Abstract
We have recently demonstrated a role of the vascular endothelium in peripheral pain mechanism by disrupting endothelial cell function using intravascular administration of octoxynol-9, a non-selective membrane active agent. As an independent test of the role of endothelial cells in pain mechanisms, we evaluated the effect of homocysteine, an agent that damages endothelial cell function. Mechanical stimulus-induced enhancement of endothelin-1 hyperalgesia in the gastrocnemius muscle of the rat was first prevented then enhanced by intravenous administration of homocysteine, but was only inhibited by its precursor, methionine. Both homocysteine and methionine significantly attenuated mechanical hyperalgesia in two models of ergonomic muscle pain, induced by exposure to vibration, and by eccentric exercise, and cutaneous mechanical hyperalgesia in an ischemia-reperfusion injury model of Complex Regional Pain Syndrome type I, all previously shown responsive to octoxynol-9. This study provides independent support for a role of the endothelial cell in pain syndromes thought to have a vascular basis, and suggests that substances that are endothelial cell toxins can enhance vascular pain.
Keywords: Vascular pain, homocysteine, methionine endothelium, muscle pain
Introduction
We recently discovered a novel phenomenon that drives an active contribution of vascular endothelial cells to peripheral pain mechanisms (Joseph et al., 2011). This phenomenon, referred to as stimulus-dependent hyperalgesia, is elicited by two potent vasoactive compounds: endothelin-1 (ET-1) and epinephrine. ET-1 and epinephrine act at their cognate receptors on the endothelial cell to produce a state in which mechanical stimulation produces enhanced release of ATP, which acts on the P2X3 purinergic receptor on sensory neurons to produce stimulus-dependent hyperalgesia (Joseph et al., 2013). Stimulus-dependent hyperalgesia is distinct from the direct hyperalgesic effect of ET-1 and epinephrine mediated by their cognate receptors on the peripheral terminals of the nociceptor (Joseph et al., 2011; Joseph and Levine, 2012a). The discovery of the role of vascular endothelial cells in stimulus-dependent hyperalgesia was made possible by adaptation of a method from the cardiovascular and renal vascular literature using the intravenous administration of octoxynol-9 to attenuate endothelial cell function (Connor and Feniuk, 1989; Jamal et al., 1992; Sun et al., 1997). We found that administration of octoxynol-9 eliminated ET-1–induced stimulus-dependent hyperalgesia it being dependent on the function of the endothelium; the direct hyperalgesic effect of ET-1 acting on the nociceptor was unaffected by octoxynol-9 administration. Since octoxynol-9 is a non-selective membrane active agent, in the present study we employed an alternative method to impair endothelial cell function. It has been demonstrated in rats as well as in humans that hyperhomocysteinemia produces endothelial dysfunction (Edirimanne et al., 2007; Kanani et al., 1999). Since hyperhomocysteinemia affects several functions of endothelial cells (Abahji et al., 2007; Pushpakumar et al., 2014), we tested whether homocysteine or its precursor, L-methionine (Edirimanne et al., 2007; Kanani et al., 1999) affects ET-1-induced stimulus-dependent hyperalgesia. We found that homocysteine, in a time-dependent manner, both inhibited and enhanced stimulus-dependent hyperalgesia, while methionine only produced inhibition. Both substances also inhibited preclinical models of vascular pain syndromes previously shown to be attenuated by octoxynol-9 (Joseph et al., 2013; Joseph and Levine, 2012a). Our data provide independent support for a role of the endothelial cell in mechanical stimulus dependent hyperalgesia and vascular pain syndromes.
Materials and Methods
Animals
Experiments were performed on adult male Sprague Dawley rats, (200–250 g; Charles River, Hollister, CA). Animals were housed three per cage, under a 12 h light/dark cycle, in a temperature- and humidity-controlled environment. Food and water were available ad libitum. All behavioral nociceptive testing was performed between 10:00 AM and 4:00 PM. Rats were acclimatized to the testing environment, by bringing them to the experimental area, in their home cages, left in the home cage for 15–30 min, after which they were placed in restrainers, cylindrical acrylic tubes that have side openings that allow extension of the hind limbs from the restrainer, for nociceptive testing. Rats were left undisturbed in the restrainer for another 15–30 min before nociceptive testing was started.
Nociceptive threshold was defined as the mean of three readings taken at 5 min intervals. All experimental protocols were approved by the University of California, San Francisco Committee on Animal Research and conformed to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Nociceptive testing
Cutaneous nociception
The nociceptive flexion reflex was quantified with an Ugo Basile Analgesymeter (Stoelting, Wood Dale, IL), which applies a linearly increasing mechanical force to the dorsum of the rat’s hind paw. Nociceptive threshold was defined as the force, in grams, at which the rat withdrew its hind paw from the stimulus. Hyperalgesia was defined as a decrease in mechanical nociceptive threshold, here presented as percentage change from baseline. Both paws of the same rat received the same treatment, and paw withdrawal thresholds were averaged for each rat. Each experiment was performed on separate groups of rats. These animals acted as their own controls, with a test agent injected either intradermally, into the dorsal surface of the hind paws, or intravenously before the intradermal administration of ET-1. Paw-withdrawal thresholds before and after drug treatment were compared.
Muscle nociception
Mechanical nociceptive threshold in the gastrocnemius muscle was quantified using a Chatillon digital force transducer (model DFI2; Ametek, Berwyn, PA) (Dina et al., 2008; Khasar et al., 2008). In lightly restrained rats (as described above), a 6-mm-diameter probe, attached to the force transducer, was applied to the skin overlying the gastrocnemius muscle, to deliver an increasing compression force. This probe width allows for evaluation of muscle nociceptive threshold without interference from nociceptive threshold of the overlying cutaneous nociceptive afferents (Murase et al., 2010). The nociceptive threshold was defined as the force (in milliNewtons) at which the rat withdrew its hind limb; results are presented as percentage change from baseline. Each hind limb (gastrocnemius muscle) is treated as an independent measure, and each experiment is performed on a separate group of rats.
Preclinical models
Vibration-induced hyperalgesia
It has been suggested that musculoskeletal pain induced by exposure to vibrating devices used in various occupations may have a vascular component (Ogasawara et al., 1997; Dowd et al., 1998). We demonstrated previously that exposure to vibration produces chronic muscle pain in the rat (Chen et al., 2010). The rat’s hind limb was vibrated with a Digital Vortex Genie II laboratory Vortex mixer (Thermo Fisher Scientific, Waltham, MA) that has a variable-speed motor with a real-time digital readout of the vibration speed. Rats were anesthetized with 3% isoflurane in oxygen and one hind limb affixed to the platform with Micropore surgical tape (3M, St. Paul, MN) so that the knee and ankle joint angles were both 90°, and without rotational torque on the leg. The leg was vibrated at a frequency of 60–80 Hz, with a 5-mm peak-to-peak displacement amplitude. These vibration frequencies are within the ranges produced by hand-held power tools (35–150 Hz) (Radwin et al., 1990). In previous studies in the rat, more intense hind limb vibration at 80 Hz for 5 h daily for 2 d did not cause muscle necrosis (Lundborg et al., 1990). In the present experiments, hind limbs were vibrated once for 15 min.
Eccentric exercise-induced hyperalgesia
The method used to eccentrically exercise the rat hind limb (Alvarez et al., 2010) is similar to that described by Kano et al. (2004) and Taguchi et al. (2005). Briefly, isoflurane-anesthetized rats were placed in the supine position and the right hind paw was affixed to the foot bracket of the exercise apparatus (model RU-72; NEC Medical Systems, Tokyo, Japan) with Micropore surgical paper tape, such that the angle of the knee and ankle joints was at 90° (with the paw 30° from vertical). The gastrocnemius muscle was stimulated via subcutaneous needle-type electrodes attached to a model DPS-07 stimulator (Dia Medical System, Tokyo, Japan) that delivered trains of rectangular pulses (100 Hz, 700 ms, 3 V) every 3 s to give a total of 300 contractions. During these electrical stimulus-induced contractions of the gastrocnemius muscle, the electromotor system rotated the foot to produce extension of the gastrocnemius muscle.
Ischemia reperfusion-induced hyperalgesia
We used an ischemia-reperfusion injury model of Complex Regional Pain Syndrome (CRPS) type I (Coderre et al., 2004; Millecamps et al., 2010; Ragavendran et al., 2013). Rats were anesthetized with isoflurane and placed on a heating pad to maintain body temperature, and ophthalmic ointment used to prevent the corneas from drying out. A nitrile O-ring (5.5 mm internal diameter, durometer rating 70 Shore A, Grainer, Inc., San Francisco, CA) was placed around a hind limb, proximal to the ankle joint, to reduce arterial blood flow to the hind paw for 3 h, after which the O-ring was removed and the rats were allowed to recover from anesthesia. Nociceptive testing was performed 3 days after ischemia-reperfusion procedure.
Drugs
Endothelin (ET-1), homocysteine, and methionine (Sigma Chemical Co., St. Louis, MO) were dissolved in saline. Drugs administered by intravenous injections (via tail vein) were given in a volume of 1 ml/kg body weight, and drugs administered by intradermal injection were given in a volume of 2.5μl/paw and 5 μl/muscle. Doses of the drugs employed in this study were based on the result of dose response studies done during this or prior studies.
Statistical analyses
Group data are represented as mean ± SEM. Statistical significance was determined by one- or two-way repeated-measures ANOVA, followed by Dunnett’s multiple comparison post hoc test. P <0.05 was considered statistically significant.
Results
Effect of intravenous homocysteine and methionine on stimulus-dependent hyperalgesia
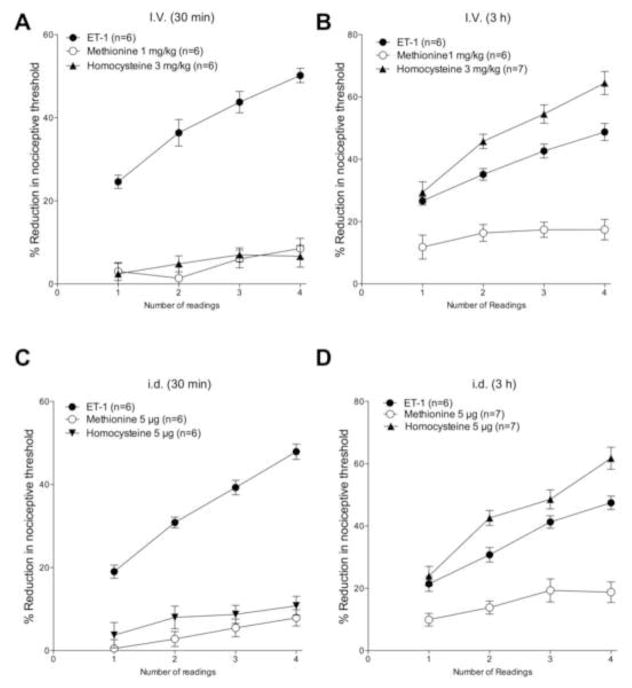
We studied the effect of systemic (intravenous, i.v.) administration of homocysteine and methionine on ET-1 induced mechanical hyperalgesia and mechanical stimulus-dependent enhancement of this hyperalgesia. Intradermal (i.d.) administration of ET-1 (100 ng) on the dorsum of the hind paw produced mechanical hyperalgesia, which increased in response to further mechanical stimulation, when threshold nociceptive mechanical stimuli were presented at 5 min intervals (i.e., stimulus-dependent hyperalgesia (Joseph et al., 2011)). When administered 30 min before ET-1, both homocysteine (3 mg/kg i.v.) and methionine (1 mg/kg i.v.) attenuated ET-1 induced mechanical hyperalgesia (first reading; Fig. 1A, 2-way ANOVA with Dunnet’s multiple comparisons test, P < 0.0001, N = 6) and stimulus-induced enhancement of ET-hyperalgesia. Both homocysteine and methionine also completely attenuated ET-1–induced stimulus-dependent hyperalgesia (Fig. 1A, P = N.S. for both, 1st reading vs. 4th reading, paired Student’s t-test, N = 6 for both). Of note, neither intravenous homocysteine nor intravenous methionine alone affected baseline nociceptive threshold (data not shown). In contrast, when administered 3 h before ET-1, neither homocysteine nor methionine alone affected the magnitude of ET-1 induced mechanical hyperalgesia (first reading; Fig. 1B, P = N.S., methionine N = 6, homocysteine N = 7). However, methionine administered 3 h before ET-1 still completely attenuated ET-1–induced stimulus-dependent hyperalgesia (Fig. 1B, P=N.S. for both, 1st reading vs. 4th reading, paired Student’s t-test, N = 6). In contrast, homocysteine administered 3 h before ET-1 significantly enhanced stimulus-dependent hyperalgesia (P < 0.05, 2-way repeated measures ANOVA, Dunnett’s multiple comparisons test N = 7).
Figure 1. Effect of methionine and homocysteine on ET-1 hyperalgesia and stimulus-dependent hyperalgesia cutaneous cutaneously.
ET-1 (100 ng, i.d. on dorsum of the hind paw) induced mechanical hyperalgesia (reading 1, 15 min post-administration) that was enhanced by repeat threshold intensity stimuli applied at the site of nociceptive testing (i.e. stimulus-dependent hyperalgesia).
A. Intravenous administration of methionine (1 mg/kg) or homocysteine (3 mg/kg), 30 min before ET-1, markedly attenuated the ET-1 hyperalgesia, and completely eliminated stimulus-dependent hyperalgesia.
B. Intravenous administration methionine (1 mg/kg) or homocysteine (3 mg/kg), 3 h before ET-1 did not affect ET-1 hyperalgesia. Stimulus-dependent hyperalgesia was completely eliminated by methionine but not by homocysteine at the 3 h pretreatment time point.
C. Intradermal administration methionine (1 μg) or homocysteine (5 μg) 30 min before ET-1 markedly attenuated ET-1 hyperalgesia, and completely eliminated stimulus-dependent hyperalgesia.
D. Intradermal administration methionine (1 μg) or homocysteine (5 μg) 3 h before ET-1 did not affect ET-1 hyperalgesia. Stimulus-dependent hyperalgesia was completely eliminated by methionine but enhanced by homocysteine, at the 3 h pretreatment time point.
Intradermal homocysteine and methionine
Similar to intravenous administration, intradermal administration of both homocysteine or methionine (at the site of nociceptive testing) 30 min before ET-1 attenuated ET-1 induced mechanical hyperalgesia (1st reading; Fig. 1C, 2-way ANOVA with Dunnet’s multiple comparisons test, P < 0.0001, N = 6), and mechanical stimulus-induced enhancement of ET-1 hyperalgesia (Fig. 1C, P = N.S. for both, 1st vs. 4th readings, paired Student’s t-test, N = 6 for both). Of note, neither i.d. homocysteine nor i.d. methionine affected baseline nociceptive threshold (data not shown). When administered 3 h before ET-1, neither methionine nor homocysteine affected ET-1 hyperalgesia (1st reading, 1D, P = N.S., methionine N = 6, homocysteine N = 7). Furthermore, homocysteine did not attenuate ET-1–induced stimulus-dependent hyperalgesia (Fig. 1D, 2-way repeated measures ANOVA, Dunnett’s multiple comparisons test, P = N.S.), while methionine significantly attenuated ET-1–induced stimulus-dependent hyperalgesia (P < 0.05, 1-way repeated measures ANOVA, Dunnett’s multiple comparisons test).
Ergonomic models
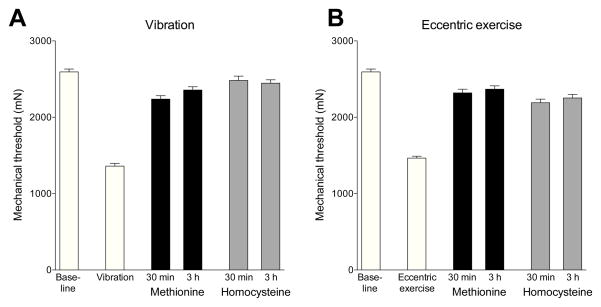
We studied the effect of methionine and homocysteine administration on muscle hyperalgesia produced by two ergonomic models: hind limb vibration and eccentric exercise (Alvarez et al., 2010; Dina et al., 2010). Both hind limb vibration (Fig. 2A) and eccentric exercise (Fig. 2B) produced a marked decrease in mechanical nociceptive threshold (both P < 0.0001, paired Student’s t-test, N = 12). Vibration- and eccentric exercise-induced muscle hyperalgesia was significantly attenuated at both 30 min and 3 h after administration of methionine (1 mg/kg, i.v.) or homocysteine (3 mg/kg, i.v.) (treatment versus vibration alone or eccentric exercise alone, P < 0.0001, one-way ANOVA, Tukey’s multiple comparisons test, N = 12).
Figure 2. Effect of intravenous methionine and homocysteine on muscle hyperalgesia and stimulus-dependent hyperalgesia produced by ischemia-reperfusion injury.
Exposure to either hind limb vibration (A) or eccentric exercise (B) produced a marked decrease in mechanical nociceptive threshold in the ipsilateral gastrocnemius muscle. Intravenous administration of either methionine (1 mg/kg) or homocysteine (3 mg/kg) prevented this hyperalgesia when evaluated 30 min and 3 h after administration.
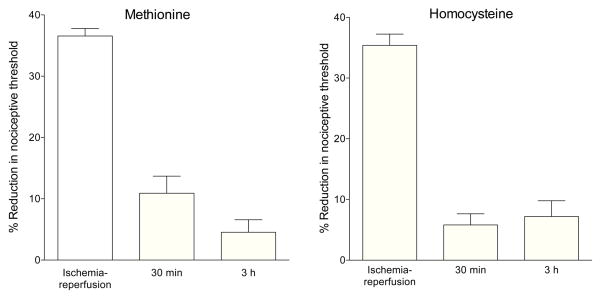
Ischemia-reperfusion injury
Ischemia-reperfusion injury (CRPS-I model) produced a marked decrease in mechanical nociceptive threshold in the gastrocnemius muscle evaluated 3 days later (Fig. 3A, B), and this hyperalgesia was significantly attenuated at both 30 min and 3 h after administration of methionine (1 mg/kg, i.v.) and after homocysteine (3 mg/kg, i.v.) (both treatments versus ischemia-reperfusion injury alone, P < 0.0001, one-way repeated measures ANOVA, Tukey’s multiple comparisons test, N = 6).
Figure 3. Effect of intravenous methionine and homocysteine on muscle hyperalgesia and stimulus-dependent hyperalgesia produced by vibration and by eccentric exercise.
Intravenous administration of: (A) methionine (1 mg/kg) or (B) homocysteine (3 mg/kg), markedly attenuated the hyperalgesia induced by ischemia reperfusion, evaluated 30 min and 3 h after administration.
Discussion
Treatment of a wide spectrum of vascular pain syndromes is a critical unmet medical need. The development of effective treatments for these common, debilitating pain syndromes is, however, hampered by our limited understanding of the role of the blood vessel in either acute or chronic pain. Recently, it has been appreciated that the vascular endothelium, the single-celled interface between the vascular lumen (containing circulating immune cells, inflammatory mediators, and hormones) and the vascular nociceptor (the origin of the vascular pain signal), also participates in signaling cascades that can enhance nociceptor function (Joseph et al., 2011; Joseph et al., 2013; Joseph and Levine, 2012a; Joseph and Levine, 2012b). Endothelial cells respond to vasoactive mediators, such as endothelin (Davenport and Maguire, 2006) to, in turn, release other pronociceptive mediators that can act at the peripheral terminal of the nociceptor (Burnstock, 1989; Burnstock, 1996; Burnstock, 2009; Joseph et al., 2011; Joseph et al., 2013). Specifically, we have shown that ET-1 and epinephrine (but not several other pronociceptive inflammatory mediators) act at their cognate receptors on endothelial cells to activate a mechanism by which further mechanical stimulation can now induce enhanced release of pronociceptive mediators (which includes ATP (Yegutkin et al., 2000),) from the endothelial cell to act on receptors on the primary afferent nociceptor. This mechanical stimulus-induced release of pronociceptive mediators from endothelial cells is enhanced by mechanical stimulation, stimulation-dependent hyperalgesia, a potentially important aspect of many vascular pain syndromes. Since stimulus-dependent hyperalgesia is not mediated by receptors for ET-1 on the peripheral terminal of the nociceptor, since spinal intrathecal administration of oligodeoxynucleotide antisense to mRNA for these receptors blocks ET-1- and epinephrine-induced hyperalgesia without affecting stimulus-dependent hyperalgesia (Joseph and Levine, 2012a), we hypothesize that stimulus-dependent hyperalgesia depends critically on endothelial cells, with ET-1 acting at its receptors on the vascular endothelial cell, to produce stimulus-dependent hyperalgesia. This hypothesis is supported by the observation that stimulus-dependent hyperalgesia induced by ET-1 is attenuated by octoxynol-9, which impairs endothelial cell function (Joseph and Levine, 2012a), without affecting hyperalgesia that is produced by action of ET-1 on its receptors on the nociceptor.
Although a role for endothelial cells in peripheral pain mechanisms was first suggested many years ago (Burnstock, 1999), technical challenges prevented a direct test of the hypothesis. However, we recently adapted a technique employed by cardiovascular and renal vascular researchers, the impairment of endothelial cell function using intravascular administration of the plasma membrane active compound, octoxynol-9 (Castro-Chaves et al., 2006; Mink et al., 2007; Sun et al., 1997), to directly assess its role in peripheral pain mechanisms, and showed that the endothelial cell plays a critical role in models of diverse pain syndromes (Joseph et al., 2013). In the current study, we used a method that produces endothelial cell dysfunction, based on the clinical observation in patients with hyperhomocysteinemia (Bellamy et al., 1998; Chao et al., 2000; Upchurch et al., 1996), and in the rats receiving the precursor, methionine in their diet or acutely administered to induce hyperhomocysteinemia and endothelial cell dysfunction (De Vriese et al., 2004; Sharma et al., 2013). We observed that administration of homocysteine intravenously produced a time-dependent attenuation and then enhancement of stimulus-dependent hyperalgesia. The sequence of actions of homocysteine to first attenuate and then enhance stimulus-dependent hyperalgesia remain to be elucidated. While 15 days of hyperhomocysteinemia produces an increase in ET-1 receptor (ET(A)) density (de Andrade et al., 2009), further studies are need to determine whether acute intravenously administered homocysteine can induce such a rapid upregulation of ET-1 receptors, although it has been observed that ET-1 receptors on blood vessels are rapidly upregulated when rat coronary arteries are isolated for short-term organ culture (Skovsted et al., 2012). How methionine attenuated stimulus-dependent hyperalgesia at both early and late time points after its administration might be explained by differences in the concentration of homocysteine over time. A very detailed dose-response and time-effect study of homocysteine and methionine effects on stimulus-dependent hyperalgesia would be required to address this bimodal effect of homocysteine.
We observed that hyperalgesia produced in preclinical models of pain syndromes, in which there is believed to be a vascular component, namely vibration- and eccentric exercise-induced muscle hyperalgesia, as well an ischemia-reperfusion model of CRPS-I, could each be reversed by both intravenous methionine and homocysteine. Of note, octoxynol-9 has previously been shown to reverse muscle hyperalgesia following hind limb vibration and eccentric exercise (Joseph et al., 2013), providing additional support for a key role for the endothelial cell in vascular pain syndromes. In contrast to the effect of homocysteine on ET-1-induced stimulus-dependent hyperalgesia, there was no enhancement of hyperalgesia 3 h after homocysteine administration in these three preclinical models, suggesting that different mediators may be released in these models. Future experiments evaluating selective receptor antagonists are needed to understand the mechanisms underlying this differential effect of homocysteine administration.
In summary, ET-1 and endothelial dysfunction are thought to play a role in vascular pain syndromes. The current study, in which we induce hyperhomocysteinemia to impair endothelial cell function, extends our earlier studies using octoxynol-9 to modulate stimulus-dependent and mechanical hyperalgesia induced in the hind limb vibration and eccentric exercise ergonomic models. The vascular endothelial cell provides an interface between circulating cells involved in inflammation and vascular sensory nerve fibers that signal pain. We have shown that the endothelium can play an important role in peripheral pain mechanisms, with the potent vasoactive peptide, ET-1, producing pain by acting both on endothelial cells and pain sensory neurons. A greater understanding of the role of the endothelial cell and its pronociceptive mediators that contribute to vascular pain will provide a rational basis for the treatment of vascular pain. For example, elevated levels of homocysteine have been reported in unstable angina (Guo et al., 2009), acute coronary syndrome (Turgan et al., 1999) and cardiac symptom X (Timurkaynak et al., 2008), presumably related to its effect on vascular function (Bhagwat et al., 2009; Nakamura and Yoshizawa, 2002). Furthermore, experimental angina is more severe in rats with endothelial dysfunction, induced by hyperhomocysteinemia (Han et al., 2007). Of note, administration of folic acid lowers homocysteine levels (Armitage et al., 2010; Guo et al., 2009), reduces the frequency of chest pain, after chronic folic acid therapy, in cardiac syndrome X patients (Alroy et al., 2007). Taken together, these data support the suggestion that the endothelial lining of blood vessels plays an important role in pain and that further investigation of homocysteine lowering therapies in vascular pain is warranted.
Highlights.
Homocysteine (i.v.) first prevented then enhanced mechanical stimulus-induced enhancement of endothelin-1 hyperalgesia
Methionine (i.v.) only prevented mechanical stimulus-induced enhancement of endothelin-1 hyperalgesia
Both homocysteine and methionine attenuated mechanical hyperalgesia induced by exposure to vibration, and eccentric exercise
Both homocysteine and methionine significantly attenuated mechanical hyperalgesia induced by a model of CRPS I
This study provides support for a role of the endothelial cell in pain syndromes thought to have a vascular basis
Acknowledgments
This work was supported by NIH grant NS085831.
Footnotes
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abahji TN, Nill L, Ide N, Keller C, Hoffmann U, Weiss N. Acute hyperhomocysteinemia induces microvascular and macrovascular endothelial dysfunction. Arch Med Res. 2007;38:411–416. doi: 10.1016/j.arcmed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Alroy S, Preis M, Barzilai M, Cassel A, Lavie L, Halon DA, Amir O, Lewis BS, Flugelman MY. Endothelial cell dysfunction in women with cardiac syndrome X and MTHFR C677T mutation. Isr Med Assoc J. 2007;9:321–325. [PubMed] [Google Scholar]
- Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. Eur J Neurosci. 2010;32:819–825. doi: 10.1111/j.1460-9568.2010.07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Sleight P, Peto R, Collins R. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010;303:2486–2494. doi: 10.1001/jama.2010.840. [DOI] [PubMed] [Google Scholar]
- Bellamy MF, McDowell IF, Ramsey MW, Brownlee M, Bones C, Newcombe RG, Lewis MJ. Hyperhomocysteinemia after an oral methionine load acutely impairs endothelial function in healthy adults. Circulation. 1998;98:1848–1852. doi: 10.1161/01.cir.98.18.1848. [DOI] [PubMed] [Google Scholar]
- Bhagwat VR, Yadav AS, Rathod IM. Homocysteine, lipid indices and antioxidants in patients with ischaemic heart disease from Maharashtra, India. Singapore Med J. 2009;50:418–424. [PubMed] [Google Scholar]
- Burnstock G. The role of adenosine triphosphate in migraine. Biomed Pharmacother. 1989;43:727–736. doi: 10.1016/0753-3322(89)90161-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 1996;347:1604–1605. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic regulation of vascular tone and remodelling. Auton Autacoid Pharmacol. 2009;29:63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- Castro-Chaves P, Roncon-Albuquerque RJ, Leite-Moreira AF. Endothelin ETA receptors and endothelium partially mediate the positive inotropic and lusitropic effects of angiotensin II. Eur J Pharmacol. 2006;544:91–96. doi: 10.1016/j.ejphar.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Chao CL, Kuo TL, Lee YT. Effects of methionine-induced hyperhomocysteinemia on endothelium-dependent vasodilation and oxidative status in healthy adults. Circulation. 2000;101:485–490. doi: 10.1161/01.cir.101.5.485. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 2004;112:94–105. doi: 10.1016/j.pain.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Connor HE, Feniuk W. Influence of the endothelium on contractile effects of 5-hydroxytryptamine and selective 5-HT agonists in canine basilar artery. Br J Pharmacol. 1989;96:170–178. doi: 10.1111/j.1476-5381.1989.tb11797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport AP, Maguire JJ. Endothelin. Handb Exp Pharmacol. 2006:295–329. doi: 10.1007/3-540-32967-6_9. [DOI] [PubMed] [Google Scholar]
- de Andrade CR, Leite PF, Montezano AC, Casolari DA, Yogi A, Tostes RC, Haddad R, Eberlin MN, Laurindo FR, de Souza HP, Correa FM, de Oliveira AM. Increased endothelin-1 reactivity and endothelial dysfunction in carotid arteries from rats with hyperhomocysteinemia. Br J Pharmacol. 2009;157:568–580. doi: 10.1111/j.1476-5381.2009.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vriese AS, Blom HJ, Heil SG, Mortier S, Kluijtmans LA, Van de Voorde J, Lameire NH. Endothelium-derived hyperpolarizing factor-mediated renal vasodilatory response is impaired during acute and chronic hyperhomocysteinemia. Circulation. 2004;109:2331–2336. doi: 10.1161/01.CIR.0000129138.08493.4D. [DOI] [PubMed] [Google Scholar]
- Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms mediating vibrationinduced chronic musculoskeletal pain analyzed in the rat. J Pain. 2010;11:369–377. doi: 10.1016/j.jpain.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edirimanne VE, Woo CW, Siow YL, Pierce GN, Xie JY, OK Homocysteine stimulates NADPH oxidase-mediated superoxide production leading to endothelial dysfunction in rats. Can J Physiol Pharmacol. 2007;85:1236–1247. doi: 10.1139/Y07-112. [DOI] [PubMed] [Google Scholar]
- Guo H, Chi J, Xing Y, Wang P. Influence of folic acid on plasma homocysteine levels & arterial endothelial function in patients with unstable angina. Indian J Med Res. 2009;129:279–284. [PubMed] [Google Scholar]
- Han YL, Cheng C, Tan HM, Wu WK, Wu YL, Sun HL, Sun J, Chen JL. Effect of Tongxinluo superfine on experimental anginal model (contraction of collaterals) in rat with endothelial dysfunction. Zhongguo Zhong Yao Za Zhi. 2007;32:2404–8. 2426. [PubMed] [Google Scholar]
- Jamal A, Bendeck M, Langille BL. Structural changes and recovery of function after arterial injury. Arterioscler Thromb. 1992;12:307–317. doi: 10.1161/01.atv.12.3.307. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Green PG, Bogen O, Alvarez P, Levine JD. Vascular endothelial cells mediate mechanical stimulation-induced enhancement of endothelin hyperalgesia via activation of P2X2/3 receptors on nociceptors. J Neurosci. 2013;33:2849–2859. doi: 10.1523/JNEUROSCI.3229-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Gear RW, Levine JD. Mechanical stimulation enhances endothelin-1 hyperalgesia. Neuroscience. 2011;178:189–195. doi: 10.1016/j.neuroscience.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Role of endothelial cells in antihyperalgesia induced by a triptan and beta-blocker. Neuroscience. 2012a;232:83–89. doi: 10.1016/j.neuroscience.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Sexual dimorphism in endothelin-1 induced mechanical hyperalgesia in the rat. Exp Neurol. 2012b;233:505–512. doi: 10.1016/j.expneurol.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanani PM, Sinkey CA, Browning RL, Allaman M, Knapp HR, Haynes WG. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation. 1999;100:1161–1168. doi: 10.1161/01.cir.100.11.1161. [DOI] [PubMed] [Google Scholar]
- Millecamps M, Laferriere A, Ragavendran JV, Stone LS, Coderre TJ. Role of peripheral endothelin receptors in an animal model of complex regional pain syndrome type 1 (CRPS-I) Pain. 2010;151:174–183. doi: 10.1016/j.pain.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink SN, Cheng ZQ, Bose R, Jacobs H, Kasian K, Roberts DE, Santos-Martinez LE, Light RB. Lysozyme, a mediator of sepsis, impairs the cardiac neural adrenergic response by nonendothelial release of NO and inhibitory G protein signaling. Am J Physiol Heart Circ Physiol. 2007;293:H3140–H3149. doi: 10.1152/ajpheart.00502.2007. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yoshizawa H. Homocysteine as a risk factor for ischemic heart disease. Rinsho Byori. 2002;50:807–814. [PubMed] [Google Scholar]
- Pushpakumar SB, Kundu S, Sen U. Endothelial dysfunction: The link between homocysteine and hydrogen sulfide. Curr Med Chem. 2014 doi: 10.2174/0929867321666140706142335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragavendran JV, Laferriere A, Xiao WH, Bennett GJ, Padi SS, Zhang J, Coderre TJ. Topical combinations aimed at treating microvascular dysfunction reduce allodynia in rat models of CRPS-I and neuropathic pain. J Pain. 2013;14:66–78. doi: 10.1016/j.jpain.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Singh M, Sharma PL. Mechanism of hyperhomocysteinemia-induced vascular endothelium dysfunction - possible dysregulation of phosphatidylinositol-3-kinase and its downstream phosphoinositide dependent kinase and protein kinase B. Eur J Pharmacol. 2013;721:365–372. doi: 10.1016/j.ejphar.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Skovsted GF, Pedersen AF, Larsen R, Sheykhzade M, Edvinsson L. Rapid functional upregulation of vasocontractile endothelin ETB receptors in rat coronary arteries. Life Sci. 2012;91:593–599. doi: 10.1016/j.lfs.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Sun ZW, Wang XD, Deng XM, Wallen R, Gefors L, Hallberg E, Andersson R. The influence of circulatory and gut luminal challenges on bidirectional intestinal barrier permeability in rats. Scand J Gastroenterol. 1997;32:995–1004. doi: 10.3109/00365529709011216. [DOI] [PubMed] [Google Scholar]
- Timurkaynak T, Balcioglu S, Arslan U, Kocaman SA, Cengel A. Plasma homocysteine level in cardiac syndrome X and its relation with duke treadmill score. Saudi Med J. 2008;29:364–367. [PubMed] [Google Scholar]
- Turgan N, Boydak B, Habif S, Apakkan S, Ozmen D, Mutaf I, Bayindir O. Plasma homocysteine levels in acute coronary syndromes. Jpn Heart J. 1999;40:729–736. doi: 10.1536/jhj.40.729. [DOI] [PubMed] [Google Scholar]
- Upchurch GRJ, Welch GN, Loscalzo J. Homocysteine, EDRF, and endothelial function. J Nutr. 1996;126:1290S–1294S. doi: 10.1093/jn/126.suppl_4.1290S. [DOI] [PubMed] [Google Scholar]
- Yegutkin G, Bodin P, Burnstock G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Br J Pharmacol. 2000;129:921–926. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]