Abstract
The definition of tumor deposits (TD) in colonic adenocarcinoma has been modified in different editions of AJCC/TNM staging system. Studies have shown that the presence of TD is associated with advanced tumor growth and poor prognosis. Most of these data were obtained in patients with simultaneous lymph node (LN) metastases. Reports focusing on the impact of TD in patients without LN metastasis are limited. We retrospectively restaged all right-sided colonic adenocarcinoma over a 10-year period using criteria from the 5th, 6th, and 7th AJCC edition. We compared the number of tumor nodule interpreted as LN and TD in each edition, and evaluated the stage migration caused by TD definition change. We then assessed clinical significance of TD in AJCC 7th edition by comparing 5-year overall survival of N1c patients vs. other N category (N0, N1, N2) patients with similar T and M status. We showed that average number of tumor nodule interpreted as LN per case and number of cases with positive LN were significantly decreased with 7th edition compared to 5th/6th; however, numbers of cases with TD and <12 LN were significantly increased with 7th edition compared to 5th/6th. These changes, however, resulted in minimal effects on the final stage grouping. Our survival analysis showed that N1c patients had significantly worse survival compared to N0 patients. Although not statistically significant, the hazard ratios indicated that N1c group might have worse survival than N1 group and better survival than N2 group. Therefore, we conclude that TD predict patient outcome at least similarly to positive LN.
Keywords: tumor deposits, lymph node, colonic adenocarcinoma, staging, survival
INTRODUCTION
Tumor deposits (TD) in the pericolorectal adipose tissue of patients with colorectal adenocarcinoma have been recognized since early in the 20th century. (1) The major origins of TD include discontinuous tumor spread, venous invasion with extravascular spread, or a totally replaced lymph node (LN). Over the years, the definition and clinical impact of TD have been discussed among pathologists, surgeons, and clinical oncologists. The definition of TD vs. LN has been revised from the 5th, the 6th, to the most recent 7th edition of the American Joint Committee on Cancer (AJCC)/Tumor Node Metastasis (TNM) staging system (Figure 1). TD were first introduced in the 5th edition of AJCC/TNM staging system in 1997 (2–3), in which tumor nodules greater than 3mm in diameter without histologic evidence of a residual LN were classified as regional LN metastasis; however, tumor nodules 3mm or less were classified in the T category as a discontinuous extension. In the next edition (6th, 2002) (4–5), the 3 mm size rule was withdrawn, and the new classification was based on the contour. If the tumor nodules had the form and smooth contour of a LN, they were classified as regional LN metastasis; if the nodules had an irregular contour, they were classified as discontinuous extension. In the most recent 7th edition (2010), (6–7) TD were defined by identifying features of residual LN architecture instead of using a specific size and contour rule. Discrete foci of tumor in the pericolorectal fat showing no evidence of residual LN tissue are considered to be peritumoral TD, and their number should be recorded in the pathology report. In addition, TD were moved from their prior involvement of T category to the formation of a new nodal subclassification of N1c, if there is no concurrent positive LN.
Figure 1.
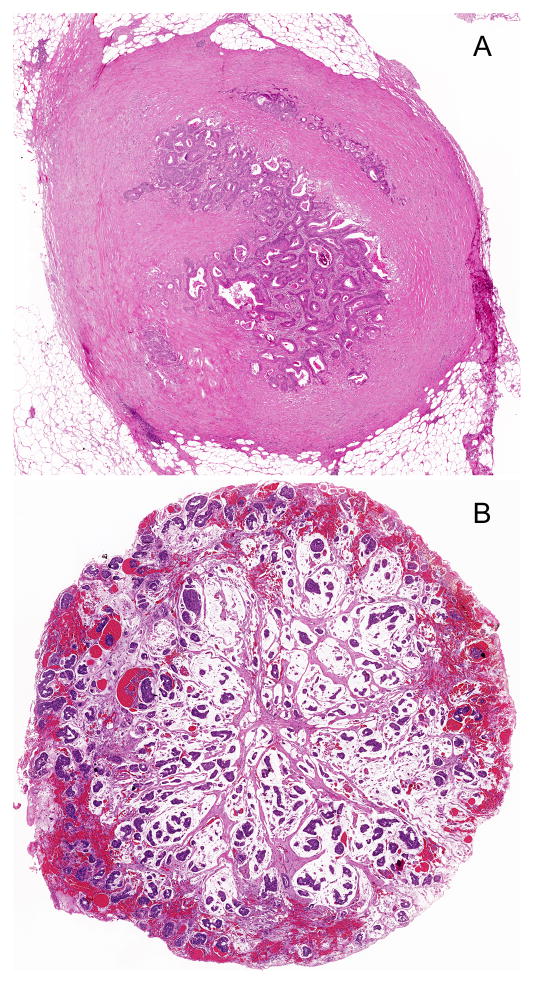
Two Examples of TD vs. LN in 5th, 6th, and 7th editions of AJCC/TNM staging system. A and B: Both tumor nodules would be classified as LN in the 5th edition because their sizes are greater than 3mm; they would be classified as LN in the 6th edition because they have round shape; and they would be classified as TD in the 7th edition because there is no definite residual LN (Hematoxylin-eosin stain, original magnification × 20).
The goal of cancer staging is to provide evidence based guidelines for clinical decision making in treatment plans, clinical trial candidacy, and estimating prognosis. Studies have shown that presence of TD is associated with advanced tumor growth and poor prognosis. (8–10) Most of these data were obtained in patients with simultaneous LN metastases. Reports focusing on the impact of TD in patients without LN metastasis are limited but vital to determining the importance of TD.
In this study, we retrospectively collected and restaged all right-sided colonic adenocarcinoma over a 10-year period from our institution using criteria from each AJCC edition. We compared the number of LN and TD in each edition, and evaluated the stage migration caused by TD definition change. We then assessed the clinical significance of TD by comparing the 5-year overall survival of N1c patients vs. other N category patients with similar T and M status. The new category of N1c in the 7th edition was created to allow data collection and outcome analysis to be performed to better understand the clinical significance of TD. We aim to provide more evidence and rationale for future AJCC/TNM staging system.
MATERIALS AND METHODS
Patient Selection
All right-sided primary colonic adenocarcinomas (438 cases) with available slides over a 10-year period (2001–2010) at the Ohio State University Wexner Medical Center were retrieved and reviewed by pathologists with an interest in gastrointestinal pathology.
For five year survival comparisons between N1c tumors with N0, N1 (N1a and N1b) and N2 tumors, we first excluded all Tis, T1 and T2 patients (n=97) since only two cases were T2N1c and none of T1 cases were N1c. In addition, 57 cases with positive M status were also excluded, as well as one case with a survival time of zero. Therefore, our comparison groups similarly included T3 and T4 tumors without distant metastasis. From the original cohort of 438 adenocarcinomas, there were 283 T3 or T4 and M0 cases that were used in survival calculations.
Staging Using AJCC 5th, 6th, and 7th Editions
All cases were restaged using AJCC 5th, 6th, and 7th edition criteria with particular attention paid to the precise definitions of TD, metastatic LN, and the depth of invasion. Stage migration due to TD definition change between 6th and 7th editions was assessed by comparing TNM stage and stage grouping status in each case.
Statistical Methods for Staging and Survival
Both TD and LN were compared between the three different editions. The average LN per case was estimated with 95% confidence intervals (CI) for each edition separately using repeated measures ANOVA. From the model, differences in the average number of LN between the 5th and 6th editions with the 7th edition were assessed. McNemar’s test was used to compare the proportions of patients with TD, <12 LN, and at least one positive LN in the 5th and 6th editions with the 7th edition.
For five year survival analysis, age and gender were compared between the four N groups (N1c, N0, N1, and N2) within the survival cohort using ANOVA and a chi-square test, respectively. Kaplan-Meier estimates of the survival function were produced to assess crude differences in five year survival, and the log-rank test was performed to test for differences in survival functions. Estimated hazard ratios (HR) and 95% CI for N1c vs. N0, N1, and N2 were calculated from both univariable (unadjusted) and age-adjusted Cox proportional hazards models. Note that gender and year of resection were initially considered as adjustment factors in addition to age; however, due to lack of evidence of being either significant predictors or confounders/effect modifiers of the relationship between N group and survival, they were not included in the final adjusted model. For both the univariable and age-adjusted models, the proportional hazards assumption for each variable in the model was assessed both graphically and by including the interaction with time (natural log scale); no serious deviations were observed. All analyses were performed using SAS/STAT software version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Staging Using AJCC 5th, 6th, and 7th Editions
A comparison summary of both LN and TD for our 438 cases by edition is shown in Table 1. The estimated average number of tumor nodule interpreted as LN per case based on the 7th edition was 21.2 (95% CI: 20, 22.3), significantly lower than the estimated LN per case according to both the 5th (estimated LN per case = 22.2; 95% CI: 21, 23.4) and 6th (estimated LN per case =21.9; 95% CI: 20.8, 23) editions (p<0.001 for both comparisons). Similarly, the number of cases with positive LN was significantly lower in the 7th edition compared to both the 5th and 6th editions (161 in the 7th edition vs. 180 for both the 5th and 6th editions; p<0.001 for both comparisons). Conversely, the number of cases with TD and the number of cases with <12 LN were both significantly increased in the 7th compared to 5th/6th editions (for TD: 123 in the 7th edition vs. 53 and 65 in the 5th and 6th editions, respectively; for <12 LN: 71 in the 7th edition vs. 50 and 55 in the 5th and 6th editions, respectively; p<0.001 for all comparisons).
Table 1.
Comparison of LN and TD Using AJCC 5th, 6th, 7th Edition
| AJCC edition | Total LN | Total +LN | Average LN per case (95% CI) | # of cases with + LN (%) | # of cases with TD (%) | # of cases <12 LN (%) |
|---|---|---|---|---|---|---|
| 5th | 9737 | 1120 | 22.2(21, 23.4) | 180 (41%) | 53 (12%) | 50 (11%) |
| 6th | 9606 | 977 | 21.9(20.8, 23) | 180 (41%) | 65 (15%) | 55 (13%) |
| 7th | 9264 | 630 | 21.2(20, 22.3) | 161 (37%) | 123 (28%) | 71 (16%) |
LN: lymph node; TD: tumor deposits; CI: confidence intervals
Nineteen (4%) of 438 cases showed a stage grouping migration between 6th and 7th editions due to TD definition change. The stage grouping migration in all 19 cases was caused primarily by an N category change. Of these 19 cases, 13 down-migrated from IIIC in the 6th to IIIA/B in the 7th due to a change from N2 in the 6th to N1 (N1a, N1b, and N1c) in the 7th; and 6 up-migrated from IIA/B in the 6th to IIIB/C in the 7th due to a change from N0 in the 6th to N1c in the 7th. No stage grouping migration in these 19 cases was caused by a T category change. Nineteen (4%) of 438 cases had a N category change due to TD definition change, but did not cause final stage grouping migration because of concurrent M1 status.
Thirty-two (7%) of 438 cases had stage grouping migration due to expanded subclassification in AJCC 7th edition.
Survival Analysis
Of the 438 patients in our study, 283 were T3 or T4 with M0 status. These patients were separated into four groups based on N status (Table 2): 17 were N1c, 162 were N0, 69 were N1 (N1a and N1b), and 35 were N2. There were no significant differences in gender (p=0.326) between different N groups; for age, it appears as though N1 patients were significantly younger compared to N0 patients (mean age 62.5 vs. 68.5 years; p=0.020). No other age differences between groups were noted.
Table 2.
Demographic Summary of N1c and Other N Groups
| Characteristic | N0 (n=162) | N1 (n=70) | N1c (n=17) | N2 (n=35) |
|---|---|---|---|---|
| Male: N (%) | 92 (57%) | 37 (53%) | 10 (59%) | 14 (40%) |
| Age: mean (SD) (range) | 68.5 (14.2) (32 – 97) | 62.5 (15.9) (24 – 93) | 66.9 (14.5) (33–83) | 67.6 (12.5) (38 – 90) |
SD: standard deviation
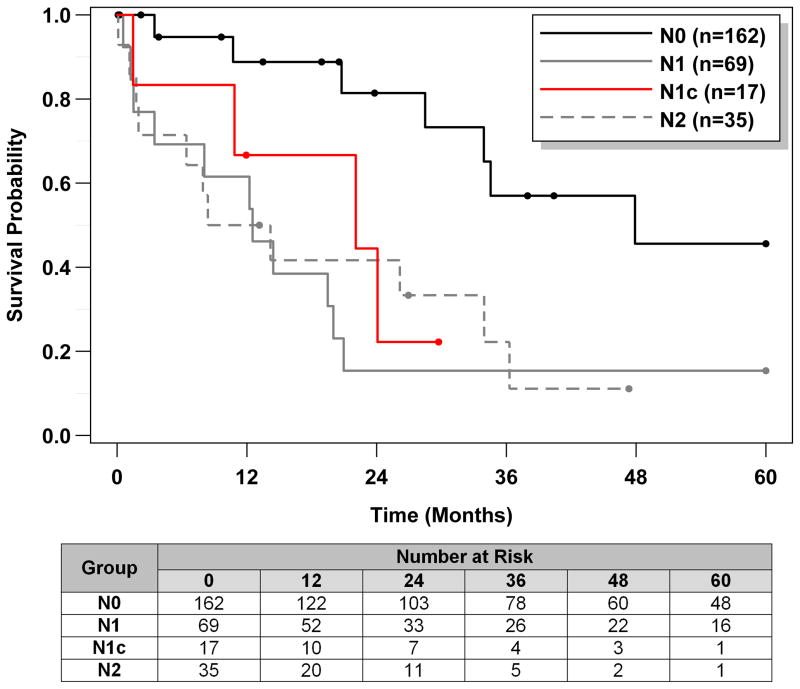
Overall five year survival of patients with N1c tumor was compared to that of N0, N1, and N2. One hundred thirty-seven of 283 (48%) of the patients died within five years of resection. Kaplan-Meier estimates of the survival function for each N group are shown in Figure 2, and the median survival times with 95% CI and log-rank test results for each N group are given in Table 3. Note that 67% of the N0 patients were censored at five years; consequently, the median survival time could not be calculated for this group. Based on the survival curves, N1c patients appeared to have worse survival compared to N0 patients (log-rank p<0.001). Although the median survival time of N1c (23.5 months; 95% CI: 11.5, 41.0) was shorter than that of N1 (31.4 months; 95% CI: 19.5, 48.1; p=0.208) and longer than that of N2 (16.7 months; 95% CI: 7.9, 24.8; p=0.179), the confidence intervals were wide, reflecting the relatively small sample sizes and differences were not statistically significant.
Figure 2.
Kaplan-Meier estimates of the survival function for N1c and other N groups.
Table 3.
Median Survival Times of N1c vs. Other N Groups
| Group | N | Deaths (%) | Median survival time (months), with 95 %CI | Log-rank test vs. N1c |
|---|---|---|---|---|
| N0 | 162 | 54 (33%) | --- | <.001 |
| N1 | 69 | 41 (59%) | 31.4 (19.5, 48.1) | 0.208 |
| N1c | 17 | 13 (76%) | 23.5 (11.5, 41.0) | --- |
| N2 | 35 | 29 (83%) | 16.7 (7.9, 24.8) | 0.179 |
CI: confidence intervals
The univariable (unadjusted) HR for N0, N1, and N2 vs. N1c with 95% CI from a Cox proportional hazards model are shown in Table 4. Compared to N1c, N0 was protective in terms of survival: the HR was 0.32 (<1), meaning that the death rate throughout the follow-up period for patients with N0 was roughly one third the death rate of patients with N1c (95% CI: 0.18, 0.61; p<0.001). Similarly, the HR for N1 vs. N1c was also less than 1, although not statistically significant (estimated HR = 0.66; 95% CI: 0.36, 1.28; p=0.192). The HR for N2 vs. N1c was greater than 1, implying that the rate of death was greater for N2 compared to N1 throughout the follow-up period; again this difference was not statistically significant (p=0.231). Results from the age-adjusted model were similar to the univariable model.
Table 4.
Cox Model Results of N1c vs. Other N Groups
| Model | Comparison | Estimated HR | 95% CI | p-value |
|---|---|---|---|---|
|
| ||||
| Univariable (unadjusted) | N0 vs. N1c | 0.32 | (0.18, 0.61) | <.001 |
| N1 vs. N1c | 0.66 | (0.36, 1.28) | 0.192 | |
| N2 vs. N1c | 1.49 | (0.79, 2.97) | 0.231 | |
| N1 vs. N2 | 0.48 | (0.32, 0.72) | <.001 | |
|
| ||||
| Adjusted for age | N0 vs. N1c | 0.29 | (0.16, 0.56) | <.001 |
| N1 vs. N1c | 0.74 | (0.41, 1.43) | 0.340 | |
| N2 vs. N1c | 1.61 | (0.85, 3.20) | 0.158 | |
| N1 vs. N2 | 0.39 | (0.26, 0.59) | <.001 | |
HR: hazard ratio; CI: confidence intervals
The log-rank test as well as the univariable and age-adjusted Cox models all indicated that there were significant survival differences between N1 (N1a, N1b) and N2, which validated the existing TNM staging rationale.
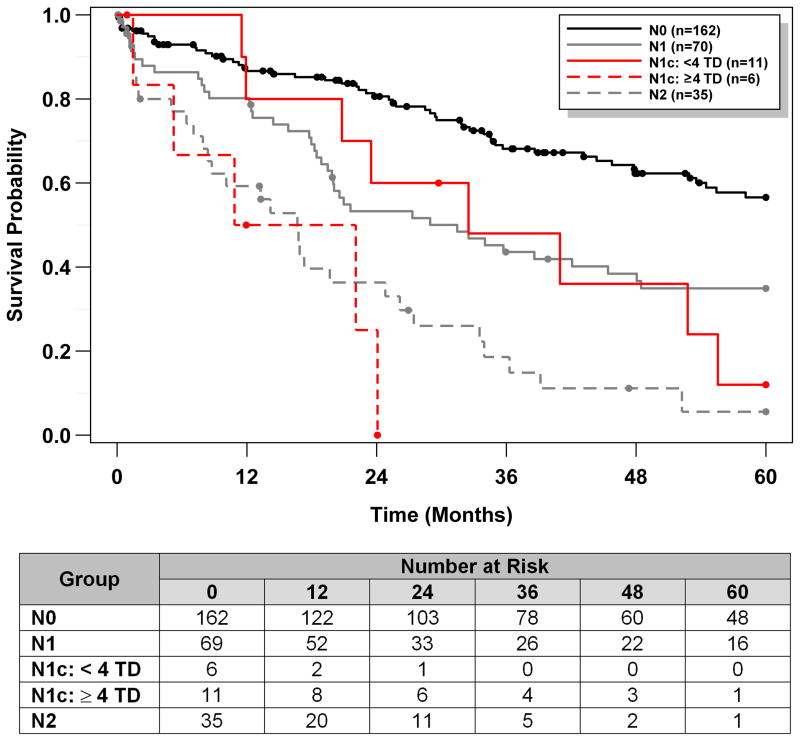
The number of TD in our 17 N1c cases ranged from 1 to >20. To evaluate the potential impact of TD number on N1c patient survival, we divided these patients into two groups based on the number of TD; the cut point of 4 TD was chosen based on N-stage system of N1 (positive LN <4, N1a and N1b) and N2 (positive LN ≥4). As a sensitivity analysis, we explored different cut points based on our data (Table 5). Note that there were no cases with 6 or 7 TD, and therefore we could not consider 6, 7 TD as potential cut points. In addition, there are only 4 patients with ≥ 8 TD, making higher cut points not feasible. Cut points of 4 TD and 5 TD both resulted in significant differences in survival, based on the log-rank test. Note however that the confidence intervals for median survival times are very wide, reflecting the fact that we only had information on 17 patients. Although our data are limited, these results do appear to provide some support of the cut point chosen based on N-stage system of N1 (positive LN <4, N1a and N1b) and N2 (positive LN ≥4). Kaplan-Meier estimates of the survival function using cut point of 4 TD are shown in Figure 3, and median survival times with 95% CI are presented in Table 5. Note that the median survival time for N1c patients with <4 TD was 32.5 months (95% CI: 11.5, 55.6) compared to only 16.5 months for N1c patients with ≥4 TD (95% CI: 1.5, 24.1). Estimates from the univariable Cox model using cut point of 4 TD are shown in Table 6. While not statistically significant, it appeared as though patients with N1c: <4 TD might have superior survival compared to N1c: ≥4 TD (estimated HR=0.37; 95% CI: 0.12, 1.25; p=0.087).
Table 5.
Median Survival Times of N1c Subgroups
| Cut point | Groups | Median Survival Time (95% CI) | Log-rank p-value |
|---|---|---|---|
| 2 | TD < 2 (n=8) TD 2+ (n=9) |
32.5 (11.5, 55.6) 23.5 (1.5, 52.8) |
0.317 |
| 3 | TD < 3 (n=9) TD 3+ (n=8) |
36.7 (11.5, 55.6) 22.1 (1.5, 24.1) |
0.117 |
| 4 | TD < 4 (n=11) TD 4+ (n=6) |
32.5 (11.5, 55.6) 16.5 (1.5, 24.1) |
0.025 |
| 5 | TD < 5 (n=12) TD 5+ (n=5) |
32.5 (11.9, 55.6) 10.9 (1.5, 24.1) |
0.033 |
| 8* | TD < 8 (n=13) TD 8+ (n=4) |
28.0 (11.5, 52.8) 17.5 (1.5, 24.1) |
0.162 |
TD: tumor deposits; CI: confidence intervals
6 and 7 were not included since no cases contained this number of tumor deposits
Figure 3.
Kaplan-Meier estimates of the survival function for each N group, with further separation of N1c into two subgroups of N1c: <4 TD, and N1c: ≥4 TD.
Table 6.
Cox Model Results of N1c Subgroups
| Model | Comparison | Estimated HR | 95% CI | p-value |
|---|---|---|---|---|
|
| ||||
| Univariable (unadjusted) | N1 vs. N1c: <4 TD | 0.87 | (0.43, 1.99) | 0.709 |
| N1c: <4 TD vs. N1c: ≥4 TD | 0.37 | (0.12, 1.25) | 0.087 | |
| N2 vs. N1c: ≥4 TD | 0.74 | (0.31, 2.18) | 0.532 | |
TD: tumor deposits; CI: confidence intervals
DISCUSSION
In this study we included all right-sided primary colonic adenocarcinomas with available slides over a 10-year period in our institution. We limited our analysis to right side colon cancers to minimize any potential effects on outcome due to tumor site. We avoided any rectal or even low sigmoid cancer that may have had pretreatment. Rectal cancer patients usually receive neoadjuvant therapy preoperatively, and the prognostic significance of TD in these patients may be different from that in the right-sided colon cancer patients without neoadjuvant therapy. (8, 11) A recent study has shown that TD in rectal adenocarcinoma after neoadjuvant chemoradiation has been associated with poor prognosis. (12)
We found that the development of the new N1c category and changes to the definition of TD in the AJCC 7th edition affected the number of tumor nodule interpreted as LN per case, and the number cases with positive LN, TD, and <12 LNs after extensive LN search. In our study, the incidence of TD was 29% (123/438), and the incidence of LN metastasis was 37% (161/438) using 7th edition. These data were similar or within the range of previous reports. (9, 13) Oncologists and surgeons should expect the number of cases with <12 LNs to significantly increase since some of those previously counted as LNs are now considered TDs. This will likely have a minimal clinical impact since all of these patients will be considered at least N1 and staged as III, and likely receive additional treatment.
The TD definition change resulted in only minimal effects on the final stage grouping between 6th and 7th editions. Only 4% (19/438) showed a stage grouping migration due to TD definition change between 6th and 7th editions. The stage grouping migration was present in both directions, and was caused primarily by N category change. None of the stage grouping migration was caused by T category change. Although the stage down-migration from IIIC to IIIA/B probably would not make much difference in clinical management; the stage up-migration from IIA/B to IIIB/C likely results in additional treatment. Nagtegaal et al (9) reported that every change in edition of TNM led to a stage migration of between 33% and 64% in patients with TD. Several study design differences can explain the lower percentage of stage grouping migration in our result. Firstly, we included all the colon cancer patients for calculation; Nagtegaal et al only included the patients with TD. When we limited our cohort to consider only the patients with TD, 15% (19 of 123) of cases with TD showed final stage migration due to TD definition changes. Also, another 15% (19 of 123) of cases with TD had N category change due to TD definition change, but did not cause stage grouping migration because of concurrent M1 status. Secondly, our result was calculated from the migration change only due to TD definition change, and the changes due to expanded subclassification were not included. There were 7% (32 of 438) of our total cases with stage grouping migration due to expanded subclassification in AJCC 7th edition. Lastly, the patient population in the previous study was from Europe; while our patient cohort was from the United States.
Studies have shown that presence of TD was associated with advanced tumor growth including higher T stage, LN and distant metastases, positive circumferential resection margin, poor differentiation, and extramural vascular invasion as well as poor prognosis. (8–10, 13–17) Most of these data were obtained in patients with simultaneous LN metastases. Whether this still holds true for patients without LN metastases has not been well studied. Only limited reports from China (16) and the Netherlands (18) have discussed the prognostic value of TD in patients without LN metastasis and suggested that the 7th edition of TNM staging satisfactorily predicts patients’ outcome for those without LN metastasis. To better address the sole impact of TD on survival in our population, our survival analysis focused on N1c patients vs. N0 or N1 (N1a and N1b) or N2 patients with similar T and M status. Our study showed that N1c patients had significantly worse outcome compared to N0 patients. Although no statistically significant differences were found between the groups of N1c and N1 or N1c and N2, the HR indicated that the N1c group might have worse survival compared to the N1 group and better survival than the N2 group. Our data suggest that TD with no LN metastasis may behave similarly to positive LN, which supports the creation of the new category of N1c in 7th edition since it would up-grade patients into stage III and likely result in additional chemotherapy. Since inter-observer variability exists, (19) some recent studies have proposed to classify TD as LN to decrease the subjectivity in assessments of TD. (17, 20)
We further investigated if the number of TD affects patient survival. Our analysis showed that the cut points of 4 TD and 5 TD resulted in significant differences in survival based on the log-rank test. Although it was difficult to estimate survival in the TD subgroups as shown by the wide confidence intervals for median survival times, we were pleased to note that our data, while limited, do appear to provide some support of the cut point chosen based on N-stage system of N1 (positive LN <4, N1a and N1b) and N2 (positive LN ≥4). Note that our results should be interpreted with caution due to the small number of patients with N1c; however, we believe that our results provide some additional evidence that TD might affect survival in a similar fashion as positive LN. The association between TD characteristics and prognosis varied in previous studies. Tong et al (16) showed that the number of TD was not an independent prognostic parameter in the TNM staging system. However, Ueno et al (13) concluded that an increasing number of TD was significantly relevant to adverse survival outcome in both of their cohorts.
Our study was a single-institution retrospective study, and therefore our results may not be generalizable to the general population. Although we observed certain trends in survival mentioned above, we were not able to fully explore potential group differences and draw firm conclusions due to the small sample sizes in some groups. In particular, further stratification of T3 and T4 within the N1c group was not possible due to the very limited number of T4N1c. Another limitation in our work is that we did not evaluate whether other characteristics of TD, such as location, diameter, and shape, played a role in prognosis because the sample size for N1c was too small. Some of these characteristics might be associated with TD origin, which can be difficult to determine in some cases. Dr. Puppa proposed one of the types of TD to be a conceptual model of in-transit carcinoma, which heralds a high risk of local and systemic recurrence. (21–24) Additional studies with larger numbers of cases may be helpful to address these issues.
In summary, our study showed that average number of tumor nodule interpreted as LN per case and the number of cases with positive LN were significantly decreased with 7th edition compared to 5th/6th edition; however, the numbers of cases with TD and <12 LN were significantly increased with 7th edition compared to 5th/6th edition. These changes, however, resulted in only minimal effects on the final stage grouping. In addition, our results revealed that N1c patients had significantly worse survival compared to N0 patients. Although no statistically significant differences were found between the groups of N1c and N1 or N1c and N2, the hazard ratios indicated that the N1c group might have worse survival than the N1 group and better survival than the N2 group. Therefore, we conclude that TD predict patient outcome at least similarly to positive LN. Many questions remain about the definition and origin of TD. Optimal classification will likely evolve as more outcome studies are completed.
Acknowledgments
supported in part by the Vanderbilt SPORE in Gastrointestinal Cancer (MKW) (NCI 5 P50 CA95103-12).
References
- 1.Gariel WB, Dukes CE, Bussey HJR. Lymphatic spread in cancer of the rectum. Br J surg. 1935;23:395–413. [Google Scholar]
- 2.Fleming ID, Cooper JS, Hensen DE, et al. AJCC Cancer Staging Manual. 5. Philadelphia, PA: Lippincott-Raven Publishers; 1997. pp. 83–88. [Google Scholar]
- 3.Sobin LH, Wittekind C. IUCC TNM Classification of Malignant Tumours. 5. Hoboken, NJ: John Wiley & Sons; 1997. [Google Scholar]
- 4.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6. New York, NY: Springer-Verlag; 2002. pp. 113–124. [Google Scholar]
- 5.Sobin LH, Wittekind C. IUCC TNM Classification of Malignant Tumours. 6. Hoboken, NJ: John Wiley & Sons; 2002. pp. 72–76. [Google Scholar]
- 6.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7. New York, NY: Springer-Verlag; 2010. pp. 143–164. [Google Scholar]
- 7.Sobin LH, Gospodarowicz M, Wittekind C. IUCC TNM Classification of Malignant Tumours. 7. Hoboken, NJ: Wiley-Blackwell; 2009. pp. 100–105. [Google Scholar]
- 8.Nagtegaal ID, Quirke P. Colorectal tumour deposits in the mesorectum and pericolon; a critical review. Histopathology. 2007 Aug;51(2):141–149. doi: 10.1111/j.1365-2559.2007.02720.x. [DOI] [PubMed] [Google Scholar]
- 9.Nagtegaal ID, Tot T, Jayne DG, et al. Lymph nodes, tumor deposits, and TNM: are we getting better? J Clin Oncol. 2011 Jun;29(18):2487–2492. doi: 10.1200/JCO.2011.34.6429. [DOI] [PubMed] [Google Scholar]
- 10.Ueno H, Hashiguchi Y, Shimazaki H, et al. Peritumoral deposits as an adverse prognostic indicator of colorectal cancer. Am J Surg. 2014 Jan;207(1):70–77. doi: 10.1016/j.amjsurg.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Song JS, Chang HJ, Kim DY, et al. Is the N1c category of the new American Joint Committee on cancer staging system applicable to patients with rectal cancer who receive preoperative chemoradiotherapy? Cancer. 2011 Sep;117(17):3917–3924. doi: 10.1002/cncr.25968. [DOI] [PubMed] [Google Scholar]
- 12.Gopal P, Lu P, Ayers GD, et al. Tumor deposits in rectal adenocarcinoma after neoadjuvant chemoradiation are associated with poor prognosis. Mod Pathol. 2014 Jan 17; doi: 10.1038/modpathol.2013.239. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueno H, Mochizuki H, Shirouzu K, et al. Actual status of distribution and prognostic impact of extramural discontinuous cancer spread in colorectal cancer. J Clin Oncol. 2011 Jun;29(18):2550–2556. doi: 10.1200/JCO.2010.33.7725. [DOI] [PubMed] [Google Scholar]
- 14.Greene FL. Tumor deposits in colorectal cancer: a moving target. Ann Surg. 2012 Feb;255(2):214–215. doi: 10.1097/SLA.0b013e3182430eaa. [DOI] [PubMed] [Google Scholar]
- 15.Nagayoshi K, Ueki T, Nishioka Y, et al. Tumor Deposit Is a Poor Prognostic Indicator for Patients Who Have Stage II and III Colorectal Cancer With Fewer Than 4 Lymph Node Metastases but not for Those With 4 or More. Dis Colon Rectum. 2014 Apr;57(4):467–474. doi: 10.1097/DCR.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 16.Tong LL, Gao P, Wang ZN, et al. Is the seventh edition of the UICC/AJCC TNM staging system reasonable for patients with tumor deposits in colorectal cancer? Ann Surg. 2012 Feb;255(2):208–213. doi: 10.1097/SLA.0b013e31821ad8a2. [DOI] [PubMed] [Google Scholar]
- 17.Ueno H, Mochizuki H, Shirouzu K, et al. Multicenter study for optimal categorization of extramural tumor deposits for colorectal cancer staging. Ann Surg. 2012 Apr;255(4):739–746. doi: 10.1097/SLA.0b013e31824b4839. [DOI] [PubMed] [Google Scholar]
- 18.Belt EJ, van Stijn MF, Bril H, et al. Lymph node negative colorectal cancers with isolated tumor deposits should be classified and treated as stage III. Ann Surg Oncol. 2010 Dec;17(12):3203–3211. doi: 10.1245/s10434-010-1152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rock JB, Washington MK, Adsay NV, et al. Debating Deposits: An Interobserver Variability Study of Lymph Nodes and Pericolonic Tumor Deposits in Colonic Adenocarcinoma. Arch Pathol Lab Med. 2013 Jul 31; doi: 10.5858/arpa.2013-0166-OA. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song YX, Gao P, Wang ZN, et al. Can the tumor deposits be counted as metastatic lymph nodes in the UICC TNM staging system for colorectal cancer? PLoS One. 2012;7(3):e34087. doi: 10.1371/journal.pone.0034087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puppa G, Maisonneuve P, Sonzogni A, et al. Pathological assessment of pericolonic tumor deposits in advanced colonic carcinoma: relevance to prognosis and tumor staging. Mod Pathol. 2007 Aug;20(8):843–855. doi: 10.1038/modpathol.3800791. [DOI] [PubMed] [Google Scholar]
- 22.Puppa G, Frankel WL. Peritumoral deposits: complicating the colorectal cancer staging system. Am J Surg. 2014 Jan 16; doi: 10.1016/j.amjsurg.2013.12.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Puppa G, Sonzogni A, Colombari R, et al. TNM staging system of colorectal carcinoma: a critical appraisal of challenging issues. Arch Pathol Lab Med. 2010 Jun;134(6):837–852. doi: 10.5858/134.6.837. [DOI] [PubMed] [Google Scholar]
- 24.Puppa G, Ueno H, Kayahara M, et al. Tumor deposits are encountered in advanced colorectal cancer and other adenocarcinomas: an expanded classification with implications for colorectal cancer staging system including a unifying concept of in-transit metastases. Mod Pathol. 2009 Mar;22(3):410–415. doi: 10.1038/modpathol.2008.198. [DOI] [PubMed] [Google Scholar]