Abstract
Background
Persistent pulmonary hypertension of the newborn (PPHN) is associated with increased risk of neuro-developmental impairments. Whether relative fetal hypoxia during evolution of PPHN renders the fetal brain vulnerable to perinatal brain injury remains unclear. We hypothesized that in utero ductal constriction, which induces PPHN also impairs cerebral angiogenesis.
Methods
Fetal lambs with PPHN induced by prenatal ligation of the ductus arteriosus were compared to gestation matched twin controls. Freshly collected or fixed brain specimens were analyzed by immunohistochemistry, Western blot analysis, and RT-PCR.
Results
Cortical capillary density was decreased in PPHN lambs compared to controls (Glut-1, Isolectin B-4 and Factor VIII, n=6, p<0.05). Hypoxia inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF) protein levels were decreased in cortical cell lysates of PPHN lambs. PPHN increased angiopoetin-1 (Ang-1) and tyrosine-protein kinase receptor (Tie-2) protein expression while angiopoetin-2 (Ang-2) protein levels were decreased (n=6, p<0.05). PPHN did not change mRNA levels of these proteins significantly (n=6).
Conclusions
PPHN decreased cortical capillary density in fetal lamb brain. PPHN decreased the expression of proteins involved in angiogenesis. These findings suggest that PPHN is associated with impaired cortical angiogenesis.
Keywords: persistent pulmonary hypertension in the newborn (PPHN), cerebral angiogenesis, HIF-1α, VEGF, sheep
1. Introduction
Persistent pulmonary hypertension of the newborn (PPHN) predisposes infants to long-term neurodevelopmental impairment1,2. Up to 25% of infants that survive PPHN have neurodevelopmental impairments, including moderate-severe cerebral palsy and Bayley Mental and Psychomotor developmental indices below 70, despite advances in intensive care support1. The onset of PPHN occurs during a time of robust cerebral angiogenesis in the newborn and this process is highly sensitive to changes in blood flow and oxygen levels3,4. The clinical course of infants with PPHN suggests that their adaptation to systemic hypoxemia is impaired. It is unknown if the fetal brain undergoes appropriate vascular remodeling in the presence of PPHN and alterations in oxygen supply in utero. Our study investigated the adaptive mechanisms of cerebrovascular remodeling to PPHN in the fetal lamb brain.
PPHN occurs when the pulmonary vascular resistance fails to decrease at birth5. The affected infant fails to establish adequate oxygenation during postnatal life and may develop multi-organ dysfunction6. Hypoxemia in these infants is due to right to left extra-pulmonary shunting of blood as a result of supra-systemic pulmonary artery pressures. In utero pulmonary hypertension from ductal constriction leads to impaired development of pulmonary vasculature and decreased blood flow from right ventricle to the aortic arch, which may lead to altered cerebral blood flow. Previous studies suggested that altered autoregulation of cerebral blood flow in PPHN may cause hypoxic brain injury and contribute to cognitive deficits in this condition2. Studies that evaluated school-aged outcomes in PPHN survivors showed that these outcomes were not significantly improved, despite advances in neonatal intensive care, suggesting that prenatal factors may also predispose these infants to postnatal impairments7. Hypoxemia and decreased cerebral blood flow in utero during evolution of PPHN may confer vulnerability to neurodevelopmental impairment.
The cerebral cortex undergoes vascular remodeling in order to preserve oxygen and nutrient supply needed to support neuronal function and metabolic needs. Understanding the mechanisms that facilitate this adaptation may help understand fetal brain adaptation to impaired oxygen states. Our group has shown that fetal lambs exposed to in utero PPHN are relatively hypoxemic compared to controls at birth 8 and that maladaptation to changes in PaO2 in PPHN in the lung occurs 9,10. Sheep have been used extensively to investigate many aspects of CNS homeostasis11,12. The development of the ovine brain at birth is similar to that of the human infant with respect to completion of neurogenesis, cerebral sulcation, and detection of the cortical component of somatosensory evoked potentials13,14. For these reasons, the sheep model is ideal to test the hypothesis that cerebral angiogenesis is impaired in the lamb cerebral cortex, as it is in the lung9,10.
Microvascular remodeling occurs in the cerebral cortex in order to preserve tissue oxygen and energy supply for neuronal function in hypoxic states15. In the rodent model of chronic hypoxia, exposure to mild hypoxia results in systemic and central nervous system adaptations that allow acclimatization to changes in the microenvironment3,16-18. The first structural changes to chronic hypoxia are seen at 4-7 days and continue for up to 3 weeks3,18. The structural changes not only include increased capillary density, but also capillary length and diameter16,17. Angiogenesis is a highly complex and coordinated process requiring multiple angiogenic and regulatory factors, receptors, and intracellular signaling pathways15. There appears to be at least two major pathways and likely a number of other redundant pathways responsible for brain angiogenesis: hypoxia-inducible factor-1 (HIF-1) dependent upregulation of vascular endothelial growth factor (VEGF) and HIF-1 independent, cyclooxygenase-2 (COX-2) dependent process with upregulation of angiopoietin-2 (Ang-2)15. Brain vascular remodeling in response to changes to oxygen availability depends on the balance between these two angiogenic pathways. We hypothesized that dysregulation of these intersecting pathways contribute to impaired cerebral angiogenesis in PPHN.
2. Material and Methods
2.1 Creation of PPHN model
This study was approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee (IACUC) and conformed to the current guidelines of NIH Care and Use of Laboratory Animals. PPHN was induced by fetal ductus arteriosus constriction at 128 ± 2 days gestation (term gestation ≈ 145 days) in twin gestation sheep as we described previously. 8,19 Control fetal lambs received sham operation. After 8 days of ductal constriction, fetal lambs were delivered by Cesarean section, euthanized, weighed, gender determined, and the brain was quickly removed and weighed. One half of the brain was removed and frozen for protein analysis. The parietal lobe was sectioned, formalin-fixed, and paraffin-embedded for immunohistochemistry.
2.2 Immunohistochemistry and determination of capillary density
Brain samples from normotensive and hypertensive lambs from six separate experiments were fixed in 10% neutral-buffered formalin, processed, and embedded in paraffin. Coronal serial sections (5μm) were obtained and mounted on positively charged glass slides (Fisher). For isolectin B4, slides were hydrated with deionized water, antigen retrieval with citrate buffer (pH 6, Dako, Carpinteria, CA, s1699) at 90°C for 20 minutes was performed, and then slides were cooled in citrate buffer for 20 minutes. All slides were stained using a Dako Autostainer Plus using programmed steps for peroxidase blocking and incubation with biotinylated primary antibody (1:200, Vector, Burlingame, CA, b-1205) for 60 minutes in room temperature. Following incubation with antibody overnight at 4°C, slides were rinsed in TBST (Thermo Scientific, Fremont, CA, TA-999-TT), then Dako tertiary with Streptavidin HRP (1:300, Dako, P039701-2) and DAB application (Dako, K346811-2) was performed and the slides were counterstained with hematoxylin (Dako, s33093-2) followed by 0.1% Ammonium hydroxide solution. For Glut-1 and factor VIII a Leica Bond Max Immunostainer was used. Both antibodies required antigen retrieval using Leica HI antigen retrieval reagent (AR9961), with Glut-1 needing 10 minutes and Factor VIII, 20 minutes. Both antibodies were detected and visualized using Bond Polymer Refine Detection System (DS9800) with the addition of a DAB enhancer (AR9436), using the MOD F protocol/software from Leica. An optimal concentration of 1:900 was determined for Glut-1 (Thermo-Fisher, MS-10637) and 1:100 for Factor VIII (Biocare, CP039). All slides were counter-stained with hematoxylin and coverslipped using a synthetic mounting media. Omission of the primary antibody served as negative control. Quantification analysis was performed by a researcher blinded to the treatment groups using a computer-assisted method with Visiomorph™ (Visiopharm, Denmark).
2.3 Quantitative Analysis of Image Data
Microscope slides of the histological staining were scanned at 40×, 20×, 10×, and 5× magnification using a Nanozoomer HT system (Hamamatsu, Japan) in the Pediatric BioBank & Analytical Tissue Core at the Children’s Research Institute at the Medical College of Wisconsin. The resulting digital images were used for quantitative analysis using commercial image post-processing software (Microimager and Visiomorph, Visiopharm, Denmark). Brown, DAB-positive pixels, blue (Hematoxylin) pixels, and white (background) pixels were classified by the software user using linear Bayesian Classification. Counts and area of DAB- positive events (<5μm and >800μm were excluded) and area of Hematoxylin-positivity were determined and exported to an Excel worksheet, where the number of DAB-positive events (=micro vessels or capillaries) per square millimeter tissue was calculated as count DAB/sum of areas DAB and Hematoxylin.
2.4 Preparation of Whole Cell Lysate
Frozen brain cortex samples from normotensive and hypertensive lambs from six separate experiments were dissected and homogenized in ice-cold lysis buffer (modified RIPA) containing 1% protease inhibitor cocktail (Roche, Nutley, NJ) and centrifuged to remove cell debris. Total protein concentrations of the homogenates were determined with a biccinchoninic acid protein assay (BCA, Pierce, Rockford, IL).
2.5 Western Blot Detection and Quantification of Protein
An aliquot of protein (30μg) was fractionated by SDS-PAGE electrophoresis and transferred onto PVDF membranes (0.2 micron, Bio-Rad Laboratories, Hercules, CA). Membranes were incubated with primary antibodies: HIF-1α (mouse monoclonal, 1:250, Invitrogen, Camarillo, CA), VEGF (mouse monoclonal, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA), COX-2 (rabbit polyclonal, Cayman Chemical, Ann Arbor, MI), Ang-1(goat polyclonal, 1:500, Santa Cruz Biotechnology), Ang-2 (rabbit polyclonal, 1:500, abcam, Cambridge, MA), and Tie-2 (rabbit polyclonal, 1:200, Santa Cruz Biotechnology). Membranes were then blotted with HRP-conjugated secondary antibody (1:10,000) appropriate for each primary antibody. The membranes were exposed to Hyperfilm ECL (Phenix, Candler, NC) after treatment with SuperSignal West Pico (Pierce). Band intensity was analyzed with NIH ImageJ (US NIH, Bethesda, Maryland, USA, http://imagej.nih.gov). The final values represent an average of the densitometric values obtained from two to three different immunoblots. The densitometric values were presented as a ratio to the densitometric values of β-actin used as a housekeeping protein.
2.6 Quantification of mRNA Abundance
RNA was extracted from frozen tissue from normotensive and hypertensive lambs from six separate experiments using the Aurum Total RNA Fatty and Fibrous Tissue Kit (Bio-Rad). cDNA was synthesized from the extracted RNA using the iScript cDNA synthesis kit (Bio-Rad). The PCR primers were designed for sheep using Primer3 as previously described 20 and shown in Table 1. Real-time RT-PCR was performed using the iQ5 multicolor real-time PCR detection system (Bio-Rad). The PCR cycle was started at 95°C for 3 minutes followed by 40 cycles of 95°C for 10 s and then 58°C for 1 min. Melting temperatures were monitored for each pair of primers, and single-peak melting temperature was observed for all of the primer pairs. Relative mRNA level was calculated per equation previously described by Pfaffl 21for each gene of interest as 2-ΔΔCt with control condition as 1 and the fold increase from this value for PPHN condition shown as mean ± 1 standard deviation from six separate experiments.
Table 1.
The sequence for primers used in RT-PCR
| Primer Name | Sequence |
|---|---|
| HIF-lα Forward | CAGAAGAACTTTTGGGCCGC |
| HIF-lα Reverse | TACAATGCACTGGGGCTGAG |
| VEGF Forward | TGCTCTCTTGGGTGCATTGG |
| VEGF Reverse | GAAGCTGCGCTGGTAGACAT |
| COX-2 Forward | TGTATCCCGCCCTTCTGGTA |
| COX-2 Reverse | CCGGCTTCTACCATGGTCTC |
| Ang-1 Forward | CATGCTCGAGATAGGCACCA |
| Ang-1 Reverse | TCCAGCAGTTGTATTTCAAGTCG |
| Ang-2 Forward | AGAAGCAACTGGTGACTGCC |
| Ang-2 Reverse | GTCCTTGGCAAGCAAGGAGTA |
| B-actin Forward | CAAAGACCTCTACGCCAACAC |
| B-actin Reverse | GACTCGTCGTACTCCTGCTTG |
2.7 Statistical Analysis
Data were shown as mean ± 1 standard deviation. Normally distributed data between two groups was compared using a Student’s t-test. Data that did not pass the normality test was analyzed with a Mann-Whitney U test. Data were analyzed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com). In all cases, p<0.05 was considered statistically significant.
3. Results
3.1 Effect of PPHN on microvessel density in cerebral cortex
Cerebral capillary density was determined by immunostaining for Glut-1, isolectin B4, and factor VIII immunostaining and counting the number of positively stained capillaries per unit area (N/mm2). Quantitative analysis showed that the capillary density in the cerebral cortex was significantly decreased (p<0.05) in the PPHN fetal lambs compared to gestation matched controls by all three staining techniques. (Control vs PPHN, n=6, p<0.05, Figure 1).
Figure 1. Microvascular density in fetal lamb cerebral cortex in PPHN and gestation matched, twin controls.
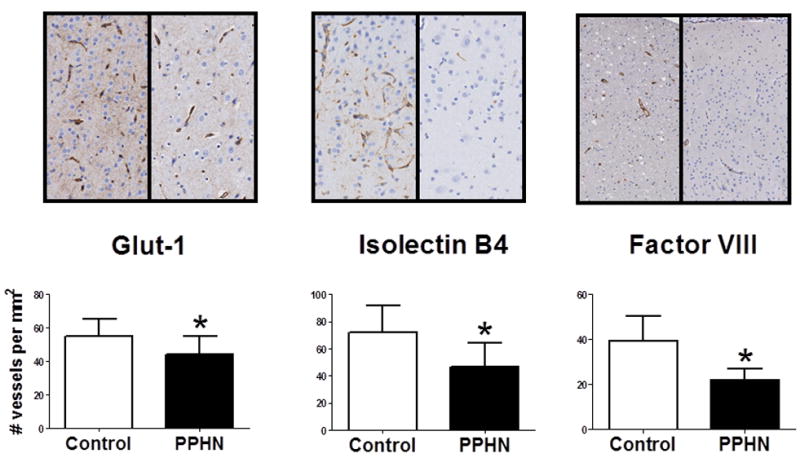
Images shown at 20× magnification for Glut-1 and isolectin B4 and 10× magnification for factor VII. Capillary density (number per square millimeters) of Glut-1, isolectin B4, and factor VIII-stained parietal lobe sections of control lambs compared to gestation matched PPHN lambs. *p<0.05 from control. Values are means ± SD; Open bars are control; Closed bars are PPHN, n=6.
3.2 Effect of PPHN on key angiogenic protein expression
HIF-1α (120 kDa) level was significantly lower in cortical cell lysates of PPHN lambs compared to twin matched controls (n=6, p<0.05, Figure 2A). VEGF (42 kDa) protein levels were also lower in cortical cell lysates of PPHN lambs compared to twin matched controls, consistent with lower HIF-1α levels in PPHN (n=6, p<0.05, Figure 2B). Ang-1 (75 kDa) and Tie-2 (125 kDa) protein levels in cortical cell lysates of PPHN samples were significantly increased compared to gestation-matched twin controls (n=6, p<0.05, Figure 2C and 2D). In contrast, Ang-2 (70 kDa) protein level was significantly lower in PPHN lambs (n=6, p<0.05, Figure 2E), while COX-2 (72 kDa) protein level was not different (n=6, p<0.05, Figure 2F).
Figure 2. Angiogenesis pathway protein levels in fetal lamb cerebral cortex in PPHN and gestation matched, twin controls.

A, HIF-1α protein optical density (OD) ratio to internal control, β-actin in frontal lobe cell lysate of control lambs compared to gestation matched PPHN lambs. Similarly, optical density ratios of angiogenesis protein to β-actin are shown for B, VEGF protein levels. C, Ang-1 protein levels. D, Tie-2 protein levels. E, Ang-2 protein levels, and F, Cox-2 protein level. *p<0.05 from control. Values are means ± SD; Open bars are control; Closed bars are PPHN, n=6.
3.3 Effect of PPHN on the mRNA levels of key angiogenic pathway genes
The mRNA levels for HIF-1α, VEGF, COX-2, Tie-2, Ang-1 or Ang-2 genes measured in the fetal cerebral cortex were not significantly different between control and PPHN lambs (n=6, figure 3)
Figure 3. Comparison of angiogenesis pathway mRNA levels from PPHN fetal lamb cerebral cortex and gestation matched, twin controls.

Relative mRNA level was calculated per equation for each gene of interest from frontal lobe as fold difference (2-ΔΔCt) with control condition as 1 and the fold increase/decrease from this value for PPHN shown as mean ± SD from six separate experiments. Each target mRNA was normalized to β-actin mRNA. Open bar indicate control condition and closed bars indicate PPHN condition.
4. Discussion
The novel findings of this study are that 1) in utero ductus arteriosus constriction is associated with decreased brain capillary density and 2) this finding is accompanied by decreases in key angiogenesis pathway proteins that mediate cerebrovascular responses to hypoxia in fetal lamb brains. The brain normally adapts to decreased oxygen supply by increasing the capillary density in an effort to maintain adequate cortical tissue oxygenation. Since PPHN is associated with episodes of postnatal hypoxia, impaired mechanisms of angiogenesis in these infants may render them more vulnerable to brain injury.
Cerebrovascular disturbances are the major causes of perinatal brain damage and contribute to the pathogenesis of cerebral palsy and developmental impairments22. An autopsy study of the frontal cortical development from fetal to adult age in humans showed that the density and diameter of vessels increase rapidly after 26 weeks gestation and peaks at 35 weeks 22. After 35 weeks of gestation, the density and diameter of vessels are the same as those seen in adults. Their observations suggest that angiogenesis is more rapid than cortical expansion but is equal to the growth of the white matter during third trimester. Therefore, neovascularization may be closely related to normal brain development and its impairment may also lead to impaired white matter development.26 This period of gestation coincides with increasing vulnerability to PPHN from ductus arteriosus constriction in the fetus as well.17, 24 In this context, our observations of decreased cortical capillary density in the PPHN exposed animals suggests that this period of angiogenesis is vulnerable to changes in fetal circulation and oxygenation.
At the time of prolific angiogenesis in the developing brain, endogenous HIF-1α regulated mechanisms are crucially involved in the survival and proliferation of neurons23,24. Several studies have shown that the immature brain is more resistant to hypoxic/ischemic insults than the mature brain, probably related to HIF-1α signaling4,25. Chronic postnatal hypoxia was associated with increased HIF-1α expression and increased oxidative metabolism and glucose transporter activity, suggesting a neuroadaptive role for HIF-126. There is also differential expression of HIF-1α and its target genes at different stages of brain development during exposure to hypoxia, which implies cellular susceptibility to hypoxic distress 4,25. Our study showed decreased expression of HIF-1α in the fetal brain exposed to PPHN; a result that supports the concept that HIF-1α is important for normal cerebral angiogenesis. HIF-1, a heterodimer of α and β subunits27, is targeted for proteosomal degradation by prolylhydroxylation of specific domains on the subunit28,29. This process is dependent on the von-Hippel-Lindau tumor suppressor 30 which serves as the recognition component of an ubiquitin ligase that promotes degradation of HIF-1α. PPHN may increase degradation of HIF-1α through this mechanism, thereby explaining the decreased levels of HIF-1α seen in our study. This possibility is supported by a lack of difference in the HIF-1α mRNA levels between control and PPHN samples. The lack of HIF-1 response in PPHN may result in impaired ability to match neuronal activity and capillary density, and explain in part the vulnerability for neurodevelopmental impairment. A recent study in which adult mice that received a HIF prolylhydroxylase inhibitor had both increased HIF-1α expression in the hippocampus and improved hippocampal memory in fear conditioning suggests that this could be a future target for pharmacologic manipulation31.
HIF-1α induction of VEGF gene expression often represents a critical rate-limiting step in angiogenesis15,32,33. HIF-1α accumulates in hypoxic states and binds to the hypoxia response element sequence, which upregulates several genes including VEGF34. VEGF functions as a neuronal survival factor and mediates both auto- and paracrine signaling in neurons in vitro 35. Astrocyte-derived VEGF mediates survival and tube stabilization of hypoxic brain microvascular endothelial cells in vitro36. VEGF has been shown to have neuroprotective effects in response to ischemia and hypoxia in postnatal rodent brain35,37. Immediate induction of VEGF in developing hypoxic brain contributes to hypoxia tolerance of neurons and astrocytes and the loss of VEGF induction may therefore, increase vulnerability to injury. We observed in this study that VEGF protein expression in the brain was decreased in PPHN, correlating with decreased capillary density in the cortical tissue. These observations may provide a mechanistic basis for increased vulnerability to neuronal injury in PPHN. VEGF mRNA levels remaining unchanged from control despite decreased protein expression may be explained by post-transcriptional gene regulation in this model of PPHN and requires further investigation38.
In the presence of VEGF, capillary destabilization occurs in hypoxic states as a result of Ang-2 binding to its receptor, Tie-2. Under normal oxygen states, Ang-1 is bound to the Tie-2 receptor, resulting in stabilization of the capillary structure and plays a crucial role in mediating reciprocal interactions between the endothelium and surrounding matrix and mesenchyme. Increased levels of Ang-2 can bind Tie-2 and decrease its availability for interaction with Ang-1 during reduced oxygen availability. This can lead to release of pericytes and destabilization of the capillary, a required step in sprouting angiogenesis39. COX-2 signaling is the main pathway for Ang-2 induction in hypoxia40. We showed that Ang-1 protein was increased in our model and Ang-2 protein level was decreased in PPHN, suggesting that the vessel remains in a stable state with halted capillary development in PPHN. Astrocytes appear to be a major source of VEGF and Ang production in the brain. Interestingly, dexamethasone treatment of astrocytic cells in vitro showed similar increased expression of Ang-1 and decreased expression of VEGF suggesting a novel role of astrocytes in blood-brain barrier (BBB) stabilization41. Our findings may reflect an adaptive mechanism for BBB stabilization in the fetal brain that subsequently could impair adaptive responses to postnatal hypoxia. These speculations require further investigation
Our data taken together shows significant brain vascular remodeling in response to in utero exposure to PPHN. This suggests that late gestation is a vulnerable time for vascular development in the brain. These findings are supported by other models of in utero oxidative stress such as intrauterine growth restriction where altered expression of key angiogenic factors occurs42. One limitation of our study is that we were unable to measure cerebral blood flow during our studies due to the fragile nature of the ductal ligation model. Further manipulation of the fetus before and after ductal ligation leads to compromised survival of the fetus in our experience. However, our findings of impaired cerebral angiogenesis and its association with our previous report of impaired angiogenesis in the lung in PPHN are novel9,10. These observations may suggest a mechanism for vulnerability to impaired neurodevelopmental outcomes observed in PPHN patients. We were also unable to find significant differences in mRNA levels to correlate transcriptional differences with the significant translational difference of key angiogenic proteins. This result is not so surprising since typically ~30 to even ~85% of the variation in protein levels in prokaryotic and eukaryotic organisms can be attributed to variation in mRNA expression. The other 15 to 70% of the variation is explained by post-transcriptional and post-translation regulation and by measurement errors43. In fact, the most significant measurements of protein vs. mRNA concentrations collected in tissue and cell culture may center on R2 = 0.443. That is, agreement between the two measurements occurs less than 40% of the time. For these reasons, our protein expression differences require further investigation of organism-specific regulation of translation and protein degradation to better understand the microvascular changes we see with this model.
5. Conclusions
In conclusion, we observed reduced cortical capillary density in fetal lambs exposed to PPHN induced by in utero ductal constriction. This study is among the first to examine blood vessel formation in the brain associated with PPHN. These findings in the brain are associated with decreased expression of proteins important for angiogenesis, including HIF-1α, VEGF, and Ang-2. Our results are consistent with findings seen in the fetal lung, and suggest that impaired angiogenesis extends beyond the lung in PPHN. Impaired angiogenesis and reduced HIF-1α and VEGF expression are typically associated with impaired white matter development and decreased neuroprotective mechanisms in the presence of hypoxia. Our observations suggest that evolution of PPHN is associated with inhibition of normal microvascular development in the brain by down-regulation of angiogenic proteins. The mechanism of decreased cerebral angiogenesis protein expression in PPHN requires further investigation.
Highlights.
PPHN predisposes infants to long-term neurologic impairments
PPHN is associated with impaired angiogenesis in the brain
Impaired cerebral angiogenesis may lead to maladaptation to hypoxic injury
Acknowledgments
This study was financially supported by NIH: T32 HL 7792-18 (SC), RO3 HD 073274 (RJT); RO1 HL 057268 and RO3 HD 059076 (GGK) and the MCW Department of Pediatrics. We would like to acknowledge the mentorship of David R Harder, PhD and the guidance of Robert H. Lane, MD MS in the preparation of manuscript. High-resolution microscope slide scanning and quantitative image analysis was performed in the Pediatric BioBank & Analytical Tissue Core at the Children’s Research Institute and the Medical College of Wisconsin, Milwaukee, WI.
Abbreviations
- CNS
Central nervous system
- PPHN
Persistent pulmonary hypertension in the newborn
- PDA
Patent ductus arteriosus
- Glut-1
Glucose transporter-1
- HIF-1
Hypoxic inducible factor-1
- VEGF
Vascular endothelial growth factor
- COX-2
Cyclooxygenase-2
- Ang-1
Angiopoetin-1
- Ang-2
Angiopoetin-2
- Tie-2
Tyrosine-protein kinase receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Konduri GG, Vohr B, Robertson C, et al. Early inhaled nitric oxide therapy for term and near-term newborn infants with hypoxic respiratory failure: neurodevelopmental follow-up. The Journal of pediatrics. 2007;150:235–40. 40 e1. doi: 10.1016/j.jpeds.2006.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berti A, Janes A, Furlan R, Macagno F. High prevalence of minor neurologic deficits in a long-term neurodevelopmental follow-up of children with severe persistent pulmonary hypertension of the newborn: a cohort study. Italian journal of pediatrics. 2010;36:45. doi: 10.1186/1824-7288-36-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dore-Duffy P, LaManna JC. Physiologic angiodynamics in the brain. Antioxidants & redox signaling. 2007;9:1363–71. doi: 10.1089/ars.2007.1713. [DOI] [PubMed] [Google Scholar]
- 4.Trollmann R, Gassmann M. The role of hypoxia-inducible transcription factors in the hypoxic neonatal brain. Brain & development. 2009;31:503–9. doi: 10.1016/j.braindev.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Konduri GG, Kim UO. Advances in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatric clinics of North America. 2009;56:579–600. doi: 10.1016/j.pcl.2009.04.004. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapointe A, Barrington KJ. Pulmonary Hypertension and the Asphyxiated Newborn. The Journal of pediatrics. 2011;158:e19–e24. doi: 10.1016/j.jpeds.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg AA, Lee NR, Vaver KN, et al. School-age outcomes of newborns treated for persistent pulmonary hypertension. Journal of perinatology : official journal of the California Perinatal Association. 2010;30:127–34. doi: 10.1038/jp.2009.139. [DOI] [PubMed] [Google Scholar]
- 8.Konduri GG, Ou J, Shi Y, Pritchard KA., Jr Decreased association of HSP90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. American journal of physiology Heart and circulatory physiology. 2003;285:H204–11. doi: 10.1152/ajpheart.00837.2002. [DOI] [PubMed] [Google Scholar]
- 9.Teng RJ, Du J, Xu H, et al. Sepiapterin improves angiogenesis of pulmonary artery endothelial cells with in utero pulmonary hypertension by recoupling endothelial nitric oxide synthase. American journal of physiology Lung cellular and molecular physiology. 2011;301:L334–45. doi: 10.1152/ajplung.00316.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teng RJ, Eis A, Bakhutashvili I, Arul N, Konduri GG. Increased superoxide production contributes to the impaired angiogenesis of fetal pulmonary arteries with in utero pulmonary hypertension. American journal of physiology Lung cellular and molecular physiology. 2009;297:L184–95. doi: 10.1152/ajplung.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Threlkeld SW, Cummings EE, et al. Ischemia–reperfusion impairs blood–brain barrier function and alters tight junction protein expression in the ovine fetus. Neuroscience. 2012;226:89–100. doi: 10.1016/j.neuroscience.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Heuij LG, Mathai S, Davidson JO, et al. Synergistic white matter protection with acute-on-chronic endotoxin and subsequent asphyxia in preterm fetal sheep. Journal of neuroinflammation. 2014;11:89. doi: 10.1186/1742-2094-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Back SA, Riddle A, Hohimer AR. Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury. Journal of child neurology. 2006;21:582–9. doi: 10.1177/08830738060210070101. [DOI] [PubMed] [Google Scholar]
- 14.Bernhard CG, Kolmodin GM, Meyerson BA. On the prenatal development of function and structure in the somesthetic cortex of the sheep. Progress in brain research. 1967;26:60–77. doi: 10.1016/S0079-6123(08)61419-3. [DOI] [PubMed] [Google Scholar]
- 15.Benderro GF, Lamanna JC. Hypoxia-induced angiogenesis is delayed in aging mouse brain. Brain research. 2011;1389:50–60. doi: 10.1016/j.brainres.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mironov V, Hritz MA, LaManna JC, Hudetz AG, Harik SI. Architectural alterations in rat cerebral microvessels after hypobaric hypoxia. Brain research. 1994;660:73–80. doi: 10.1016/0006-8993(94)90840-0. [DOI] [PubMed] [Google Scholar]
- 17.Stewart PA, Isaacs H, LaManna JC, Harik SI. Ultrastructural concomitants of hypoxia-induced angiogenesis. Acta neuropathologica. 1997;93:579–84. doi: 10.1007/s004010050654. [DOI] [PubMed] [Google Scholar]
- 18.Harik N, Harik SI, Kuo NT, Sakai K, Przybylski RJ, LaManna JC. Time-course and reversibility of the hypoxia-induced alterations in cerebral vascularity and cerebral capillary glucose transporter density. Brain research. 1996;737:335–8. doi: 10.1016/0006-8993(96)00965-1. [DOI] [PubMed] [Google Scholar]
- 19.Konduri GG, Bakhutashvili I, Eis A, Pritchard K., Jr Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. American journal of physiology Heart and circulatory physiology. 2007;292:H1812–20. doi: 10.1152/ajpheart.00425.2006. [DOI] [PubMed] [Google Scholar]
- 20.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 21.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mito T, Konomi H, Houdou S, Takashima S. Immunohistochemical study of the vasculature in the developing brain. Pediatric neurology. 1991;7:18–22. doi: 10.1016/0887-8994(91)90100-y. [DOI] [PubMed] [Google Scholar]
- 23.Milosevic J, Maisel M, Wegner F, et al. Lack of hypoxia-inducible factor-1 alpha impairs midbrain neural precursor cells involving vascular endothelial growth factor signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:412–21. doi: 10.1523/JNEUROSCI.2482-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan X, Heijnen CJ, van der Kooij MA, Groenendaal F, van Bel F. The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain research reviews. 2009;62:99–108. doi: 10.1016/j.brainresrev.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Trollmann R, Schneider J, Keller S, et al. HIF-1-regulated vasoactive systems are differentially involved in acute hypoxic stress responses of the developing brain of newborn mice and are not affected by levetiracetam. Brain research. 2008;1199:27–36. doi: 10.1016/j.brainres.2007.12.069. [DOI] [PubMed] [Google Scholar]
- 26.Lai JC, Tranfield EM, Walker DC, et al. Ultrastructural evidence of early endothelial damage in coronary arteries of rat cardiac allografts. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2003;22:993–1004. doi: 10.1016/s1053-2498(02)01163-4. [DOI] [PubMed] [Google Scholar]
- 27.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7987–92. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 31.Adamcio B, Sperling S, Hagemeyer N, Walkinshaw G, Ehrenreich H. Hypoxia inducible factor stabilization leads to lasting improvement of hippocampal memory in healthy mice. Behavioural brain research. 2010;208:80–4. doi: 10.1016/j.bbr.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocrine reviews. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 34.LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. The Journal of experimental biology. 2004;207:3163–9. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- 35.Ogunshola OO, Antic A, Donoghue MJ, et al. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. The Journal of biological chemistry. 2002;277:11410–5. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- 36.Chow J, Ogunshola O, Fan SY, Li Y, Ment LR, Madri JA. Astrocyte-derived VEGF mediates survival and tube stabilization of hypoxic brain microvascular endothelial cells in vitro. Brain research Developmental brain research. 2001;130:123–32. doi: 10.1016/s0165-3806(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Jin K, Xie L, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. The Journal of clinical investigation. 2003;111:1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang SH, Hla T. Post-transcriptional gene regulation by HuR and microRNAs in angiogenesis. Current opinion in hematology. 2014;21:235–40. doi: 10.1097/MOH.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 39.Pichiule P, Chavez JC, LaManna JC. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. The Journal of biological chemistry. 2004;279:12171–80. doi: 10.1074/jbc.M305146200. [DOI] [PubMed] [Google Scholar]
- 40.LaManna JC, Sun X, Ivy AD, Ward NL. Is cycloxygenase-2 (COX-2) a major component of the mechanism responsible for microvascular remodeling in the brain? Advances in experimental medicine and biology. 2006;578:297–303. doi: 10.1007/0-387-29540-2_47. [DOI] [PubMed] [Google Scholar]
- 41.Kim H, Lee JM, Park JS, et al. Dexamethasone coordinately regulates angiopoietin-1 and VEGF: a mechanism of glucocorticoid-induced stabilization of blood-brain barrier. Biochemical and biophysical research communications. 2008;372:243–8. doi: 10.1016/j.bbrc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 42.Venhoranta H, Bauersachs S, Taponen J, et al. Fetal growth restriction caused by MIMT1 deletion alters brain transcriptome in cattle. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2013;31:463–7. doi: 10.1016/j.ijdevneu.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 43.de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Molecular bioSystems. 2009;5:1512–26. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]


