Abstract
The dietary compound capsaicin is responsible for the “hot and spicy” taste of chili peppers and pepper extracts. It is a valuable pharmacological agent with several therapeutic applications in controlling pain and inflammation. Emerging studies show that it displays potent anti-tumor activity in several human cancers. On a more basic research level, capsaicin has been used as a ligand to activate several types of ion-channel receptors. The pharmacological activity of capsaicin-like compounds is dependent on several factors like the dose, the route of administration and most importantly on its concentration at target tissues. The present review describes the current knowledge involving the metabolism and bioavailability of capsaicinoids in rodents and humans. Novel drug delivery strategies used to improve the bioavailability and therapeutic index of capsaicin are discussed in detail. The generation of novel capsaicin-mimetics and improved drug delivery methods will foster the hope of innovative applications of capsaicin in human disease.
1. Introduction
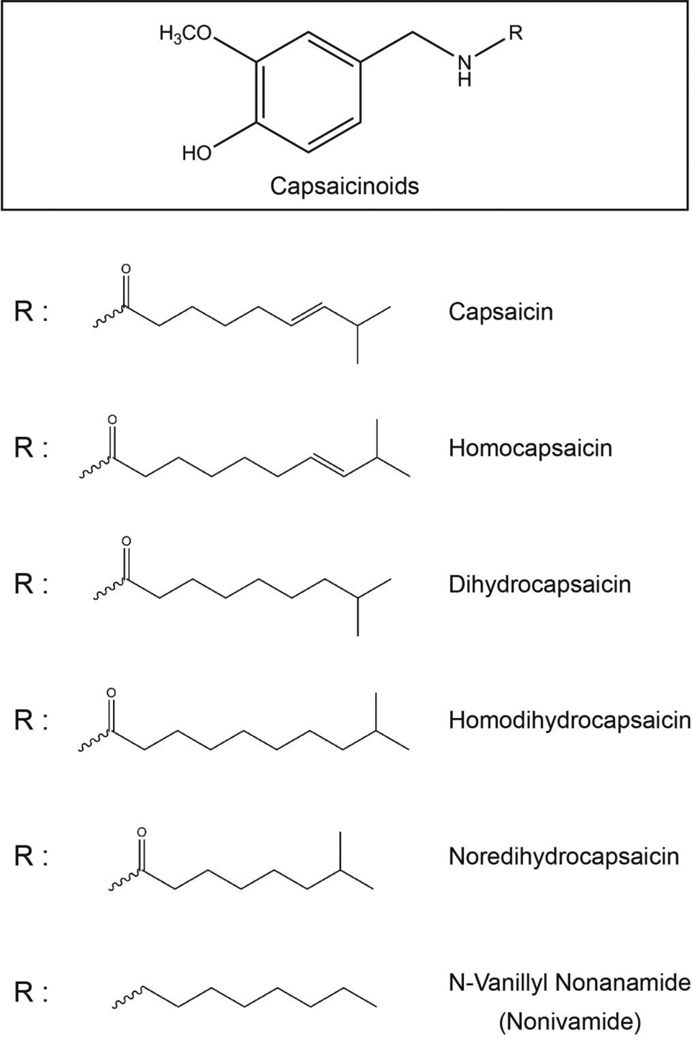
Capsaicinoids are a group of compounds responsible for the spicy, pungent taste of hot chili peppers (Capsicum annuum and Capsicum frutescens) [1, 2]. The family of capsaicinoids is primarily comprised of capsaicin, dihydrocapsaicin, nondihydrocapsaicin, homohydrocapsaicin, homodihydrocapsaicin and nonivamide (Fig. 1) [2]. The “heat-sensation” of capsaicin arises due to the binding of capsaicin to transient receptor potential vanilloid (TRPV) ion-channel receptors [3]. Capsaicin functions as a high affinity agonist of the TRPV1 receptor [4]. Other capsaicinoids besides capsaicin also produce the “heat-sensation” via the TRPV1 receptor. However, some of the biological activities of capsaicin, like its anti-neoplastic, cardioprotective effects, have been found to be independent of the TRPV1 receptor. More research is required to elucidate the molecular mechanism underlying these TRPV1-independent effects of capsaicinoids.
Fig. 1.
Structure of capsaicin and capsaicinoids.
2. Pharmacological effects of capsaicinoids
2.1 Effects of high-dose capsaicinoids
The biological effects of capsaicinoids are dependent on the dose of these compounds administered and the time of exposure [5]. Exposure to high doses of capsaicin (above 100 mg capsaicin per kg body weight) for a prolonged time causes peptic ulcers, accelerates the development of prostate, stomach, duodenal, and liver cancers and enhances breast cancer metastasis [5, 6].
2.2 Effects of low-dose capsaicinoids
2.2.1 Anti-cancer effects
Several convergent studies indicate that low-doses of capsaicin display a cancer-chemopreventive, anti-neoplastic activity [7] [8]. Capsaicin induces robust apoptosis in multiple types of human cancer cells both in vitro and in mice models. Recent studies have focused on the potential of capsaicin as a viable anti-cancer drug applicable to the management and treatment of human small cell lung cancer, breast cancer, prostate cancer and colon cancer. Data from our lab as well from other researchers show that low doses of capsaicin are well tolerated in mice; weight, food intake and water intake are identical between vehicle-treated mice and capsaicin-treated mice [7, 9].
Nutritional compounds have been investigated in combination with standard chemotherapeutic drugs like cisplatin for treatment of several human cancers [10]. Capsaicin potentiates the apoptotic activity of cisplatin in human stomach cancer and attenuates cisplatin-induced renal toxicity in rodent models [11, 12].
2.2.2 Analgesic effects
Capsaicinoids have been extensively studied for their analgesic activity [4, 13]. The oral or local administration of capsaicin reduces inflammation and pain from rheumatoid arthritis, fibromyalgia and chemical hyperalgesia [14]. Low concentrations of capsaicin are included in over-the-counter analgesic creams. High concentrations of capsaicin have been explored as treatment for neuropathic pain (e.g., Qutenza/NGX-4010), postoperative pain (e.g. Adlea; Anesiva Inc.) and cluster headaches (e.g., Civamide; Winston Laboratories) [15, 16]. The reader is referred to several excellent reviews on this subject [4, 14, 17–19].
2.2.3 Other pharmacological effects
Capsaicin has been found to display cardioprotective activity [4]. Capsaicinoids block platelet aggregation and activity of clotting factors VIII and IX [20]. The anti-platelet and anti-coagulant activity of capsaicin was independent of TRPV1 [21]. Capsaicin inhibits oxidation of LDL (low density lipoproteins), reduces total serum cholesterol and lipid peroxide levels in rat models [13]. The administration of low dose capsaicinoids exerts beneficial effects on the gastrointestinal system such as gastric epithelium restitution, repair of gastric mucosa and increase of mucosal blood flow [13]. Finally, capsaicinoids show anti-obesity activity by enhancing energy expenditure of the body [22].
The pharmacological effects of capsaicinoids are dependent on their attaining a suitable concentration at the target organ site. The bioavailability of capsaicin has been studied in several animal models [13]. However, since most of the studies exploring the anti-cancer and analgesic activity of capsaicinoids has been studied in mice (or rat) models, the present review will focus on the bioavailability of capsaicinoids in rodents and humans. Subsequently, we will describe the current strategies used by researchers to improve the delivery of capsaicin to target tissues, thereby increasing the scope of improving its therapeutic index.
3. Biotransformation of capsaicin in vitro
3.1 Liver
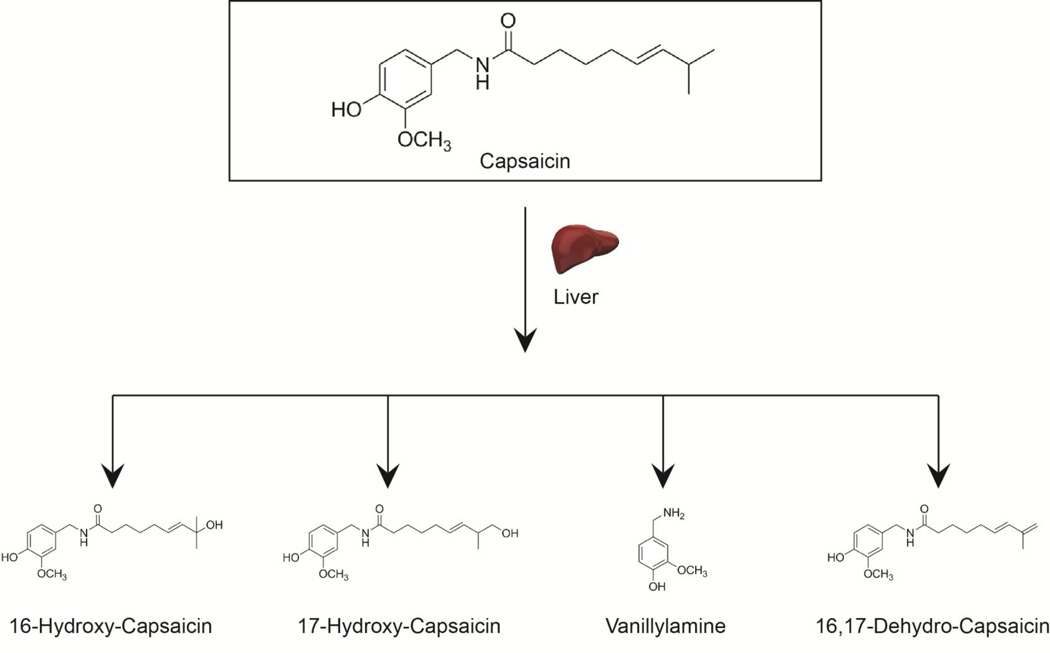
Early studies established that the majority of capsaicin is metabolized in the liver (Fig. 2) [23]. Several laboratories investigated the hepatic metabolism of capsaicinoids in vitro using hepatic microsomes and S9 fractions [24]. The metabolism of capsaicin was faster in the rat and human liver microsomes than the corresponding 9000g supernatant (S9 fraction). Chanda et al. (2008) observed that capsaicin was completely metabolized within 20 minutes in rat and human microsomes. The most abundant hepatic metabolite was 16-hydroxycapsaicin, followed by 16,17-dehydrocapsaicin. In human liver microsomes, five metabolites were detected, the most abundant being 16-hydroxycapsaicin, 17-hydroxycapsaicin and 16,17-dehydrocapsaicin (Fig. 2) [25].
Fig. 2.
A schematic diagram showing the metabolites of capsaicin in rodent and human liver.
The metabolism of capsaicin was slower in the S9 fractions compared to hepatic microsomes and different metabolites were obtained [25]. Chanda et al., (2008) reported that the major metabolites detected in the S9 fractions from the rats were vanillylamine, 16-hydroxycapsaicin and 16,17-dehydrocapsaicin [25]. In contrast, Reilly et al., (2003) did not detect any vanillylamine or vanillin metabolites in the S9 fraction [24]. In a recent publication Reilly et al., (2013) have identified 5,5′-dicapsaicin as a novel metabolite of capsaicin in the liver [26]. This is an intriguing finding because 5,5′-dicapsaicin and 4′-O-5-dicapsaicin ether metabolites are usually generated during peroxidase-mediated capsaicinoid metabolism [27]. Data from Reilly et al., (2013) seems to suggest that P450 enzymes can oxidize capsaicin to generate free radical intermediates. No dicapsaicin metabolites were detected by Chanda et al., (2008). This may be explained by the fact that Reilly and his co-workers used a much longer incubation period of 60 minutes and a 100 times higher concentration of 100 µM capsaicin compared to Chanda et al., (2008) [24]. Similarly, the concentration of capsaicin used by Reilly et al., (2013) in the dicapsaicin experiments was 500 µM [26]. Such higher concentrations of capsaicin may have impacted metabolic pathways as capsaicin has been reported to inhibit some CYP isozymes of human P450 [24, 28].
The hepatic metabolism of dihydrocapsaicin was studied in vitro by using extracts of Wistar rat livers [29, 30]. The dihydrocapsaicin was metabolized to vanillylamine and 8-methylnonanoic acid. The vanillylamine was subsequently transformed to vanillin.
3.2 Lung
Studies by Reilly et al., (2003) showed that the metabolism of capsaicin in lung microsomes was 20–40 fold slower than the liver microsomes in both rat and humans [24]. The metabolites obtained using human lung microsomes were similar to the liver microsomes. Such slower metabolism of capsaicinoids in the lung suggests a tissue-specific nature of metabolic pathways. Alternately, it may be possible that the respiratory tissues have low amounts of enzymes which metabolize capsaicin. The authors also observed that the IC50 values for cytotoxicity assays involving capsaicin are two-fold higher in BEAS-2B and A549 lung epithelial cells as compared to HepG2 hepatic epithelial cells [24]. However, it must be noted that the cytotoxic effects of capsaicin are mediated in part by TRPV receptors on target cells. Therefore, the relative expression of TRPV receptors on lung tissue (versus liver tissue) would be an important determinant in the cytotoxic activity of capsaicin in human lung and liver cells. Studies by Athaniousou et al., (2007) have shown that capsaicin causes apoptosis in H460 human lung adenocarcinoma cells, by regulating enzymes involved in mitochondrial respiration (in a TRPV-independent manner). Again, the levels of these mitochondrial enzymes may vary between lung and liver tissue which could explain the results obtained by Reilly and his colleagues [24].
3.3 Skin
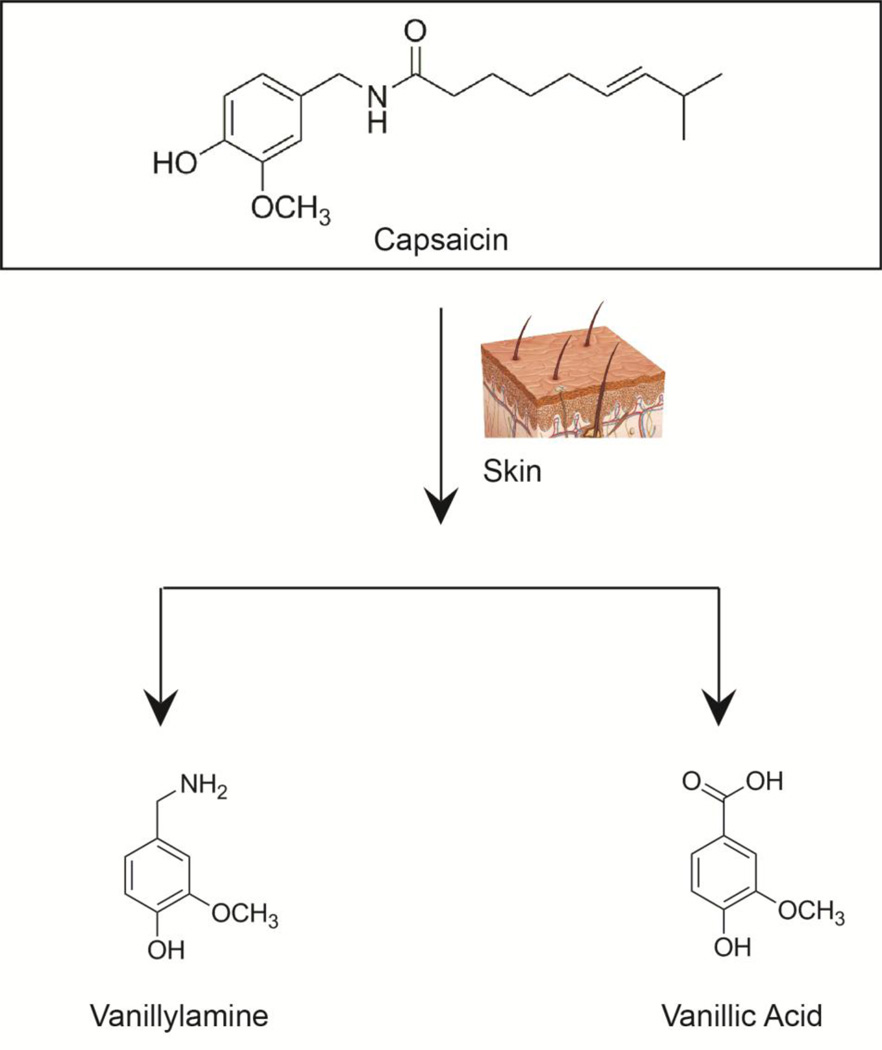
The metabolism of capsaicin is very slow in the skin. Capsaicin is metabolized in human skin by about 20 hours, and the predominant metabolites include vanillylamine and vanillic acid (Fig. 3) [25]. Wang et al., (2001) studied the skin absorption of capsaicin from capsaicin-containing commercial creams. The transport of capsaicin was dependent on the amount of capsaicin present in the cream and follows first order kinetics across the skin barrier [31].
Fig. 3.
A schematic diagram showing the metabolites of capsaicin in human skin.
3.4 Intestine
The intestinal absorption of capsaicin in vitro was done using everted intestinal sacs isolated from rats [32]. It was observed that capsaicin was robustly absorbed both into intestinal tissues, jejunum and serosal fluid. Kawada et al., (1984) studied the in situ metabolism of capsaicin using ligated loops of stomach, jejunum and ileum [30]. The application of 1 mM capsaicin led to rapid absorbance of the compound in the lumen within one hour; the absorbance rate was 50% in the stomach, 80% in the jejunum and 70% in the ileum. This indicated that capsaicin was absorbed better in the jejunum and ileum as compared to the stomach. The authors repeated these studies with dihydrocapsaicin and obtained similar results [30].
4. Bioavailability and metabolism of capsaicinoids in vivo
4.1 Topical administration in skin
Capsaicin is robustly absorbed from the skin upon topical administration [4]. Several capsaicin-based creams or patches are available as over-the-counter medications [33]. These contain 0.025%-1% capsaicin and are commonly used for pain relief. Pershing et al., (2004) evaluated the metabolism of capsaicin in the human stratum corneum following a single topical exposure of a 3% solution containing 55% capsaicin [34]. The uptake of capsaicin into the stratum corneum was very rapid, at one minute after the application. The experiment was repeated using dihydrocapsaicin (35% dihydrocapsaicin in 3% solution) and a mixture of capsaicinoids (10% capsaicinoids in 3% solution), and similar results were obtained. The capsaicinoids were comprised of nordihydrocapsaicin, homodihydrocapsaicin, homocapsaicin and nonivamide. The authors also tried three different vehicles, namely mineral oil (MO), propylene glycol (PG) and isopropyl alcohol (IPA). IPA delivered three times greater amount of capsaicin and dihydrocapsaicin to the skin than PG and MO [34]. These observations may be explained by the fact that capsaicin and dihydrocapsaicin are more soluble in alcohol-based vehicles like IPA rather than non-polar viscous solvents like PG and MO. The volatile nature of IPA delivers a greater amount of capsaicin in the stratum corneum, relative to non-volatile viscous vehicles like PG and MO. Taken together, the maximal concentration of capsaicinoids in the skin was dependent on the relative solubility of these compounds in the vehicle.
The effects of intradermal capsaicin injection (0.1 µg – 250 µg) in humans, were dependent on the dose injected and the frequency of injections [4]. The injection site was primarily the forearms and the feet. The patients reported heat-sensitivity, visual flare at the injection site, primary hyperalgesia and secondary hyperalgesia [4]. Other effects of intradermal capsaicin injection include secondary tactile allodynia, increase in blood flow, increase of temperature and vasodialation.
4.2 Oral and gastrointestinal administration
Previous studies show that capsaicin is rapidly absorbed from the stomach and the intestine following oral administration. Such absorption of capsaicin does not involve active transporter proteins and is a passive process [4]. The amount of capsaicin absorbed ranges from 50%-90% depending on the experimental model used. Suresh and Srinivasan, (2010) administered capsaicin by oral gavage at a dose of 30 mg capsaicin/kg body weight in Wistar albino rats [35]. The distribution of capsaicin was studied in the liver, kidney, intestine, serum and blood by HPLC analysis. Blood and intestine showed the peak concentration of capsaicin at one hour [35]. The liver and kidney displayed maximal amounts of capsaicin in 3 hours and 6 hours, respectively. The authors added up the concentrations of capsaicin found in tissues (liver, kidney, intestine, serum and blood) one hour post administration and observed that about 24.4% of the initially administered capsaicin was localized in these tissues. However, after 24 hours this percentage dropped down from 24.4% to 1.24% and after 48 hours it further decreased to 0.057%. No capsaicin was detected four days after oral gavage. These results suggest that capsaicin is rapidly metabolized in all the above tissues.
Emerging studies show that capsaicin displays potent anti-tumor activity in animal models of lung cancer, breast cancer, colon cancer and gastric cancer [36–39]. An established model to investigate the anti-cancer activity of compounds in vivo is the nude mouse model (athymic mouse model). In these experiments human cancer cell lines are injected subcutaneously in the scapula or the flank of immunodeficient nude mice. A nude mouse contains a mutation which causes a disruption of the Foxn1 gene or the HNF-3/forkhead homolog 11 gene [40]. The genetic mutation of the Foxn1 gene results in a lack of body hair and an inhibited immune system due to a greatly reduced number of T cells [40]. Almost all of the rodent-based studies involving the bioavailability of capsaicin are done in rats, not in mice. We investigated the bioavailability of capsaicin in nude mice after oral administration. We chose oral administration because nutritional compounds like capsaicin are often administered via dietary methods.
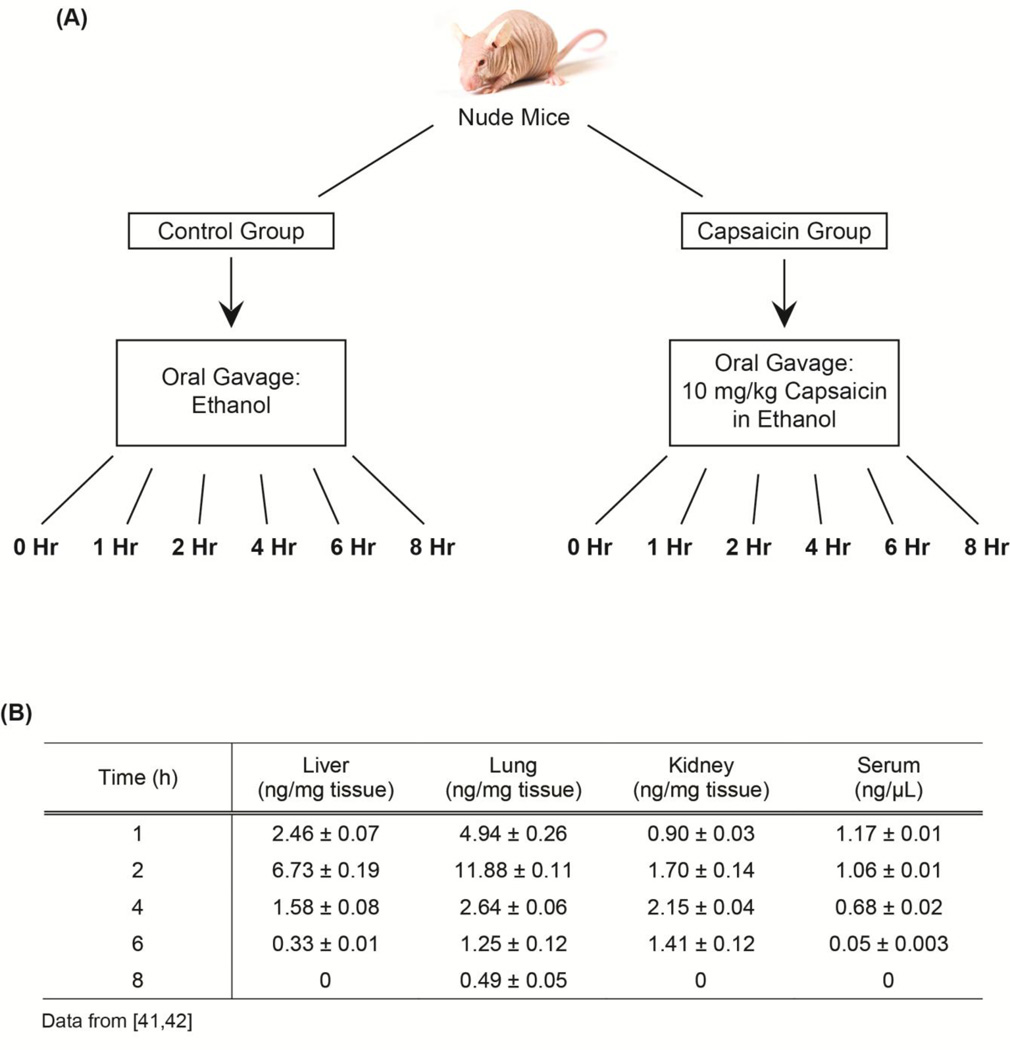
Four week old male nude mice were obtained from Charles River Laboratories and acclimatized for one week [41, 42]. They were housed in autoclaved cages with ad libitum access to food and water in HEPA-filtered racks and closely monitored by animal facility staff. All procedures involving nude mice were conducted according to the Animal Care and Use guidelines in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International and were approved by the Institutional Animal Care and Use Committee (IACUC) of Joan C. Edwards School of Medicine, Marshall University (protocol #515).
After acclimatization, the mice were divided into twelve groups comprised of eight mice each [41, 42]. The schema of the study is shown in Fig. 4A. The protocol of the study is based on the method of Zhang et al., (2010) [43]. The mice in the treatment group were administered 10 mg capsaicin per kg body weight (dissolved in ethanol) by oral gavage. The control group was administered ethanol only. The capsaicin-treated mice and the corresponding control-mice were euthanized at 1, 2, 4, 6 and 8 hours post oral gavage (Figure 4A). The lung, liver, blood and kidney of each mouse was collected and snap frozen in liquid nitrogen. Subsequently, 50 mg of the organs were used to make lysates using T-Per lysis buffer (Pierce Biotechnology), according to manufacturer’s protocol [44]. An aliquot of 100 µg of protein was used to measure the capsaicin content using the Beacon Capsaicin Plate kit (Beacon Analytical Systems, ME). Capsaicin was robustly detected in the serum at 1 hour after administration of capsaicin (Figure 4B). The liver, lungs and kidney contained the highest level of capsaicin at 2 hours after capsaicin oral gavage. The lung contained a higher level of unchanged capsaicin from 1 hour up to 8 hours post administration. In contrast, no capsaicin was detected in the liver, kidney or serum at 8 hours after oral gavage [41, 42]. The results of our studies agreed with those of other published reports which showed that the lung metabolized capsaicin slower than liver or blood [24, 30]. Our laboratory is investigating the growth-inhibitory effects of capsaicin in human lung cancer. The fact that capsaicin is metabolized slowly in the lung implies that there is a higher amount of unmodified capsaicin which can exert potent anti-tumor activity on lung tumors. We believe that the results of our experiments are relevant for all researchers working in the field of drug discovery and cancer.
Fig. 4.
(A) A flow-chart representing the experiment involving the bioavailability of capsaicin in nude mouse (B). A table summarizing the tissue distribution of orally administered capsaicin in nude mouse.
The metabolism of dihydrocapsaicin following oral administration (at a dose of 20 mg dihydrocapsaicin per kg body weight using a stomach feeding tube) was studied in Wistar Rats. The analysis of urine specimens revealed that dihydrocapsaicin was metabolized to vanillyl alcohol (37.6%), vanillic acid (19.2%) or vanillylamine (4.7%) as free forms or as their glucuronides. Furthermore, 8.7% of unchanged dihydrocapsaicin was detected in the urine. The authors also observed that the metabolism of dihydrocapsaicin was maximal in the liver, then the kidney, followed by the lung and was very low in the brain [29]. This data is analogous with the pattern of capsaicin metabolism in vitro (using microsomal preparations), where the lung was found to metabolize capsaicin slower than the liver [24].
The gastrointestinal absorption of capsaicin and dihydrocapsaicin has been studied in male Wistar rats. All the studies report that the 85–95% of the administered capsaicin is rapidly absorbed from the gastrointestinal lumen [30]. Most of the capsaicin is metabolized in the liver. A small amount of capsaicin was found to be hydrolyzed in the small intestine in vivo. The absorbed capsaicin was detected in the portal blood and partly metabolized to 8-methyl nonanoic acid [29]. Donnerer et al., (1990) administered capsaicin intragastrically to anesthetized male Sprague-Dawley rats [45]. Similar to the results of Kawada et al., (1984), Donnerer et al., (1990) observed that capsaicin was readily absorbed across the gastrointestinal tract and detected in portal blood. In both these studies, the amount of intact capsaicin (or dihydrocapsaicin) in the portal blood was higher when the capsaicinoids were absorbed solely from the stomach, as compared to the stomach and intestine [29, 45]. This data seem to suggest that the majority of the capsaicin was absorbed from the stomach and the intestine plays a minor role in this process.
Chaiyasit et al., (2009) studied the pharmacokinetics of orally administered capsaicinoids (C. frutescens extract) in humans [46]. Twelve healthy volunteers were administered 5 g of C. frutescens extract. The pharmacokinetic parameters were measured in the blood after the administration of capsaicinoids in the form of a gel capsule. Blood was collected from the volunteers every 10 minutes for the first one hour and every fifteen minutes for the next one hour [46]. The half-life of capsaicin in the blood was found to be about 25 minutes. The peak plasma concentration of capsaicin (Tpeak) was 2.5 ng/mL (~8.2 nM) at 45 minutes. After 105 minutes, no capsaicin was detected in the blood of the volunteers.
4.3 Systemic administration of capsaicin
Donnerer et al., (1990) compared the tissue-specific distribution of capsaicin after intravenous and subcutaneous injection of capsaicin [45]. The amount of unchanged capsaicin in the brain and spinal cord was five-fold higher than that of the trunk blood upon intravenous administration [4, 23, 45]. Ninety minutes after subcutaneous injection of capsaicin, the levels of unmodified capsaicin were lower in the brain, spinal cord and the trunk blood, relative to intravenous administration of capsaicin. They did not examine the levels of capsaicin in the liver. This void in knowledge was filled by Saria et al., (1982) who observed that intravenous injection of capsaicin in rats led to rapid metabolism of capsaicin within 3 minutes in the liver and the blood [23]. An interesting finding of these studies was that intravenous capsaicin administration leads to the rapid entry of capsaicin in the central nervous system. However, the levels of capsaicin in the brain, spinal cord, liver and blood are greatly decreased 10 minutes after administration. The authors hypothesized that such decrease of unchanged capsaicin may be due to formation of conjugates or its redistribution into the adipose tissue [23]. A second point to note is that the brain and spinal cord metabolized capsaicin much more slowly than the liver upon intravenous administration of capsaicin. This pattern of metabolism is similar to the one observed after oral administration of capsaicin [35].
Saria et al., (1982) also studied the bioavailability of capsaicin after a single subcutaneous injection of 50 mg capsaicin/kg body weight in Sprague Dawley rats [23]. The earliest time point that capsaicin was detected in the kidney, blood, brain and spinal cord was about ten minutes. The absorption of capsaicin in adipose tissue was a slower process and was only detected at 30 minutes post injection. The levels of capsaicin in all of these above-mentioned tissues steadily increased from ten minutes and attained maximum values at about 5 hours. The fact that capsaicin was detected early, yet its level increased very slowly (over several hours) suggests a slow diffusion process from the site of injection. It is known that several drugs display a slow rate of diffusion after subcutaneous injection. The reason for this is that the drug has to travel through several cell membranes and organ capillaries before it reaches the systemic circulation. In contrast, oral gavage, intragastric routes and intravenous injections leads to direct entry of capsaicin into the systemic bloodstream.
4.4 Miscellaneous administration of capsaicin
Kawada et al., (1985) analyzed the metabolism of capsaicin after intraperitoneal (IP) injection in anesthetized male Wistar rats. They observed that capsaicin was rapidly metabolized after injection. The maximal amount of capsaicin was detected in the thigh venous blood 16 minutes after the IP injection and the amount steadily decreasing until about 40 minutes post injection. The half-life of capsaicin was about 12 minutes [47].
Another major application of capsaicin is in pepper sprays. Reilly et al., (2002) used anesthetized Wistar rats to recapitulate exposures to pepper sprays in animals [48]. The rats were placed in nose-only exposure apparatus and exposed to aerosols generated from solutions of capsaicinoids for 15 minutes. The solutions used were 0, 1, 5 and 50 mg/ml capsaicinoids containing 55% capsaicin, 35% dihydrocapsaicin, 2.8% nonivamide, 4.3% nonhydrocapsaicin, 1.5% homodihydrocapsaicin and 1.4% homocapsaicin. The authors analyzed capsaicin, dihydrocapsaicin and nonivamide in the liver, lung and blood of the rats. The concentrations of capsaicinoids were found to be dependent on the dose received by the rat. At the highest dose (50 mg/ml), the metabolism of capsaicin was found to be maximal in the liver, followed by blood and least in the lung [48]. In contrast, the metabolism of dihydrocapsaicin and nonivamide were found to be slowest in the blood.
An interesting fact to note is that the bioavailability and half-life of capsaicin is quite low in the plasma, irrespective of the route of administration. This has led to extensive research aimed at devising strategies to improve the drug-delivery properties of capsaicin, enhance its half-life and augment its bioavailability in target tissues and organs (see Section 5).
5. Advanced Drug Delivery Systems
One of the most prevalent pharmacological applications of capsaicin is pain management. Topical capsaicin formulations like creams, lotions, and patches are used for relief of musculoskeletal and neuropathic pain [14, 18]. It is not surprising that the majority of drug delivery studies have aimed to improve the transdermal delivery of capsaicin. We provide a description of advanced drug delivery strategies involving capsaicin below.
5.1. Enhancers, sustained release patches, and gel-formulations
Zi et al., (2008) investigated the delivery properties of capsaicin using hydroxypropyl-beta-cyclodextrin (HPbetaCD) as a solubilizer and a permeation enhancer. HPbetaCD formed a hydrophilic inclusion complex with capsaicin. It was observed that capsaicin formulated with 2% HP-beta-CD displayed increased percutaneous absorption and penetration flux through isolated rat skin [49].
Other methods which increase the transport of the capsaicin analog nonivamide across the skin are electroporation and iontophoresis [50]. Electroporation was found to increase the permeation of nonivamide across isolated mouse skin. Transdermal iontophoresis refers to the transport of charged ions in a medium across the skin with the help of an applied electric field. Transdermal iontophoresis has been investigated in conjunction with other physical enhancement techniques such as low frequency ultrasound and erbium:YAG (yttrium-aluminum-garnet) laser to increase the transdermal delivery of nonivamide [50]. The combination of erbium:YAG laser treatment and ultrasound along with iontophoresis increased the skin permeation of nonivamide relative to vehicle-treated controls. In addition, the use of the erbium:YAG laser treatment method enabled the authors to precisely and reversibly control the removal of the stratum corneum.
A high concentration capsaicin dermal patch (capsaicin content = 179 mg capsaicin per patch) called NGX-4010 has been developed to treat patients with neuropathic pain associated with postherpetic neuralgia (PHN) [4]. Clinical trials have shown that a 60-minute application of NGX-4010 provided rapid and sustained relief to patients suffering from PHN. The concentration of capsaicin in the plasma of patients 60 minutes after application was about 1.38 ng/ml [28]. As mentioned earlier, the highest amount of capsaicin found in the plasma of patients was about 17.8 ng/mL or 58 nM. The elimination half-life of capsaicin in the plasma was found to be 1.34 hours [51, 52]. This is considerably higher than the t1/2 value of 25 minutes observed by Chaiyasit et al., (2009) [46]. The heat-sensation and erythema produced by the NGX-4010 patch were described by the patients as mild to moderate.
Cho et al., (2012) performed a double-blind randomized controlled clinical trial testing the efficacy of a 0.1% capsaicin hydrogel patch for relief of chronic myofascial neck pain in patients [53]. Fifty seven patients were part of this study. The authors reported that there was a decrease in neck disability index (NDI), visual analog scale (VAS) and Beck’s depression index (BDI) in the intervention group at two and four weeks after the trial was commenced. The authors did not find any difference in the final outcomes between the placebo group and the treatment group. The authors suggest that the lack of outcome measures may have been due to irritation and pain caused by the placebo patch. Another issue was the proper placement of both the placebo patch and the capsaicin patch. Further patient-oriented studies are required to clarify the feasibility of capsaicin in the management of chronic myofascial neck pain in patients.
Clinical trials have also investigated the ability of a 0.025% capsaicin patch to decrease the pain associated with diabetic neuropathy [54]. Although the patch was well tolerated in patients, it provided no relief from pain. The abovementioned data seem to suggest that those transdermal patches containing high amounts of capsaicin provide better pain relief in patients than low concentration capsaicin dermal patches.
Wang et al., (2001) have evaluated the skin absorption of capsaicin formulated as chitosan and carboxymethyl-chitosan (CMC)-sodium hydrogels. They found that the rate of permeation of capsaicin across the skin in vitro is greater from hydrogels than capsaicin-containing creams. Previous studies have shown that capsaicin penetrates the skin via a lipoidal pathway. Therefore the partitioning of the capsaicin from the hydrogel to the stratum corneum depends on the polarity of the hydrogel. The chitosan and CMC-sodium hydrogels are more hydrophilic than creams (which are oil in water formulations). Therefore, the lipophilic capsaicin would preferentially partition into the non-polar stratum corneum. This is in agreement with the observation that the rate of permeation of capsaicin across the skin depends on the nature of the hydrogel; non-ionic hydrogels showed slower rate of capsaicin transport than cationic or anionic hydrogels [31]. In cases of non-ionic hydrogels, the solubility of capsaicin may also be important in governing its rate of release from the polymer. Wang et al., (2001) also noticed that increasing the cross-linking of the hydrogels decreased the release rate of capsaicin from the polymer-matrix. This phenomenon may be explained by polymer entanglement and reduction of pore size of the hydrogel, as the crosslinking of the polymer increases. The lower pore size and higher viscosity of the hydrogel will retard the rate at which capsaicin diffuses out of the matrix.
The thermosensitive hydrogel polyethylene glycol-polylactic-co-glycolic acid (PEG-PLGA) was used a vehicle for capsaicin in rat models of bladder irritation [55]. The advantage of this capsaicin-thermosensitive hydrogel is that it is a liquid at body temperature and could be administered intravesically into the bladder. The capsaicin-hydrogel was well tolerated and safe. It significantly decreased bladder contraction compared to capsaicin solution in ethanol. The advantage of the thermosensitive hydrogel is its amphiphilic nature, biodegradability and biocompatibility. The polymer was designed to be chemically robust so that it would be unaffected by urine constituents such as urea and electrolytes. However, this polymer does degrade slowly in aqueous environments, which makes it attractive for systemic administration and long-acting, repeatable formulations of capsaicin.
Peng et al., (2010) used controlled release cubic phase gels made from glycerol monooleate (MO), propylene glycol (1,2-propanediol, PG), and water for transdermal delivery of capsaicin. The amount of capsaicin used in these cells was about 2.5 mg/g of gel. The authors studied the release rate of capsaicin from these cubic gels in vitro using HPLC analysis [56]. They found that capsaicin was efficiently released from these cubic gels; however the release rate of capsaicin was dependent on the amount of water in the gel formulation.
5.2. Films, microemulsions, vesicles and liposomes
The capsaicin analog nonivamide has been encapsulated in film-forming emulsions containing Eudragit® NE 30D (NE) and/or Eudragit RS30D® (RS) [57]. The permeability of nonivamide contained in Eudragit® NE 30D (NE) was found to rise with increase in the amount of NE added to the film-forming emulsions in in vitro permeability experiments [57]. However, this result was not observed in permeability experiments using pig ear skin. Chen et al., (2010) prepared capsaicin-chitosan microsphere (CCMS) tablets for sustained delivery of capsaicin to the intestine [58]. Eudragit® L 100 was used to prepare the CCMS-enteric-coated tablets by the process of wet granulation. The CCMS tablets released capsaicin efficiently according to first order kinetics. Tan et al., (2014) recently tested the anti-obesity effects of CCMS microspheres in obese rats [59]. The CCMS tablets were well tolerated by the rats and there was no evidence of gross toxicity or discomfort. The CCMS tablets produced a greater decrease in body weight, body mass index, organ index, body fat, proportion of fat to body weight, and serum lipids, relative to capsaicin alone. Most interestingly, the anti-obesity effect of CCMS tablets was better than the Orlistat, which is the standard of care for reducing obesity in patients. The favorable drug delivery and safety profile of CCMS enteric coated tablets could have potential applications in the systemic administration of capsaicin in patients.
Huang et al., (2008) have explored microemulsion techniques to improve the transdermal delivery of the capsaicinoid nonivamide [60]. They prepared mixed surfactant-based microemulsions comprising of Tween-80 and Span 20. They used ethanol as a cosurfactant, isopropyl myristate as the oil phase and water as the external phase. Experiments showed that these mixed surfactant microemulsions increased the transdermal delivery of nonivamide by 3.7–7.1 fold relative to controls [60].
Other sustained release formulations of capsaicin include encasement in flexible membrane vesicles (FMVs) made of phospholipids and surfactants. The capsaicin-FMVs were mixed with a Carbopol® 934 hydrogel to facilitate topical application. Raza et al., (2011) compared the pharmacological activity of capsaicin-FMV-gel to capsaicin containing liposomes. The capsaicin-FMV-gel showed better permeability across the skin of LACA mice ex vivo. They also displayed improved analgesic activity in the radiant tail flick mouse model. The capsaicin-FMV-gel also caused less redness, burning and itching sensation on the skin, compared to capsaicin containing creams. It would have been interesting to compare the sensory profile of capsaicin-FMV-gel to capsaicin-liposomes. However, the authors have not performed this experiment in their published report. Zhu et al., have attempted to encapsulate capsaicin in mixed polymeric micelles consisting of polyvinylpyrrolidome (PVP)/sodium cholate/phospholipid mixed system. Pharmacokinetic experiments in rats revealed that these capsaicin-loaded polymeric micelles displayed a prolonged plasma half and a two-fold higher oral bioavailability, relative to free capsaicin. In addition, this formulation was well tolerated and caused no gastric irritation in rats [61].
Niosomal carriers have been explored for transdermal drug delivery of capsaicin. Niosomes are non-ionic surfactant-based vesicles generated from the self-assembly of non-ionic amphiphiles. The closed bilayer structure, biocompatibility, biodegradability and non-immunogenic nature of niosomes makes them excellent drug carriers. Data by Tavano et al., (2011) reveal that transdermal delivery of capsaicin was greater with capsaicin-niosomes than with capsaicin-microemulsion formulation. Similarly, the percutaneous permeation ability of capsaicin-niosmes was superior to that of capsaicin-microemulsions. The authors measured the rate of capsaicin released over 12 hours from both these formulations. The amount of capsaicin released from niosomes was greater than microemulsions. This can be explained by the fact that the microemulsions were considerably more viscous than the niosomes. The capsaicin diffuses faster from the niosomal matrix than the microemulsions, where the capsaicin molecule has to overcome the immiscible oil phase to reach the skin [62].
Linderroth et al., (2009) have designed a unique drug-targeting system which combines liposomes and the prodrug strategy. They designed a liposome made from glycerophospholipids which was activated by the enzyme secretory phospholipase-2 (sPLA2). This enzyme sPLA2 is over expressed on most human cancer cells. Therefore, the authors decided to encapsulate capsaicin (which has anti-cancer activity) in these liposomes. The treatment of these liposomes with sPLA2 hydrolyzed the liposome and released the capsaicin. MALDI-TOF experiments showed that sPLA2 released 90% of the capsaicin within 24 hours. The colon cancer cell line Colo 205 releases robust amounts of sPLA2 in the supernatant. The authors used the Colo 205 supernatant and showed that all the capsaicin within the liposome was released within 24 hours. It would be interesting to see if these capsaicin-loaded prodrug-liposomes display potent anti-tumor activity in colon tumor xenografts in nude mouse models [63].
5.3. Nanotechnology based delivery systems
A diverse array of nanotechnologies has been used to improve the bioavailability of capsaicinoids. Contri et al., (2011) encapsulated capsaicin and dihydrocapsaicin in polymeric nanocapsules. The encapsulation efficiency for both of these capsaicinoids was close to 100%, and the formulation had a shelf life of about 90 days. A similar study was conducted by Kim et al., (2011) who tested the release profile of capsaicin from poly(d,l-lactide-co-glycolide) [PLGA]-nanoparticles. The authors observed that the loading efficiency and release profile of capsaicin was sensitive to slight changes in the oil water ratio used during the loading of the PLGA-nanoparticles [64]. This can be explained by the fact that capsaicin is water-insoluble and any attempt to increase the amount of water in the oil-water emulsion will result in lower loading efficiency and decreased release of capsaicin. The authors compared the capsaicin-release profiles from three types of nanoparticles, namely poly(L-lactide) [PLLA]-nanoparticles, PLGA-nanoparticles and poly-ε-caprolactone (PCL)-nanoparticles. The PLLA-nanoparticles showed higher capsaicin-encapsulation efficiency, improved sustained release profiles and better shelf life than the other two matrices. The difference in release pattern of capsaicin between all three polymers seems to result from the difference in the crystallinity between them. The degree of crystallinity of PLLA is greater than PLGA or PCL. The capsaicin gets trapped in the crystalline matrix of PLLA and defuses out at a steady sustained rate, with progressive degradation of the matrix. However, a major caveat of these studies is that they have not tested the delivery of capsaicin-nanoparticles in any biological experimental system.
Solid-lipidic nanoparticles (SLN) and nanostructured lipid carriers (NLC) have been explored for the transdermal delivery of capsaicin [65]. SLNs are comprised of crystallized lipid droplets and are organized in a highly-ordered crystalline structure. The cargo drug (in this case capsaicin) is considered to be a part of the lipid crystalline matrix of SLNs. In contrast, NLCs have a less ordered crystalline structure. The relatively more flexible structure of NLCs makes them superior drug delivery agents to the SLNs. Capsaicin-loaded NLCs showed superior permeation through skin and better retention in the stratum corneum than capsaicin-containing SLNs. The in vivo pharmacological efficacy of capsaicin-loaded NLCs was evaluated in LACA mice model. The capsaicin-NLC was formulated into a gel using stearic acid. The capsaicin-NLC gel showed greater permeability and retention across mouse skin, compared to conventional capsaicin-containing creams. The analgesic activity of capsaicin-NLC gel (as measured by the radiant mouse tail flick experiment) was also superior to that of capsaicin-containing cream [65].
Nano-vesicular ethosomal formulations of capsaicin have been investigated for the treatment of inflammatory musculoskeletal diseases like arthritis [66, 67]. The permeability of these nano-ethosomal formulations was found be greater than capsaicin containing creams in ex vivo human skin models. Experiments in rat models revealed that ethosomal capsaicin decreased edema in rat paws. The ethosomal capsaicin also displayed improved anti-arthritic activity and analgesic activity compared to the capsaicin-containing cream. The same research group did a subsequent study where they created an ethosomal formulation of the capsaicinoid extract of the Bhut Jolokia (the hottest chili pepper in the world) and tested its anti-arthritic activity [66]. They observed that the topical ethosomal formulation of Bhut Jolokia caused substantial decrease in joint swelling, hyperalgesia, edema and pain-sensation in Freund’s adjuvant inducted arthritis in Wistar albino rats. The anti-arthritic activity of capsaicin was also explored in nano-transfersomes and similar results were obtained as those of the ethosomal formulations of capsaicin.
Nanofiber mats have been investigated for transdermal delivery of capsaicinoids. Opanasopit et al., (2013) compared the capsaicin-release profiles of two types of nanofiber mats: one made from polyvinylalcohol (PVA) and the other from cellulose acetate (CA) [68]. The nanofiber mats loaded varying amounts (0.5–2%) of capsicum extract (CE). The authors observed that the release rate and permeability of capsaicin using shed snake skin showed that the transport of capsaicin across the skin was higher in CE-loaded PVA-nanofibers compared to CE-loaded CA-nanofibers. The higher release rate and permeability of CE-PVA nanofibers was ascribed to the highly erosive nature of PVA. PVA has a high susceptibility to release its cargo in aqueous media. As the CE-loaded PVA-nanofibers encounter the aqueous microenvironment of the skin, the PVA fibers start releasing capsaicin at a rapid pace. The release rate of CE-loaded PVA-nanofibers is about 60-fold higher than CE-loaded CA-nanofibers. The higher amounts of capsaicin in the skin microenvironment lead to improved permeation rates across the skin.
Another approach used by researchers has been to encapsulate capsaicin in nanoparticles followed by incorporation of chitosan hydrogel to improve its permeability across the skin [69]. The capsaicin-nanoparticles showed increased rates of adhesion and retention (of capsaicin) in human skin relative to free capsaicin mixed with chitosan hydrogel. Such increased retention in the epidermis and dermis led to more efficient diffusion and delivery of capsaicin to the deeper layers of the skin.
An important question which arises from these data is whether the sensory effects of capsaicin-nanoparticles are better than existing capsaicin formulations. Nanovesicle formulations (transferosomes and ethosomes) of Bhut Jolokia chili peppers displayed better tolerability and compliance in patients than Thermagel (a commercial formulation of capsaicin). Toxicological studies have showed that nano-ethosomal formulations of capsaicin showed no signs of skin irritation, redness or gross toxicity in rat models. Similarly, capsaicin-containing SLNs and NLCs showed no changes in the histopathology of skin upon topical administration. Overall, the side- effect prolife of nano-formulations of capsaicin were much milder than free capsaicin [64, 65, 67, 69].
6. Conclusions and future directions
The nutritional compound capsaicin is widely used as a topical analgesic [4]. Recent studies have shown that capsaicin has anti-cancer, cardioprotective and anti-obesity effects [7, 13]. The half-life of capsaicin in the lung and the skin is higher than that of the liver [24, 25, 34]. This implies that capsaicin-based drugs may prove to be more efficacious in lung or skin ailments.
A major challenge in the clinical application of capsaicin is its short half-life and low bioavailability. Another caveat of capsaicin (as a drug) is that it produces a burning sensation, and the resulting discomfort limits patient compliance. Several strategies have been explored to improve the bioavailability of capsaicin. These include hydrogel formations, encapsulation in liposomes and iontophoresis [62, 70, 71]. More recently, nanotechnology has been used to produce continuous release formulations of capsaicin [64, 65, 67, 69, 72]. The advantage of such sustained-release formulations is two-fold. First, they induce a more continuous and prolonged release of capsaicin to the target organ site. For example, most of the sustained-release topical formulations display better skin permeability than capsaicin-creams. Whereas most of the long-acting capsaicin formulations have been primarily designed for transdermal delivery, it is probable that they could be adapted for other forms of administration [59, 61]. Second, such a “reservoir effect” may decrease the dosage of capsaicin required for treatment, and this will lead to fewer side effects in patients. This is especially true for nanoparticle-based capsaicin formulations which cause minimum discomfort in animal models. Capsaicin-nanoparticles are also compatible with other drugs and may be used in combination therapy. Desai et al., (2013) found that capsaicin-nanoparticles showed synergism with TNF-α-siRNA to decrease skin inflammation in mouse models [72]. Systematic structure-activity studies will lead to the identification of second- generation capsaicin-mimetics which will not have the unpleasant side effects of capsaicin and will possess greater pharmacological activity than capsaicin. Long-acting capsaicin analogs and capsaicin-mimetics will form the basis of novel pharmacological interventions against a wide array of human diseases like chronic pain, atherosclerosis and cancer.
Acknowledgements
We thank Dr. Srikumar Chellappan and his laboratory for their help and support. This work was supported by the NIH R15 AREA grant (1R15CA161491-01A1) and an AICR research grant to PDG. MAV is supported by grants from the West Virginia IDeA Network of Biomedical Research Excellence (P20RR016477 and P20GM103434).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no conflict of interest.
References
- 1.Govindarajan VS, Sathyanarayana MN. Capsicum--production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. Critical reviews in food science and nutrition. 1991;29:435–474. doi: 10.1080/10408399109527536. [DOI] [PubMed] [Google Scholar]
- 2.Reyes-Escogido Mde L, Gonzalez-Mondragon EG, Vazquez-Tzompantzi E. Chemical and pharmacological aspects of capsaicin. Molecules. 2011;16:1253–1270. doi: 10.3390/molecules16021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annual review of neuroscience. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill J, Brock C, Olesen AE, Andresen T, Nilsson M, Dickenson AH. Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacological reviews. 2012;64:939–971. doi: 10.1124/pr.112.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bley K, Boorman G, Mohammad B, McKenzie D, Babbar S. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicologic pathology. 2012;40:847–873. doi: 10.1177/0192623312444471. [DOI] [PubMed] [Google Scholar]
- 6.Mozsik G, Past T, Abdel Salam OM, Kuzma M, Perjesi P. Interdisciplinary review for correlation between the plant origin capsaicinoids, non-steroidal antiinflammatory drugs, gastrointestinal mucosal damage and prevention in animals and human beings. Inflammopharmacology. 2009;17:113–150. doi: 10.1007/s10787-009-0002-3. [DOI] [PubMed] [Google Scholar]
- 7.Lau JK, Brown KC, Dom AM, Dasgupta P. Capsaicin: Potential Applications in Cancer Therapy. London, United Kingdom: Bentham Press Inc; 2012. [Google Scholar]
- 8.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Tharakan ST, Sung B, Anand P. Potential of spice-derived phytochemicals for cancer prevention. Planta Med. 2008;74:1560–1569. doi: 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- 9.Lau JK, Brown KC, Dom AM, Witte TR, Thornhill BA, Crabtree CM, Perry HE, Brown JM, Ball JG, Creel RG, Damron CL, Rollyson WD, Stevenson CD, Hardman WE, Valentovic MA, Carpenter AB, Dasgupta P. Capsaicin induces apoptosis in human small cell lung cancer via the TRPV6 receptor and the calpain pathway. Apoptosis. 2014;19:1190–1201. doi: 10.1007/s10495-014-1007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valentovic MA, Santanam NS. Nutrition, oxidative stress and cancer. London, United Kingdom: Bentham Press Inc; 2012. [Google Scholar]
- 11.Jung SH, Kim HJ, Oh GS, Shen A, Lee S, Choe SK, Park R, So HS. Capsaicin ameliorates cisplatin-induced renal injury through induction of heme oxygenase-1. Molecules and cells. 2014;37:234–240. doi: 10.14348/molcells.2014.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valentovic MA, Ball JG, Brown JM, Terneus MV, McQuade E, Van Meter S, Hedrick HM, Roy AA, Williams T. Resveratrol attenuates cisplatin renal cortical cytotoxicity by modifying oxidative stress. Toxicology in vitro : an international journal published in association with BIBRA. 2014;28:248–257. doi: 10.1016/j.tiv.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo XJ, Peng J, Li YJ. Recent advances in the study on capsaicinoids and capsinoids. European journal of pharmacology. 2011;650:1–7. doi: 10.1016/j.ejphar.2010.09.074. [DOI] [PubMed] [Google Scholar]
- 14.Sharma SK, Vij AS, Sharma M. Mechanisms and clinical uses of capsaicin. European journal of pharmacology. 2013;720:55–62. doi: 10.1016/j.ejphar.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 15.Remadevi R, Szallisi A. Adlea (ALGRX-4975), an injectable capsaicin (TRPV1 receptor agonist) formulation for longlasting pain relief. IDrugs : the investigational drugs journal. 2008;11:120–132. [PubMed] [Google Scholar]
- 16.Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain research reviews. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Smith H, Brooks JR. Capsaicin-based therapies for pain control, Progress in drug research. Fortschritte der Arzneimittelforschung. Progres des recherches pharmaceutiques. 2014;68:129–146. doi: 10.1007/978-3-0348-0828-6_5. [DOI] [PubMed] [Google Scholar]
- 18.Peppin JF, Pappagallo M. Capsaicinoids in the treatment of neuropathic pain: a review. Therapeutic advances in neurological disorders. 2014;7:22–32. doi: 10.1177/1756285613501576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laslett LL, Jones G. Capsaicin for osteoarthritis pain, Progress in drug research. Fortschritte der Arzneimittelforschung. Progres des recherches pharmaceutiques. 2014;68:277–291. doi: 10.1007/978-3-0348-0828-6_11. [DOI] [PubMed] [Google Scholar]
- 20.Adams MJ, Ahuja KD, Geraghty DP. Effect of capsaicin and dihydrocapsaicin on in vitro blood coagulation and platelet aggregation. Thrombosis research. 2009;124:721–723. doi: 10.1016/j.thromres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Mittelstadt SW, Nelson RA, Daanen JF, King AJ, Kort ME, Kym PR, Lubbers NL, Cox BF, Lynch JJ., 3rd Capsaicin-induced inhibition of platelet aggregation is not mediated by transient receptor potential vanilloid type 1. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2012;23:94–97. doi: 10.1097/MBC.0b013e32834ddf18. [DOI] [PubMed] [Google Scholar]
- 22.Reinbach HC, Smeets A, Martinussen T, Moller P, Westerterp-Plantenga MS. Effects of capsaicin, green tea and CH-19 sweet pepper on appetite and energy intake in humans in negative and positive energy balance. Clinical nutrition. 2009;28:260–265. doi: 10.1016/j.clnu.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Saria A, Skofitsch G, Lembeck F. Distribution of capsaicin in rat tissues after systemic administration. The Journal of pharmacy and pharmacology. 1982;34:273–275. doi: 10.1111/j.2042-7158.1982.tb04245.x. [DOI] [PubMed] [Google Scholar]
- 24.Reilly CA, Ehlhardt WJ, Jackson DA, Kulanthaivel P, Mutlib AE, Espina RJ, Moody DE, Crouch DJ, Yost GS. Metabolism of capsaicin by cytochrome P450 produces novel dehydrogenated metabolites and decreases cytotoxicity to lung and liver cells. Chemical research in toxicology. 2003;16:336–349. doi: 10.1021/tx025599q. [DOI] [PubMed] [Google Scholar]
- 25.Chanda S, Bashir M, Babbar S, Koganti A, Bley K. In vitro hepatic and skin metabolism of capsaicin. Drug metabolism and disposition: the biological fate of chemicals. 2008;36:670–675. doi: 10.1124/dmd.107.019240. [DOI] [PubMed] [Google Scholar]
- 26.Reilly CA, Henion F, Bugni TS, Ethirajan M, Stockmann C, Pramanik KC, Srivastava SK, Yost GS. Reactive intermediates produced from the metabolism of the vanilloid ring of capsaicinoids by p450 enzymes. Chemical research in toxicology. 2013;26:55–66. doi: 10.1021/tx300366k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernal MA, Barcelo AR. 5,5'-Dicapsaicin, 4'-O-5-dicapsaicin ether, and dehydrogenation polymers with high molecular weights are the main products of the oxidation of capsaicin by peroxidase from hot pepper. Journal of agricultural and food chemistry. 1996;44:3085–3089. [Google Scholar]
- 28.Babbar S, Chanda S, Bley K. Inhibition and induction of human cytochrome P450 enzymes in vitro by capsaicin. Xenobiotica; the fate of foreign compounds in biological systems. 2010;40:807–816. doi: 10.3109/00498254.2010.520044. [DOI] [PubMed] [Google Scholar]
- 29.Kawada T, Iwai K. In vivo and in vitro metabolism of dihydrocapsaicin, a pungent principle of hot pepper, in rats. Agric Biol Chem. 1985;49:441–448. [Google Scholar]
- 30.Kawada T, Suzuki T, Takahashi M, Iwai K. Gastrointestinal absorption and metabolism of capsaicin and dihydrocapsaicin in rats. Toxicology and applied pharmacology. 1984;72:449–456. doi: 10.1016/0041-008x(84)90121-2. [DOI] [PubMed] [Google Scholar]
- 31.Wang YY, Hong CT, Chiu WT, Fang JY. In vitro and in vivo evaluations of topically applied capsaicin and nonivamide from hydrogels. International journal of pharmaceutics. 2001;224:89–104. doi: 10.1016/s0378-5173(01)00755-4. [DOI] [PubMed] [Google Scholar]
- 32.Monsereenusorn Y. In vitro intestinal absorption of capsaicin. Toxicology and applied pharmacology. 1980;53:134–139. doi: 10.1016/0041-008x(80)90390-7. [DOI] [PubMed] [Google Scholar]
- 33.Hayman M, Kam P. Capsaicin: a review of its pharmacology and clinical applications. Curr Anaesth Crit Care. 2008;19:338–343. [Google Scholar]
- 34.Pershing LK, Reilly CA, Corlett JL, Crouch DJ. Effects of vehicle on the uptake and elimination kinetics of capsaicinoids in human skin in vivo. Toxicology and applied pharmacology. 2004;200:73–81. doi: 10.1016/j.taap.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Suresh D, Srinivasan K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. The Indian journal of medical research. 2010;131:682–691. [PubMed] [Google Scholar]
- 36.Ziglioli F, Frattini A, Maestroni U, Dinale F, Ciufifeda M, Cortellini P. Vanilloid-mediated apoptosis in prostate cancer cells through a TRPV-1 dependent and a TRPV-1-independent mechanism. Acta Biomed. 2009;80:13–20. [PubMed] [Google Scholar]
- 37.Chow J, Norng M, Zhang J, Chai J. TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells--Mechanisms behind a possible new "hot" cancer treatment. Biochim Biophys Acta. 2007;1773:565–576. doi: 10.1016/j.bbamcr.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Lu HF, Chen YL, Yang JS, Yang YY, Liu JY, Hsu SC, Lai KC, Chung JG. Antitumor activity of capsaicin on human colon cancer cells in vitro and colo 205 tumor xenografts in vivo. J Agric Food Chem. 2010;58:12999–123005. doi: 10.1021/jf103335w. [DOI] [PubMed] [Google Scholar]
- 39.Thoennissen NH, O’Kelly J, Lu D, Iwanski GB, La DT, Abbassi S, Leiter A, Karlan B, Mehta R, Koeffler HP. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene. 2010;29:285–296. doi: 10.1038/onc.2009.335. [DOI] [PubMed] [Google Scholar]
- 40.Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372:103–107. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- 41.Rollyson W, Stover C, Brown KC, Perry H, Stevenson C, Crabtree C, Dom AM, Lau JK, Witte T, Hardman WE, Dasgupta P. The anti-cancer dietary compound capsaicin shows higher bioavailability in the lung than other organs. The FASEB Journal. 2014;281(Supplement 644.2) [Google Scholar]
- 42.Stover C, Crabtree C, Dom AM, Lau JK, Brown KC, Shiflett B, Witte T, Hardman WE, Dasgupta P. Capsaicin: a novel dietary therapeutic agent in human small cell lung cancers. The FASEB Journal. 2013;271(Supplement 1166.1) [Google Scholar]
- 43.Zhang L, Angst E, Park JL, Moro A, Dawson DW, Reber HA, Eibl G, Hines OJ, Go VL, Lu QY. Quercetin aglycone is bioavailable in murine pancreas and pancreatic xenografts. Journal of agricultural and food chemistry. 2010;58:7252–7257. doi: 10.1021/jf101192k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinkade R, Dasgupta P, Carie A, Pernazza D, Carless M, Pillai S, Lawrence N, Sebti SM, Chellappan S. A small molecule disruptor of Rb/Raf-1 interaction inhibits cell proliferation, angiogenesis, and growth of human tumor xenografts in nude mice. Cancer Res. 2008;68:3810–3818. doi: 10.1158/0008-5472.CAN-07-6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donnerer J, Amann R, Schuligoi R, Lembeck F. Absorption and metabolism of capsaicinoids following intragastric administration in rats. Naunyn-Schmiedeberg’s archives of pharmacology. 1990;342:357–361. doi: 10.1007/BF00169449. [DOI] [PubMed] [Google Scholar]
- 46.Chaiyasit K, Khovidhunkit W, Wittayalertpanya S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2009;92:108–113. [PubMed] [Google Scholar]
- 47.Kawada T, Watanabe T, Katsura K, Takami H, Iwai K. Formation and metabolism of pungent principle of Capsicum fruits. XV. Microdetermination of capsaicin by high-performance liquid chromatography with electrochemical detection. Journal of chromatography. 1985;329:99–105. doi: 10.1016/s0021-9673(01)81899-9. [DOI] [PubMed] [Google Scholar]
- 48.Reilly CA, Crouch DJ, Yost GS, Fatah AA. Determination of capsaicin, nonivamide, and dihydrocapsaicin in blood and tissue by liquid chromatography-tandem mass spectrometry. Journal of analytical toxicology. 2002;26:313–319. doi: 10.1093/jat/26.6.313. [DOI] [PubMed] [Google Scholar]
- 49.Zi P, Yang X, Kuang H, Yang Y, Yu L. Effect of HPbetaCD on solubility and transdermal delivery of capsaicin through rat skin. International journal of pharmaceutics. 2008;358:151–158. doi: 10.1016/j.ijpharm.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Fang JY, Hwang TL, Huang YB, Tsai YH. Transdermal iontophoresis of sodium nonivamide acetate. V. Combined effect of physical enhancement methods. International journal of pharmaceutics. 2002;235:95–105. doi: 10.1016/s0378-5173(01)00972-3. [DOI] [PubMed] [Google Scholar]
- 51.Babbar S, Marier JF, Mouksassi MS, Beliveau M, Vanhove GF, Chanda S, Bley K. Pharmacokinetic analysis of capsaicin after topical administration of a high-concentration capsaicin patch to patients with peripheral neuropathic pain. Therapeutic drug monitoring. 2009;31:502–510. doi: 10.1097/FTD.0b013e3181a8b200. [DOI] [PubMed] [Google Scholar]
- 52.Backonja M, Wallace MS, Blonsky ER, Cutler BJ, Malan P, Jr, Rauck R, Tobias J, Group N-CS. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet neurology. 2008;7:1106–1112. doi: 10.1016/S1474-4422(08)70228-X. [DOI] [PubMed] [Google Scholar]
- 53.Cho JH, Brodsky M, Kim EJ, Cho YJ, Kim KW, Fang JY, Song MY. Efficacy of a 0.1% capsaicin hydrogel patch for myofascial neck pain: a double-blinded randomized trial. Pain medicine. 2012;13:965–970. doi: 10.1111/j.1526-4637.2012.01413.x. [DOI] [PubMed] [Google Scholar]
- 54.Kulkantrakorn K, Lorsuwansiri C, Meesawatsom P. 0.025% capsaicin gel for the treatment of painful diabetic neuropathy: a randomized, double-blind, crossover, placebo-controlled trial. Pain practice : the official journal of World Institute of Pain. 2013;13:497–503. doi: 10.1111/papr.12013. [DOI] [PubMed] [Google Scholar]
- 55.Tyagi P, Chancellor MB, Li Z, De Groat WC, Yoshimura N, Fraser MO, Huang L. Urodynamic and immunohistochemical evaluation of intravesical capsaicin delivery using thermosensitive hydrogel and liposomes. The Journal of urology. 2004;171:483–489. doi: 10.1097/01.ju.0000102360.11785.d7. [DOI] [PubMed] [Google Scholar]
- 56.Peng X, Wen X, Pan X, Wang R, Chen B, Wu C. Design and in vitro evaluation of capsaicin transdermal controlled release cubic phase gels. AAPS PharmSciTech. 2010;11:1405–1410. doi: 10.1208/s12249-010-9481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lunter DJ, Daniels R. New film forming emulsions containing Eudragit(R) NE and/or RS 30D for sustained dermal delivery of nonivamide. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2012;82:291–298. doi: 10.1016/j.ejpb.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Chen J, Huang GD, Tan SR, Guo J, Su ZQ. The preparation of capsaicin-chitosan microspheres (CCMS) enteric coated tablets. International journal of molecular sciences. 2013;14:24305–24319. doi: 10.3390/ijms141224305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan S, Gao B, Tao Y, Guo J, Su ZQ. Antiobese effects of capsaicin-chitosan microsphere (CCMS) in obese rats induced by high fat diet. Journal of agricultural and food chemistry. 2014;62:1866–1874. doi: 10.1021/jf4040628. [DOI] [PubMed] [Google Scholar]
- 60.Huang YB, Lin YH, Lu TM, Wang RJ, Tsai YH, Wu PC. Transdermal delivery of capsaicin derivative-sodium nonivamide acetate using microemulsions as vehicles. International journal of pharmaceutics. 2008;349:206–211. doi: 10.1016/j.ijpharm.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 61.Zhu Y, Peng W, Zhang J, Wang M, Firempong CK, Feng C, Liu H, Xu X, Yu J. Enhanced oral bioavailability of capsaicin in mixed polymeric micelles: Preparation, in vitro and in vivo evaulation. J Functional Foods. 2014;8:358–366. [Google Scholar]
- 62.Tavano L, Alfano P, Muzzalupo R, de Cindio B. Niosomes vs microemulsions: new carriers for topical delivery of Capsaicin, Colloids and surfaces, B. Biointerfaces. 2011;87:333–339. doi: 10.1016/j.colsurfb.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 63.Linderoth L, Peters GH, Madsen R, Andresen TL. Drug delivery by an enzyme-mediated cyclization of a lipid prodrug with unique bilayer-formation properties. Angewandte Chemie. 2009;48:1823–1826. doi: 10.1002/anie.200805241. [DOI] [PubMed] [Google Scholar]
- 64.Kim S, Kim JC, Sul D, Hwang SW, Lee SH, Kim YH, Tae G. Nanoparticle formulation for controlled release of capsaicin. Journal of nanoscience and nanotechnology. 2011;11:4586–4591. doi: 10.1166/jnn.2011.3636. [DOI] [PubMed] [Google Scholar]
- 65.Raza K, Shareef MA, Singal P, Sharma G, Negi P, Katare OP. Lipid-based capsaicin-loaded nano-colloidal biocompatible topical carriers with enhanced analgesic potential and decreased dermal irritation. Journal of liposome research. 2014 doi: 10.3109/08982104.2014.911314. [DOI] [PubMed] [Google Scholar]
- 66.Sarwa KK, Das PJ, Mazumder B. A nanovesicle topical formulation of Bhut Jolokia (hottest capsicum): a potential anti-arthritic medicine. Expert opinion on drug delivery. 2014;11:661–676. doi: 10.1517/17425247.2014.891581. [DOI] [PubMed] [Google Scholar]
- 67.Sarwa KK, Mazumder B, Rudrapal M, Verma VK. Potential of capsaicin-loaded transfersomes in arthritic rats. Drug delivery. 2014 doi: 10.3109/10717544.2013.871601. [DOI] [PubMed] [Google Scholar]
- 68.Opanasopit P, Sila-On W, Rojanarata T, Ngawhirunpat T. Fabrication and properties of capsicum extract-loaded PVA and CA nanofiber patches. Pharmaceutical development and technology. 2013;18:1140–1147. doi: 10.3109/10837450.2012.727004. [DOI] [PubMed] [Google Scholar]
- 69.Contri RV, Katzer T, Ourique AF, da Silva AL, Beck RC, Pohlmann AR, Guterres SS. Combined effect of polymeric nanocapsules and chitosan hydrogel on the increase of capsaicinoids adhesion to the skin surface. Journal of biomedical nanotechnology. 2014;10:820–830. doi: 10.1166/jbn.2014.1752. [DOI] [PubMed] [Google Scholar]
- 70.Chen X, Sun X, Ren K, Zhang X, Zhang Z, Gong T. Enhanced aqueous solubility and bioavailability of capsaicin by the preparation of an inclusion complex. Arzneimittel-Forschung. 2010;60:571–574. doi: 10.1055/s-0031-1296327. [DOI] [PubMed] [Google Scholar]
- 71.Raza K, Singh B, Mahajan A, Negi P, Bhatia A, Katare OP. Design and evaluation of flexible membrane vesicles (FMVs) for enhanced topical delivery of capsaicin. Journal of drug targeting. 2011;19:293–302. doi: 10.3109/1061186X.2010.499464. [DOI] [PubMed] [Google Scholar]
- 72.Desai PR, Marepally S, Patel AR, Voshavar C, Chaudhuri A, Singh M. Topical delivery of anti-TNFalpha siRNA and capsaicin via novel lipid-polymer hybrid nanoparticles efficiently inhibits skin inflammation in vivo. Journal of controlled release : official journal of the Controlled Release Society. 2013;170:51–63. doi: 10.1016/j.jconrel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]