYPERTENSIONAHA.107.100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.do Carmo JM, Bassi M, da Silva AA, Hall JE. Systemic but not central nervous system nitric oxide synthase inhibition exacerbates the hypertensive effects of chronic melanocortin-3/4 receptor activation. Hypertension. 2011;57:428–434. doi: 10.1161/HYPERTENSIONAHA.110.163931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo JJ, da Silva AA, Hall JE. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension. 2003;41:768–774. doi: 10.1161/01.HYP.0000048194.97428.1A. [DOI] [PubMed] [Google Scholar]
- 19.da Silva AA, Kuo JJ, Hall JE. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension. 2004;43:1312–1317. doi: 10.1161/01.HYP.0000128421.23499.b9. [DOI] [PubMed] [Google Scholar]
- 20.Kuo JJ, da Silva AA, Tallam LS, Hall JE. Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation. Hypertension. 2004;43:370–375. doi: 10.1161/01.HYP.0000111836.54204.93. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension. 2010;55:644–651. doi: 10.1161/HYPERTENSIONAHA.109.145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett CJ, Ramchandra R, Guild SJ, Lala A, Budgett DM, Malpas SC. What sets the long-term level of renal sympathetic nerve activity: a role for angiotensin II and baroreflexes? Circ Res. 2003;92:1330–1336. doi: 10.1161/01.RES.0000078346.60663.A0. [DOI] [PubMed] [Google Scholar]
- 23.Barrett CJ, Guild SJ, Ramchandra R, Malpas SC. Baroreceptor denervation prevents sympathoinhibition during angiotensin II-induced hypertension. Hypertension. 2005;46:168–172. doi: 10.1161/01.HYP.0000168047.09637.d4. [DOI] [PubMed] [Google Scholar]
- 24.Carroll RG, Lohmeier TE, Brown AJ. Chronic angiotensin II infusion reduces renal norepinephrine overflow in the conscious dog. Hypertension. 1987;6:675–681. doi: 10.1161/01.hyp.6.5.675. [DOI] [PubMed] [Google Scholar]
- 25.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest. 2012;122:3960–3964. doi: 10.1172/JCI64583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramchandra R, Barrett CJ, Guild SJ, McBryde F, Malpas SC. Role of renal sympathetic nerve activity in hypertension induced by chronic nitric oxide inhibition. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1479–R1485. doi: 10.1152/ajpregu.00435.2006. [DOI] [PubMed] [Google Scholar]
- 27.Dos Santos FM, Martins Dias DP, da Silva CA, Fazan R, Jr, Salgado HC. Sympathetic activity is not increased in L-NAME hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R89–R95. doi: 10.1152/ajpregu.00449.2009. [DOI] [PubMed] [Google Scholar]
- 28.Young CN, Fisher JP, Gallagher KM, Whaley-Connell A, Chaudhary K, Victor RG, Thomas GD, Fadel PJ. Inhibition of nitric oxide synthase evokes central sympatho-excitation in healthy humans. J Physiol. 2009;587:4977–4986. doi: 10.1113/jphysiol.2009.177204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodson AR, Leibold JM, Gutterman DD. Inhibition of nitric oxide synthesis augments centrally induced sympathetic coronary vasoconstriction in cats. Am J Physiol Heart Circ Physiol. 1994;267:H1272–H1278. doi: 10.1152/ajpheart.1994.267.4.H1272. [DOI] [PubMed] [Google Scholar]
- 30.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;303:H197–H206. doi: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Silva AA, do Carmo JM, Wang Z, Hall JE. The brain melanocortin system, sympathetic control, and obesity hypertension. Physiology (Bethesda) 2014;29:196–202. doi: 10.1152/physiol.00061.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P, Cui BP, Zhang LL, Sun HJ, Liu TY, Zhu GQ. Melanocortin 3/4 receptors in paraventricular nucleus modulate sympathetic outflow and blood pressure. Exp Physiol. 2013;98:435–443. doi: 10.1113/expphysiol.2012.067256. [DOI] [PubMed] [Google Scholar]
- 33.Iwasa M, Kawabe K, Sapru HN. Activation of melanocortin receptors in the intermediolateral cell column of the upper thoracic cord elicits tachycardia in the rat. Am J Physiol Heart Circ Physiol. 2013;305:H885–H893. doi: 10.1152/ajpheart.00443.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152:612–619. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]