Abstract
The roseoloviruses, human herpesvirus-6A -6B and -7 (HHV-6A, HHV-6B and HHV-7) cause acute infection, establish latency, and in the case of HHV-6A and HHV-6B, whole virus can integrate into the host chromosome. Primary infection with HHV-6B occurs in nearly all children and was first linked to the clinical syndrome roseola infantum. However, roseolovirus infection results in a spectrum of clinical disease, ranging from asymptomatic infection to acute febrile illnesses with severe neurologic complications and accounts for a significant portion of healthcare utilization by young children. Recent advances have underscored the association of HHV-6B and HHV-7 primary infection with febrile status epilepticus as well as the role of reactivation of latent infection in encephalitis following cord blood stem cell transplantation.
Introduction
Roseoloviruses include human herpesvirus-6A -6B and -7 (HHV-6A, HHV-6B and HHV-7), which constitute the Betaherpesviridae subfamily of human herpesviruses along with human cytomegalovirus (HCMV). HHV-6 was first isolated from immunocompromised adults in 1986 by Salahuddin and colleagues [1]. Initially two distinct variants of HHV-6 were identified, HHV-6A and B with HHV-6B causing disease in developed countries. The two variants were officially classified as separate viruses in 2012[2].
As with all human herpesviruses, following primary infection HHV-6 and -7 establish latent or persistent infection in different cell types, have the ability to reactivate, and may be intermittently shed in bodily fluids [3]. Unlike other human herpesviruses, HHV-6A and HHV-6B are also found integrated into the host genome (ciHHV-6). Integration has been documented in 0.2-1% of the general population and along with latency has confounded the ability to correlate the presence of viral nucleic acid with active disease [4].
The syndrome known as roseola infantum was reported as early as 1809 by Robert Willan in his textbook “On cutaneous diseases” [5]. This clinical entity is also commonly referred to as exanthem subitum and early published descriptions of the disease still hold true. It is an illness that affects children by the age of three and is marked by the abrupt development of high fever lasting 3-5 days. The hallmark maculopapular rash appears as the fever subsides, and there may be few, if any, associated symptoms. Despite knowledge of this common disease of infancy, the etiologic agent was not identified until 1988 by Yaminishi and colleagues [6]. They demonstrated both the presence of circulating virus in peripheral blood mononuclear cells (PBMCs) during acute roseola and subsequent seroconversion during convalescence in four infants in Japan. It was nearly a decade later before our understanding of the full clinical spectrum of HHV-6 primary infection was expanded past roseola.
Recognition of primary infection with HHV-6 is important because the high prevalence of infection and its association with fever leads to substantial healthcare utilization. Primary infection in childhood is also strongly associated with neurologic complications, and reactivation of the latent virus under immunosuppressive conditions has been associated with significant morbidity. This review discusses the spectrum of clinical disease associated with roseolovirus primary infection, highlighting recent advances.
Epidemiology
The ubiquitous nature of infection with HHV-6 is evidenced by the fact that all newborns have passive maternal antibody to HHV-6 which typically wanes by 4-6 months of age, with primary infection occurring fairly soon thereafter [7-9]. The young age of primary HHV-6 infection was demonstrated in a prospective study by Hall and colleagues of children with fever seen in the emergency department (ED) in Rochester, NY [7]. Utilizing viral isolation and seroconversion, HHV-6B was identified as the causative agent of illness in 159 of 1553 children less than 24 months of age, while only one child out of 100 at 25-36 months of age had fever due to primary HHV-6B infection. The peak age of infection was 6-9 months [7]. Zerr and colleagues conducted a population-based prospective cohort study of HHV-6 primary infection in children from birth through two years of age in Seattle, WA. Based on persistent shedding of HHV-6B DNA in saliva, they noted a peak incidence of primary infection from nine to 21 months of age among children in the community, which is slightly older than the ED-based study. This shift in age of acquisition is also reflected in a 40% cumulative incidence of infection by 12 months of age, but the vast majority of children (77%) still acquired the virus by 24 months of age [10].
While HHV-6A DNA has been identified in umbilical cord blood mononuclear cells and in approximately one third of individuals with ciHHV-6, its role in subsequent active disease has not yet been established [11]. Clinical disease in North America, Europe and Asia has almost exclusively been linked to HHV-6B infection [2]. This contrasts with one region of sub-Saharan Africa, where HHV-6A DNA was detected in a majority of infants in an HIV-1 endemic region [12].
Transmission
The exact modes of transmission of HHV-6 have yet to be definitely determined. It is presumed that HHV-6 can be transmitted from the saliva of asymptomatic adults and older children because of the rapid and reliable transmission of virus to susceptible infants and the lack of recognized outbreaks [3]. It does seem clear that close contact is required for transmission, supported by the observations that having older siblings and parents who share saliva are associated with virus acquisition, but attending daycare is not [10, 13]. Recently, transmission of HHV-6 via respiratory droplets has been suggested by the identification of viral DNA in nasal mucosa and olfactory bulb specimens. Olfactory-ensheathing cells, specialized glial cells present in the nasal cavity, are also capable of being infected in vitro with HHV-6A suggesting that the olfactory pathway may be a route of entry of HHV-6 into the CNS [14].
Congenital infection with HHV-6 also occurs in approximately 1% of newborns [11]. While this rate is similar to congenital transmission of CMV, 86% of congenital infections are transmitted via chromosomally-integrated virus (ciHHV-6) while a minority (14%) is transmitted through presumed transplacental infection [15]. Chromosomal integration with germline transmission is a mechanism unique to HHV-6 and has not been demonstrated for HHV-7 or any other human herpesvirus. Infants with ciHHV-6 have measurable HHV-6-specific antibody, but it is unknown whether this is protective, whether the virus is actively replicating and the long term effects of congenitally-acquired HHV-6[4, 15, 16].
Clinical presentation
Symptoms
The most common finding in children with HHV-6 primary infection is fever (Table 1). Compared to other febrile illnesses in children under two years of age evaluated in an emergency department setting, HHV-6 infection has been shown to cause a significantly higher mean temperature (39.6°C compared to 38.9°C), with the great majority of children exhibiting temperatures greater than 39°C. In the study in Rochester NY, fevers remained high for the first three days with 15% of children remaining febrile for six or more days. Children with primary HHV-6 infection also presented earlier into the illness for medical care than children with other febrile illnesses (2.1 vs. 2.9 days) [7].
Table 1.
Signs and Symptoms Associated with Primary HHV-6 Infection.
| Range | Vianna et al.[46] | Zerr et al.[10] | Caserta et al.[43] | Hall et al.[7] | Asano et al.[17] | Pruksananonda et al.[18] | |
|---|---|---|---|---|---|---|---|
| Year Published | 1992-2008 | 2008 | 2005 | 1998 | 1994 | 1994 | 1992 |
| Number of Subjects | 626 | 97 | 130 | 29 | 160 (1094 evaluated)* | 179 | 34 |
| Inclusion Criteria | Children with rash and/or roseola | Out-patient cohort | Children with fever | Children evaluated in the Emergency Dept. | Children with rash and/or roseola | Children evaluated in the Emergency Dept. | |
| % of patients with symptom(s) when reported | |||||||
| Asymptomatic | 6 | 6 | |||||
| Fever (T>38° C) | 58-98 | 94 | 58 | 100 | 100 [87 (T>39)] | 98 | 100 [65 (T>40)] |
| Rash (generalized) | 18-48 | 91 | 31 | 48 | 18 | ||
| Roseola | 17-24 | 24 | 17 | ||||
| Gastrointestinal Symptoms (general) | 3-34 | 34 | 30 | 3 | |||
| vomiting | 8-21 | 21 | 8 | 21 | |||
| diarrhea | 24-68 | 24 | 26 | 68 | 27 | ||
| Upper Respiratory Symptoms | 3-41 | 41 | 3 | ||||
| rhinorrhea | 56-66 | 61 | 66 | 56 | |||
| Lower Respiratory Symptoms | 24 | 24 | |||||
| Cough | 27-62 | 62 | 34 | 27 | |||
| Cervical Adenopathy | 31-34 | 34 | 31 | ||||
| Pharyngeal Papules | 65 | 65 | |||||
| Tonsillitis | 29 | 29 | |||||
| Conjunctivitis | 26 | 26 | |||||
| Acute Otitis Media/Inflammed Tympanic Membranes | 8-62 | 8 | 30 | 62 | |||
| Eyelid Edema | 30 | 30 | |||||
| Fussiness/Irritability | 69-82 | 70 | 69 | 82 | |||
| Seizures | 0-17 | 1 | 0 | 17 | 13 | 8 | 3 |
| Bulging Anterior Fontanelle | 26 | 26 | |||||
| Prompted Outpatient Visit | 39 | 39 | |||||
| Prompted Hospitalization | 13-17 | 17 | 13 | ||||
None of the additional 582 infants with non-febrile illness evaluated in the Emergency Department or the 352 infants without an acute illness seen in ambulatory clinics had evidence of primary HHV-6 infection.
While studies from Japan have strongly linked HHV-6 to the clinical syndrome of roseola, this may be a reflection of study design and subject inclusion criteria [6, 17]. Prospective studies in the US have revealed that the classic syndrome of roseola accompanies only a minority of primary HHV-6B infections. The hallmark rash of roseola was observed in only 6% of the children at initial presentation when febrile and in another 17% at the time of defervescence in the study by Hall and colleagues [7]. Similarly, rash was only present in approximately 20% of children during primary HHV-6 infection in the community based study in Seattle, WA [10]. This highlights that roseola infantum is identified in less than a quarter of children with primary HHV-6 infection in the United States.
Fever, fussiness and rhinorrhea are present in over half of children with primary HHV-6B infection while diarrhea, rash and roseola are all significantly more common during primary HHV-6B infection than other periods of illness [10]. Additionally, febrile children with HHV-6B infection are less likely to present with cough or other symptoms of lower respiratory tract infection [7].
Healthcare Utilization
HHV-6B primary infection is a common cause of acute medical care visits accounting for 10% of physician office visits and 10-17% of acute febrile ED visits in children up to 36 months of age [7, 10, 18, 19]. Remarkably, primary infection has been identified in 24% of children from six to nine months of age presenting to the ED with an acute febrile illnesses (Figure 1) [7]. Additionally, children with primary HHV-6B infection are more likely to present with signs of serious systemic illness, irritability, and inflamed tympanic membranes and are commonly diagnosed with a presumed serious bacterial infection or otitis media, often resulting in unnecessary antibiotic use. Hospitalization due to concern for serious infection has been documented in one-third of children less than six months of age with primary infection seen in an ED [7, 18]. These data indicate that acute HHV-6B infection is associated with a high level of healthcare utilization. While the majority of children have a relatively benign clinical course, the acute clinical presentation may be concerning to both parents and healthcare providers alike.
Figure 1.
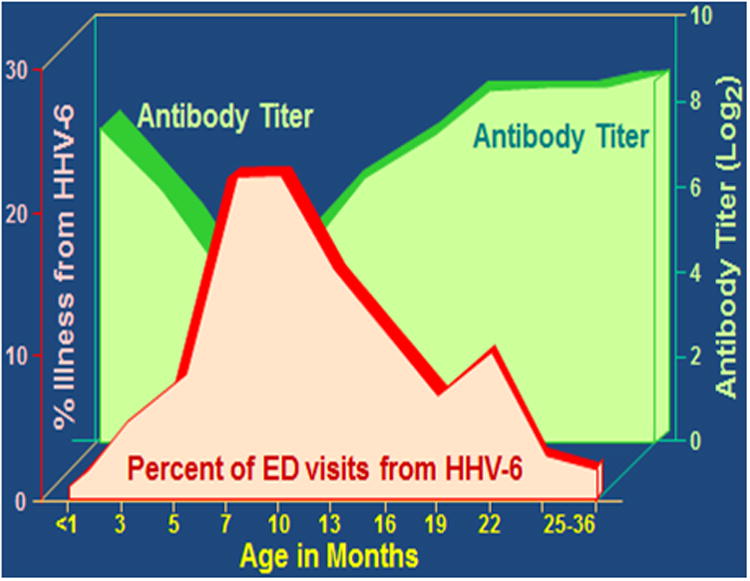
HHV-6 Antibody Titer in 0-36 Month Old Children and Acquisition of Primary Infection.
Data from N Engl J Med, Hall CB et al., Human Herpesvirus-6 Infection in Children: A Prospective Study of Complication and Reactivation. 331: 432-8. Copyright (1994) Massachusetts Medical Society. Reprinted with permission.
Complications
Case reports and small case series have linked primary HHV-6 infection with a wide range of potential complications including myocarditis, rhabdomyolysis, thrombocytopenia, Guillain-Barre syndrome and hepatitis/fulminant hepatic failure [20-22]. Many of these studies used the presence of HHV-6 DNA in the target organ, PBMCs, or other body fluids as evidence of active HHV-6 infection. However, detection of viral nucleic acid can represent active infection, latent infection or ciHHV-6. While such laboratory studies are not widely available, active replication of the virus or protein production should be identified to correlate infection with the clinical syndrome observed. The absence or presence of HHV-6-specific antibody can then be used to help determine whether there is a primary infection or reactivation, respectively.
Neurologic Complications and Sequelae
Seizures
Neurologic complications, manifested as seizures or encephalopathy, have long been associated with roseola. However, the true prevalence of seizures complicating HHV-6 primary infection has been difficult to determine due to the wide variation in study designs and populations. A literature review encompassing studies from 1994 to 2004 found that 17% of children seeking medical attention with primary HHV-6 infection had seizures as a complication [23]. Children from 12-15 months of age may be at particular risk with a documented rate of febrile seizures of 36% among children presenting to the ED with acute HHV-6B infection. Overall, primary HHV-6B infection accounts for approximately 25 to 33% of the febrile seizures observed in children less than 24 months of age in the emergency department setting [7, 24]. Additionally, data form the United Kingdom identified HHV-6B & HHV-7 infection in 17% of cases of suspected encephalitis or severe febrile seizures in young children [25].
While the majority of febrile seizures are considered to have a benign clinical course, 5-8% meet criteria for status epilepticus, and an estimated 5,000 to 10,000 cases of febrile status epilepticus (FSE) occur annually in the United States. Febrile seizures are the most common cause of status epilepticus in previously healthy children, accounting for over 70% of status epilepticus during the second year of life [26, 27]. The long term consequences of FSE are still not completely understood. There is a potential but controversial link to the future development of intractable temporal lobe epilepsy and hippocampal sclerosis, which is the most common reason for epilepsy surgery in adults [28, 29]. Recent data from the multicenter prospective study, Consequences of Prolonged Febrile Seizures in Childhood (FEBSTAT), has substantially expanded our current understanding of FSE [30]. This study has provided ongoing detailed evaluation of 200 children from ages one month through five years who presented with FSE in order to study the casual relationship between FSE and temporal lobe epilepsy. HHV-6 and HHV-7 virologic studies were performed to determine the frequency of roseolovirus-associated FSE and to determine if roseolovirus-associated FSE is more likely to cause subsequent hippocampal injury and temporal lobe epilepsy. There were 44 cases of primary roseolovirus infection and 14 cases of reactivation as determined by serology and reverse transcriptase PCR. Together, HHV-6B and HHV-7 accounted for one-third of the cases of FSE in the study with HHV-6B causing the majority. There were no differences in acute temporal lobe (hippocampal) injury between children with HHV-6 or HHV-7 infection and those without at the time of infection, and the subsequent development of hippocampal sclerosis is still under active investigation. Therefore, while roseoloviruses may cause hippocampal injury, it appears they may be no more likely than other viruses to do so during the acute illness [30]. HHV-6B has been found in temporal lobe specimens of patients with intractable temporal lobe epilepsy, but the causal relationship between HHV-6B reactivation and hippocampal injury remains undefined [31-33].
Encephalitis and other neurologic disorders
HHV-6B reactivation is an established cause of limbic encephalitis in immune compromised persons following hematopoetic stem cell transplantation, as initially described by Wainwright and colleagues [34] (please refer to the accompanying review by Zerr and Hill). More recently, the receipt of cord blood stem cells has been highly associated with HHV-6 reactivation and encephalitis [35, 36]. HHV-6B, and rarely HHV-7, primary infection has also been associated with encephalitis in immune competent individuals [37]. There appears to be a distinct geographic distribution, with the highest incidence occurring in Japan. Surveys estimate that 60 cases of roseola per year are complicated by encephalitis in Japan, making it the second most common cause of infection-related encephalitis. Severe neurologic sequelae such as acute necrotizing encephalitis, hemorrhagic shock and acute encephalopathy with biphasic seizures complicate nearly half of those cases [38, 39]. Evidence suggests that this may be a cytokine-mediated disorder [40]. HHV-6B has also been implicated in triggering potentially fatal neurologic deterioration in children with an underlying mitochondrial disorder involving polymerase gamma gene (POLG) mutations, suggesting that underlying host factors may contribute to the severity of HHV-6-associated neurological disease [41].
HHV-7 Primary infection
HHV-7 was first isolated from CD4+ lymphocytes in 1990 by Frenkel and colleagues and subsequently found to be a distinct virus closely related to HHV6-A and HHV-6B and an additional cause of roseola [42]. Infection is highly prevalent worldwide and also causes universal infection in childhood. However, HHV-7 tends to infect slightly older children when compared to primary HHV-6B infection. A small case series identified 8 cases of primary HHV-7 infection out of 250 children presenting to the ED with fever. The median age of presentation was 26 months and only one child was less than 13 months old. The clinical presentation was indistinguishable from that of HHV-6B infection, and notably six of the eight children presented with seizures [43]. Suga and colleagues in Japan also found that HHV-7 infection was comparable to HHV-6 in a slightly older child, although seizure activity was only observed in one of fifteen cases of HHV-7 primary infection [44]. While these studies are relatively small in size, it appears that HHV-7 primary infection has the potential for severe complications similar to HHV-6. Recent evidence has also linked delayed HHV-7 primary infection with severe neurologic complications, including encephalitis and Guillain-Barre syndrome [45].
Summary/Research Priorities
Primary infection with roseoloviruses is nearly universal in early childhood. While the majority of infections are self-limited, the large number of infections coupled with the characteristic fever leads to significant healthcare utilization and possible antibiotic misuse. New methods for sensitive, specific and timely diagnosis of acute infection could potentially mitigate some of the healthcare expenditures and antimicrobial overuse (please refer to the accompanying review on diagnostics by Hill et al). Additionally, the universal nature of infection with roseoloviruses, along with the recognition of ciHHV-6, creates unique challenges in investigating the true burden of disease and research is most urgently needed to determine methodology and criteria for distinguishing a causal relationship between roseoloviruses and pathology. Although primary infection has been directly linked to a spectrum of neurologic complications, most notably febrile status epilepticus, the full spectrum of complications and their clinical burden remain important research questions. The identification of potential biomarkers to predict individuals at high risk for complications and the possible benefits of antiviral treatment in select populations are related research priorities.
Highlights.
HHV-6B primary infection occurs in nearly all children by three years of age.
HHV-6 can integrate into the host genome and be passed via germline transmission.
Only a quarter of HHV-6B primary infections manifest as roseola infantum in the US.
HHV-6B or HHV-7 account for 1/3 of febrile status epilepticus cases in children.
Delayed HHV-7 primary infection may be associated with more severe neurologic complications.
Acknowledgments
This review was presented in part at the NIAID-sponsored conference entitled “Roseoloviruses: Clinical Impact, Interventions, and Research Needs”, June 2, 2014, Natcher Center, NIH, Bethesda, MD.
Dr. Epstein's contribution was supported in part by a grant from the National Institute of Neurological Disorders and Stroke [NINDS grant NS43209 (P.I. S. Shinnar, M.D.,Ph.D)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Contributor Information
Brenda L. Tesini, Email: Brenda_tesini@urmc.rochester.edu.
Leon G. Epstein, Email: L-epstein@northwestern.edu.
References
- 1.Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M, Halligan G, Biberfeld P, Wong-Staal F, Kramarsky B, et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- 2.Ablashi D, Agut H, Alvarez-Lafuente R, Clark DA, Dewhurst S, DiLuca D, Flamand L, Frenkel N, Gallo R, Gompels UA, et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol. 2014;159(5):863–70. doi: 10.1007/s00705-013-1902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suga S, Yoshikawa T, Kajita Y, Ozaki T, Asano Y. Prospective study of persistence and excretion of human herpesvirus-6 in patients with exanthem subitum and their parents. Pediatrics. 1998;102(4 Pt 1):900–4. doi: 10.1542/peds.102.4.900. [DOI] [PubMed] [Google Scholar]
- 4.Hall CB, Caserta MT, Schnabel KC, Shelley LM, Carnahan JA, Marino AS, Yoo C, Lofthus GK. Transplacental congenital human herpesvirus 6 infection caused by maternal chromosomally integrated virus. J Infect Dis. 2010;201(4):505–7. doi: 10.1086/650495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altschuler EL. Oldest description of roseola and implications for the antiquity of human herpesvirus 6. Pediatr Infect Dis J. 2000;19(9):903. doi: 10.1097/00006454-200009000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988;1(8594):1065–7. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 7.Hall CB, Long CE, Schnabel KC, Caserta MT, McIntyre KM, Costanzo MA, Knott A, Dewhurst S, Insel RA, Epstein LG. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med. 1994;331(7):432–8. doi: 10.1056/NEJM199408183310703. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa T, Suga S, Asano Y, Yazaki T, Kodama H, Ozaki T. Distribution of antibodies to a causative agent of exanthem subitum (human herpesvirus-6) in healthy individuals. Pediatrics. 1989;84(4):675–7. [PubMed] [Google Scholar]
- 9.Ward KN, Gray JJ, Fotheringham MW, Sheldon MJ. IgG antibodies to human herpesvirus-6 in young children: changes in avidity of antibody correlate with time after infection. J Med Virol. 1993;39(2):131–8. doi: 10.1002/jmv.1890390209. [DOI] [PubMed] [Google Scholar]
- 10.Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang ML, Wald A, Rhoads MP, Nguy L, Bornemann R, Morrow RA, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352(8):768–76. doi: 10.1056/NEJMoa042207. [DOI] [PubMed] [Google Scholar]
- 11.Hall CB, Caserta MT, Schnabel KC, Boettrich C, McDermott MP, Lofthus GK, Carnahan JA, Dewhurst S. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7) J Pediatr. 2004;145(4):472–7. doi: 10.1016/j.jpeds.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Bates M, Monze M, Bima H, Kapambwe M, Clark D, Kasolo FC, Gompels UA. Predominant human herpesvirus 6 variant A infant infections in an HIV-1 endemic region of Sub-Saharan Africa. J Med Virol. 2009;81(5):779–89. doi: 10.1002/jmv.21455. [DOI] [PubMed] [Google Scholar]
- 13.Rhoads MP, Magaret AS, Zerr DM. Family saliva sharing behaviors and age of human herpesvirus-6B infection. J Infect. 2007;54(6):623–6. doi: 10.1016/j.jinf.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Harberts E, Yao K, Wohler JE, Maric D, Ohayon J, Henkin R, Jacobson S. Human herpesvirus-6 entry into the central nervous system through the olfactory pathway. Proc Natl Acad Sci U S A. 2011;108(33):13734–9. doi: 10.1073/pnas.1105143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall CB, Caserta MT, Schnabel K, Shelley LM, Marino AS, Carnahan JA, Yoo C, Lofthus GK, McDermott MP. Chromosomal integration of human herpesvirus 6 is the major mode of congenital human herpesvirus 6 infection. Pediatrics. 2008;122(3):513–20. doi: 10.1542/peds.2007-2838. [DOI] [PubMed] [Google Scholar]
- 16*.Gravel A, Hall CB, Flamand L. Sequence analysis of transplacentally acquired human herpesvirus 6 DNA is consistent with transmission of a chromosomally integrated reactivated virus. J Infect Dis. 2013;207(10):1585–9. doi: 10.1093/infdis/jit060. HHV-6 DNA sequence analysis of two mother-infant pairs in which the mothers had ciHHV-6 and infants had transplacentally-acquired HHV-6 suggests that reactivation of maternal ciHHV-6 is a potential mode of congenital HHV-6 infection. [DOI] [PubMed] [Google Scholar]
- 17.Asano Y, Yoshikawa T, Suga S, Kobayashi I, Nakashima T, Yazaki T, Kajita Y, Ozaki T. Clinical features of infants with primary human herpesvirus 6 infection (exanthem subitum, roseola infantum) Pediatrics. 1994;93(1):104–8. [PubMed] [Google Scholar]
- 18.Pruksananonda P, Hall CB, Insel RA, McIntyre K, Pellett PE, Long CE, Schnabel KC, Pincus PH, Stamey FR, Dambaugh TR, et al. Primary human herpesvirus 6 infection in young children. N Engl J Med. 1992;326(22):1445–50. doi: 10.1056/NEJM199205283262201. [DOI] [PubMed] [Google Scholar]
- 19**.Colvin JM, Muenzer JT, Jaffe DM, Smason A, Deych E, Shannon WD, Arens MQ, Buller RS, Lee WM, Weinstock EJ, et al. Detection of viruses in young children with fever without an apparent source. Pediatrics. 2012;130(6):e1455–62. doi: 10.1542/peds.2012-1391. Recent prospective study of children less than three years of age presenting to ED with undifferentiated fever. HHV-6 DNA was detected in 17% of febrile children, identifying it as a major cause of fever and healthcare utilization in this age group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujino M, Ohashi M, Tanaka K, Kato T, Asano Y, Yoshikawa T. Rhabdomyolysis in an infant with primary human herpesvirus 6 infection. Pediatr Infect Dis J. 2012;31(11):1202–3. doi: 10.1097/INF.0b013e318266b3c9. [DOI] [PubMed] [Google Scholar]
- 21.Yoshikawa T, Ihira M, Suzuki K, Suga S, Kito H, Iwasaki T, Kurata T, Tanaka T, Saito Y, Asano Y. Fatal acute myocarditis in an infant with human herpesvirus 6 infection. J Clin Pathol. 2001;54(10):792–5. doi: 10.1136/jcp.54.10.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshikawa T, Morooka M, Suga S, Niinomi Y, Kaneko T, Shinoda K, Muraki Y, Takahashi K, Sugaya N, Asano Y. Five cases of thrombocytopenia induced by primary human herpesvirus 6 infection. Acta Paediatr Jpn. 1998;40(3):278–81. doi: 10.1111/j.1442-200x.1998.tb01928.x. [DOI] [PubMed] [Google Scholar]
- 23.Millichap JG, Millichap JJ. Role of viral infections in the etiology of febrile seizures. Pediatr Neurol. 2006;35(3):165–72. doi: 10.1016/j.pediatrneurol.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Barone SR, Kaplan MH, Krilov LR. Human herpesvirus-6 infection in children with first febrile seizures. J Pediatr. 1995;127(1):95–7. doi: 10.1016/s0022-3476(95)70263-6. [DOI] [PubMed] [Google Scholar]
- 25.Ward KN, Andrews NJ, Verity CM, Miller E, Ross EM. Human herpesviruses-6 and -7 each cause significant neurological morbidity in Britain and Ireland. Arch Dis Child. 2005;90(6):619–23. doi: 10.1136/adc.2004.062216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hesdorffer DC, Benn EK, Bagiella E, Nordli D, Pellock J, Hinton V, Shinnar S, Team FS. Distribution of febrile seizure duration and associations with development. Ann Neurol. 2011;70(1):93–100. doi: 10.1002/ana.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, Garnett L, Fortner CA, Ko D. A prospective,population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46(4):1029–35. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- 28.Falconer MA, Serafetinides EA, Corsellis JA. Etiology and Pathogenesis of Temporal Lobe Epilepsy. Arch Neurol. 1964;10:233–48. doi: 10.1001/archneur.1964.00460150003001. [DOI] [PubMed] [Google Scholar]
- 29.Shinnar S, Hesdorffer DC, Nordli DR, Jr, Pellock JM, O'Dell C, Lewis DV, Frank LM, Moshe SL, Epstein LG, Marmarou A, et al. Phenomenology of prolonged febrile seizures: results of the FEBSTAT study. Neurology. 2008;71(3):170–6. doi: 10.1212/01.wnl.0000310774.01185.97. [DOI] [PubMed] [Google Scholar]
- 30**.Epstein LG, Shinnar S, Hesdorffer DC, Nordli DR, Hamidullah A, Benn EK, Pellock JM, Frank LM, Lewis DV, Moshe SL, et al. Human herpesvirus 6 and 7 in febrile status epilepticus: the FEBSTAT study. Epilepsia. 2012;53(9):1481–8. doi: 10.1111/j.1528-1167.2012.03542.x. A multicenter, prospective study of children presenting with febrile status epilepticus associated HHV-6 or HHV-7 infection with one-third of the cases. The possible link to the development of hippocampal injury is under current investigation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li JM, Lei D, Peng F, Zeng YJ, Li L, Xia ZL, Xia XQ, Zhou D. Detection of human herpes virus 6B in patients with mesial temporal lobe epilepsy in West China and the possible association with elevated NF-kappaB expression. Epilepsy Res. 2011;94(1-2):1–9. doi: 10.1016/j.eplepsyres.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Niehusmann P, Mittelstaedt T, Bien CG, Drexler JF, Grote A, Schoch S, Becker AJ. Presence of human herpes virus 6 DNA exclusively in temporal lobe epilepsy brain tissue of patients with history of encephalitis. Epilepsia. 2010;51(12):2478–83. doi: 10.1111/j.1528-1167.2010.02741.x. [DOI] [PubMed] [Google Scholar]
- 33.Theodore WH, Epstein L, Gaillard WD, Shinnar S, Wainwright MS, Jacobson S. Human herpes virus 6B: a possible role in epilepsy? Epilepsia. 2008;49(11):1828–37. doi: 10.1111/j.1528-1167.2008.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wainwright MS, Martin PL, Morse RP, Lacaze M, Provenzale JM, Coleman RE, Morgan MA, Hulette C, Kurtzberg J, Bushnell C, et al. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol. 2001;50(5):612–9. doi: 10.1002/ana.1251. [DOI] [PubMed] [Google Scholar]
- 35*.Hill JA, Koo S, Guzman Suarez BB, Ho VT, Cutler C, Koreth J, Armand P, Alyea EP, 3rd, Baden LR, Antin JH, et al. Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transplant. 2012;18(11):1638–48. doi: 10.1016/j.bbmt.2012.04.016. A retrospective cohort study of hematopoietic stem cell transplant recipients identified the receipt of cord blood stem cells as a major risk factor for the development of HHV-6-associated posttransplantation acute limbic encephalitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheurer ME, Pritchett JC, Amirian ES, Zemke NR, Lusso P, Ljungman P. HHV-6 encephalitis in umbilical cord blood transplantation: a systematic review and meta-analysis. Bone Marrow Transplant. 2013;48(4):574–80. doi: 10.1038/bmt.2012.180. [DOI] [PubMed] [Google Scholar]
- 37.Ward KN. Child and adult forms of human herpesvirus 6 encephalitis: looking back, looking forward. Curr Opin Neurol. 2014;27(3):349–55. doi: 10.1097/WCO.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 38.Yoshikawa T, Ohashi M, Miyake F, Fujita A, Usui C, Sugata K, Suga S, Hashimoto S, Asano Y. Exanthem subitum-associated encephalitis: nationwide survey in Japan. Pediatr Neurol. 2009;41(5):353–8. doi: 10.1016/j.pediatrneurol.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Hoshino A, Saitoh M, Oka A, Okumura A, Kubota M, Saito Y, Takanashi J, Hirose S, Yamagata T, Yamanouchi H, et al. Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev. 2012;34(5):337–43. doi: 10.1016/j.braindev.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Kawamura Y, Yamazaki Y, Ohashi M, Ihira M, Yoshikawa T. Cytokine and chemokine responses in the blood and cerebrospinal fluid of patients with human herpesvirus 6B-associated acute encephalopathy with biphasic seizures and late reduced diffusion. J Med Virol. 2014;86(3):512–8. doi: 10.1002/jmv.23788. [DOI] [PubMed] [Google Scholar]
- 41.Al-Zubeidi D, T M, Pathak S, Cai C, Schlaggar BL, Storch GA, Grange DK, Watson ME. Fatal Human Herpes Virus-6 Associated Encephalitis in Two Boys with Underlying POLG Mitochondrial Disorders. Pediatric Neurology. 2014 doi: 10.1016/j.pediatrneurol.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Frenkel N, Schirmer EC, Wyatt LS, Katsafanas G, Roffman E, Danovich RM, June CH. Isolation of a new herpesvirus from human CD4+ T cells. Proc Natl Acad Sci U S A. 1990;87(2):748–52. doi: 10.1073/pnas.87.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caserta MT, Hall CB, Schnabel K, Long CE, D'Heron N. Primary human herpesvirus 7 infection: a comparison of human herpesvirus 7 and human herpesvirus 6 infections in children. J Pediatr. 1998;133(3):386–9. doi: 10.1016/s0022-3476(98)70275-6. [DOI] [PubMed] [Google Scholar]
- 44.Suga S, Yoshikawa T, Nagai T, Asano Y. Clinical features and virological findings in children with primary human herpesvirus 7 infection. Pediatrics. 1997;99(3):E4. doi: 10.1542/peds.99.3.e4. [DOI] [PubMed] [Google Scholar]
- 45*.Schwartz KL, Richardson SE, Ward KN, Donaldson C, MacGregor D, Banwell B, Mahant S, Bitnun A. Delayed primary HHV-7 infection and neurologic disease. Pediatrics. 2014;133(6):e1541–7. doi: 10.1542/peds.2013-3344. Identified three adolescents with severe neurologic disease (encephalitis and Guillaine-Barre syndrome) and evidence of acute HHV-7 primary infection based on CSF PCR and serology. This suggests that delayed acquisition of virus may be linked to severe neurologic complications. [DOI] [PubMed] [Google Scholar]
- 46.Vianna RA, de Oliveira SA, Camacho LA, Knowles W, Brown D, Pereira AC, Velarde LG, Siqueira MM. Role of human herpesvirus 6 infection in young Brazilian children with rash illnesses. Pediatr Infect Dis J. 2008;27(6):533–7. doi: 10.1097/INF.0b013e3181673c50. [DOI] [PubMed] [Google Scholar]


