Abstract
In response to infection and trauma, exquisite control of the innate inflammatory response is necessary to promote an anti-microbial response and minimize tissue injury. Over the course of the host response, activated leukocytes are essential for the initial response and can later become unresponsive or undergo apoptosis. Leukocytes, along the continuum of activation to apoptosis, have been shown to generate microvesicles. These vesicles can range in size from 0.1 to 1.0 µm and can retain proteins, RNA and DNA of their parent cells. Importantly, neutrophil-derived microvesicles (NDMV) are robustly increased under inflammatory conditions. The aim of this review is to summarize the research to date upon NDMVs. This will include describing under which disease states NDMVs are increased, mechanisms underlying formation, and the impact of these vesicles upon cellular targets. Altogether, increased awareness of NDMVs during the host innate response may allow for diagnostic tools as well as potential novel therapies during infection and trauma.
Keywords: Apoptosis, inflammatory, immune response, microvesicles, neutrophil derived microvesicles, sepsis
INTRODUCTION
Microvesicles (MVs) are small intact vesicles ranging in size from 0.1–1.0 µm. These extracellular vesicles have been observed to bleb off the surface of a number of cell types including erythrocytes, endothelial cells, lymphocytes, platelets, and myeloid cells [1]. Microvesicles have been implicated in a variety of disease processes including atherosclerosis [2], thrombosis [3], rheumatoid arthritis [4], cancer [5], inflammation [5, 6], transfusion-related acute lung injury [7], and sepsis [6]. Chargaff and West first described MPs in 1946 as a precipitable factor in platelet free plasma [8]. Later, in 1967 Wolf described lipid-rich MVs separated by ultracentrifugation from fresh plasma as “platelet dust” [9].
Cells have been shown to generate MV after activation and during events leading to apoptosis [1]. These MV can contain membrane, cytoplasmic and nuclear components of the parent cell [1] and typically retain surface markers of their parent cells [10], but are distinct in composition and size from other subcellular structures, such as apoptotic bodies or exosomes [1]. Current literature suggests that MV generation is the end point of an activation cascade and / or the beginning of apoptosis requiring increases in intracellular calcium concentration, rearrangement of the cytoskeleton, and the budding of these vesicles from the plasma membrane [11], and as such functions as a terminal stepof cell signaling.
Microvesicles express extracellular facing proteins that are known to be involved in a variety of important cellular processes and thus can act as bioactive effectors [12]. In fact, MVs can exert biological effects to host cells by stimulating receptors of the target cell, or by transferal of vesicle content, such as protein, lipids, mRNA, or microRNA, to the target cell [13]. Additionally, circulating microvesicles can act together with soluble mediators to enhance biological processes, such as coagulation [14]. Microvesicle compositions, even from the same cell type, can vary depending on the stimuli or disease state. Examples include: 1) endothelial cells, stimulated by plasminogen activator inhibitor-1 or TNF-α, generate microvesicles with distinct protein compositions [15]. 2) Platelet derived microvesicles derived just prior to cell apoptosis rather than cell activation, can differentially modulate cellular and physiological functions [16]. 3) Cancer cell microvesicles can transfer oncogenic content to bystander cells [17]. In this way, microvesicles act as distinct packets of information that transfer information between parent cells to target cells depending on the cellular environment or stimulus.
In general, microvesicles can mediate the immune response under pathophysiological conditions. Of interest, neutrophil derived microvesicles (NDMVs) are currently one of the least studied microvesicle populations, despite the neutrophil being an integral part of the immune response. Similar to the cell from which they originate, NDMVs are involved in a number of distinct autocrine and paracrine immunological processes. The remainder of this review will focus only upon NDMVs, specifically, increased numbers during disease, generation during inflammatory conditions, impact on target cells, and potential future directions.
NUMBERS OF NDMV ARE INCREASED IN DISEASE STATES
Neutrophil derived microvesicles are present in very small amounts in normal physiologic conditions [18]. However, NDMVs are reported to be elevated in a variety of inflammatory disorders [18a, 18c, 19] (Table 1). The increase of NDMV numbers during pathophysiological processes could be used, advantageously, as diagnostic indicator of inflammation. For example, an early report that investigated patients with various kidney disorders, found NDMV populations were significantly increased in patients with acute vasculitis, chronic vasculitis, IgA nephropathy, and tubulointerstitial nephritis, as well as patients who were undergoing hemodialysis [19a]. Another study demonstrated that elevated circulating NDMVs alone can be an independent predictor of atherosclerosis burden [19b]. NDMVs from patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis have elevated levels compared to normal counterparts and have a pro-inflammatory effect on endothelial cells, thus contributing to the disease process [20]. Additionally, in patients with systemic lupus erythematosus (SLE) and anti-phospholipid syndrome, both autoimmune conditions that affect the vasculature, NDMVs are also increased and associated with increased plasmin generation [19c]. Because of these associations, quantification of NDMVs could be used as a prognostic indicator of vascular diseases.
Table 1.
Presence of NDMVs in human disease.
| Human Disease | Source | References |
|---|---|---|
| Cardiovascular Disease/Atherosclerosis | Platelet-poor plasma (PRP) | [19b, 51] |
| Systemic Lupus Erythematosus, Anti-phospholipid Syndrome | PRP | [51] |
| ANCA-vasculitis | PolymorphPrep (Blood) | [20] |
| Pneumonia/ARDS | BAL / PRP / Blood | [6, 19a, 22] |
| Tubulo-interstitial nephritis, Vasculitis, IgA Nepropathy | Blood | [19a] |
| Sepsis | Blood / Peritoneal Lavage | [6, 18] |
| Localized Infection | Blister exudate | [18c] |
Abbreviations: NDMV – Neutrophil Derived Microvesicle, ARDS – acute respiratory distress syndrome, BAL – Bronchoalveolar lavage (BAL).
During infectious conditions, systemic NDMV numbers are observed to be elevated. Plasma from patients with community acquired pneumonia showed a statistically significant increase in circulating NDMVs, compared to healthy individuals [18c]. During meningococcal sepsis, the level of circulating NDMVs is significantly increased compared to baseline levels, while other microvesicle populations are not significantly changed [18a]. Neutrophils from trauma patients more robustly generated microvesicles than did neutrophils from healthy individuals, leading to elevated NDMVs in these patients [21]. The question of whether the robust increase in NDMVs continually or preferentially during the early or latter stages of disease pathophysiology remains to be answered.
In the circulation, platelet derived microvesicles are the largest microvesicle population observed [19c]. In contrast, at the sites of infection or inflammation, NDMVs have been shown to predominate [6, 18c, 22]. One study demonstrated that NDMVs were over 14-fold more concentrated in a blister vs. human serum, despite the serum containing 10-fold more neutrophils than the blister [18c]. Consistent with this, NDMVs were significantly increased in BAL samples from patients with pneumonia and increased in abdominal washings from patients with abdominal sepsis. NDMVs also predominated as the primary MV population at infectious foci [6]. Acute Respiratory Distress Syndrome (ARDS) is a condition usually seen in patients with sepsis or severe trauma characterized by lung inflammation, which leads to impaired gas exchange and worsening inflammation. It was observed that neutrophil derived microvesicles were the most numerous MV populations in bronchial washings in patients with ARDS [22]. Interestingly in this same study, ARDS patients who had a higher NDMV level had a survival benefit [22]. We speculate that in lung inflammation, NDMVs have an anti-inflammatory effect upon leukocytes and this suppression of the host immune response would limit impaired gas exchange, leading to improved outcomes.
Altogether, the presence of NDMVs in these conditions could be used as a novel and rapid marker of inflammation or infection. While this is an interesting prospect, the aforementioned studies quantifying NDMV levels in patients utilized multiple methods of enumerating NDMVs. Some of these studies measured NDMVs directly from patient samples, while others isolated and stimulated neutrophils to generate NDMVs, which were subsequently quantified. Until future studies develop a standardized method of collection and analysis [10, 23], interpretation of these data in a day-today clinical setting will be problematic.
Microvesicle Stimulation and Mechanisms of formation
Similar to other global microvesicle populations, neutrophil derived microvesicles can be elicited from both apoptotic and activated cells by a large number of stimuli. Microparticle composition appears to be different based on stimuli used for generation. This is illustrated by a recent study by Dalli et al., demonstrating different NDMV protein composition depending on neutrophil state, and discrete impact endothelial cell gene expression profiles, depending on stimulus used for NDMV generation [18c]. For organizational purposes, we will separate these compounds into 3 broad categories: bacterial byproducts, host factors/cytokines, and exogenous compounds. In general, bacteria have been observed to cause neutrophils to spawn NDMVs [18b, 24]. Strikingly, these microvesicles are subsequently observed to curtail bacterial growth [18b]. Bacterial byproducts such as endotoxin [25] and fMLP [26] are potent inducers of NDMV generation. In terms of host mediators, TNF-α [27], complement [26e, 28], IL-8 [26a], and Platelet activating factor [20] have all been demonstrated to increase NDMVs generation. Other exogenous compounds such as the protein kinase C activator, PMA [20, 26a, 26b], ionomycin [26a, 26b], and nitric oxide synthase inhibitor, L-NAME [29], have also been found to increase NDMV production. A complete list of compounds, and references to date, dealing with the generation neutrophil derived microvesicles can be found in Table 2.
Table 2.
Stimuli for NDMV generation.
| NDMV Induction Stimulus |
Concentration | Incubation Time |
Species: (H)uman (M)ouse (R)ats |
Quantification Method |
Notes | References |
|---|---|---|---|---|---|---|
| Bacterial Byproducts | ||||||
| - Bacteria | n/a | 20 min | H, R | FACS, EM | [18b, 24] | |
| - LPS | 5 ug/mL | 90 min | H | FACS | [25] | |
| - fMLP | 10 uM – 1 M | 5 min-2 hr | H, M | FACS | [19a, 20–22, 26, 29, 35, 37, 42,52] | |
| Host Factors | ||||||
| - Spontaneous | n/a | 15 min | H | FACS | a. | [21–22, 52b, 52c, 53] |
| - TNF-α | 1–10 ng/mL | 20 min | H, M | FACS | b. | [19a, 20, 27, 54] |
| - Complement | 6–9 ug/mL, 100 ng/mL |
20–25 min | M | EM | [26e, 28] | |
| - IL-8 | 100 ng/mL | 2 hr | H | FACS | [26d] | |
| - PAF | 500 nM | 15–30 min | H | FACS | [25b] | |
| - Calcium | 1 mM | 15–30 min | H | FACS | [25b] | |
| - ANCA | 200 ug/mL | 1 hr | H | FACS | c. | [20] |
| Exogenous Compounds | ||||||
| - PMA | 10 nM – 5 M | 20 min | H | FACS, EM | [18b, 25b, 26a, 26b] | |
| - Ionomycin | 0.5–5 mM | 20 min | H | FACS | [26a, 26b] | |
| - L-NAME | 30 uM | 1 hr | H | FACS | [29] | |
Samples taken from patients with ARDS [22]
Neutrophils pre-incubated overnight for synchronization [54]
Used TNF-α to prime neutrophils [20]
Abbreviations: FACS - Fluorescent Activated Cell Sorting, EM - Electron Microscopy, LPS – Lipopolysaccharide, PAF – Platelet Activating Factor, L-NAME – NG-nitro-l-arginine methyl ester, ANCA – anti-neutrophil cytoplasmic antibody
Currently the methodologies utilized to investigate mechanisms underlying NDMV generation are quite varied. For example, a number of papers “prime” neutrophils prior to the incubation with the molecule of interest, while others do not. In addition, there is great variability on incubation lengths for the stimulus of interest. Furthermore, NDMV quantification is varied in a number of ways, such by protein amount or absolute or indirect counting by flow cytometry. There is no consensus on which cell surface marker(s) should be utilized to identify NDMVs. Indeed, neutrophil specific antibody titration curves should be presented to show specificity of the antibody for NDMV. Annexin positivity (to detect extracellular expression of phosphatidylserine) or negativity as it relates to NDMV enumeration is also something in which there is no consensus in the field. It is quite possible that MV expression of phosphatidylserine will vary depending on the timing of MV derivation; ie., during the early or latter stages of the disease state [30]. However, without standardized methodology to characterize and enumerate NDMVs, it will be difficult to temporally compare differences of NDMV generation with different stimuli.
A number of papers have evaluated intracellular molecular mechanisms of NDMV generation. Multiple studies have looked at NDMVs being generated through cell activation specifically investigating compounds that increase intracellular calcium concentration [18b, 25b, 26a, 26b]. Increased intracellular calcium is known to increase calpain activity [31]. The enzyme calpain is normally responsible for the degradation of talin, an essential structural component of the neutrophil cytoskeleton and the shedding of membrane-derived vesicles [32]. Because of the relationship of calcium, calpain, and microvesicle formation, most studies have evaluated microvesicle formation in the presence of reagents that increase intracellular calcium, namely, fMLP, PMA (a protein kinase C activator), and L-NAME. Consistent with this, calpain inhibition decreased NDMV generation after neutrophil activation [25a, 29]. In terms of NDMV generation during cellular during the events leading to apoptosis, TNF-α alone has been shown to be sufficient to generate NDMVs, through an NF-kB and/or Caspase 8 dependent pathway [27]. Caspase 8 activates Caspase 3, and caspase 3 can activate Calpain via cleavage of the calpain inhibitor calpastatin [33]. Altogether, the majority of these studies investigating NDMV generation utilize in vitro systems. One challenge going forward is to verify that these in vitro elucidated mechanisms using in vivo models.
NDMV Impact on Target Cells
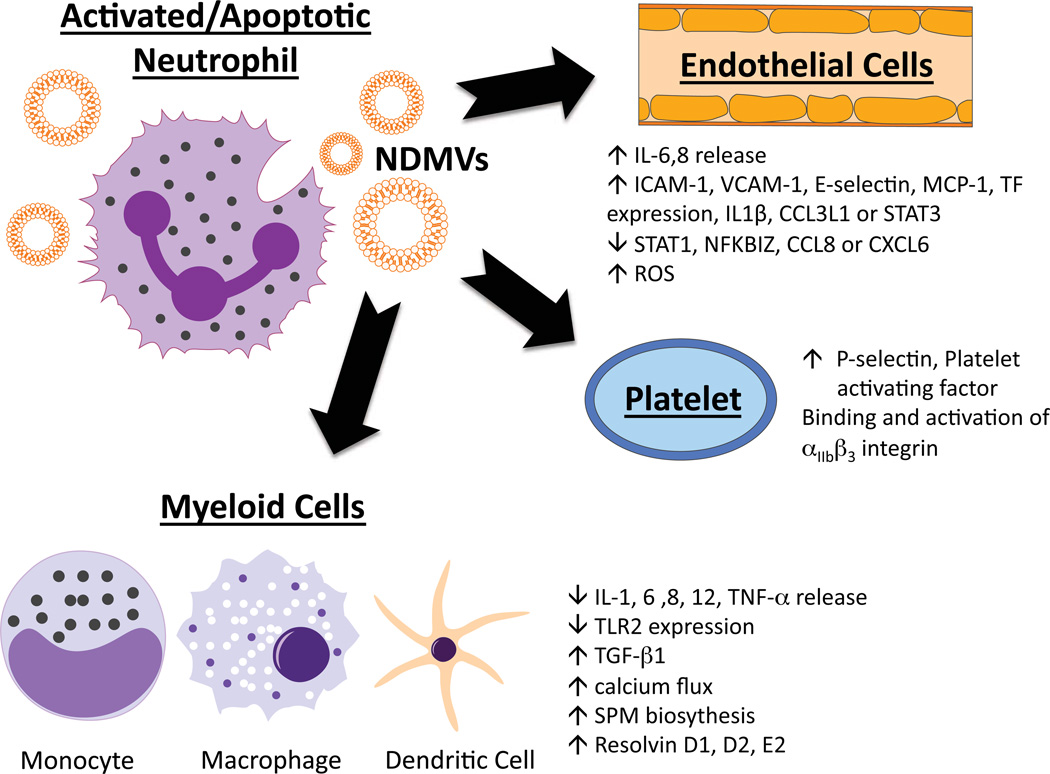
The number of NDMVs can be increased in a number of inflammatory disease states, and their presence appears to be able to influence the immunological process (Fig. 1). Although there have been a number of in vitro studies describing how NDMVs can mediate target cell function, there is a paucity of reports describing how manipulating NDMV numbers in vivo could impact the pathophysiology being studied. It remains to be determined whether NDMVs can augment or depress the inflammatory response. Currently, the literature suggests that this response is dependent upon the state of neutrophil/how the NDMV is generated, as well as dependent upon the cellular target. The effects of NDMVs on cells can be seen on Table 3, and are discussed below.
Fig. 1. Impact of NDMVs on various cells.
Abbreviations: SPM - Specialized pro-resolving mediator.
Table 3.
NDMV impact upon host cell.
| Target Cell | NDMV Source | Cell Impact | Relevant Mechanisms | References |
|---|---|---|---|---|
| Endothelial cells | fMLP, IL-8, TNF-α, MPO |
↑ IL-6,8 release ↑ ICAM-1, VCAM-1, E-selectin, MCP-1, TF expression, IL1β, CCL3L1 or STAT3 ↓ STAT1, NFKBIZ, CCL8 or CXCL6 ↑ ROS |
Induction of JNK1 pathway NDMPs bind to Endothelial cells via CD18 |
[18c, 20, 26c, 26d] |
| Macrophage | fMLP, C5a | ↓ IL-1, 6 ,8, 10, 12, TNF-α release ↓ TLR2 expression ↑ TGF-β1 ↑ calcium flux ↑ SPM biosythesis ↑ Resolvin D1, D2, E2 |
Induction of MerTK-dependent anti- inflammatory pathway Activates PI3K/Akt pathway |
[18c, 26e, 37–38, 40a] |
| Immature macrophages |
fMLP | ↑ TGF-β1, ↓CD40, CD80, CD83, CD86, and HLA-DP DQ DR expression ↓ IL-8, IL-10, IL-12, and TNF-α release |
[40a, 42] | |
| Neutrophils | fMLP | ↓ cell/cell adhesion | Annexin-1 presence on MP implicated in decreased in adhesion |
[35] |
| Platelets | PMA, LPS | ↑ P-selectin, Platelet activating factor | Binding and activation of αIIbβ3 integrin | [25] |
| Monocyte | Neat - BAL/Serum | ↑ phagocytocic activity ↑ or ↓ cell activationa |
[27] | |
| Bacteria | S. Aureus | ↓ bacterial growth | Increased bacterial clumping | [18b] |
Increased activity if NDMP phagocytosed by THP-1 cell, decreased if NDMP interacts with cell, but is not phagocytosed
Abbreviations: MPO - Myeloperoxidase, ROS - Reactive Oxygen Species, SPM - Specialized pro-resolving mediator, THP1 - Human acute monocytic leukemiacell line, BAL - Bronchoalveolar Lavage
Interestingly, NDMVs can have an autocrine impact with respect to neutrophil chemotaxis. This is consistent with concept that microvesicle formation is intertwined with uropod formation of a polarized cell and the content of the MV [34]. It has been demonstrated that NDMVs can enhance chemotaxis due to expression of the surface adhesion molecules L-selectin and P-selectin glycoprotein 1 on the NDMV [29]. In contrast, it has been shown that NDMVs inhibit chemotaxis dependent upon annexin 1 expression [35]. It is likely that the explanation for these seemingly disparate results is due to the mechanism of NDMV generation. In the former study, NDMVs were generated by fMLP stimulation coupled with L-NAME incubation. In the latter case, NDMVs were generated without the L-NAME incubation. These studies implicate the importance of nitric oxide upon MV function during formation.
NDMVs can exert a pro-inflammatory response on platelets. It has also been observed that NDMVs can cause an increase in platelet expression of P-selectin via binding and activation of αIIbβ3 integrin [25b]. NDMVs isolated from septic humans activated JNK1-specific signaling in endothelial cells resulting in the release of IL-6 and MCP-1 [26d]. Consistent with NDMV-mediated increases in inflammation, another study demonstrated that NDMVs caused endothelial cells to generate IL-6 and IL-8, as well as increased expression of adhesion molecules [26c]. Additionally, NDMVs were found to bind to endothelial cells via CD18, resulting in increased ICAM-1 expression as well as increased reactive oxygen species generation by endothelial cells [20]. NDMVs were also shown to increase endothelial cell tissue factor expression, which was associated with the generation of a factor Xa-dependent procoagulant response [26d]. While these examples are ways in which NDMVs would enhance the inflammatory response, NDMVs generated from neutrophils co-incubated with endothelial cells were shown to down-regulate STAT1, NF-κB, CCL8, and CXCL6 expression in endothelial cells, which would result in an anti-inflammatory processes or decreased pro-inflammatory actions [18c]. Interestingly, Lim et al., recently demonstrated that inhibition of NDMV generation resulted in increase in increased vascular leakage, suggesting that NDMVs, in normal physiologic conditions, are in part, responsible for maintaining vascular integrity [36].
NDMVs have been demonstrated to exert an overall anti-inflammatory or inflammation-resolving effect on cells of a myeloid lineage. In mature macrophages, NDMV-macrophage cell contact is sufficient for blocking macrophage phagocytosis [26e]. NDMVs also block the inflammatory response of macrophages to zymosan and LPS [26e]. Specifically NDMVs, block the zymosan activation of macrophages by inhibiting NF-κB phosphorylation and nuclear translocation [37]; this inhibition was found to be through NDMV activation of macrophage MerTK (Mer-receptor tyrosine kinase) and PI3K/Akt pathways [37]. Phosphatidylserine expression on NDMVs is thought to be the mechanism for MerTK activation on macrophages, as PS blockade inhibits MerTK activation [38]. Interestingly, PS expression on other cell-derived microvesicles did not result in activation of this pathway [38]. In addition, NDMVs from both apoptotic and zymogen stimulated neutrophils cause macrophages to generate distinct lipid pro-resolving mediators [39], which would also serve to contribute to a counter inflammatory response. Alterations in TGF-β1 production have been observed after incubation with NDMVs [26e, 40]. TGF-β1 can enhance regulatory T-Cell differentiation [41], as well as block macrophage and lymphocyte activation. More recently, NDMVs have been shown to decrease production of IFN-γ and TNF-α, while increasing release of TGF-β1in IL-2 activated natural killer cells [40b].
Similar to mature macrophages, in monocyte-derived dendritic cells, NDMVs caused a change in morphology consistent with reduced phagocytic activity and increased release of TGF-β1 [42]. In this same study, when NDMVs were co-incubated with monocyte-derived dendritic cells in the presence of LPS, activation marker expression decreased, inflammatory cytokine production decreased, and macrophages demonstrated a reduced capacity to induce T cell proliferation [42]. Finally, the presence of NDMVs was observed to decrease neutrophil-HUVEC adherence [35]. We speculate that these aforementioned actions would blunt proinflammatory actions of myeloid cells during the immune response.
Consistent with other models [43], one constant observation concerning NDMV, regardless of target cell, is that when the NDMV is annexin V positive, the impact upon the immune response is invariably immune suppressive. Ingestion of phosphatidylserine expressing neutrophils by macrophages leads to alterations in PGE-2 [44] and IL-10 [45] levels. Additionally, blockage of PS expression on NDMVs attenuated the anti-inflammatory response in NK cells [40b]. Changes in PGE-2 could serve to modulate the macrophage immune response decreasing inflammatory cytokine secretion [46]. IL-10 can suppress the antigen-presenting capacity of antigen presenting cells, regulate phagocytosis on macrophage subsets, and decrease inflammatory cytokine production by neutrophils [47]. Thus, we speculate that increased NDMV numbers generated from apoptotic cells may act as a brake on pro-inflammatory functions.
NDMV generation during sepsis may illustrate the way NDMV numbers would influence the pathophysiology of a disease. Sepsis is the leading cause of morbidity and mortality in surgical intensive care units [48]. This disease is characterized by an overwhelming acute inflammatory response and impaired immune responses [49], as well as endothelial dysfunction, and a systemic prothrombotic state. These all often culminate in end-organ hypo-perfusion or micro-vessel thrombosis, which can lead to multi-organ failure and eventually death. In a patient who is septic, NDMV generating mediators, as seen in Table 1, whether bacteria, bacterial byproducts or host-derived mediators, are abundant. We speculate that these mediators result in NDMV production that is excessive. Elevated NDMVs then can cause increased coagulation [26d] and platelet adhesion [25b] leading to micro-thrombosis, increased inflammation of the vasculature [26c, 26d] and increase reactive oxygen species [20, 26c] leading to impaired vascular function. Taken together, we postulate that a sustained overabundance of NDMVs may be contributing to an anti-inflammatory immune status. This would be detrimental during sepsis, as an immunosuppressed phenotype has been shown to be associated with an inability to clear infections and to worsened outcomes [50].
CONCLUSION
The field of NDMVs remains an area of biology where much remains to be elucidated. The impact of microvesicles on the host inflammatory response highlights the need for more research into this area. NDMVs as biomarkers for disease and targets for medical intervention holds much promise, however, a standardized methodology for NDMV qualification and quantification needs to be agreed upon. Additionally, more work is needed to elucidate the molecular mechanisms of NDMV generation and their subsequent impact on target cells. By understanding the effects NDMVs have on target cells, and by identifying molecular targets involved in NDMV generation, the clinician will potentially be able to modulate the immune system through control of NDMV production.
ACKNOWLEDGEMENTS
Declared none.
FUNDING
NIH RO1, GM100913-01.
NIH T32, GM8478-20.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Ardoin SP, Shanahan JC, Pisetsky DS. The role of microparticles in inflammation and thrombosis. Scand. J. Immunol. 2007;66(2–3):159–165. doi: 10.1111/j.1365-3083.2007.01984.x. [DOI] [PubMed] [Google Scholar]
- 2.Leroyer AS, Tedgui A, Boulanger CM. Role of microparticles in atherothrombosis. J. Intern. Med. 2008;263(5):528–537. doi: 10.1111/j.1365-2796.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- 3.Rautou PE, Vion AC, Amabile N, Chironi G, Simon A, Tedgui A, Boulanger CM. Microparticles, vascular function, and atherothrombosis. Circ. Res. 2011;109(5):593–606. doi: 10.1161/CIRCRESAHA.110.233163. [DOI] [PubMed] [Google Scholar]
- 4.Jungel A, Distler O, Schulze-Horsel U, Huber LC, Ha HR, Simmen B, Kalden JR, Pisetsky DS, Gay S, Distler JH. Microparticles stimulate the synthesis of prostaglandin E(2) via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum. 2007;56(11):3564–3574. doi: 10.1002/art.22980. [DOI] [PubMed] [Google Scholar]
- 5.Anderson HC, Mulhall D, Garimella R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab. Invest. 2010;90(11):1549–1557. doi: 10.1038/labinvest.2010.152. [DOI] [PubMed] [Google Scholar]
- 6.Prakash PS, Caldwell CC, Lentsch AB, Pritts TA, Robinson BR. Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J. Trauma Acute Care Surg. 2012;73(2):401–406. doi: 10.1097/TA.0b013e31825a776d. discussion 406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saas P, Angelot F, Bardiaux L, Seilles E, Garnache-Ottou F, Perruche S. Phosphatidylserine-expressing cell byproducts in transfusion: A pro-inflammatory or an anti-inflammatory effect? Transfus. Clin. Biol. 2012;19(3):90–97. doi: 10.1016/j.tracli.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946;166(1):189–197. [PubMed] [Google Scholar]
- 9.Wolf P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967;13(3):269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 10.Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb. Haemost. 2011;105(3):396–408. doi: 10.1160/TH10-09-0595. [DOI] [PubMed] [Google Scholar]
- 11.Distler JH, Pisetsky DS, Huber LC, Kalden JR, Gay S, Distler O. Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis. Rheum. 2005;52(11):3337–3348. doi: 10.1002/art.21350. [DOI] [PubMed] [Google Scholar]
- 12.Carpintero R, Gruaz L, Brandt KJ, Scanu A, Faille D, Combes V, Grau GE, Burger D. HDL interfere with the binding of T cell microparticles to human monocytes to inhibit pro-inflammatory cytokine production. PLoS One. 2010;5(7):e11869. doi: 10.1371/journal.pone.0011869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 14.Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122(11):1873–1880. doi: 10.1182/blood-2013-04-460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson DB, Sander T, Kaul S, Wakim BT, Halligan B, Twigger S, Pritchard KA, Jr., Oldham KT, Ou JS. Comparative proteomic analysis of PAI-1 and TNF-alpha-derived endothelial microparticles. Proteomics. 2008;8(12):2430–2446. doi: 10.1002/pmic.200701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Connor DE, Exner T, Ma DD, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb, Haemost. 2010;103(5):1044–1052. doi: 10.1160/TH09-09-0644. [DOI] [PubMed] [Google Scholar]; (b) Perez-Pujol S, Marker PH, Key NS. Platelet microparticles are heterogeneous and highly dependent on the activation mechanism: studies using a new digital flow cytometer. Cytometry A. 71(1):38–45. doi: 10.1002/cyto.a.20354. [DOI] [PubMed] [Google Scholar]; (c) Vasina EM, Cauwenberghs S, Staudt M, Feijge MA, Weber C, Koenen RR, Heemskerk JW. Aging- and activation-induced platelet microparticles suppress apoptosis in monocytic cells and differentially signal to proinflammatory mediator release. Am. J. Blood Res. 2013;3(2):107–123. [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008;10(5):619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]; (b) Lima LG, Oliveira AS, Campos LC, Bonamino M, Chammas R, Werneck C, Vicente CP, Barcinski MA, Petersen LC, Monteiro RQ. Malignant transformation in melanocytes is associated with increased production of procoagulant microvesicles. Thromb. Haemost. 2011;106(4):712–723. doi: 10.1160/TH11-03-0143. [DOI] [PubMed] [Google Scholar]
- 18.Nieuwland R, Berckmans RJ, McGregor S, Boing AN, Romijn FP, Westendorp RG, Hack CE, Sturk A. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95(3):930–935. [PubMed] [Google Scholar]; (b) Timar CI, Lorincz AM, Csepanyi-Komi R, Valyi-Nagy A, Nagy G, Buzas EI, Ivanyi Z, Kittel A, Powell DW, McLeish KR, Ligeti E. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood. 2013;121(3):510–518. doi: 10.1182/blood-2012-05-431114. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Dalli J, Montero Melendez T, Norling LV, Yin X, Hinds C, Haskard D, Mayr M, Perretti M. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol. Cell. Proteomics. 2013;12(8):2205–2219. doi: 10.1074/mcp.M113.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniel L, Fakhouri F, Joly D, Mouthon L, Nusbaum P, Grunfeld JP, Schifferli J, Guillevin L, Lesavre P, Halbwachs-Mecarelli L. Increase of circulating neutrophil and platelet microparticles during acute vasculitis and hemodialysis. Kidney Int. 2006;69(8):1416–1423. doi: 10.1038/sj.ki.5000306. [DOI] [PubMed] [Google Scholar]; (b) Chironi G, Simon A, Hugel B, Del Pino M, Gariepy J, Freyssinet JM, Tedgui A. Circulating leukocyte-derived microparticles predict subclinical atherosclerosis burden in asymptomatic subjects. Arterioscler. Thromb. Vasc. Biol. 2006;26(12):2775–2780. doi: 10.1161/01.ATV.0000249639.36915.04. [DOI] [PubMed] [Google Scholar]; (c) Lacroix R, Plawinski L, Robert S, Doeuvre L, Sabatier F, Martinez de Lizarrondo S, Mezzapesa A, Anfosso F, Leroyer AS, Poullin P, Jourde N, Njock MS, Boulanger CM, Angles-Cano E, Dignat-George F. Leukocyte- and endothelial-derived microparticles: a circulating source for fibrinolysis. Haematologica. 2012;97(12):1864–1872. doi: 10.3324/haematol.2012.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong Y, Eleftheriou D, Hussain AA, Price-Kuehne FE, Savage CO, Jayne D, Little MA, Salama AD, Klein NJ, Brogan PA. Anti-neutrophil cytoplasmic antibodies stimulate release of neutrophil microparticles. J. Am. Soc. Nephrol. 2012;23(1):49–62. doi: 10.1681/ASN.2011030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujimi S, Ogura H, Tanaka H, Koh T, Hosotsubo H, Nakamori Y, Kuwagata Y, Shimazu T, Sugimoto H. Increased production of leukocyte microparticles with enhanced expression of adhesion molecules from activated polymorphonuclear leukocytes in severely injured patients. J. Trauma. 2003;54(1):114–9. doi: 10.1097/00005373-200301000-00014. discussion 119–20. [DOI] [PubMed] [Google Scholar]
- 22.Guervilly C, Lacroix R, Forel JM, Roch A, Camoin-Jau L, Papazian L, Dignat-George F. High levels of circulating leukocyte microparticles are associated with better outcome in acute respiratory distress syndrome. Crit. Care. 2011;15(1):R31. doi: 10.1186/cc9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Lacroix R, Judicone C, Mooberry M, Boucekine M, Key NS, Dignat-George F, The ISSCW. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J. Thromb. Haemost. 2013 doi: 10.1111/jth.12207. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Stagnara J, Garnache Ottou F, Angelot F, Mourey G, Seilles E, Biichle S, Saas P, Racadot E. Correlation between platelet-derived microparticle enumeration by flow cytometry and phospholipid-dependent procoagulant activity in microparticles: the centrifugation step matters! Thromb. Haemost. 2012;107(6):1185–1187. doi: 10.1160/TH11-07-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi J, Fujieda H, Kokubo Y, Wake K. Apoptosis of neutrophils and their elimination by Kupffer cells in rat liver. Hepatology. 1996;24(5):1256–1263. doi: 10.1053/jhep.1996.v24.pm0008903407. [DOI] [PubMed] [Google Scholar]
- 25.(a) Watanabe J, Marathe GK, Neilsen PO, Weyrich AS, Harrison KA, Murphy RC, Zimmerman GA, McIntyre TM. Endotoxins stimulate neutrophil adhesion followed by synthesis and release of platelet-activating factor in microparticles. J. Biol. Chem. 2003;278(35):33161–33168. doi: 10.1074/jbc.M305321200. [DOI] [PubMed] [Google Scholar]; (b) Pluskota E, Woody NM, Szpak D, Ballantyne CM, Soloviev DA, Simon DI, Plow EF. Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood. 2008;112(6):2327–2335. doi: 10.1182/blood-2007-12-127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hess C, Sadallah S, Hefti A, Landmann R, Schifferli JA. Ectosomes released by human neutrophils are specialized functional units. J. Immunol. 1999;163(8):4564–4573. [PubMed] [Google Scholar]; (b) Gasser O, Hess C, Miot S, Deon C, Sanchez JC, Schifferli JA. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 2003;285(2):243–257. doi: 10.1016/s0014-4827(03)00055-7. [DOI] [PubMed] [Google Scholar]; (c) Mesri M, Altieri DC. Endothelial cell activation by leukocyte microparticles. J. Immunol. 161(8):4382–4387. [PubMed] [Google Scholar]; (d) Mesri M, Altieri DC. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J. Biol Chem. 1999;274(33):23111–23118. doi: 10.1074/jbc.274.33.23111. [DOI] [PubMed] [Google Scholar]; (e) Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104(8):2543–2548. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 27.Johnson BL, 3rd, Goetzman HS, Prakash PS, Caldwell CC. Mechanisms underlying mouse TNF-alpha stimulated neutrophil derived microparticle generation. Biochem. Biophys. Res. Commun. 2013;437(4):591–596. doi: 10.1016/j.bbrc.2013.06.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(a) Morgan BP, Dankert JR, Esser AF. Recovery of human neutrophils from complement attack: removal of the membrane attack complex by endocytosis and exocytosis. J. Immunol. 1987;138(1):246–253. [PubMed] [Google Scholar]; (b) Campbell AK, Morgan BP. Monoclonal antibodies demonstrate protection of poly-morphonuclear leukocytes against complement attack. Nature. 1985;317(6033):164–166. doi: 10.1038/317164a0. [DOI] [PubMed] [Google Scholar]
- 29.Nolan S, Dixon R, Norman K, Hellewell P, Ridger V. Nitric oxide regulates neutrophil migration through microparticle formation. Am. J. Pathol. 2008;172(1):265–273. doi: 10.2353/ajpath.2008.070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.(a) Ayers L, Kohler M, Harrison P, Sargent I, Dragovic R, Schaap M, Nieuwland R, Brooks SA, Ferry B. Measurement of circulating cell-derived microparticles by flow cytometry: sources of variability within the assay. Thromb. Res. 2011;127(4):370–377. doi: 10.1016/j.thromres.2010.12.014. [DOI] [PubMed] [Google Scholar]; (b) Freyssinet JM, Toti F. Formation of procoagulant microparticles and properties. Thromb. Res. 2010;125(Suppl 1):S46–S48. doi: 10.1016/j.thromres.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 31.Pasquet JM, Dachary-Prigent J, Nurden AT. Calcium influx is a determining factor of calpain activation and microparticle formation in platelets. Eur. J. Biochem. 1996;239(3):647–654. doi: 10.1111/j.1432-1033.1996.0647u.x. [DOI] [PubMed] [Google Scholar]
- 32.Miyoshi H, Umeshita K, Sakon M, Imajoh-Ohmi S, Fujitani K, Gotoh M, Oiki E, Kambayashi J, Monden M. Calpain activation in plasma membrane bleb formation during tert-butyl hydroperoxide-induced rat hepatocyte injury. Gastroenterology. 1996;110(6):1897–1904. doi: 10.1053/gast.1996.v110.pm8964416. [DOI] [PubMed] [Google Scholar]
- 33.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003;4(7):552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 34.(a) Shen B, Fang Y, Wu N, Gould SJ. Biogenesis of the posterior pole is mediated by the exosome/microvesicle protein-sorting pathway. J. Biol. Chem. 2011;286(51):44162–44176. doi: 10.1074/jbc.M111.274803. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yang JM, Gould SJ. The cis-acting signals that target proteins to exosomes and microvesicles. Biochem. Soc. T. 2013;41(1):277–282. doi: 10.1042/BST20120275. [DOI] [PubMed] [Google Scholar]
- 35.Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112(6):2512–2519. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- 36.Lim K, Sumagin R, Hyun YM. Extravasating Neutrophil-derived Microparticles Preserve Vascular Barrier Function in Inflamed Tissue. Immune Netw. 2013;13(3):102–106. doi: 10.4110/in.2013.13.3.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eken C, Martin PJ, Sadallah S, Treves S, Schaller M, Schifferli JA. Ectosomes released by polymorphonuclear neutrophils induce a MerTK-dependent anti-inflammatory pathway in macrophages. J. Biol. Chem. 2010;285(51):39914–39921. doi: 10.1074/jbc.M110.126748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eken C, Sadallah S, Martin PJ, Treves S, Schifferli JA. Ectosomes of polymorphonuclear neutrophils activate multiple signaling pathways in macrophages. Immunobiology. 2013;218(3):382–392. doi: 10.1016/j.imbio.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–e72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.(a) Hoffmann PR, Kench JA, Vondracek A, Kruk E, Daleke DL, Jordan M, Marrack P, Henson PM, Fadok VA. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo . J. Immunol. 2005;174(3):1393–1404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]; (b) Pliyev BK, Kalintseva MV, Abdulaeva SV, Yarygin KN, Savchenko VG. Neutrophil microparticles modulate cytokine production by natural killer cells. Cytokine. 2014;65(2):126–129. doi: 10.1016/j.cyto.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eken C, Gasser O, Zenhaeusern G, Oehri I, Hess C, Schifferli JA. Polymorphonuclear neutrophil-derived ectosomes interfere with the maturation of monocyte-derived dendritic cells. J. Immunol. 2008;180(2):817–824. doi: 10.4049/jimmunol.180.2.817. [DOI] [PubMed] [Google Scholar]
- 43.(a) Frey B, Gaipl US. The immune functions of phosphatidylserine in membranes of dying cells and microvesicles. Seminars in immunopathology. 2011;33(5):497–516. doi: 10.1007/s00281-010-0228-6. [DOI] [PubMed] [Google Scholar]; (b) Lima LG, Chammas R, Monteiro RQ, Moreira ME, Barcinski MA. Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer letters. 2009;283(2):168–175. doi: 10.1016/j.canlet.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 44.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 1998;101(4):890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390(6658):350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 46.Medeiros A, Peres-Buzalaf C, Fortino Verdan F, Serezani CH. Prostaglandin E2 and the suppression of phagocyte innate immune responses in different organs. Mediators Inflamm. 2012;2012:327–568. doi: 10.1155/2012/327568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lalani I, Bhol K, Ahmed AR. Interleukin-10: biology, role in inflammation and autoimmunity. Ann. Allergy Asthma Immunol. 1997;79(6):469–483. doi: 10.1016/S1081-1206(10)63052-9. [DOI] [PubMed] [Google Scholar]
- 48.Antonopoulou A, Giamarellos-Bourboulis EJ. Immunomodulation in sepsis: state of the art and future perspective. Immunotherapy. 2011;3(1):117–128. doi: 10.2217/imt.10.82. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler AP, Bernard GR. Treating patients with severe sepsis. N. Engl. J. Med. 1999;340(3):207–214. doi: 10.1056/NEJM199901213400307. [DOI] [PubMed] [Google Scholar]
- 50.Torgersen C, Moser P, Luckner G, Mayr V, Jochberger S, Hasibeder WR, Dunser MW. Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesth. Analg. 2009;108(6):1841–1847. doi: 10.1213/ane.0b013e318195e11d. [DOI] [PubMed] [Google Scholar]
- 51.Lacroix R, Plawinski L, Robert S, Doeuvre L, Sabatier F, Martinez de Lizarrondo S, Mezzapesa A, Anfosso F, Leroyer A, Poullin P, Jourde N, Njock MS, Boulanger C, Angles-Cano E, Dignat-George F. Leukocyte- and endothelial-derived microparticles: a circulating source for fibrinolysis. Haematologica. 2012;97(12):1864–1872. doi: 10.3324/haematol.2012.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.(a) Gasser O, Schifferli JA. Microparticles released by human neutrophils adhere to erythrocytes in the presence of complement. Exp. Cell Res. 2005;307(2):381–387. doi: 10.1016/j.yexcr.2005.03.011. [DOI] [PubMed] [Google Scholar]; (b) Fujimi S, Ogura H, Tanaka H, Koh T, Hosotsubo H, Nakamori Y, Kuwagata Y, Shimazu T, Sugimoto H. Activated polymorphonuclear leukocytes enhance production of leukocyte microparticles with increased adhesion molecules in patients with sepsis. J. Trauma. 2002;52(3):443–448. doi: 10.1097/00005373-200203000-00005. [DOI] [PubMed] [Google Scholar]; (c) Itakura Sumi Y, Ogura H, Tanaka H, Koh T, Fujita K, Fujimi S, Nakamori Y, Shimazu T, Sugimoto H. Paradoxical cytoskeleton and microparticle formation changes in monocytes and polymorphonuclear leukocytes in severe systemic inflammatory response syndrome patients. J. Trauma. 2003;55(6):1125–1132. doi: 10.1097/01.TA.0000096663.21402.5C. [DOI] [PubMed] [Google Scholar]
- 53.Nusbaum P, Laine C, Seveau S, Lesavre P, Halbwachs-Mecarelli L. Early membrane events in polymorphonuclear cell (PMN) apoptosis: membrane blebbing and vesicle release, CD43 and CD16 down-regulation and phosphatidylserine externalization. Biochem. Soc. T. 2004;32(Pt3):477–479. doi: 10.1042/BST0320477. [DOI] [PubMed] [Google Scholar]
- 54.Nusbaum P, Laine C, Bouaouina M, Seveau S, Cramer EM, Masse JM, Lesavre P, Halbwachs-Mecarelli L. Distinct signaling pathways are involved in leukosialin (CD43) down-regulation, membrane blebbing, and phospholipid scrambling during neutrophil apoptosis. J. Biol. Chem. 2005;280(7):5843–5853. doi: 10.1074/jbc.M413405200. [DOI] [PubMed] [Google Scholar]