Abstract
AR-12 has been evaluated in clinical trials as an anti-cancer agent but also has demonstrated host-directed, broad-spectrum clearance of bacteria. We have previously shown that AR-12 has activity in vitro against Salmonella enterica serovar Typhimurium and Francisella species by inducing autophagy and other host immune pathways. AR-12 treatment of S. Typhimurium-infected mice resulted in a 10-fold reduction in bacterial load in the liver and spleen and an increased survival time. However, AR-12 treatment did not protect mice from death, likely due poor formulation. In the current study, AR-12 was encapsulated in a microparticulate carrier formulated from the novel degradable biopolymer acetalated dextran (Ace-DEX) and subsequently evaluated for its activity in human monocyte-derived macrophages (hMDMs). Our results show that hMDMs efficiently internalized Ace-DEX microparticles (MPs), and that encapsulation significantly reduced host cell cytotoxicity compared to unencapsulated AR-12. Efficient macrophage internalization of AR-12 loaded MPs (AR-12/MPs) was further demonstrated by autophagosome formation that was comparable to free AR-12 and resulted in enhanced clearance of intracellular Salmonella. Taken together, these studies provide support that Ace-DEX encapsulated AR-12 may be a promising new therapeutic agent to control intracellular bacterial pathogens of macrophages by targeting delivery and reducing drug toxicity.
Keywords: Autophagy, Salmonella, Macrophage, Pathogens, Microparticles, Drug resistance
1. Introduction
Salmonella is a Gram-negative facultative intracellular bacterial pathogen that is responsible for approximately 1.3 billion human infections annually (Coburn et al., 2007). Among the over 2500 serovars of S. enterica identified, Salmonella enterica serovar Typhi (S. Typhi), the etiologic agent of typhoid fever, is a human specific disease with an estimated 21 million new infections a year resulting in approximately 200,000 deaths annually worldwide (Crump et al., 2004). Once inside host cells, Salmonella utilizes various mechanisms to evade killing by macrophages. One such mechanism is the formation of Salmonella-containing vacuoles (SCVs) where bacteria proliferate by exploiting cellular nutrition through an alteration in host cell vesicle trafficking, and by evading the phagocyte oxidase complex (Coburn et al., 2007). In parallel, the macrophage uses various innate immune mechanisms to defend against intracellular invasion of Salmonella including the formation of autophagosomes (Birmingham and Brumell, 2006; Birmingham et al., 2006). After escaping from damaged SCVs, bacteria have been shown within autophagosomes and are eliminated by the fusion of lysosomes with these vesicles (Birmingham and Brumell, 2006; Birmingham et al., 2006).
Not only is Salmonella evasive to clearance by the immune system, but the emergence of multi-drug resistant strains illustrates its ability to overcome common antibiotic therapies (Zaki and Karande, 2011). Although resistance is more common in non-typhoidal salmonellae, S. Typhi resistance to ceftriaxone has been documented as emerging in Bangladesh and Kuwait for up to 15 years (Afzal et al., 2013). Indeed, a recent study in Pakistan and India concludes that 73.7% (n=80), 56.2%, and 52.5% of bacterial isolates had developed resistance to sulfonamide, ampicillin, or streptomycin, respectively (Afzal et al., 2013), with 58.7% of the overall isolates having multi-drug resistance. Resistant strains have also been observed in Kenya with resistance against cotrimoxazole in 66.7% (22 individuals) of the S. Typhi clinical isolates (Onyango et al., 2008) and in the United States where sulfamethoxazole resistance was observed in 56% of food-borne S. Typhi isolates (Glynn et al., 2004). To better combat this evasive pathogen, host-cell directed therapy is an attractive option. Host-cell directed therapy has been developed to treat infection due to several pathogens (Collier et al., 2013) [e.g., Mycobacterium tuberculosis (Zumla et al., 2013), viral diseases (Moore et al., 2013), malaria (Achtman et al., 2012)] in an effort to lessen the pathogens’ ability to evade clearance by the immune system as well as to limit the pathogens’ development of resistance.
Here we apply host-targeted AR-12 to combat S. Typhi within infected macrophages. AR-12 is a small molecule derived from a COX-2 inhibitor, but which now lacks COX-2 activity. It has been shown to suppress tumor cell viability through multiple mechanisms including activation of endoplasmic reticulum stress, inhibition of PDK-1/Akt signaling and the induction of autophagy (Gao et al., 2008; Kucab et al., 2005; Park et al., 2008). Moreover, it has received Food and Drug Administration Investigational New Drug (FDA-IND) approval and completed clinical trials for cancer treatment (#NCT00978523). With respect to bacterial infection, unencapsulated (free) AR-12 has displayed broad-spectrum activity in vitro against Salmonella enterica Typhimurium (S. Typhimurium) and Francisella tularensis (Chiu et al., 2009a; Chiu et al., 2009b) wherein bacterial burdens were significantly reduced in the host macrophage, in vitro. Our previous studies showed that free AR-12 is capable of enhancing clearance of S. Typhimurium in mouse cells via increased autophagy and other host immune activating pathways. Induction of autophagy by pharmacological agents has been shown to reduce the survival of various intracellular pathogens (Abdulrahman et al., 2011; Amer and Swanson, 2002; Amer and Swanson, 2005; Birmingham and Brumell, 2006; Birmingham et al., 2006; Biswas et al., 2008; Campbell and Spector, 2012; Chiu et al., 2009a; Gutierrez et al., 2004; Nakagawa et al., 2004). In vivo AR-12 treatment of S. Typhimurium infection resulted in a 10-fold reduction in bacterial load in the liver and spleen as well as a significant increase in survival time of infected mice (Chiu et al., 2009a). Despite these promising results, AR-12 treatment did not prevent mice lethality, likely due to the inability to achieve a sufficient in vivo concentration because of the hydrophobicity of this drug.
To increase the intracellular concentration of AR-12, we have encapsulated it into a microparticulate carrier comprised of the novel biodegradable polymer acetalated dextran (Ace-DEX) (Bachelder et al., 2008a; Broaders et al., 2009a; Kauffman et al., 2012a). Ace-DEX is unique among other commonly used biodegradable polymers, including polyesters such as poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL), because it creates pH neutral degradation products of dextran and extremely low levels of both ethanol and acetone, a metabolic product. Moreover, it has tunable degradation kinetics that can range from hours to days (Broaders et al., 2009a) due to the polymer’s acid-sensitivity which offers advantages for exposure to the bacterium in a relatively acidic phagosomal environment (Bachelder et al., 2008a). By encapsulating AR-12 in an Ace-DEX microparticle (MP), we can overcome the poor water solubility of the drug and facilitate macrophage passive targeting since non-phagocytic cells are not able to efficiently internalize particles larger than 100nm (Foged et al., 2005; Hirota et al., 2007). Thus, encapsulation of AR-12 should enhance its delivery by providing a triggered release of the drug intracellularly within the phagosome.
Here, we encapsulated AR-12 within Ace-DEX MPs and evaluated these particles for drug loading, size and shape. Additionally, we assessed the uptake of fluorescently labeled Ace-DEX MPs and determined the cytotoxicity and drug concentration of AR-12-loaded Ace-DEX MPs (AR-12/MPs) in macrophages. To evaluate the encapsulated drug’s effect, the ability of AR-12/MPs to modulate autophagy and clearance of S. Typhimurium was measured. Taken together, the studies described herein provide support that Ace-DEX encapsulated AR-12 may be a promising new therapeutic agent to control intracellular bacterial pathogens of macrophages.
2. Materials and Methods
2.1. Reagents and antibodies
AR-12 was provided by Arno Therapeutics, Inc. (Flemington, NJ). Lipopolysaccharide (LPS) and IFN-γ were purchased from EMD Millipore (Billerica, MA). The following antibodies were used in this study: anti-LC3 antibody (Abcam, Cambridge, MA, USA), anti-S. Typhi antibody (Biodesign, Lake Forest, CA), Alexa Red-conjugated goat anti-mouse IgG, and Alexa Green goat anti-rabbit IgG (Invitrogen, Carlsbad, CA). All chemicals, except where indicated, were purchased from Sigma (St. Louis, MO) and used as purchased.
2.2. Fabrication of Ace-DEX
Acetalated dextran (Ace-DEX) was reacted as previously outlined (Kauffman et al., 2012a). In brief, lyophilized 71k dextran was reacted in acidic and anhydrous conditions in the presence of 2-ethoxypropene (Waterstone, Carmel, IN). The reaction was quenched with the addition of triethylamine (TEA) and purified via filtration. The end product was resuspended in water made basic (pH ~9) through the addition of TEA, lyophilized, washed in ethanol, re-lyophilized and weighed to get an overall yield of 94–98%. The cyclic acetal coverage was characterized via NMR (Broaders et al., 2009a) and determined to be 50–56% for all polymers used in this study.
2.3. Encapsulation of AR-12 in Ace-DEX MPs
The production of MPs was performed as described in our previous publications (Broaders et al., 2009b; Kauffman et al., 2012b) with the addition that dishes and glassware were soaked in 0.5 M sodium hydroxide overnight, rinsed with isopropanol and dried prior to use. Two mg of the AR-12 was added to 100 mg of Ace-DEX (2% wt/wt loading), dissolved in dichloromethane (DCM) and 3% wt/wt polyvinyl alcohol (PVA) in phosphate buffer saline (PBS, pH 7.4) was added to the solution. The mixture was sonicated using a Misonix Ultrasonic Liquid Processor (Farmingdale, NY; 60 W, duty cycle 50%) while chilled in an ice bath. The emulsion was then poured into a 0.3% PVA solution in PBS and stirred for 2h to evaporate the organic solvent. The suspension was filtered using a sterile MidiKros tangential flow filtration system (Spectruam Labs, Rancho Dominguez, CA) to remove un-encapsulated drug and PVA. The particles were then collected and lyophilized. For fluorescently loaded particles, 1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate MPs (DTP) was loaded at 1% weight loading in the same manner outlined above. Also, the same procedure was used to fabricate blank particles without adding drug. The AR-12 loaded particles (AR-12/MPs) and blank particles were confirmed to have LPS levels below detection limits, prior to use as assessed using a ToxinSensor ™ Chromogenic LAL Endotoxin Assay Kit (GenScript USA Inc, Piscataway, NJ).
2.4. Quantification of AR-12 in Microparticles and Macrophages
To quantify the amount of AR-12 in Ace-DEX MPs, AR-12/MPs were prepared in a 1 gL-1 solution of dimethyl sulfoxide (DMSO). A standard curve of unencapsulated AR-12 in DMSO was then prepared in a solvent resistant 96-well plate (Polypropylene; Thermo Fisher Scientific, Waltham, MA) with triplicate samples of broken-down, previously encapsulated AR-12. These samples were then read in a plate reader at λex 280nm/λem 380nm and used to calculate the amount of drug encapsulated. The encapsulation efficacy was then determined as the amount of drug encapsulated divided by the amount of drug loaded (the theoretical drug loading).
The amount of AR-12 in macrophages was also quantified. RAW 264.7 macrophages (5×104 cells/well) were left to adhere 2–3 hours in a 96-well plate with Thermo Scientific HyClone DMEM (Dulbecco's Modified Eagle's Medium)/high glucose with 5% Fetal Bovine Serum and 1% penicillin/streptomycin. Cells were then cultured for 24 hours with either free AR-12 in DMSO or AR-12/MPs. After culturing, cells were washed twice with chilled PBS, lysed with the addition of 200 µl of the mobile phase solution (by volume) and centrifuged at 4000 rpm for 10 minutes. The mobile phase solution consisted of acetonitrile (55%), acetic acid (0.5%), Triton X-100 (0.1%), and ddH2O (44.4%). Lysates from each well were transferred to an Amicon Ultra Centrifugal Filter (Millipore). The filtrate from each tube was chilled overnight at 4°C and quantification of AR-12 was determined by high-performance liquid chromatography (HPLC). AR-12 concentration was determined using an Agilent 1100 series HPLC with a 1260 diode array (Santa Clara, CA). Samples were run through a ZORBX Eclipse XDB-C8 4.6 mm × 150 mm (Agilent), pore size 5 µm column, with a C8-guard column and passed through the column at a flow rate of 1 ml/min in the mobile phase. AR-12 was detected at a wavelength of 267 nm with a retention time of 2.9 minutes. Sample peaks were compared to a standard curve and analyzed using Agilent Chemstation software.
2.5. Characterization of Microparticle Size
Dynamic light scattering (DLS) samples were prepared in basic water (pH 9.0) and diluted to reduce the average count rate to ~100 kilocounts/min when analyzed with a BI-200SM DLS system (Brookhaven Instruments, Holtsville, NY). The reading was performed using a laser wavelength of 633nm, pinhole setting of 200 µm and detector angle of 90°. The average of five readings was taken and reported.
Particle size and morphology was obtained using a NOCA Nano SEM 400 (Field Emission, Inc., Hillsboro OR). Particles were suspended at a concentration of 10gL−1 in basic water (pH 9.0) and pipetted onto an aluminum pin stub specimen mount (Ted Pella; Redding, CA) and allowed to air dry at room temperature. Using a Sputter Coater 108 (Cressington Scientific Instruments, Watford, England), the samples were coated with a layer of gold-palladium alloy for two minutes and images were acquired with the SEM.
2.6. Isolation of human monocyte-derived macrophage (hMDMs)
Human monocyte-derived macrophages (hMDMs) were isolated from human blood via venipuncture (Schlesinger et al., 1990) from healthy donors following protocols approved by the Ohio State University Institutional Review Board. Written informed consent was provided by study participants. Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood over a Ficoll cushion (GE Heathcare Bio-Science, Piscataway, NJ). PBMCs were then cultured in sterile screw-cap Teflon wells in RPMI 1640 plus L-glutamine (Gibco-Life Technologies, Grand Island, NY) with 20% autologous human serum at 37°C in a humidified incubator containing 5% CO2 for 5 days. PBMCs were then recovered from Teflon wells by chilling them on ice, re-suspending the cells in RPMI 1640 with 10% autologous serum, and allowing them to attach in 6-well or 24-well tissue culture plates for 2–3h at 37°C in a humidified incubator containing 5% CO2. Lymphocytes were then washed away leaving hMDM monolayers at a density of approximately 2.0×105 cells/well for 24-well plates or 8.0×105 cells/well for 6-well plates for S. Typhi infection or for collection of cell lysates, respectively.
2.7. Bacterial cultures and analysis of bacterial growth in hMDMs
Wild-type S. Typhi TY2, a gift from Dr. Carolyn Hardegree (FDA), was cultured in Luria-Bertani (LB) broth (Difco, Detroit, MI) at 37°C. Analysis of bacterial growth in hMDMs was described previously (Chiu et al., 2009a). Briefly, overnight cultures of S. Typhi were prepared for infection of hMDMs by sub-culture (1:50) in fresh LB broth and incubated for 4h at 37°C. Bacteria were then collected by centrifugation at 3,000g for 10 min and suspended in phosphate buffer saline (PBS) to an optical density of 0.6 at 600nm which was equivalent to 5×108 CFUmL−1. hMDMs were infected with S. Typhi at a multiplicity of infection (MOI) of 20 in the presence of 2% autologous serum in RPMI 1640 (Gibco-Life Technologies). Two hours after infection, extra-cellular bacteria were removed by addition of 100µgmL-1 gentamicin to the culture medium for 1h and then the cell layer was thoroughly washed three times with pre-warmed RPMI 1640. The infected hMDMs were then treated with different concentrations of free AR-12 or AR-12/MPs in fresh culture medium containing 2% autologous serum. Gentamicin was also added at 10µgmL−1 to eliminate potential re-infection by extracellular bacteria. At different time points, the infected cells were lysed with 0.1% Triton X-100 (Calbiochem, San Diego, CA) in PBS for 15 min. The cell lysates were then serially diluted with PBS and spread on LB agar plates. The surviving intracellular bacteria were determined by enumerating CFU after 24h incubation at 37°C.
2.8. Cytotoxicity assay
The effects of free AR-12 and AR-12/MPs on hMDM viability were assessed by using a lactate dehydrogenase (LDH) assay (Roche Applied Science, Indianapolis, IN). Prior to the experiment, hMDM cells were seeded into 24-well plates at 2×105 cells/well (3 wells per test group) with RPMI 1640 supplemented with 20% autologous serum. The medium from each well was removed and replaced with fresh 2% autologous serum in RPMI 1640 containing different concentrations of free AR-12 dissolved in dimethyl sulfoxide (DMSO; final concentration of 0.1%), AR-12/MPs, blank MPs or DMSO vehicle control. The positive control received 0.1% Triton X-100 at the time of harvesting supernatants. After 72h of treatment, supernatants were collected, centrifuged to remove cells, and subjected to the LDH assay (as per manufacturer’s instructions). Absorbance at 570 nm was determined via a plate reader and the cytotoxicity was calculated as a percentage compared to the positive control treated cells.
2.9. Fluorescence microscopy
For visualizing the internalization of MPs into hMDMs, DTP (λex 550 nm; λem 567 nm) Ace-DEX microparticles were prepared (DTP/MPs). hMDMs were cultured with DTP/MPs at a MP concentration equivalent to 2.5µM AR-12. At different time points, cells were washed, fixed and examined for DTP/MP internalization into hMDMs. The percentage of DTP/MP positive hMDMs was determined by dividing DTP/MP positive cells by the total number of hMDMs randomly counted from at least three epifluorescence microscope viewing fields (≥ 300cells/test group).
Epifluorescence microscopy was used to visualize intracellular S. Typhi, autophagosomes and microparticles. The ability of AR-12/MPs to induce autophagosomes was examined using a Cyto-ID® Autophagy Detection kit (Enzo Life Science, NY). Briefly, 2×105 hMDMs on coverslips were treated with either 1.25µM free AR-12 in DMSO or AR-12/MPs for 18h, the cells were then washed three times with warm PBS and then one time with 1× assay buffer. The autophagosomes and nuclei were labeled with Cyto-ID® Autophagy Detection and Hoechst 33342 Nuclear stain, respectively, at 37°C in 5% CO2 incubator. After a 30 min incubation, the cells were washed with 1× assay buffer and fixed with 4% formaldehyde for 20 min at room temperature. The stained cells were visualized using epifluorescence microscopy with a FITC filter (λex 480 nm, λem 530nm) and the nuclear Hoechst 33342 with a DAPI filter (λex 340nm, λem 480nm).
The co-localization of intracellular S. Typhi with autophagosomes was examined as described previously (Chiu et al., 2009a) with minor modification. Briefly, hMDMs were infected with S. Typhi as described (MOI = 20) for 2h. After removal of extracellular bacteria, the infected cells were treated with 1.25µM AR-12/MPs. At different time points, the infected cells were washed three times with PBS, fixed with 4% paraformaldehyde in PBS for 20 min, permeabilized with ice-cold methanol for 10 sec and blocked with 3% bovine serum albumin (BSA) in PBS overnight at 4°C. After three washes with PBS, the cells were incubated with a mixture of primary antibodies [1:100 mouse S. Typhi antibody and 1:100 rabbit LC3 antibody] in 5% goat serum in PBS. After a 2h incubation at room temperature, the cells were blocked with 3% BSA for 1h and incubated with a mixture of fluorescently-labeled secondary antibodies of goat anti-rabbit Fluor 488 and goat anti-mouse Fluor 546 (Invitrogen, Carlsbad). Cells were washed three times with PBS after a 2h incubation and macrophage nuclei were stained with DAPI.
To examine if S. Typhi co-localizes with AR-12/MPs, hMDMs were infected with GFP-expressing S. Typhi (MOI = 100) for 1h and then treated with AR-12/MPs at an MP concentration equivalent to 2.5µM AR-12/MPs. At different time points, cells were washed, fixed with 4% paraformaldehyde and macrophage nuclei were stained with DAPI.
2.10. Immunoblotting
Western blots were performed to examine the autophagy protein LC3 and its derivative form LC3-II as previously described (Chiu et al., 2009a; Chiu et al., 2009b). Briefly, after treatment with free AR-12 or AR-12/MPs, cells were washed with ice-cold PBS, lysed and incubated on ice for 10 min. After centrifugation at 14,000×g for 10 min, equivalent amounts of lysate supernatant were mixed with 4× Laemmli buffer and heated to 95°C for 5 min. 20µg protein equivalents were resolved by SDS-PAGE (12%) and transferred onto a 0.2µm nitrocellulose membrane. The membrane was blocked with 5% skim milk in Tris-buffered saline (TBS) for 1h and rinsed with 0.5%Tween-20 in TBS (TBST), incubated overnight at 4°C with rabbit anti-LC3 antibody (1:1000) in 5% skim milk dissolved in TBST, washed six times with TBST and incubated with HRP-conjugated goat anti-IgG secondary antibody (1:2000) in 5% skim milk/TBST for 1h. After six washes with TBST, the immune-positive bands were visualized by enhanced chemiluminescence (GE Amersham, Piscataway, NJ) on X-ray film.
2.11. Statistical analysis
Data are presented as mean ± standard deviation (SD). p-values were calculated using one-way ANOVA for multiple comparisons and adjusted with Bonferroni’s correction or using a non-paired Student’s t test where two group means were compared; *p<0.05; **p<0.01; ***p<0.001; NS, not significant. Statistical analysis was performed using GraphPad Prism 5.
3. Results
3.1. Encapsulation of AR-12 into Ace-DEX microparticles
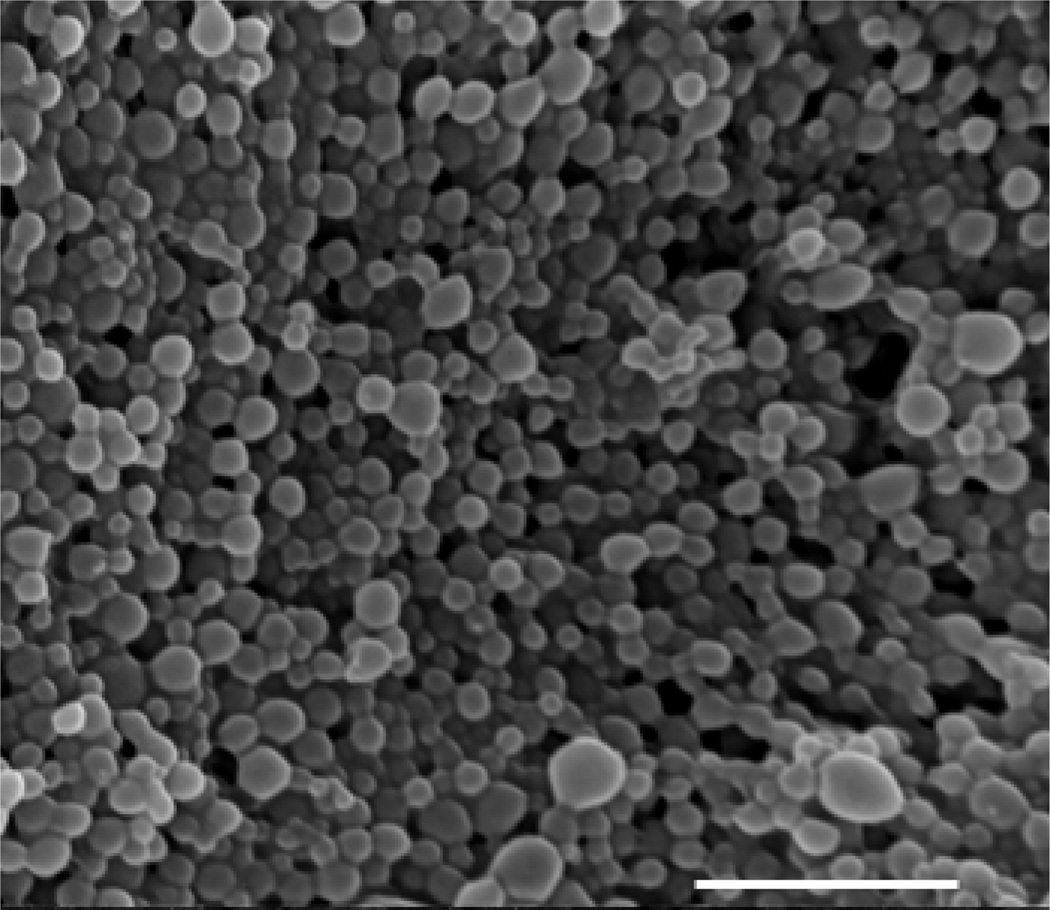
To address the proposed enhanced clearance of intracellular Salmonella by encapsulation of the autophagy inducer AR-12, MPs were produced to contain the drug as cargo. Fig. 1 displays a representative scanning electron micrograph of AR-12-loaded Ace-DEX MPs (AR-12/MPs), illustrating that the particles are spherical in nature. As the DLS and SEM micrographs indicate, the particles are polydispersed in nature with a size of 255 ± 45 nm (n=5). The encapsulation efficiency is 44.7% and the drug loading is 8.95 µg AR-12/mg Ace-DEX.
Fig. 1.
Representative scanning electron micrograph of Ace-DEX microparticles (MPs) encapsulating AR-12, scale bar represents 5 µM.
3.2. Cytotoxicity of AR-12 in Ace-DEX microparticles in macrophages
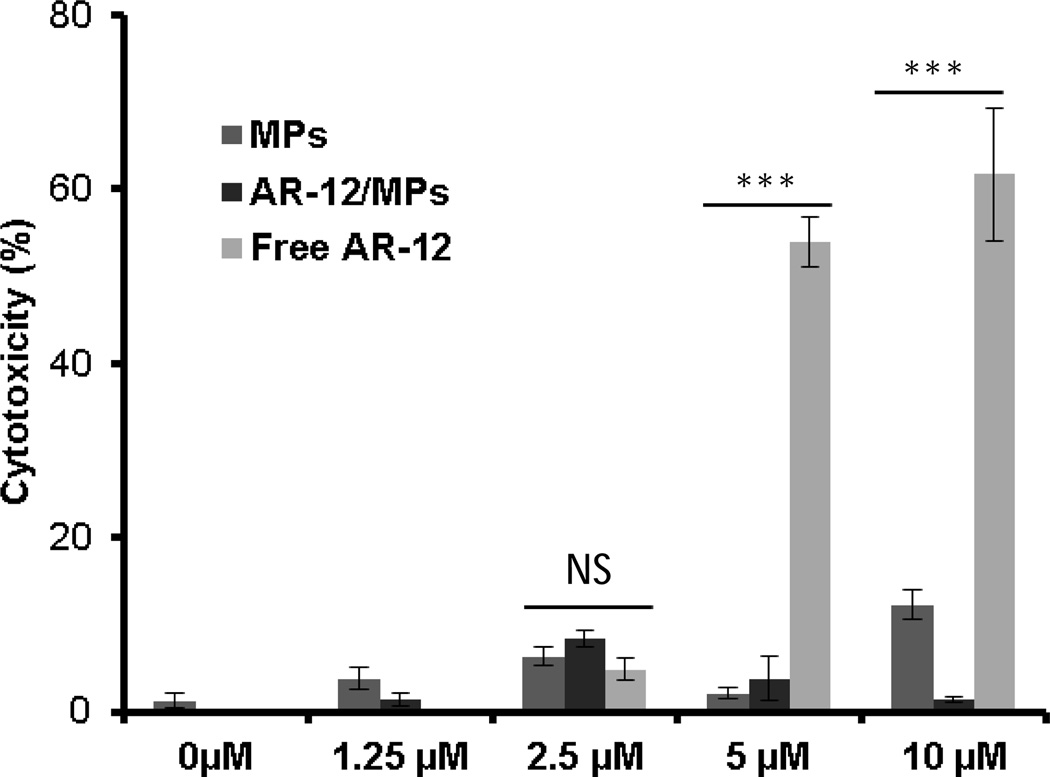
From a clinical perspective, treatment of infectious diseases caused by intracellular pathogens may require prolonged treatment regimens and the ability for agents to access intracellular sites with limited toxicity. Thus to examine cytotoxicity, hMDMs, which serve as the primary target in vivo for S. Typhi, were treated with empty Ace-DEX particles, free AR-12 or AR-12/MPs for 72h. LDH release was monitored in the supernatants as an indicator of cell toxicity. For each concentration, the blank particle group represents the same mass of particles that corresponds to the given mass of AR-12/MPs for that particular AR-12 concentration. To equalize AR-12 concentrations between free AR-12 and AR-12/MPs, the amount of AR-12 from AR-12/MPs was determined using the known AR-12 encapsulation efficiency. The percentage of cytotoxicity is significantly higher (p<0.001) in the free AR-12 group compared to the AR-12/MPs or MP group at the 5µM and 10µM concentrations at the 72h time point (Fig. 2). The empty MP groups at any of the concentrations exerted minimal or no toxicity with respect to the cells only control. These results provide strong evidence that encapsulation of AR-12 into Ace-DEX MPs reduces toxicity of the drug for human macrophages.
Fig. 2.
Cytotoxicity of human monocyte-derived macrophages (hMDMs) cultured in the absence or presence of AR-12 for 72h. AR-12 = un-encapsulated drug, AR-12/MPs = encapsulated AR-12, and MPs = empty Ace-DEX microparticles. Cytotoxicity is presented as relative LDH release compared to positive control (0.2% Triton X-100). Data are presented as average ± SD (***P ≤ 0.001).
3.3. Internalization of AR-12/MPs into macrophages
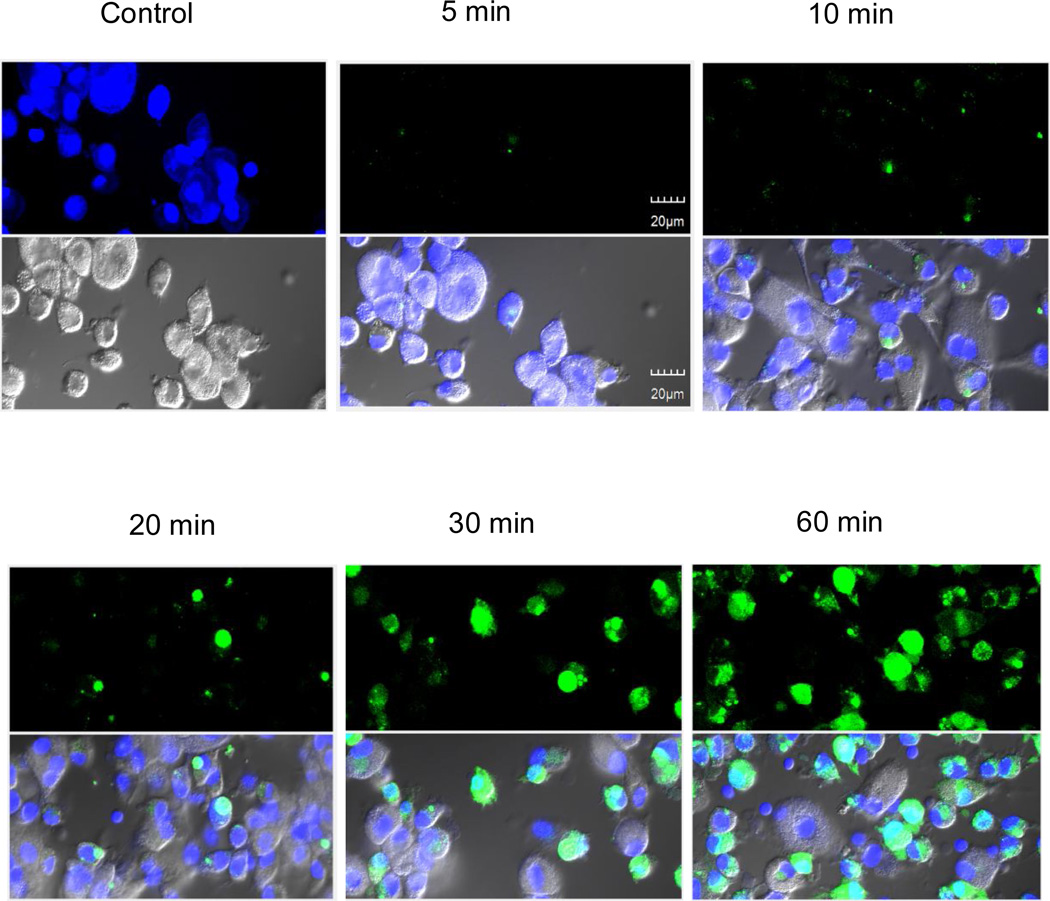
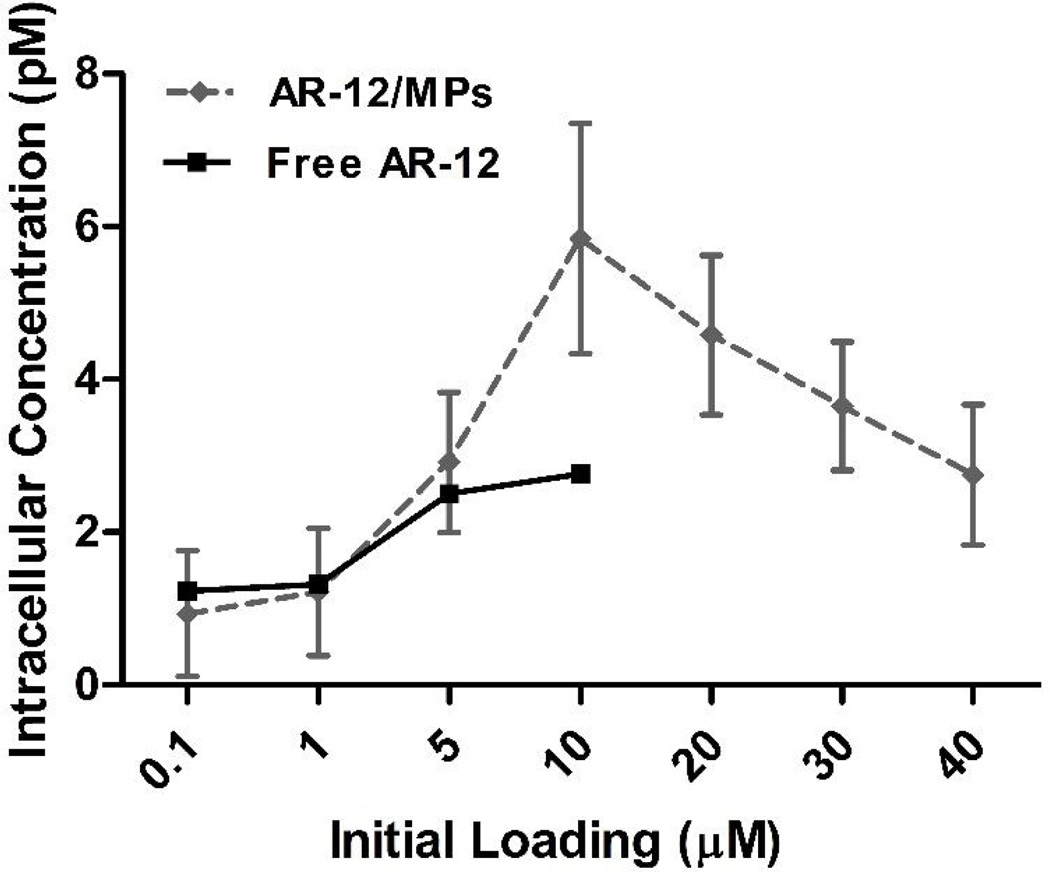
Given the reduction in cytotoxicity for hMDMs observed in Fig. 2, we addressed the formal possibility that AR-12/MPs were poorly internalized into macrophages by examining the ability of AR-12/MPs to be phagocytosed by macrophages. 1,1′-Dioctadecyl-3,3,3′,3′- tetramethylindocarbocyanine perchlorate (DTP; λex 550 nm; λem 567 nm) was encapsulated into Ace-DEX MPs (DTP/MPs) and hMDMs were cultured with DTP/MPs at a MP concentration equivalent to 2.5µM AR-12. Internalization of DTP/MPs was examined by epifluorescence microscopy at different time points. As shown in Fig. 3, DTP/MPs were efficiently internalized into hMDMs. By the 60 min time point, 97.8±2.62% of the hMDMs had internalized MPs. We next compared the intracellular concentration of AR-12 between free AR-12- and AR-12/MP-treated RAW 264.7 cells. The drug concentration was measured in cell lysates at 24h after treatment using HPLC. As shown in Fig. 4, encapsulation of AR-12 increased cell loading of the drug, reaching peak intracellular concentrations at 10 µM initial encapsulated AR-12, while free AR-12 plateaued at 5 µM initial loading, where significant cell death occurred with the unencapsulated drug (Fig 2). Thus, encapsulation of AR-12 not only reduces cell cytotoxicity but also increases the intracellular drug concentration.
Fig. 3.
Efficient internalization of 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate MPs (DTP/MPs) into hMDMs at a concentration of 2.5 µM AR-12. The cells were visualized using epifluorescence microscopy. Nuclei were stained with DAPI (blue). Green represents DTP/MPs. The results are representative of two experiments.
Fig. 4.
Intracellular drug concentration in RAW 264.7 macrophages cultured with un-encapsulated (Free) or Ace-DEX microparticles encapsulating AR-12 (AR-12/MPs). Drug concentration was measured in cell lysates by HPLC. The decrease in cell concentration at 10 µM is due to cytotoxicity, which is high above 5 µM of free drug.
3.4. AR-12/MP-induced autophagosome formation is comparable to free AR-12
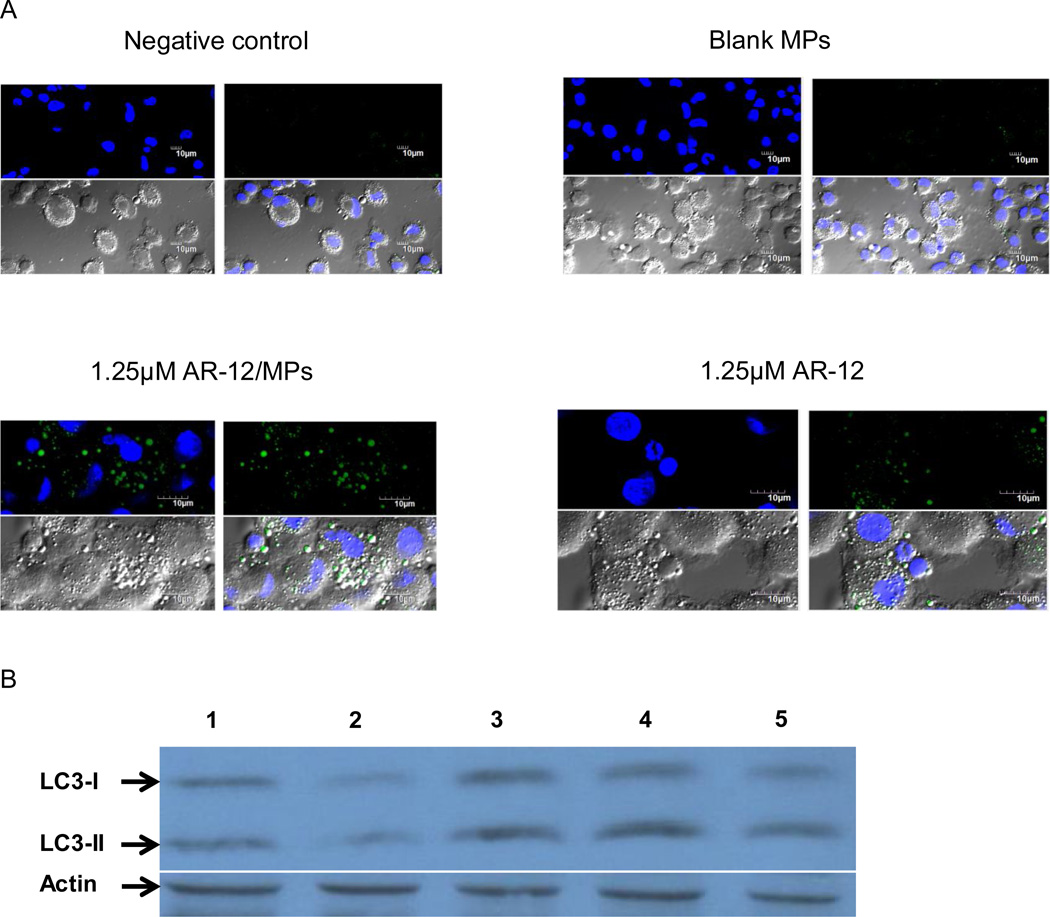
Ace-DEX MP encapsulated AR-12 is efficiently internalized by hMDMs, resulting in an increased intracellular concentration of the drug. Therefore the ability of AR-12/MPs to induce autophagosome formation in hMDMs was next assessed and compared with that of free AR-12. hMDMs were exposed to 1.25µM of either free AR-12 or AR-12/MPs for 18h and evaluated for evidence of autophagy by microscopy and Western blot analysis. Autophagosomes in the cytosol were labeled using a Cyto-ID Autophagy Detection Kit and visualized using epifluorescence microscopy. As shown in Fig. 5A, AR-12/MPs induced autophagosome formation comparable to free AR-12 in hMDMs as evidenced by characteristic speck formation (green). Additionally, during autophagy the cytoplasmic protein microtubule-associated protein light chain 3-I (LC3-I) is cleaved and recruited to the autophagosomes where LC3-II, a specific autophagosome marker, is generated via site-specific lipidation (Deretic et al., 2013). As shown in Fig. 5B, immunoblotting of the LC3-II protein in lysates from AR-12/MP-treated macrophages showed an increase in LC3-II levels comparable to that in free AR-12-treated macrophages. Together, the results indicate that AR-12/MPs are capable of inducing autophagy in hMDMs similar to free AR-12.
Fig. 5.
AR-12/MP-induced autophagosome formation in hMDMs is greater than or equal to free AR-12. (A) Nuclei were stained with DAPI (blue) and autophagosomes were labeled with a cationic amphiphilic tracer dye (green). The results shown are representative of two experiments. (B) Western blot using anti-LC3 antibody. LC3 cleavage was induced by AR-12/MPs; (1) Medium control; (2) Equivalent amount of blank MPs; (3) 1.25µM free AR-12; (4) 1.25 µM AR-12/MPs; (5) 100nM LPS and 100nM IFN-γ as a positive control. The results are representative of five experiments.
3.5. AR-12/MPs reduce S. Typhi growth in hMDMs
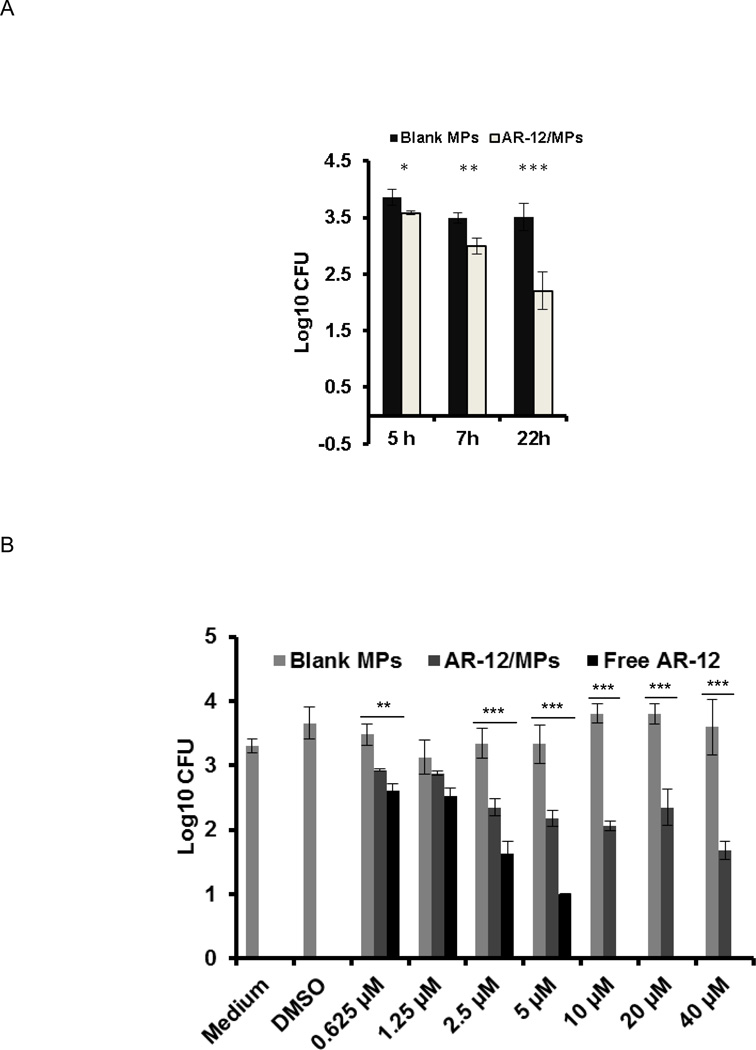
Our previous studies demonstrated that free AR-12 inhibited the growth of intracellular bacteria in macrophages via induction of autophagy and other immune mechanisms (Chiu et al., 2009a; Chiu et al., 2009b). To examine whether AR-12/MPs are also capable of inhibiting S. Typhi growth in macrophages, hMDMs were infected with S. Typhi (MOI = 20) for 2h, washed and treated with different concentrations of free AR-12 or AR-12/MPs. The intracellular survival of S. Typhi in hMDMs was determined by CFUs of cell lysates. We first determined the growth curve of S. Typhi in hMDMs in the presence of 20µM AR-12/MPs and found that AR-12/MPs inhibit bacterial growth in a time-dependent fashion. The maximal growth inhibition was obtained at 22h post treatment compared to the vehicle control (Fig. 6A). We next compared bacterial growth inhibition by free AR-12 and AR-12/MPs at 22h post treatment. As shown in Fig. 6B, free AR-12 had a stronger inhibitory effect on S. Typhi growth in hMDMs. However, like free AR-12, AR-12/MPs displayed a dose-dependent effect consistent with the specific activity of AR-12. It is possible that encapsulation makes the effect entirely related to internal cellular drug action like autophagy whereas free AR-12 has additive effects at the cost of toxicity.
Fig. 6.
Inhibition of intracellular growth of S. Typhi in macrophages by AR-12/MPs. (A) Time-dependent effects of 20µM AR-12/MPs on intracellular growth of S. Typhi. hMDMs were incubated with S. Typhi for 2h. After the removal of extracellular bacteria, the infected cells were treated with 20µM AR-12/MPs. The bacterial load (CFU) was determined by plating cell lysates. (B) AR-12/MPs retained ability to enhance the clearance of S. Typhi by hMDMs. Infected hMDMs were treated with different doses of unencapsulated AR-12 or AR-12/MPs. The bacterial load (CFU) was determined at 22h post treatment. AR-12 = un-encapsulated drug, AR-12/MPs = encapsulated AR-12, and AP = empty Ace-DEX microparticles. The data are presented as average ± SD (P ≤ 0.05). AR-12 demonstrates hMDM cytotoxicity at a concentration ≥ 10µM.
3.6. AR-12/MPs do not exert direct antimicrobial activity towards S. Typhi
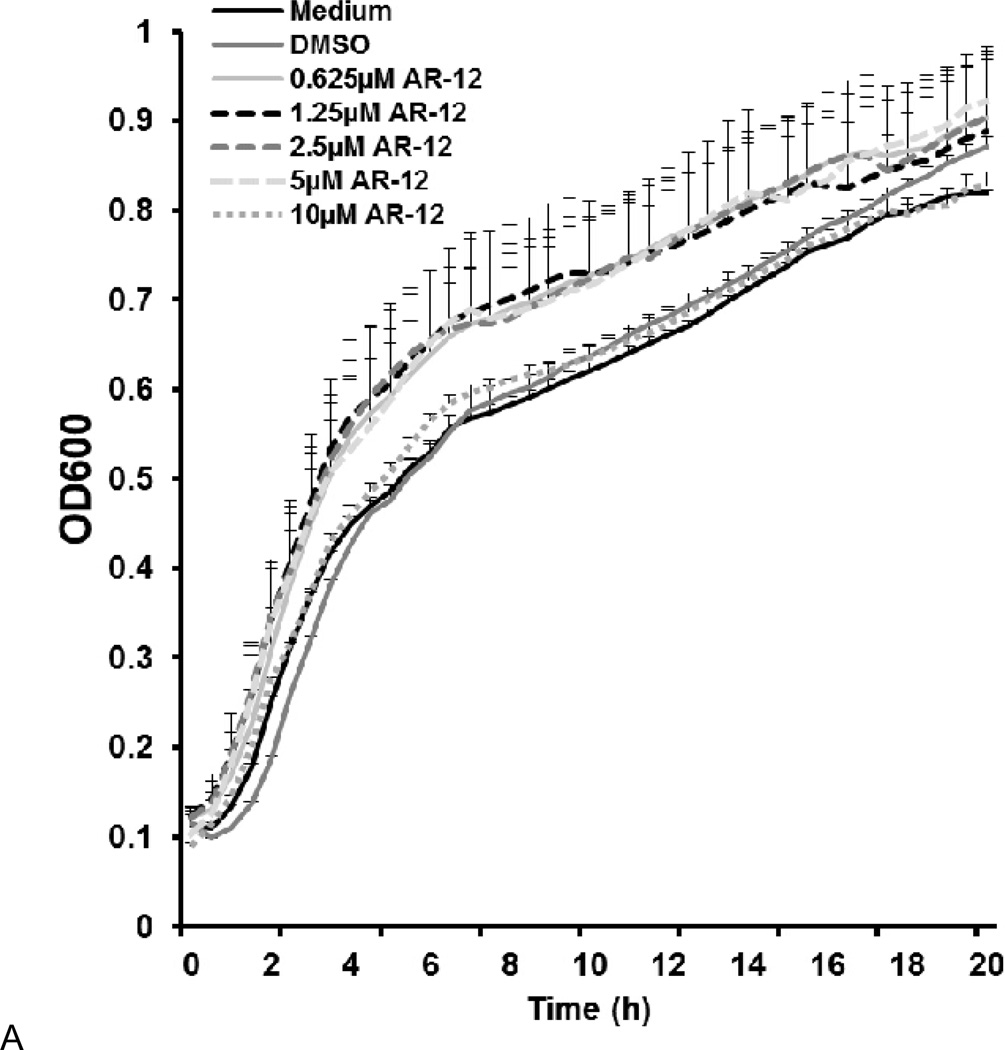
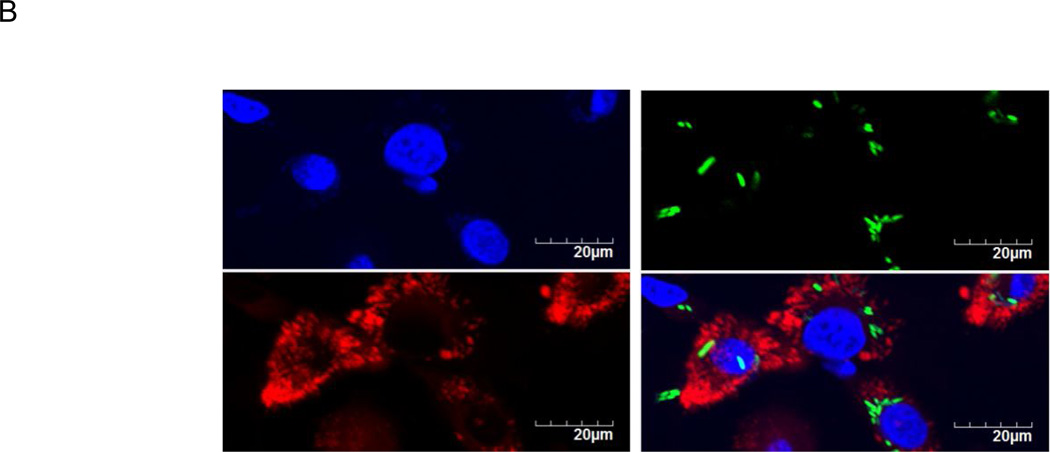
AR-12 may exert direct antimicrobial activity against S. Typhi in macrophages in addition to its host-directed effects. Previous studies have shown that free AR-12 does not have direct antimicrobial activity against Salmonella serovar Typhimurium (Chiu et al., 2009a). Consistent with the previous findings, free AR-12 concentrations as high as 10µM did not exhibit direct antimicrobial activity against S. Typhi over 24h (Fig. 7A). Additionally, we examined the potential co-localization of Salmonella with MPs inside the macrophages. Green fluorescent protein (GFP)-expressing S. Typhi was used to infect hMDMs for 1h (MOI= 100). The infected macrophages were then cultured with 2.5 µM DTP-labeled MPs at a concentration equivalent to 2.5µM of AR-12. GFP-expressing S. Typhi and DTP-labeled MPs were visualized using epifluorescence microscopy. As shown in Fig. 7B, we did not observe co-localization of GFP-expressing S. Typhi and DTP-labeled MPs. This finding, together with the lack of direct killing in vitro, suggests that AR-12 released from MPs intracellularly does not exert direct killing of S. Typhi.
Fig. 7.
AR-12 does not exert direct killing against S. Typhi in vitro and in the macrophages. (A) Growth curve of S. Typhi for the indicated concentrations of AR-12. (B) S. Typhi does not co-localize with MPs in hMDMs post-infection. Green = S. Typhi expressing GFP; Red = DTP/MPs; Blue = nuclei stained with DAPI. The results are representative of two experiments.
3.7. Co-localization of S. Typhi with autophagosomes induced by AR-12/MPs
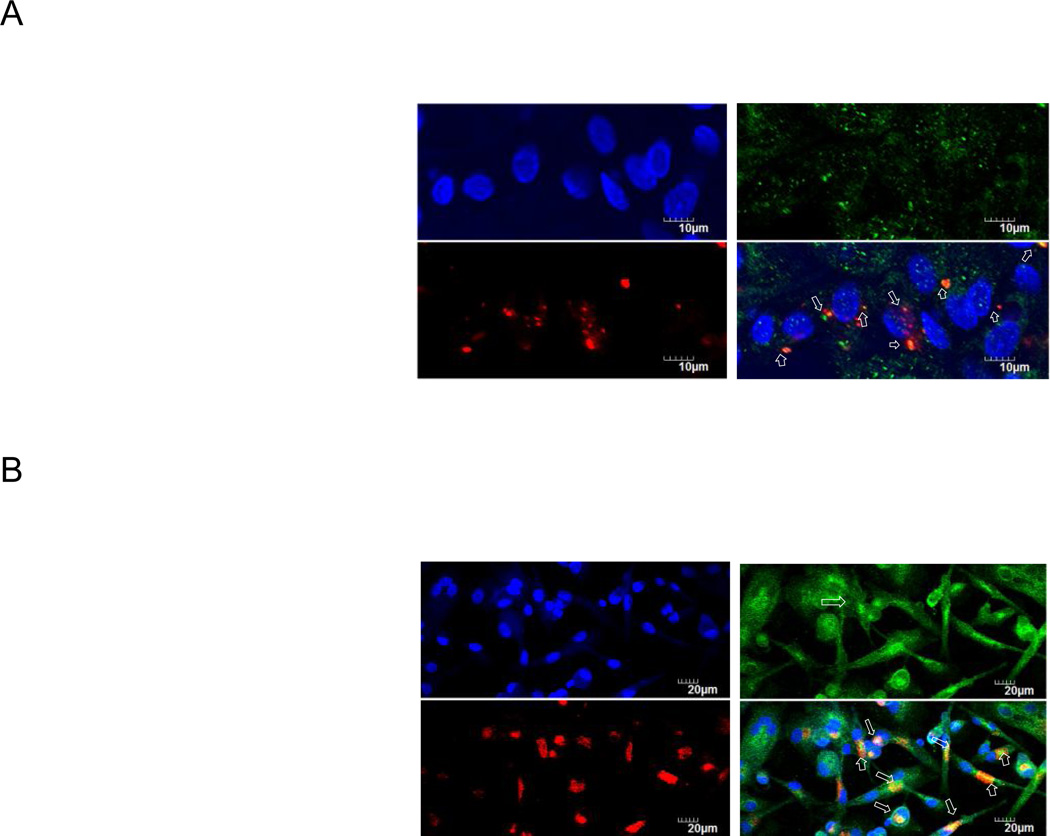
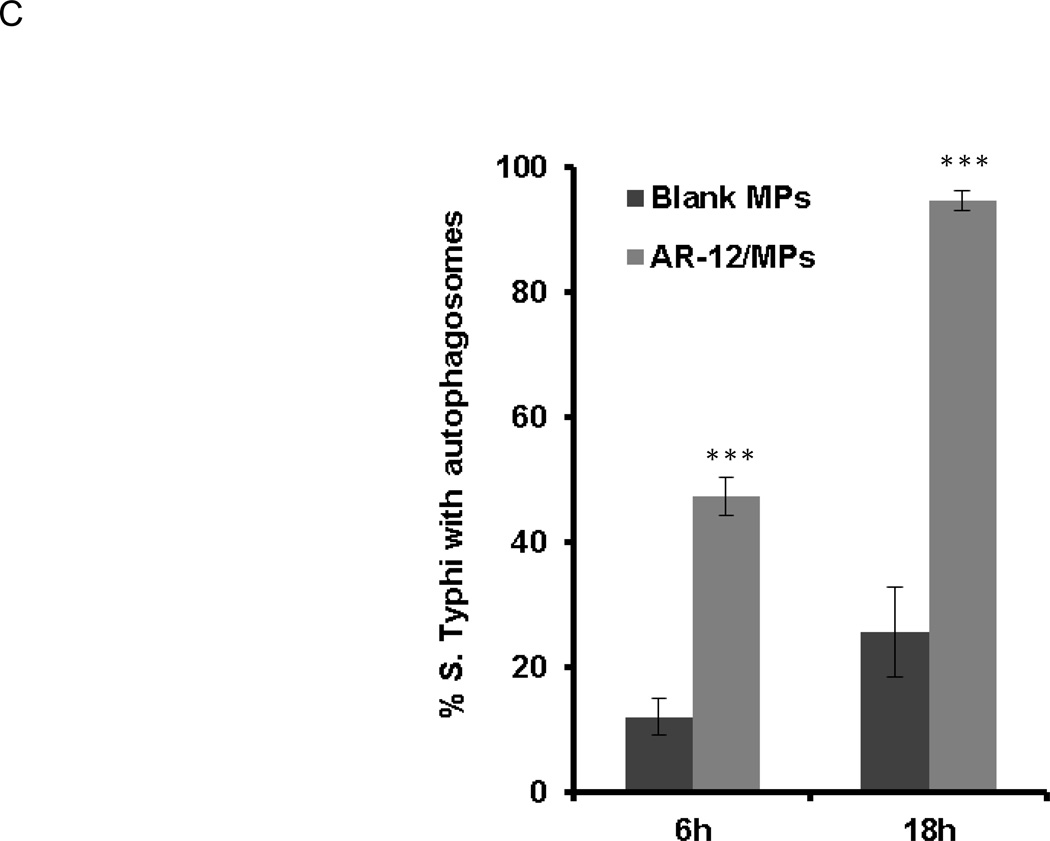
Because AR-12/MPs are also capable of inducing autophagy and inhibiting S. Typhi intracellular growth, we assessed the effects of AR-12/MPs on the co-localization of S. Typhi with autophagosomes in the infected hMDMs using epifluorescence microscopy. The results showed that treatment of the infected hMDMs with 1.25 µM AR-12/MPs for 6h resulted in approximately 50% bacteria co-localized with LC3-positive puncta compared to approximately 12% in the cells treated vehicle alone (Fig. 8A and C). Eighteen hours after AR-12/MP treatment, extensive co-localization of bacteria/bacterial debris with LC3-positive puncta was observed in all infected hMDMs (Fig. 8B and C).
Fig. 8.
AR-12 increases the co-localization of intracellular S. Typhi with autophagosomes in hMDMs. Co-localization (white arrows) of S. Typhi with autophagosomes induced by AR-12/MPs at (A) 6 h and (B) 18 h post treatment. (C) Percentage of S.Typhi co-localized with autophagosomes at 6h and 18h post treatment compared with vehicle treat control. Nuclei were stained with DAPI (blue); labeled S. Typhi is red; and autophagosomes are green. The results are representative of three experiments and presented as the average ± SD (***P ≤ 0.001).
4. Discussion
The incidence of Salmonella infections and the rapid emergence of multidrug resistant S. Typhi (Parry et al., 2002) highlight the important worldwide threat of this pathogen and the need for new approaches to therapeutic intervention. Activation of host defense as well as targeting host factors modulated by microbes are emerging approaches to control intracellular pathogens (Finlay and Hancock, 2004; Schwegmann and Brombacher, 2008). The induction of autophagy is known to result in the elimination of intracellular pathogens, and AR-12 is a pharmacological agent capable of inducing autophagy in various cell types (Chiu et al., 2009a; Chiu et al., 2009b) where it inhibits intracellular bacterial growth within infected macrophages (Chiu et al., 2009a; Chiu et al., 2009b).
While our previous work showed that AR-12 helped to clear bacteria from macrophages, it did not protect mice from lethality, possibly due to limited drug availability as a result of the drug’s hydrophobicity, a situation that can also promote drug resistance. To overcome the solubility issue and to increase delivery, we have encapsulated AR-12 in degradable acetalated dextran (Ace-DEX) microparticles that target phagocytes. The size of the MPs, as presented in Figure 1, is approximately 255 nm in diameter. For parenteral administration of MPs targeting macrophages, the ideal size is between 100–1,000nm. Particles less than 100 nm (Foged et al., 2005; Hirota et al., 2007) are not efficiently internalized by macrophages and larger particles (1 −2 µm) are predominately transported by dendritic cells from the injection site to the lymph node (Manolova et al., 2008). Not only have we formulated MPs of a size ideal for delivering compounds to target host cells, but we have encapsulated the above mentioned novel, FDA-IND-approved small molecule therapeutics, AR-12. AR-12 was encapsulated at a modest efficiency of 44.7% with a 2% initial weight loading. The loss of drug in the emulsion process is likely due to its partitioning into the aqueous phase. Overall, we have formulated particles that are approximately 9% AR-12 per polymer.
Use of encapsulated AR-12 for the delivery of drug to macrophages results in a significant decrease in drug cytotoxicity (Fig. 2) and an increase in intracellular drug concentration above what can be achieved with unencapsulated drug (Fig. 4). While this may appear paradoxical (MPs aid intracellular loading of the drug but still result in less cytotoxicity), it is likely that encapsulation reduces potential AR-12 interactions with non-specific targets at the cell surface or interior. The high amount of drug loading is not surprising since MPs are efficiently phagocytosed by hMDMs with nearly all of the macrophages containing MPs after 60 min (Fig. 3). Efficient uptake of MPs by macrophages was also observed with PLGA MPs of similar size to the Ac-DEX MPs reported here. Mathaes et al. report an approximately 70-times greater amount of fluorescence compared to media control, as determined by flow cytometry, when J774.A1 macrophages were cultured with FITC-PLGA MPs (Mathaes et al., 2014). This high amount of particle uptake also translates to a high amount of drug loading (Fig. 4) with the MP formulation resulting in increased loading compared to unencapsulated drug. The effects of high intracellular drug loading with Ace-DEX MPs has been observed previously with small molecule drugs (Bachelder et al., 2010; Peine et al., 2013), wherein a drug dose sparing is observed. Dose sparing was also observed in PLGA as part of the Peine et al. study when comparing the cytokine response of macrophages treated with PLGA and Ac-DEX MPs encapsulating Poly I:C. However, the observed production of most inflammatory cytokines was greater for the faster degrading Ac-DEX MPs encapsulating Poly I:C than for the PLGA MPs (Peine et al., 2013). The increased activity of the Ac-DEX MPs indicates that the dose sparing and diminished toxicity are likely an effect of the acid-sensitivity of Ace-DEX. Ace-DEX particles undergo tunable burst release in acidic conditions but have slower, tunable release at neutral pHs (Bachelder et al., 2008b). Thus, once engulfed by phagocytic cells, the pH sensitive Ace-DEX particles allow the burst release of their drug payload in the phagosomes thus maximizing the amount of drug released in the phagosomes while limiting release systematically. These characteristics of Ace-DEX make it a more desirable vehicle for the delivery of drugs such as AR-12 into phagocytes than other available materials.
We found that AR-12/MPs induced at least equal levels of autophagy compared to free AR-12 (Fig. 5A and B). Based on a literature search, this paper is the first of its kind to encapsulate an autophagy agent in polymeric microparticles for antibacterial action. Autophagy inhibitors have been encapsulated as cancer chemotherapeutics (Zhang et al., 2014), and tolerance (Kauffman et al., 2012b) but this area has not by and large been explored for the treatment of phagocytes to kill resident bacterial pathogens. Also, PLGA particles of similar size have been shown to increase autophagy (Halamoda Kenzaoui et al., 2012), although this could be due to LPS or other endotoxin contamination since TLR agonists have been shown to increase autophagy (Collier et al., 2013). We did not observe that Ac-DEX blank particles significantly increased autophagy (Fig 5). Furthermore, addition of AR-12/MPs resulted in growth inhibition of S. Typhi in hMDMs in a dose- and time-dependent manner, with the most marked inhibition at 22h post treatment (Fig. 6A and B) with 94% co-localization of the bacteria and autophagy vacuoles. No direct antimicrobial activity of AR-12 for S. Typhi was observed. AR-12 has been shown to induce autophagy via inhibition of Akt-1, an upstream regulator of mTOR (Chiu et al., 2009a). The host cell kinase Akt 1 is important for the intracellular survival of bacterial pathogens via multiple mechanisms and inhibition of this kinase impairs the growth of S. Typhimurium and M. tuberculosis (Kuijl et al., 2007) in host macrophages. It is notable that our efforts to induce autophagy in hMDMs using rapamycin or starvation were unsuccessful, suggesting differences in the sensitivity to conventional autophagy inducing agents between hMDMs and murine macrophages due to unknown mechanisms (data not shown). Although our current study clearly indicates an effect of AR-12 on S. Typhi clearance in hMDMs, our earlier study showed that free AR-12 displays a biphasic effect on intracellular growth of S. Typhimurium in macrophages (Chiu et al., 2009a) that is dependent on both autophagy and non-autophagic mechanisms (Chiu et al., 2009a; Chiu et al., 2009b). We did not observe a biphasic effect on inhibition of intracellular growth of S. Typhi in hMDMs, perhaps due to the controlled and intracellular release of drug from AR-12/MPs. Nonetheless, our studies indicate that AR-12/MPs are potent autophagy activators in hMDMs. In many studies, autophagy-mediated bacterial killing has been defined as the co-localization of bacteria and LC3, but it is obvious that LC3 can decorate membranous compartments other than autophagosomes including phagosomes. Our study demonstrated that AR-12/MPs increased conversion of LC3-I to LC3-II and co-localization of S. Typhi with LC3 (Fig. 5A and B and Fig. 8A and B), reinforcing that inhibition of intracellular bacterial growth was likely as a result of inducing autophagy.
5. Conclusion
Encapsulation of AR-12 in Ace-DEX particles (AR-12/MPs) facilitates the passive targeting of macrophages and improves AR-12 delivery to hMDMs. While increasing intracellular drug concentration, AR-12/MPs significantly reduced host cell cytotoxicity compared to free AR-12. The improvement of drug delivery by encapsulation in vitro was further demonstrated by its ability to induce robust autophagosome formation and the enhanced clearance of S. Typhi in infected hMDMs. Taken together, these studies provide support that Ace-DEX encapsulated AR-12 may be a promising new therapeutic agent to control the intracellular growth of bacterial pathogens.
ACKNOWLEDGMENTS
We are grateful to the Campus Microscopy and Imaging Facility at The Ohio State University for the epifluorescence microscopy studies performed. AR-12 was provided by Arno Therapeutics, Inc. This research was supported by NIH grant (R21AI102252). The investigators have received sponsored research funding from Arno Therapeutics for work outside what is contained in this manuscript.
Abbreviations
- Ace-DEX
Acetalated dextran
- ANOVA
Analysis of variance
- AR-12/MPs
AR-12 loaded MPs
- BSA
Bovine serum albumin
- CFU
Colony forming unit
- COX-2
Cyclooxygenase-2
- DAPI
4',6-diamidino-2-phenylindole
- DCM
Dichloromethane
- DLS
Dynamic light scattering
- DMEM
Dulbecco's Modified Eagle's Medium
- DMSO
Dimethyl sulfoxide
- DTP
1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- FDA
Food and Drug Administration
- FITC
Fluorescein isothiocyanate
- FDA-IND
Food and Drug Administration Investigational New Drug
- GFP
Green fluorescent protein
- hMDMs
Human monocyte-derived macrophages
- HPLC
High-performance liquid chromatography
- HRP
Horseradish peroxidase
- IFN-γ
Interferon gamma
- LB
Luria-Bertani
- LC3
Microtubule-associated protein 1A/1B–light chain 3
- LDH
Lactate dehydrogenase
- LPS
Lipopolysaccharide
- MOI
Multiplicity of infection
- MPs
Ace-DEX microparticles
- NMR
Nuclear magnetic resonance
- PBMCs
Peripheral blood mononuclear cells
- PBS
Phosphate saline buffer
- PCL
Polycaprolactone
- PLGA
Poly(lactic-co-glycolic acid)
- PVA
Polyvinyl alcohol
- RAW 264.7
Cell line murine Macrophage
- RPMI
Roswell Park Memorial Institute
- SCV
Salmonella containing vacuole
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SEM
Scanning Electron Microscopy
- TBS
Tris-buffered saline
- TEA
Triethylamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulrahman BA, Khweek AA, Akhter A, Caution K, Kotrange S, Abdelaziz DH, Newland C, Rosales-Reyes R, Kopp B, McCoy K, Montione R, Schlesinger LS, Gavrilin MA, Wewers MD, Valvano MA, Amer AO. Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis. Autophagy. 2011;7:1359–1370. doi: 10.4161/auto.7.11.17660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman AH, Pilat S, Law CW, Lynn DJ, Janot L, Mayer ML, Ma S, Kindrachuk J, Finlay BB, Brinkman FS, Smyth GK, Hancock RE, Schofield L. Effective adjunctive therapy by an innate defense regulatory peptide in a preclinical model of severe malaria. Sci Transl Med. 2012;4:135ra164. doi: 10.1126/scitranslmed.3003515. [DOI] [PubMed] [Google Scholar]
- Afzal A, Sarwar Y, Ali A, Maqbool A, Salman M, Habeeb MA, Haque A. Molecular evaluation of drug resistance in clinical isolates of Salmonella enterica serovar Typhi from Pakistan. J Infect Dev Ctries. 2013;7:929–940. doi: 10.3855/jidc.3154. [DOI] [PubMed] [Google Scholar]
- Amer A, Swanson MS. Autophagy is an immediate macrophage response to infection by Legionella pneumophila. Mol Biol Cell. 2002;13:10a–10a. doi: 10.1111/j.1462-5822.2005.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer AO, Swanson MS. Autophagy is an immediate macrophage response to Legionella pneumophila. Cell Microbiol. 2005;7:765–778. doi: 10.1111/j.1462-5822.2005.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelder EM, Beaudette TT, Broaders KE, Dashe J, Frechet JM. Acetal-derivatized dextran: an acid-responsive biodegradable material for therapeutic applications. J Am Chem Soc. 2008a;130:10494–10495. doi: 10.1021/ja803947s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelder EM, Beaudette TT, Broaders KE, Dashe J, Frechet JMJ. Acetal-derivatized dextran: An acid-responsive biodegradable material for therapeutic applications. J Am Chem Soc. 2008b;130:10494. doi: 10.1021/ja803947s. + [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelder EM, Beaudette TT, Broaders KE, Frechet JM, Albrecht MT, Mateczun AJ, Ainslie KM, Pesce JT, Keane-Myers AM. In vitro analysis of acetalated dextran microparticles as a potent delivery platform for vaccine adjuvants. Mol Pharm. 2010;7:826–835. doi: 10.1021/mp900311x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham CL, Brumell JH. Autophagy recognizes intracellular Salmonella enterica serovar Typhimurium in damaged vacuoles. Autophagy. 2006;2:156–158. doi: 10.4161/auto.2825. [DOI] [PubMed] [Google Scholar]
- Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281:11374–11383. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- Biswas D, Qureshi OS, Lee WY, Croudace JE, Mura M, Lammas DA. ATP-induced autophagy is associated with rapid killing of intracellular mycobacteria within human monocytes/macrophages. Bmc Immunol. 2008;9 doi: 10.1186/1471-2172-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broaders KE, Cohen JA, Beaudette TT, Bachelder EM, Frechet JM. Acetalated dextran is a chemically and biologically tunable material for particulate immunotherapy. Proc Natl Acad Sci U S A. 2009a;106:5497–5502. doi: 10.1073/pnas.0901592106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broaders KE, Cohen JA, Beaudette TT, Bachelder EM, Frechet JMJ. Acetalated dextran is a chemically and biologically tunable material for particulate immunotherapy. P Natl Acad Sci USA. 2009b;106:5497–5502. doi: 10.1073/pnas.0901592106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GR, Spector SA. Vitamin D Inhibits Human Immunodeficiency Virus Type 1 and Mycobacterium tuberculosis Infection in Macrophages through the Induction of Autophagy. Plos Pathogens. 2012;8 doi: 10.1371/journal.ppat.1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HC, Kulp SK, Soni S, Wang D, Gunn JS, Schlesinger LS, Chen CS. Eradication of intracellular Salmonella enterica serovar Typhimurium with a small-molecule, host cell-directed agent. Antimicrob Agents Chemother. 2009a;53:5236–5244. doi: 10.1128/AAC.00555-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HC, Soni S, Kulp SK, Curry H, Wang D, Gunn JS, Schlesinger LS, Chen CS. Eradication of intracellular Francisella tularensis in THP-1 human macrophages with a novel autophagy inducing agent. J Biomed Sci. 2009b;16:110. doi: 10.1186/1423-0127-16-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn B, Grassl GA, Finlay BB. Salmonella, the host and disease: a brief review. Immunol Cell Biol. 2007;85:112–118. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- Collier MA, Gallovic MD, Peine KJ, Duong AD, Bachelder EM, Gunn JS, Schlesinger LS, Ainslie KM. Delivery of host cell-directed therapeutics for intracellular pathogen clearance. Expert review of anti-infective therapy. 2013;11:1225–1235. doi: 10.1586/14787210.2013.845524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BB, Hancock RE. Can innate immunity be enhanced to treat microbial infections? Nat Rev Microbiol. 2004;2:497–504. doi: 10.1038/nrmicro908. [DOI] [PubMed] [Google Scholar]
- Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298:315–322. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Gao M, Yeh PY, Lu YS, Hsu CH, Chen KF, Lee WC, Feng WC, Chen CS, Kuo ML, Cheng AL. OSU-03012, a novel celecoxib derivative, induces reactive oxygen species-related autophagy in hepatocellular carcinoma. Cancer Res. 2008;68:9348–9357. doi: 10.1158/0008-5472.CAN-08-1642. [DOI] [PubMed] [Google Scholar]
- Glynn MK, Reddy V, Hutwagner L, Rabatsky-Ehr T, Shiferaw B, Vugia DJ, Segler S, Bender J, Barrett TJ, Angulo FJ. Prior antimicrobial agent use increases the risk of sporadic infections with multidrug-resistant Salmonella enterica serotype Typhimurium: a FoodNet case-control study, 1996–1997. Clin Infect Dis 38 Suppl. 2004;3:S227–S236. doi: 10.1086/381591. [DOI] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Halamoda Kenzaoui B, Chapuis Bernasconi C, Guney-Ayra S, Juillerat-Jeanneret L. Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. The Biochemical journal. 2012;441:813–821. doi: 10.1042/BJ20111252. [DOI] [PubMed] [Google Scholar]
- Hirota K, Hasegawa T, Hinata H, Ito F, Inagawa H, Kochi C, Soma G, Makino K, Terada H. Optimum conditions for efficient phagocytosis of rifampicin-loaded PLGA microspheres by alveolar macrophages. J Control Release. 2007;119:69–76. doi: 10.1016/j.jconrel.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Kauffman KJ, Do C, Sharma S, Gallovic MD, Bachelder EM, Ainslie KM. Synthesis and characterization of acetalated dextran polymer and microparticles with ethanol as a degradation product. ACS applied materials & interfaces. 2012a;4:4149–4155. doi: 10.1021/am3008888. [DOI] [PubMed] [Google Scholar]
- Kauffman KJ, Kanthamneni N, Meenach SA, Pierson BC, Bachelder EM, Ainslie KM. Optimization of rapamycin-loaded acetalated dextran microparticles for immunosuppression. Int J Pharm. 2012b;422:356–363. doi: 10.1016/j.ijpharm.2011.10.034. [DOI] [PubMed] [Google Scholar]
- Kucab JE, Lee C, Chen CS, Zhu J, Gilks CB, Cheang M, Huntsman D, Yorida E, Emerman J, Pollak M, Dunn SE. Celecoxib analogues disrupt Akt signaling, which is commonly activated in primary breast tumours. Breast Cancer Res. 2005;7:R796–R807. doi: 10.1186/bcr1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijl C, Savage NDL, Marsman M, Tuin AW, Janssen L, Egan DA, Ketema M, van den Nieuwendijk R, van den Eeden SJF, Geluk A, Poot A, van der Marel G, Beijersbergen RL, Overkleeft H, Ottenhoff THM, Neefjes J. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature. 2007;450:725–U710. doi: 10.1038/nature06345. [DOI] [PubMed] [Google Scholar]
- Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- Mathaes R, Winter G, Besheer A, Engert J. Influence of particle geometry and PEGylation on phagocytosis of particulate carriers. Int J Pharm. 2014;465:159–164. doi: 10.1016/j.ijpharm.2014.02.037. [DOI] [PubMed] [Google Scholar]
- Moore TW, Sana K, Yan D, Krumm SA, Thepchatri P, Snyder JP, Marengo J, Arrendale RF, Prussia AJ, Natchus MG, Liotta DC, Plemper RK, Sun A. Synthesis and Metabolic Studies of Host Directed Inhibitors for Anti Viral Therapy. ACS Med Chem Lett. 2013;4:762–767. doi: 10.1021/ml400166b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, Hamada S, Yoshimori T. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- Onyango D, Machioni F, Kakai R, Waindi EN. Multidrug resistance of Salmonella enterica serovars Typhi and Typhimurium isolated from clinical samples at two rural hospitals in Western Kenya. J Infect Dev Ctries. 2008;2:106–111. [PubMed] [Google Scholar]
- Park MA, Yacoub A, Rahmani M, Zhang G, Hart L, Hagan MP, Calderwood SK, Sherman MY, Koumenis C, Spiegel S, Chen CS, Graf M, Curiel DT, Fisher PB, Grant S, Dent P. OSU-03012 stimulates PKR-Like endoplasmic reticulum-dependent increases in 70-kDa heat shock protein expression, attenuating its lethal actions in transformed cells. Molecular Pharmacology. 2008;73:1168–1184. doi: 10.1124/mol.107.042697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- Peine KJ, Bachelder EM, Vangundy Z, Papenfuss T, Brackman DJ, Gallovic MD, Schully K, Pesce J, Keane-Myers A, Ainslie KM. Efficient delivery of the toll-like receptor agonists polyinosinic:polycytidylic acid and CpG to macrophages by acetalated dextran microparticles. Mol Pharm. 2013;10:2849–2857. doi: 10.1021/mp300643d. [DOI] [PubMed] [Google Scholar]
- Schlesinger LS, Bellingerkawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium-Tuberculosis Is Mediated by Human Monocyte Complement Receptors and Complement Component-C3. Journal of Immunology. 1990;144:2771–2780. [PubMed] [Google Scholar]
- Schwegmann A, Brombacher F. Host-directed drug targeting of factors hijacked by pathogens. Sci Signal. 2008;1:re8. doi: 10.1126/scisignal.129re8. [DOI] [PubMed] [Google Scholar]
- Zaki SA, Karande S. Multidrug-resistant typhoid fever: a review. J Infect Dev Ctries. 2011;5:324–337. doi: 10.3855/jidc.1405. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong Y, Zeng X, Liang X, Li X, Tao W, Chen H, Jiang Y, Mei L, Feng SS. The effect of autophagy inhibitors on drug delivery using biodegradable polymer nanoparticles in cancer treatment. Biomaterials. 2014;35:1932–1943. doi: 10.1016/j.biomaterials.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Zumla A, Nahid P, Cole ST. Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov. 2013;12:388–404. doi: 10.1038/nrd4001. [DOI] [PubMed] [Google Scholar]