Abstract
BACKGROUND
In mice, refrigerator-stored red blood cells (RBCs) are cleared by extravascular hemolysis and induce cytokine production. To enhance understanding of this phenomenon, we sought to model it in vitro.
STUDY DESIGN AND METHODS
Ingestion of refrigerator-stored murine RBCs and subsequent cytokine production were studied using J774A.1 mouse macrophage cells and primary murine splenic macrophages. Wild-type and Ccl2-GFP-reporter mice were used for RBC clearance in vivo.
RESULTS
Although J774A.1 cells and primary macrophages preferentially ingested refrigerator-stored RBCs in vitro, as compared to freshly-isolated RBCs, neither produced increased cytokines following erythrophagocytosis. In contrast, phagocytosis of refrigerator-stored RBCs in vivo induced increases in circulating monocyte chemoattractant protein-1 (MCP-1) and keratinocyte chemoattractant (KC), and correspondingly increased mRNA levels in mouse spleen and liver. In the spleen, these were predominantly expressed by CD11b+ cells. Using Ccl2-GFP-reporter mice, the predominant splenic population responsible for MCP-1 mRNA production were tissue-resident macrophages (i.e., CD45+, CD11b+, F4/80+, Ly6c+, CD11clow cells).
CONCLUSION
J774A.1 cells and primary macrophages selectively ingested refrigerator-stored RBCs by phagocytosis. Although cytokine expression was not enhanced, this approach could be used to identify the relevant receptor-ligand combination(s). In contrast, cytokine levels increased following phagocytosis of refrigerator-stored RBCs in vivo. These were primarily cleared in the liver and spleen, which demonstrated increased MCP-1 and KC mRNA expression. Finally, in mouse spleen, tissue-resident macrophages were predominantly involved in MCP-1 mRNA production. The differences between cytokine production in vitro and in vivo are not yet well understood.
Keywords: red blood cells, transfusion, macrophages, cytokines, inflammation
Introduction
Red blood cell (RBC) transfusion is the most commonly used therapeutic procedure.1 Although RBCs can be stored for up to 42 days,2 multiple studies suggest that transfusions of RBCs stored for >14–21 days may increase the risk of adverse events.3–11 Although this remains controversial,12,13 it is still important to understand the mechanisms and consequences of the rapid clearance of transfused refrigerator storage-damaged RBCs. To this end, a murine model of RBC storage and transfusion was successfully developed.14 Because mouse RBCs “age” in vitro faster than human RBCs, they have an earlier “outdate.” Thus, leukoreduced mouse RBCs stored for <14 days in citrate-phosphate-dextrose-adenine-1 (CPDA-1) have a 24-hour post-transfusion recovery (i.e., ~75%) analogous to that used to determine maximal human RBC storage intervals.14,15
Based on mouse15 and human studies,16 we developed the “iron hypothesis.”17,18 This suggests that phagocytosis of storage-damaged RBCs leads to catabolism of the ingested RBCs, rapidly producing significant amounts of intracellular “free” iron that overwhelm the iron-buffering capacity of ferritin. In mice,15 dogs,19 and, perhaps, human patients,20 clearance of refrigerator storage-damaged RBCs induces a cytokine response; this may be induced by increased levels of reactive oxygen species produced by intracellular “free” iron.21,22 However, neither the cells responsible for cytokine production, nor the mechanisms involved, have been determined. We hypothesized that macrophages, which are central to normal iron metabolism,23 and which participate in clearing refrigerator storage-damaged RBCs,15 also produce the resulting cytokines. To study this, we sought an in vitro model; although preferential phagocytosis of refrigerator storage-damaged RBCs was seen, cytokine production was not convincingly demonstrated. However, using transgenic mice, magnetic sorting, and flow cytometry, tissue-resident splenic macrophages were identified as a significant source of cytokines in this setting in vivo.
Materials and Methods
Mice
Wild-type C57BL/6, CD-1, and FVB/NJ mice were purchased. Ccl2-GFP reporter mice24 were provided by Dr. Eric G. Pamer (Memorial Sloan-Kettering Cancer Center, New York, NY). Mice, housed in a specific pathogen-free facility, were used at 8–12 weeks of age. Study protocols were approved by the Institutional Animal Care and Use Committee.
Cell culture
J774A.1 mouse macrophages (TIB-67; American Type Culture Collection, Manassas, VA) were maintained in complete medium: RPMI 1640 (Gibco, Grand Island, NY) supplemented with 2 mM L-glutamine, penicillin (50 units/ml), streptomycin (50 µg and 10% fetal bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT). Cells were seeded 48 hours before use in the presence of 5 µM all-trans retinoic acid at 3.5 × 106 cells/60 mm culture dish (for the erythrophagocytosis assay) or 0.78 × 106 cells/well in 24-well plates (for the cytokine assay), yielding ~5 × 106 cells/dish and ~1 × 106 cells/well, respectively, for the experiments. One hour before the erythrophagocytosis assay, medium was replaced with fresh complete RPMI 1640 medium and cells incubated at 37°C in the presence or absence of 10 µM cytochalasin D or 0.5 µM wortmannin (Sigma, St. Louis, MO); these inhibit actin polymerization and phosphoinositide 3-kinase, respectively.
Mouse splenic macrophages were prepared from three, fresh, pooled spleens, which were minced through a 70-µm strainer in sterile phosphate-buffered saline (PBS). The cells were centrifuged at 300 × g for 10 minutes at room temperature and re-suspended for 5 minutes in Red Blood Cell Lysis Solution (Miltenyi Biotec Inc., Auburn, CA). Resulting nucleated splenocytes were washed with PBS and re-suspended in Growth Medium: Dulbecco’s high glucose minimal essential medium (MEM) supplemented with 2 mM L-glutamine, penicillin (50 units/ml), streptomycin (50 µg/ml), 10% FBS, 1 mM HEPES, 1% MEM nonessential amino acids, 2% MEM amino acids, 0.1% β-mercaptoethanol (Gibco, Grand Island, NY), and 20% L929 cell conditioned medium. After filtration through a 30-µm nylon cell strainer, splenocytes were added to 24-well plates (for the cytokine assay) or 6-well plates (for the erythrophagocytosis assay). After 3 days, non-adherent cells were removed and the medium replaced with fresh Growth Medium, which was subsequently changed every three days; cells were used on Day 14.
RBCs
RBCs were collected and stored, as described previously.15 Briefly, cohorts of 20 mice were bled by cardiac puncture into CPDA-1. Blood was pooled, leukoreduced (Purecell Neo; Pall Corporation, Port Washington, NY), and centrifuged at 400 × g for 15 minutes. RBCs (final hemoglobin concentration: 17.0–17.5 g/dl) were stored in 15-ml Falcon tubes for defined periods.
To identify bacterial contamination, 500 µl of stored RBCs were inoculated into Peds Plus/F culture bottles (BD Diagnostic Systems) and evaluated with a BACTEC™ continuous monitoring blood culture system (BD Diagnostic Systems) for ≥5 days; this detects ≥10 colony-forming units (CFU) per milliliter with a sensitivity of 97%.25
Erythrophagocytosis
RBCs (washed twice with PBS at 50 times the RBC volume) were added to 60 mm culture dishes containing macrophage monolayers (RBC:macrophage ratio of 50:1) and incubated for 1 hour at 37°C in complete medium. Monolayers were then washed twice with ice-cold PBS, non-internalized RBCs removed by hypotonic lysis, and washed, scraped cells were transferred into tubes and pelleted at 400 × g.
RBC ingestion was quantified by measuring hemoglobin using a modified Drabkin’s method.26 Thus, macrophages were lysed on ice for 10 minutes in 100 µl of Drabkin’s reagent (Ricca Chemical Company, Arlington, TX) containing 10% Nonidet P-40 (Fluka, Chemie AG, Buchs, Switzerland). Lysates were centrifuged for 20 minutes at 16000 × g at 4°C and supernatants (20 µl) were distributed in triplicate into 384-well plates (Corning Inc., Corning, NY). Absorbance at 540 nm was compared with results using Count-a-part Cyanmethemoglobin Standards (Diagnostic Technology, Inc., Hauppauge, NY). For positive controls, rabbit IgG anti-mouse RBC antibody (625–1250 ng/ml; Rockland Immunochemicals, Inc. Gilbertsville, PA) was used to induce Fcγ receptor-mediated phagocytosis; negative controls were performed without RBCs.
Alternatively, following lysis of the non-internalized RBCs, macrophages were incubated at 37°C in incomplete RPMI 1640 medium. Culture supernatants were collected at 2-hour intervals and stored at −30°C. Monocyte chemoattractant protein [MCP]-1 (equivalently, CCL2) and keratinocyte chemoattractant [KC or, equivalently, CXCL1]) were quantified using the Cytometric Bead Array Mouse Soluble Protein Flex Kit (BD Biosciences); data acquired with an Accuri C6 flow cytometer (BD Biosciences) were analyzed using BD Accuri™ C6 software.
RBC transfusion
In brief, RBCs (400 µL) at approximately 60% hematocrit (final hemoglobin concentration: 17.0–17.5 g/dl) were transfused through the retro-orbital plexus of isoflurane-anesthetized mice, as described.15 RBC recovery was determined using a dual-labeling method.15 At defined times, mice were anesthetized with isoflurane, sacrificed, and exsanguinated. For some experiments, lipopolysaccharide (LPS; E. coli 0111:B4 (Sigma); 1 µg/gram of mouse weight) was injected intraperitoneally.
RNA isolation
Groups of five mice each were transfused with fresh or refrigerator-stored RBCs. Two hours post-transfusion, spleens, livers, kidneys, lungs, and bone marrow were harvested and frozen using RNAlater (Life Technologies, Grand Island, NY). Total RNA was isolated from spleens, livers, kidneys, and lungs using the RNeasy Mini Kit (Qiagen, Valencia, CA) and from bone marrow using the RNeasy Micro Kit (Qiagen). On-column DNase digestion was performed using the RNase-Free DNase Set (Qiagen). RNA concentration was measured by spectrophotometry. First-strand cDNA synthesis used 2.5 µg of input RNA and the RT2 First Strand Kit (Qiagen).
CD11b+ enrichment
Groups of five mice each were transfused with fresh RBCs, refrigerator-stored RBCs, or PBS. Two hours post-transfusion, spleens were harvested and pooled within groups, and single cell suspensions prepared. Splenocytes (1 × 109) were labeled with CD11b MicroBeads, and CD11b+ cells were enriched using LS Midi-MACS separation columns (Miltenyi Biotec Inc.). Eluted CD11b+ cells were passed over a fresh column and again eluted to increase the purity of the magnetically-labeled fraction. Un-separated cells, CD11b− cells (flow through), and CD11b+ cells (eluate) were processed separately.
Real-time quantitative reverse transcription-polymerase chain reaction (qPCR) analysis
Un-separated, CD11b−, and CD11b+ cells were pelleted and lysed with 1 ml of TRIzol (Invitrogen, Grand Island, NY). Chloroform (0.2 ml) was added and, following shaking and centrifugation (12000 × g for 15 minutes at 4°C), upper aqueous phases (600 µl) were collected. After adding equal volumes of ethanol to aqueous phases, total RNA was isolated using the RNeasy Micro Kit (Qiagen). Genomic DNA contamination was removed using the RNase-Free DNase Set (Qiagen). First-strand cDNA synthesis used total RNA (3 µg) and the RT2 First Strand Kit (Qiagen). The Stratagene Mx3000P QPCR System (Agilent Technologies, Inc., Santa Clara, CA) was used for real-time PCR amplification. Each reaction, in triplicate, contained 0.5 µl cDNA (from 15 ng of input RNA), 400 nM of each primer, and 12.5 µl of RT2 SYBR Green qPCR Master Mix (Qiagen) in a final volume of 25 µl. Amplification proceeded at 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. To ensure single product amplification, melting curve analyses were performed. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was the normalization control and the ΔΔCT method was used to detect fold changes in gene expression. Primers for amplifying MCP-1 (NM_011333), KC (NM_008176), and GAPDH (NM_008084) were purchased from SABiosciences.
Phagocyte depletion
Clodronate- or PBS-containing liposomes were prepared, as described.27 Briefly, 8 mg of cholesterol and 86 mg of phosphatidylcholine were dissolved in chloroform and combined, followed by chloroform removal by rotary evaporation. The film was dispersed in 0.7 M clodronate or PBS. Following sonication, liposomes were washed by centrifugation at 22000 × g for 25 min at 10°C, filtered dissolved in sterile PBS (4 ml final volume), and stored. Liposomes were injected intravenously (100 µl/mouse) two days pre-transfusion. Phagocyte depletion was confirmed by immunohistochemistry with anti–mouse F4/80 monoclonal antibody (eBioscience; San Diego, CA).
Flow cytometry
Isolated splenocytes were suspended in ice-cold Stain Buffer (BD Biosciences), incubated with anti-mouse CD16/CD32 (Mouse Fc Block, BD Biosciences), and aliquots (106 cells) stained with various antibody combinations: anti-mouse CD45 (clone 30-F11, APC), anti-mouse CD19 (clone 1D3, APC), anti-mouse CD3e (clone 145-2C11, APC), anti-mouse Ly-6G (clone 1A8, APC), anti-mouse CD11b (clone M1/70, APC), anti-mouse CD11b (clone M1/70, FITC), and anti-mouse F4/80 (clone BM8, APC). Anti-mouse IgG2b/κ, APC and anti-mouse IgG2b/κ FITC antibodies were isotype controls. Antibodies were purchased from BD Biosciences, except anti-mouse F4/80, which was from eBioscience. Stained cells were washed with ice-cold Stain Buffer, and data acquired using a BD FACSAria II Cell Sorter and analyzed using FlowJo v8.8 software (Tree Star, Inc.).
Statistical analysis
Results are presented as mean ± standard deviation unless otherwise specified. Comparisons between group means were tested by one-way ANOVA with Bonferroni post-tests for multiple comparisons. All statistical analysis was performed using Prism 5 (GraphPad Software). P values <0.05 were considered significant.
Results
Mouse macrophages in vitro preferentially ingest refrigerator-stored RBCs by phagocytosis
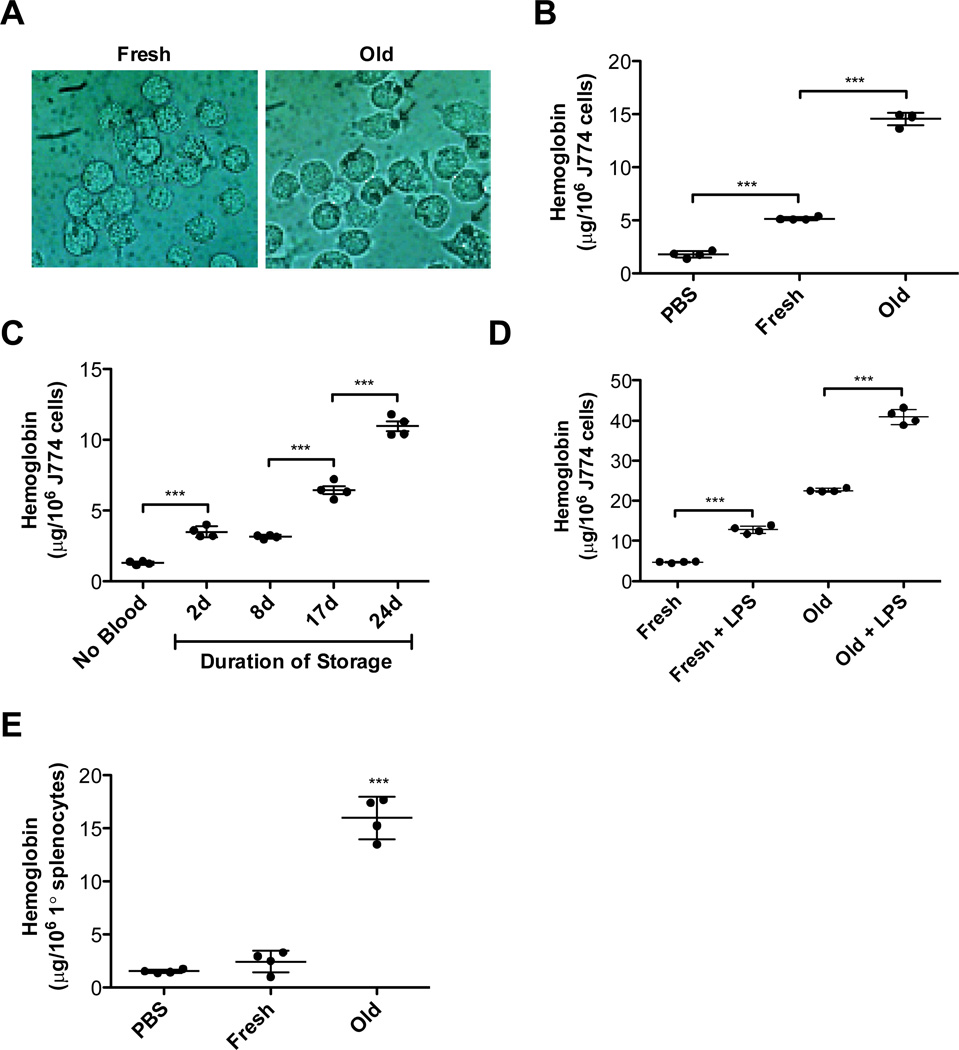
Following incubation with fresh or refrigerator-stored RBCs, J774A.1 cells preferentially ingested refrigerator-stored RBCs (Figure 1, Panel A). When RBC ingestion was quantified, hemoglobin content significantly increased following incubation with refrigerator-stored RBCs (Figure 1, Panel B). To examine whether RBC storage time affects J774A.1 ingestion, macrophage hemoglobin content was measured following incubation with RBCs stored for 2–24 days; J774A.1 hemoglobin concentration increased following exposure to RBCs stored for any time interval (Figure 1, Panel C). In addition, because LPS enhances Fcγ receptor-mediated phagocytosis,29 we tested its effects on refrigerator-stored RBCs. Indeed, LPS-treated J774A.1 cells demonstrated significantly enhanced phagocytosis of both fresh and refrigerator-stored RBCs (Figure 1, Panel D).
Figure 1. Prolonged refrigerated storage of murine RBCs leads to increased erythrophagocytosis in vitro.
Erythrophagocytosis assays were performed at 37°C in 5% CO2, in complete medium, for 1 hour, at a RBC:macrophage ratio of 50:1. Results are presented as mean ± s.d. (A) J774A.1 cells were incubated with fresh RBCs (“Fresh”) or RBCs stored at 4°C for 14 days (“Old”). Following osmotic shock to remove non-ingested RBCs, cells were photographed with a phase-contrast microscope (original magnification: 400×). Arrows indicate RBCs ingested by J774A.1 cells. (B) J774A.1 cells were incubated with PBS, fresh RBCs, or 14-day refrigerator-stored (i.e., old) RBCs. The amounts of ingested RBCs were quantified by harvesting cells following osmotic shock, lysing the resulting J774A.1 cells, and measuring hemoglobin content. (C) J774A.1 cells were incubated with PBS or with RBCs stored at 4°C for 2, 8, 17, or 24 days. The amounts of ingested RBCs were quantified, as in Panel B. (D) J774A.1 cells were incubated with fresh RBCs or 14-day refrigerator-stored RBCs in the presence or absence of LPS (100 ng/mL). The amounts of ingested RBCs were quantified, as in Panel B. (E) Primary cultures of mouse splenocytes were incubated with PBS, fresh RBCs, or 14-day refrigerator-stored RBCs. The amounts of ingested RBCs were quantified, as described in Panel B. Shown are representative examples of at least 3 experiments.
Phagocytosis experiments were also performed using primary mouse splenic macrophages. After 14 days in culture, >99% of these cells were CD11b+ by flow cytometry (not shown). Following incubation with PBS, or fresh or refrigerator-stored RBCs, their hemoglobin content was significantly increased only by refrigerator-stored RBCs (Figure 1, Panel E).
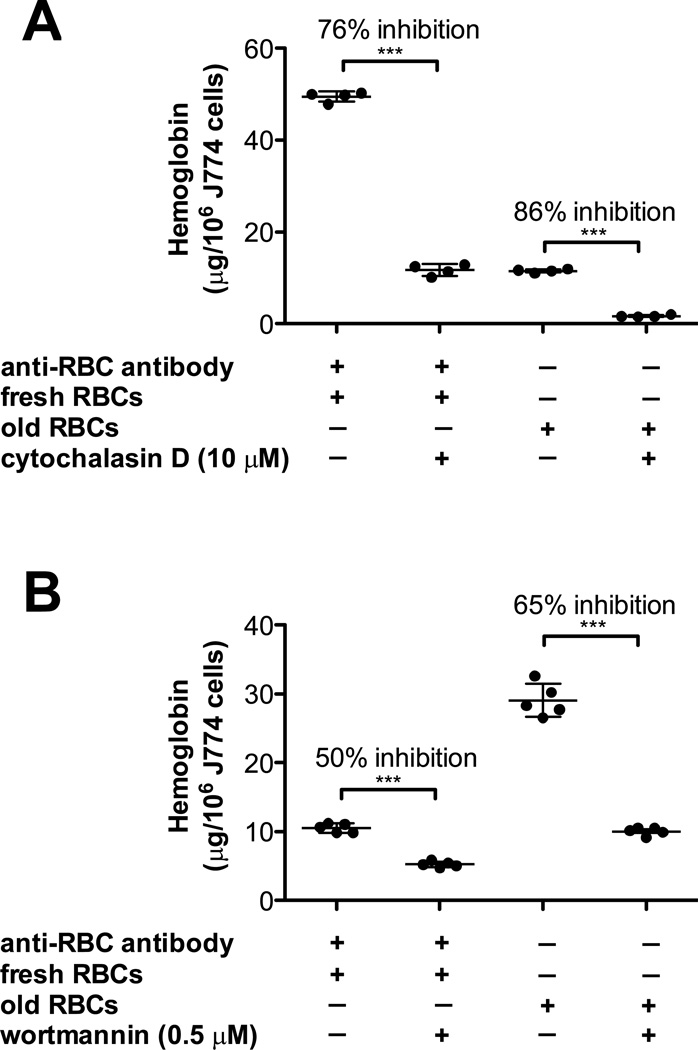
Fcγ receptor-mediated erythrophagocytosis requires actin polymerization.28 To determine whether storage-damaged RBC ingestion also involves this process, cytochalasin D was used, which inhibits actin polymerization. As a control, cytochalasin D inhibited phagocytosis of fresh RBCs coated with rabbit IgG anti-mouse RBC antibodies (Figure 2, Panel A). Similarly, cytochalasin D inhibited ingestion of refrigerator-stored RBCs (Figure 2, Panel A), suggesting that ingestion of IgG-coated or storage-damaged RBCs involves phagocytosis. Similarly, wortmannin, a phosphoinositide 3-kinase inhibitor, inhibited phagocytosis of both IgG-coated and refrigerator storage-damaged RBCs (Figure 2, Panel B). Inhibitors of phosphoinositide 3-kinase (LY294002, 5µM), p38 MAP kinase (SB203580, 30 mM), MEK (PD98059, 50 mM), and tyrosine kinases (genistein, 300 mM) all significantly inhibited phagocytosis of both IgG-coated and refrigerator storage-damaged RBCs to similar extents (not shown). These results demonstrate similarity of the signal transduction machinery required to ingest these different RBC targets.
Figure 2. The signal transduction machinery required to ingest storage-damaged RBCs is similar to IgG-mediated phagocytosis.
(A) J774A.1 cells were incubated either with fresh RBCs in the presence of a sub-agglutinating concentration of rabbit IgG anti-mouse RBC antibody (left) or with 14-day refrigerator-stored RBCs (right) in the presence or absence of 10 µM cytochalasin D, an inhibitor of actin polymerization. The amounts of ingested RBCs were quantified by harvesting cells following osmotic shock, lysing the resulting J774A.1 cells, and measuring hemoglobin content. (B) J774A.1 cells were incubated either with fresh RBCs in the presence of a sub-agglutinating concentration of rabbit IgG anti-mouse RBC antibody (left) or with 14-day refrigerator-stored RBCs (right) in the presence or absence of 0.5 µM wortmannin, an inhibitor of phosphoinositide-3 kinase. The amounts of ingested RBCs were quantified, as described in Panel A.
Phagocytosis of refrigerator-stored RBCs in vitro does not produce a cytokine response
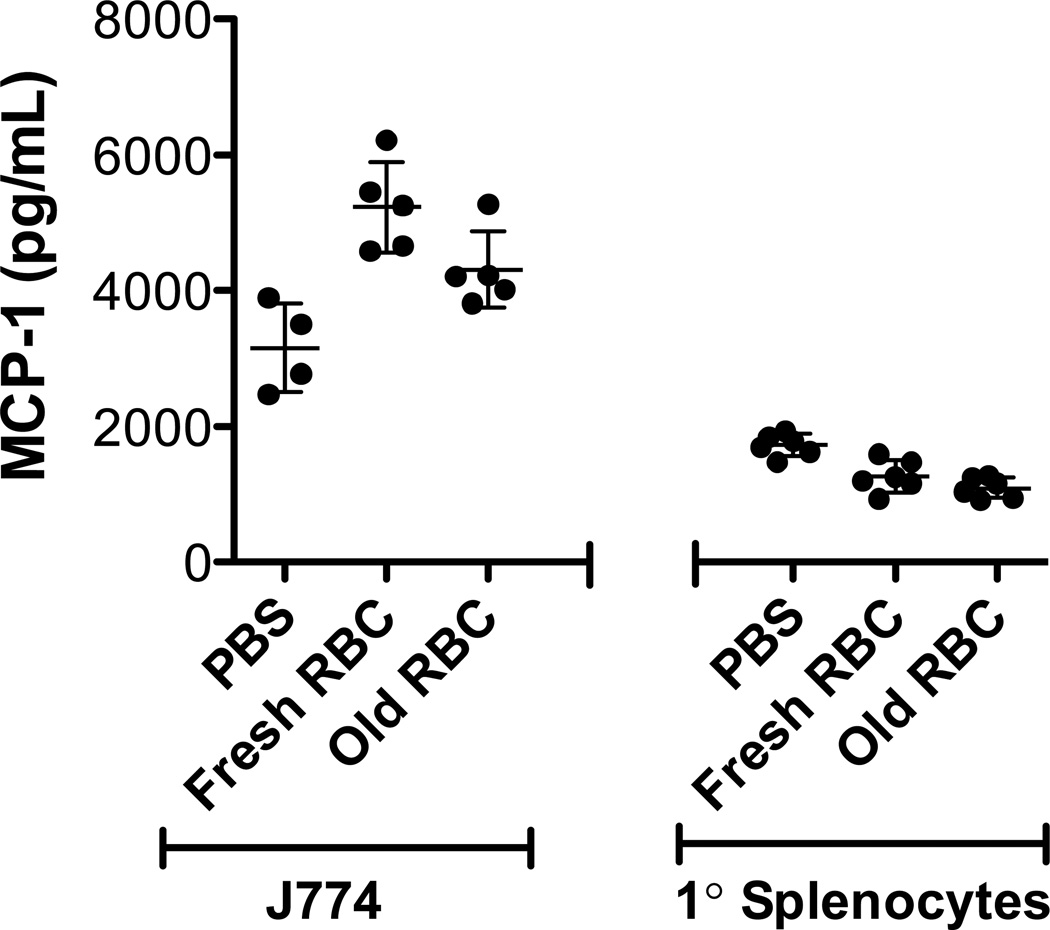
Conditioned culture medium was collected in 2-hour intervals for up to 20 hours following erythrophagocytosis. Neither J774A.1 cells nor primary macrophages (Figure 3) increased MCP-1 or KC secretion after phagocytosis of refrigerator-stored RBCs. Nonetheless, as expected,30,31 LPS stimulated cytokine secretion by both J774A.1 cells and primary macrophages (not shown); thus, these cells can up-regulate secretion of these proteins in vitro.
Figure 3. Phagocytosis in vitro of refrigerator-stored RBCs does not induce significant MCP-1 secretion by J774A.1 cells and primary splenocytes.
J774A.1 cells (left) or primary splenocytes (right) were incubated with PBS, fresh RBCs, or 14-day refrigerator-stored RBCs (“Old”) for one hour in complete medium at 37°C in 5% CO2 at a RBC:macrophage ratio of 50:1. After incubation and subsequent removal of non-ingested RBCs by brief osmotic shock, the phagocytic cells were incubated in incomplete RPMI 1640 medium for 4 hours and secreted MCP-1 was quantified, as described in Materials and Methods. Shown are representative examples of at least 3 experiments.
Phagocyte depletion prevents increased MCP-1 levels in vivo after refrigerator-stored RBC transfusions
Because erythophagocytosis did not induce cytokines in vitro, we used an in vivo model to elucidate the cell type(s) synthesizing cytokines post-transfusion, using MCP-1 as a model.
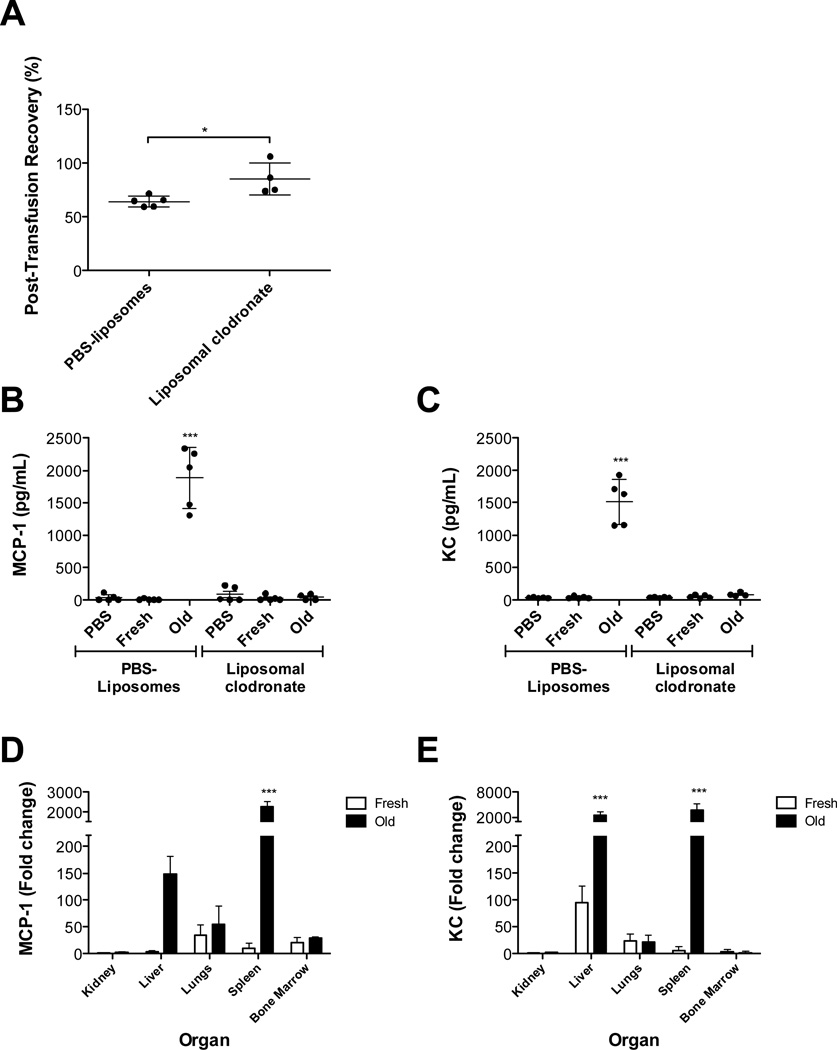
To determine if macrophage depletion affects the cytokine response, mice were treated with clodronate-containing liposomes pre-transfusion. As expected, the 24-hour RBC recovery improved significantly in liposomal clodronate-treated mice (Figure 4, Panel A).15 In addition, MCP-1 and KC levels were markedly increased in liposome control-treated mice following refrigerator-stored RBC transfusions (Figure 4, Panels B–C). However, in liposomal clodronate-treated mice, refrigerator-stored RBC transfusions did not induce increased MCP-1 or KC levels. These data, supported by the absence of hepatic and splenic macrophages in liposomal clodronate-treated mice (Hod et al.15 and not shown), suggest that phagocytic cells are required for cytokine secretion upon transfusion of refrigerator storage-damaged RBCs.
Figure 4. Depletion of macrophages in vivo reduces circulating MCP-1 levels in response to refrigerator-stored RBC transfusions. Refrigerator-stored RBC transfusions induce the highest levels of MCP-1 mRNA synthesis in the spleen.
Transfusion recipients were male C57BL/6 mice. (A) Leukoreduced fresh and 14-day refrigerator-stored (“Old”) RBCs were separately transfused into recipients previously infused intraperitoneally with liposomes containing either PBS or clodronate. The recovery of the transfused RBCs was calculated by dual-label flow cytometry at 24 hours post-transfusion. Results are presented as mean ± s.d.
Mice were infused intraperitoneally with liposomes containing either PBS or clodronate and then infused with PBS or transfused with fresh or 14-day refrigerator-stored RBCs. Mice were sacrificed 2 hours after infusion/transfusion and circulating MCP-1 levels (B) and KC levels (C) were quantified.
Mice were transfused with fresh or 14-day refrigerator-stored RBCs. Two hours after transfusion, organs were harvested and MCP-1 expression (D) and KC expression (E) were measured by a real-time qPCR primer assay. The fold increases in cytokine expression after stored RBC transfusions were calculated for all organs by normalizing to the kidney results after fresh RBC transfusions. *P<0.05, ***P=0.001 compared with fresh RBCs. Shown are representative examples of at least 2 experiments.
Refrigerator-stored RBC transfusions induce increased MCP-1 mRNA synthesis in vivo
Refrigerator-stored RBC transfusions induce increased cytokine levels,15 including MCP-1 and KC (Figure 4, Panels B–C). To determine which organs are involved, the spleen, liver, kidneys, lungs, and bone marrow were harvested from transfused mice and gene expression was evaluated. Significant increases in splenic MCP-1 and KC mRNA levels (>2000-fold) were observed 2 hours post-transfusion with refrigerator-stored RBCs (Figure 4, Panels E–F). KC mRNA expression increased 2000-fold in liver, after refrigerator-stored, but not fresh, RBC transfusions. Although a >100-fold increase in MCP-1 was observed in the liver, it did not reach statistical significance. No significant increases in MCP-1 or KC expression were seen in kidneys, lungs, or bone marrow.
CD11b+-enriched splenocytes synthesize MCP-1 mRNA in vivo following refrigerator-stored RBC transfusions
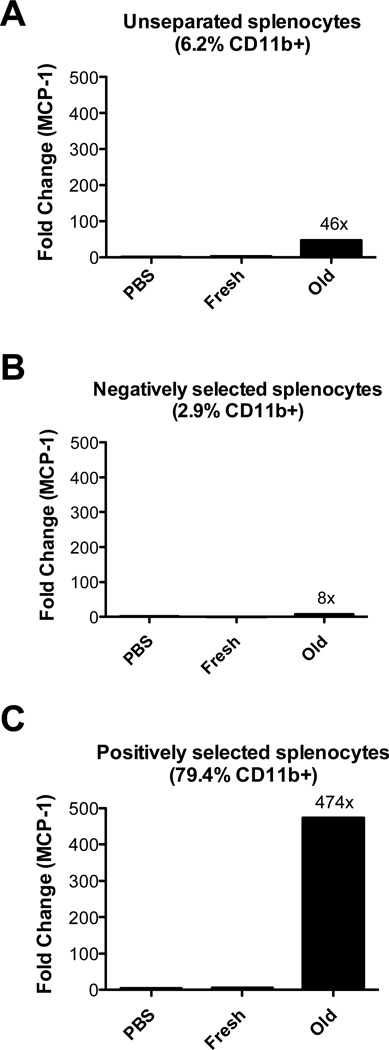
Splenocytes from transfused mice were either un-fractionated or separated into CD11b− and CD11b+ populations; mRNA expression was evaluated by a qPCR assay. Using this approach, refrigerator-stored RBC transfusions induced a statistically significant, 46-fold increase in MCP-1 mRNA expression in un-fractionated splenocytes, as compared to PBS infusion (Figure 5, Panel A). In contrast, fresh RBC transfusions only caused a 2-fold increase in MCP-1 (Figure 5, Panel A). Similar observations were seen using whole spleens (Figure 4, Panel D). In CD11b− and CD11b+ subpopulations, 6- and 474-fold increases in MCP-1 mRNA expression were seen, respectively, after refrigerator-stored RBCs transfusions (Figure 5, Panels B-C). In contrast, the corresponding fold increases following fresh RBC transfusions were 0 and 6, respectively (not shown). The un-fractionated, CD11b−, and CD11b+ pools contained approximately 6, 3, and 80% CD11b+ cells, respectively. The presence of 3% CD11b+ cells in the CD11b− population may explain the 8-fold increase in MCP-1 expression after refrigerator-stored RBC transfusions. In addition, the CD11b− population may contain other cells capable of synthesizing MCP-1. Nonetheless, these results suggest that CD11b+ cells are the predominant splenic source of MCP-1 mRNA synthesis following refrigerator-stored RBC transfusions.
Figure 5. CD11b+ cells are responsible for increases in splenic MCP-1 mRNA expression following transfusions of refrigerator-stored RBCs.
Mice were infused with PBS, or transfused with fresh or 14-day refrigerator-stored (“Old”) RBCs. Two hours after transfusion, spleens were harvested and splenocytes separated into CD11b− and CD11b+ cell subpopulations by magnetic cell sorting. MCP-1 expression was measured as in Figure 3, Panel C. (A) In un-separated splenocytes, CD11b+ cells represent 6.2% of all cells (based on flow cytometry). In this population, MCP-1 mRNA levels increased 46-fold after refrigerator-stored RBC transfusions, as compared to the PBS control. (B & C) In CD11b− and CD11b+ subpopulations, respectively, MCP-1 mRNA levels increased 8- and 474-fold, respectively, after refrigerator-stored RBC transfusions, as compared to the PBS control. Shown are representative examples of at least 2 experiments.
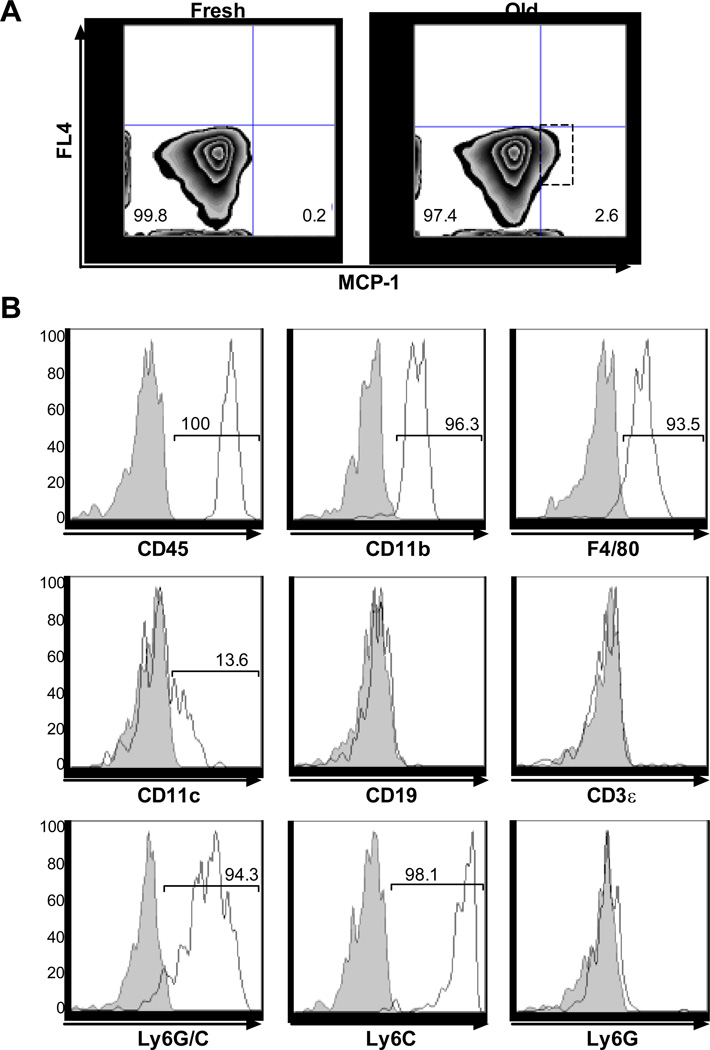
Ccl2-GFP reporter mice24 were used to investigate this issue further. They express Green Fluorescent Protein under the control of the endogenous MCP-1 promoter. Refrigerator-stored RBC transfusions specifically induced GFP expression in a small splenocyte population (2.6%; lower right quadrant; Figure 6, Panel A), which were all CD45-positive, and >90% were positive for CD11b, F4/80, and Ly6C (Figure 6, Panel B), confirming their identity as macrophages. In addition, some of these cells were CD11c+, suggesting that dendritic cells are also involved. In contrast, Ly6G+ cells (a neutrophil marker) did not express GFP. Taken together, these data suggest that, in the spleen, refrigerator-stored RBC transfusions primarily increase MCP-1 expression in tissue-resident macrophages,32 along with a small population of CD11c+, probably dendritic cells.
Figure 6. Refrigerator-stored RBC transfusions increase mRNA expression from the MCP-1 promoter in splenic CD11b+ cells of Ccl2-GFP reporter mice.
Ccl2-GFP reporter mice were transfused with fresh or 14-day refrigerator-stored (“Old”) RBCs. Splenocytes were analyzed by flow cytometry 2 hours after transfusion. (A) Transfusions of refrigerator-stored RBCs, as compared to fresh RBCs, increased the number of splenocytes with GFP expression driven by the MCP-1 promoter from 0.2 to 2.6%. (B) Splenocytes were stained for various cell surface markers, as indicated. Shown are histograms for the indicated markers gated on the splenocytes from the dashed rectangle in Panel A following transfusions with 14-day refrigerator-stored RBCs. Gray histograms: isotype-control antibodies; white histograms: test antibodies. Shown are representative examples of 2 experiments.
Discussion
In previous studies, splenic and hepatic macrophages were primarily responsible for clearing transfused, refrigerator storage-damaged RBCs in mice.15 This rapid clearance induced increased cytokine levels. In addition, intact RBCs were required; infusing cell-free supernatant or samples deliberately hemolyzed in vitro did not produce this effect,15 suggesting that neither free hemoglobin, nor products released by storage hemolysis, were responsible for this phenomenon. The current study sought to model phagocytosis of refrigerator storage-damaged RBCs in vitro to enhance understanding of the cells responsible for cytokine synthesis in vivo in murine transfusion recipients. To our knowledge, this represents the first demonstration that J774A.1 cells and primary splenic macrophages use phagocytosis to preferentially ingest refrigerator storage-damaged, as compared to fresh, murine RBCs (Figure 1, Panels A-E). Moreover, by analogy with Fcγ-receptor mediated RBC phagocytosis, which requires complex signal transduction machinery,33 various soluble inhibitors significantly diminished phagocytosis in vitro of both IgG-coated and refrigerator storage-damaged RBCs (Figure 2, Panel A–B; and not shown), suggesting parallels between the mechanisms involved in clearing these different RBC targets.34
According to the “iron hypothesis,”17,18 macrophage ingestion of a bolus of RBCs initiates catabolism of the ingested RBCs, rapidly producing significant amounts of intracellular “free” iron, which overwhelms the iron-buffering capacity of ferritin.23 Increased intracellular “free” iron can increase reactive oxygen species levels through Fenton chemistry, leading to cytokine synthesis and release.21,22 To investigate this in vitro, cytokines were measured in conditioned media after incubation of macrophages with RBCs. Although some cytokines of interest were constitutively produced, no increases in cytokines were seen after phagocytosis of refrigerator storage-damaged RBCs (Figure 3). This may be due to the relatively low amount of erythrophagocytosis (Figure 1). In addition, LPS induced cytokine secretion by J774A.1 cells, demonstrating that they were capable of regulated cytokine production. Finally, because the cytokine response produced by clearing refrigerator storage-damaged RBCs in vivo synergizes with sub-clinical endotoxinemia,15 J774A.1 macrophages were evaluated to determine whether LPS and erythrophagocytosis could synergize to enhance cytokine production in vitro. Although LPS enhanced erythrophagocytosis (Figure 1, Panel D), no synergy in cytokine secretion was seen (not shown). Thus, phagocytosis in vitro of refrigerator storage-damaged RBCs, under these conditions, neither induced nor enhanced MCP-1 or KC production.
Several concepts may explain the lack of cytokine secretion following phagocytosis in vitro of refrigerator storage-damaged RBCs. For example, there may be a threshold effect based on the number of RBCs ingested. Alternatively, hepatic and splenic macrophages may weakly produce cytokines after toll-like receptor (i.e., TLR) ligation, despite potent endocytic activity;35 this low pro-inflammatory response may relate to the role these cells play following exposure to (low levels of) circulating foreign substances. Thus, these cells may ingest particles effectively, but not produce cytokines abundantly, to enable elimination of pathogens and other targets without inducing cytokine-mediated tissue injury.
There also may be issues with the model itself. For example, methodological variables such as incubation time, macrophage:RBC ratio, and medium used may affect the cytokine response. FBS-containing medium lacks mouse hepcidin; this could enhance macrophage ferroportin expression, allowing continuous export of intracellular “free” iron. When intracellular “free” iron levels are low, cytokine synthesis is decreased, even in response to a relevant stimulus. Thus, in Hfe knockout mice, low hepcidin levels enhance macrophage ferroportin expression.36 Using both primary macrophages from Hfe knockout mice and ferroportin-transfected J774A.1 cells, the cytokine response to LPS was reduced.36 Intracellular iron chelation also reduced this response in wild-type macrophages in vitro.36 In another example, LPS induced increased cytokine expression in vitro in macrophages from Ferroportin (Fpn1) knockout mice, as compared to controls.37 In addition, altering intracellular “free” iron in Fpn1 knockout macrophages, by iron chelation or iron supplementation, inhibited or stimulated cytokine expression, respectively.37 Therefore, it would be interesting to determine whether ferroportin down-regulation by exogenous mouse hepcidin in cultured macrophages induces cytokine production after phagocytosis of refrigerator storage-damaged RBCs. A final explanation may relate to the redox status in cultured macrophages. Thus, macrophage cell lines and primary cultured macrophages, in contrast to freshly-isolated monocytes, have a reducing intracellular environment accompanied by up-regulated antioxidant pathways.38,39 This could buffer the reactive oxygen species produced by intracellular “free” iron. For example, cultured macrophages did not respond to signal transduction due to reactive oxygen species produced by TLR4 signaling, which normally enhances inflammasome function.38 However, this phenomenon may not be universal; for example, in our hands, LPS, presumably acting through TLR4, did induce MCP-1 secretion by J774A.1 cells (not shown). In addition, TLR3 signaling in cultured murine macrophages produced sufficient amounts of reactive oxygen species to activate STAT1 and NF-κB, thereby producing inflammatory mediators.40 Nonetheless, we used primary macrophages and a cell line, because it was not feasible to collect sufficient numbers of fresh monocytes to induce sufficient erythrophagocytosis in vitro. However, it would be interesting to determine if exposure of cultured macrophages to reactive oxygen species in vitro38 could overcome this obstacle. It would also be interesting to quantify reactive oxygen species in macrophages following phagocytosis of refrigerator storage-damaged RBCs.
Because erythrophagocytosis in vitro did not induce cytokines, we sought to determine the cellular origin of these cytokines in vivo. Based on our results and those of others, macrophages are important in clearing senescent, IgG-coated, and refrigerator storage-damaged RBCs (Figure 4, Panel A-C and Refs.15,41,42). In addition, IgG-coated or refrigerator storage-damaged RBC clearance in vivo induced increased cytokine levels.15,43 In the current study, splenic and hepatic MCP-1 and KC mRNA levels increased significantly following refrigerator storage-damaged RBC transfusions (Figure 4 Panels E–F); splenic up-regulation was more prominent, perhaps because there were more macrophages per gram of sample used for RNA isolation.44–46 However, given the >10-fold size difference between liver and spleen, hepatic synthesis is also likely significant.
CD11b+-enriched cells were primarily responsible for splenic MCP-1 production, at least transcriptionally (Figure 5); similar results were seen for KC. Because mouse spleen contains multiple myeloid populations, flow cytometry was used to identify MCP-1 expressing cells. Thus, in Ccl2-GFP-reporter recipients, refrigerator-stored RBC transfusions induced significantly elevated MCP-1-regulated GFP expression in splenocytes (Figure 6, Panel A); similar effects were seen with LPS (not shown). By flow cytometry (Figure 6, Panel B), most of these splenocytes were CD45+, CD11b+, F4/80+, Ly6c+, and CD11clow; this is compatible with classically-activated, M1-type tissue-resident macrophages.32,47–49 Although B-cells may also serve as antigen presenting cells,50 an MCP-1 response was not observed in CD19+ cells (Figure 6, Panel B). However, some CD11c+ cells, which are likely to be dendritic cells, expressed GFP after refrigerator-stored RBC transfusions; this is consistent with previous observations.51 A limitation of this analysis is that a full characterization of the responsive macrophage and dendritic cell subsets using multicolor antibody panels was not performed. Furthermore, although not examined, it is highly likely that tissue-resident hepatic macrophages also participate in this response. Because neither J774A.1 cells nor primary splenic macrophages exhibited this response after erythrophagocytosis in vitro, cooperation between macrophages and other cells in a tissue matrix may also be required.
Finally, because cultured macrophages preferentially ingested refrigerator-stored RBCs in vitro (Figure 1), this could provide a tractable model for identifying macrophage receptors recognizing cognate ligands that mark refrigerator storage-damaged RBCs for clearance. Although the Fcγ receptor may, or may not, be involved, future studies could directly explore its role using knock-out mice.52 Other candidates include macrophage lectins53 and scavenger receptors;54 the latter are involved in phagocytosis of oxidatively-damaged RBCs.54 The model presented herein could provide a useful platform for such studies.
Acknowledgments
The authors thank Dr. Eric Pamer for providing the Ccl2-GFP reporter mice.
This work was supported in part by grants from the NIH (R01-098014 to S.L.S., K08-HL103756 to E.A.H.) and a Louis V. Gerstner Scholars Award (to E.A.H.).
Footnotes
Authorship contributions
Contributions: B.S.W., E.A.H., R.O.F., J.C.Z., and S.L.S. designed the study. B.S.W., N.K., E.A.H., and S.B. acquired the data. B.S.W., E.A.H., R.O.F., J.C.Z., and S.L.S. controlled and analyzed the data; B.S.W., E.A.H., and S.L.S. wrote the paper; and all authors edited drafts and reviewed the final version of the manuscript.
Disclosure: The authors declare no conflicts of interest relevant to the manuscript submitted to Transfusion.
References
- 1.Pfuntner A, Wier LM, Stocks C. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): 2013. Most Frequent Procedures Performed in U.S. Hospitals, 2010: Statistical Brief # 149. [Google Scholar]
- 2.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–1195. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 5.Offner PJ, Moore EE, Biffl WL, Johnson JL, Silliman CC. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–716. doi: 10.1001/archsurg.137.6.711. [DOI] [PubMed] [Google Scholar]
- 6.Leal-Noval SR, Rincon-Ferrari MD, Garcia-Curiel A, Herruzo-Aviles A, Camacho-Larana P, Garnacho-Montero J, Amaya-Villar R. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119:1461–1468. doi: 10.1378/chest.119.5.1461. [DOI] [PubMed] [Google Scholar]
- 7.Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth. 1997;44:1256–1261. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 8.Basran S, Frumento RJ, Cohen A, Lee S, Du Y, Nishanian E, Kaplan HS, Stafford-Smith M, Bennett-Guerrero E. The association between duration of storage of transfused red blood cells and morbidity and mortality after reoperative cardiac surgery. Anesth Analg. 2006;103:15–20. doi: 10.1213/01.ane.0000221167.58135.3d. [DOI] [PubMed] [Google Scholar]
- 9.Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, Denny C, Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg JA, McGwin G, Jr, Marques MB, Cherry SA, 3rd, Reiff DA, Kerby JD, Rue LW., 3rd Transfusions in the less severely injured: does age of transfused blood affect outcomes? J Trauma. 2008;65:794–798. doi: 10.1097/TA.0b013e318184aa11. [DOI] [PubMed] [Google Scholar]
- 11.Spinella PC, Carroll CL, Staff I, Gross R, Mc Quay J, Keibel L, Wade CE, Holcomb JB. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in-hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dzik W. Fresh blood for everyone? Balancing availability and quality of stored RBCs. Transfus Med. 2008;18:260–265. doi: 10.1111/j.1365-3148.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- 13.Fergusson DA, Hebert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, Sankaran K, Tinmouth A, Blajchman MA, Kovacs L, Lachance C, Lee S, Walker CR, Hutton B, Ducharme R, Balchin K, Ramsay T, Ford JC, Kakadekar A, Ramesh K, Shapiro S. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–1451. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 14.Gilson CR, Kraus TS, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, Shaz BH, Zimring JC. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S, Sireci AN, Stephens HL, Stotler BA, Wojczyk BS, Zimring JC, Spitalnik SL. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hod EA, Spitalnik SL. Harmful effects of transfusion of older stored red blood cells: iron and inflammation. Transfusion. 2011;51:881–885. doi: 10.1111/j.1537-2995.2011.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hod EA, Spitalnik SL. Stored red blood cell transfusions: Iron, inflammation, immunity, and infection. Transfusion Clinique et Biologique. 2012;19:84–89. doi: 10.1016/j.tracli.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callan MB, Patel RT, Rux AH, Bandyopadhyay S, Sireci AN, O'Donnell PA, Ruane T, Sikora T, Marryott K, Sachais BS, Hod EA. Transfusion of 28-day-old leucoreduced or non-leucoreduced stored red blood cells induces an inflammatory response in healthy dogs. Vox Sang. 2013 doi: 10.1111/vox.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keir AK, McPhee AJ, Andersen CC, Stark MJ. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Ped Res. 2013;73:75–79. doi: 10.1038/pr.2012.144. [DOI] [PubMed] [Google Scholar]
- 21.Tsukamoto H, Lin M, Ohata M, Giulivi C, French SW, Brittenham G. Iron primes hepatic macrophages for NF-kappaB activation in alcoholic liver injury. Am J Physiol. 1999;277:G1240–G1250. doi: 10.1152/ajpgi.1999.277.6.G1240. [DOI] [PubMed] [Google Scholar]
- 22.Xiong S, She H, Takeuchi H, Han B, Engelhardt JF, Barton CH, Zandi E, Giulivi C, Tsukamoto H. Signaling role of intracellular iron in NF-kappaB activation. J Biol Chem. 2003;278:17646–17654. doi: 10.1074/jbc.M210905200. [DOI] [PubMed] [Google Scholar]
- 23.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schelonka RL, Chai MK, Yoder BA, Hensley D, Brockett RM, Ascher DP. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996;129:275–278. doi: 10.1016/s0022-3476(96)70254-8. [DOI] [PubMed] [Google Scholar]
- 26.Moore GL, Ledford ME, Merydith A. A micromodification of the Drabkin hemoglobin assay for measuring plasma hemoglobin in the range of 5 to 2000 mg/dl. Biochem Med. 1981;26:167–173. doi: 10.1016/0006-2944(81)90043-0. [DOI] [PubMed] [Google Scholar]
- 27.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 28.Nitta T, Suzuki T. Fc gamma 2b receptor-mediated prostaglandin synthesis by a murine macrophage cell line (P388D1) J Immunol. 1982;128:2527–2532. [PubMed] [Google Scholar]
- 29.Cooper PH, Mayer P, Baggiolini M. Stimulation of phagocytosis in bone marrow-derived mouse macrophages by bacterial lipopolysaccharide: correlation with biochemical and functional parameters. J Immunol. 1984;133:913–922. [PubMed] [Google Scholar]
- 30.Cavaillon JM, Fitting C, Haeffner-Cavaillon N, Kirsch SJ, Warren HS. Cytokine response by monocytes and macrophages to free and lipoprotein-bound lipopolysaccharide. Infection and Immunity. 1990;58:2375–2382. doi: 10.1128/iai.58.7.2375-2382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly KA, Hill MR, Youkhana K, Wanker F, Gimble JM. Dimethyl sulfoxide modulates NF-kappa B and cytokine activation in lipopolysaccharide-treated murine macrophages. Infection and Immunity. 1994;62:3122–3128. doi: 10.1128/iai.62.8.3122-3128.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutz HU. Naturally occurring autoantibodies in mediating clearance of senescent red blood cells. Adv Exper Med Biol. 2012;750:76–90. doi: 10.1007/978-1-4614-3461-0_6. [DOI] [PubMed] [Google Scholar]
- 35.Movita D, Kreefft K, Biesta P, van Oudenaren A, Leenen PJ, Janssen HL, Boonstra A. Kupffer cells express a unique combination of phenotypic and functional characteristics compared with splenic and peritoneal macrophages. J Leuk Biol. 2012;92:723–733. doi: 10.1189/jlb.1111566. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Johnson EE, Shi HN, Walker WA, Wessling-Resnick M, Cherayil BJ. Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J Immunol. 2008;181:2723–2731. doi: 10.4049/jimmunol.181.4.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Zhang F, An P, Guo X, Shen Y, Tao Y, Wu Q, Zhang Y, Yu Y, Ning B, Nie G, Knutson MD, Anderson GJ, Wang F. Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood. 2011;118:1912–1922. doi: 10.1182/blood-2011-01-330324. [DOI] [PubMed] [Google Scholar]
- 38.Carta S, Tassi S, Pettinati I, Delfino L, Dinarello CA, Rubartelli A. The rate of interleukin-1beta secretion in different myeloid cells varies with the extent of redox response to Toll-like receptor triggering. J Biol Chem. 2011;286:27069–27080. doi: 10.1074/jbc.M110.203398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubartelli A. Redox control of NLRP3 inflammasome activation in health and disease. J Leuk Biol. 2012;92:951–958. doi: 10.1189/jlb.0512265. [DOI] [PubMed] [Google Scholar]
- 40.Yang CS, Kim JJ, Lee SJ, Hwang JH, Lee CH, Lee MS, Jo EK. TLR3-triggered reactive oxygen species contribute to inflammatory responses by activating signal transducer and activator of transcription-1. J Immunol. 2013;190:6368–6377. doi: 10.4049/jimmunol.1202574. [DOI] [PubMed] [Google Scholar]
- 41.Mebius RE, Kraal G. Structure and function of the spleen. Nature Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 42.Weiss L. The red pulp of the spleen: structural basis of blood flow. Clin Haematol. 1983;12:375–393. [PubMed] [Google Scholar]
- 43.Hod EA, Cadwell CM, Liepkalns JS, Zimring JC, Sokol SA, Schirmer DA, Jhang J, Spitalnik SL. Cytokine storm in a mouse model of IgG-mediated hemolytic transfusion reactions. Blood. 2008;112:891–894. doi: 10.1182/blood-2008-01-132092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Furth R, Diesselhoff-den Dulk MM. Dual origin of mouse spleen macrophages. J Exp Med. 1984;160:1273–1283. doi: 10.1084/jem.160.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baratta JL, Ngo A, Lopez B, Kasabwalla N, Longmuir KJ, Robertson RT. Cellular organization of normal mouse liver: a histological, quantitative immunocytochemical, and fine structural analysis. Histochem Cell Biol. 2009;131:713–726. doi: 10.1007/s00418-009-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katano M, Miyazaki K, Aso M, Torisu M. Chemotaxis of Kupffer cells isolated from rodent liver. Microbiol Immunol. 1981;25:1059–1066. doi: 10.1111/j.1348-0421.1981.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 47.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry Part A. 2012;81:343–350. doi: 10.1002/cyto.a.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janeway CA, Jr, Ron J, Katz ME. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol. 1987;138:1051–1055. [PubMed] [Google Scholar]
- 51.Hendrickson JE, Chadwick TE, Roback JD, Hillyer CD, Zimring JC. Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood. 2007;110:2736–2743. doi: 10.1182/blood-2007-03-083105. [DOI] [PubMed] [Google Scholar]
- 52.Schirmer DA, Song SC, Baliff JP, Harbers SO, Clynes RA, Krop-Watorek A, Halverson GR, Czerwinski M, Spitalnik SL. Mouse models of IgG- and IgM-mediated hemolysis. Blood. 2007;109:3099–3107. doi: 10.1182/blood-2006-08-040139. [DOI] [PubMed] [Google Scholar]
- 53.Vaysse J, Gattegno L, Bladier D, Aminoff D. Adhesion and erythrophagocytosis of human senescent erythrocytes by autologous monocytes and their inhibition by beta-galactosyl derivatives. Proc Natl Acad Sci. 1986;83:1339–1343. doi: 10.1073/pnas.83.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terpstra V, van Berkel TJ. Scavenger receptors on liver Kupffer cells mediate the in vivo uptake of oxidatively damaged red blood cells in mice. Blood. 2000;95:2157–2163. [PubMed] [Google Scholar]