Abstract
Coexisting patellofemoral osteoarthritis (PFOA) is a common finding in patients with tibiofemoral osteoarthritis (TFOA). The purpose of this study was to elucidate whether severity of coexisting PFOA in patients with TFOA is correlated with altered sagittal-plane gait biomechanics and greater knee-specific impairments. One hundred and six patients with radiographic TFOA were stratified into three groups of no PFOA, mild PFOA, and severe PFOA. All patients completed instrumented gait analysis, quantitative quadriceps strength testing and knee range of motion assessment. Compared to patients with no PFOA, those with severe PFOA exhibited reduced loading-response knee flexion excursions (p=0.002) and increased peak single-leg stance external knee flexion moments (p<0.05). The severe PFOA group also demonstrated lower quadriceps strength compared to the no PFOA and mild PFOA groups (p<0.001). Regression analysis further revealed that quadriceps strength and knee extension range of motion were independently associated with altered sagittal-plane knee biomechanics during gait (p<0.03). Reduced loading response knee flexion excursion during gait may be an attempt to decrease patellofemoral joint loading by patients with severe PFOA but it may increase impact loading of their arthritic tibiofemoral joint. Additionally, the greater external knee flexion moments observed during the single-leg stance phase of gait can lead to an overall increase in patellofemoral joint loading and symptoms in patients with more severe PFOA. Given the association between knee-specific impairments and altered gait biomechanics in our study, addressing quadriceps muscle weakness and limited knee extension range of motion may be indicated in patients with TFOA and severe coexisting PFOA.
Keywords: Knee, Osteoarthritis, Tibiofemoral Joint, Patellofemoral Joint, Gait, Biomechanics
1. INTRODUCTION
Osteoarthritis (OA) impacts approximately 27 million adults in the United States, with the knee as one of the most commonly affected joints with 50% lifetime risk of developing symptoms [1, 2]. Although the majority of the mechanistic, prognostic and intervention studies of knee OA have focused on the disease of the tibiofemoral (TF) joint, the patellofemoral (PF) joint appears to be the most prevalent site of pathology, with 40–69% of adults with complaints of chronic knee pain having isolated or combined radiographic evidence of PFOA [3, 4]. Additionally, PFOA has been found to be independently associated with quadriceps muscle weakness, limited knee range of motion, increased pain, as well as significant functional limitations and disability [5–7]. Despite its high prevalence and clinical implications, PFOA remains an understudied aspect of chronic knee pain and an area in need of continued research.
It has been recently speculated that chronic PF pain in younger patients may be a precursor to PFOA later in life [8, 9]. Chronic PF pain in middle-aged adults has also been associated with radiographic signs of PFOA [4]. Given the plausible mechanistic link between chronic PF pain and PFOA, it stands to reason that biomechanical examination of gait patterns in patients with PF pain may provide valuable information regarding potential deviations and compensations adopted by patients with PFOA. To this end, a recent gait study revealed no differences in frontal or transverse plane gait biomechanics between individuals with mild to moderate PFOA and an age-matched control group, despite previous evidence of such alterations in younger patients with PF pain [10]. The authors suggested that normal gait may not be demanding enough to cause frontal or transverse plane gait deviation in this patient population. However, whether patients with PFOA exhibit deviations in the sagittal-plane, where the gait demands are the greatest, was not examined and has yet to be determined.
It has been previously proposed that the PF discomfort associated with high sagittal-plane demands of the gait cycle during level and ramped walking can result in compensation strategies geared towards reducing PF joint loading and pain in young patients with PF pain [11–14]. As compressive forces of the PF joint are the vector summation of the quadriceps and patellar ligament forces, the high sagittal-plane external knee flexion moments during the loading response phase of gait, which increase the force production requirements of the quadriceps, have been linked with large PF joint compressive forces [15]. Therefore, it has been hypothesized that patients with PF pain often limit their loading response knee flexion excursion as an attempt to reduce pain by limiting the external knee flexion moments and compressive loading of the PF joint [11, 12]. Although such compensatory strategy is a logical approach to decrease PF joint compression and pain, it also reduces the normal active shock absorption of the knee and may lead to deleterious impulse loading and degenerative changes of the TF joint [13, 16].
It also stands to reason that potential sagittal-plane gait deviations in patients with coexisting PFOA and TFOA may exist due to the previously reported increases in severity of knee-specific impairments such as quadriceps muscle weakness and limited knee extension range of motion [6]. For example, reduced knee flexion excursions during the loading response phase of gait have been previously reported in patients with significant quadriceps weakness following anterior cruciate ligament reconstruction and total knee arthroplasty as a strategy to limit the demands placed on weak quadriceps [17, 18]. Similar reductions in knee flexion excursion could be expected in patients with coexisting PFOA and TFOA due to their previously reported quadriceps muscle weakness [6]. Limited loading response knee flexion excursion during gait may also be caused by greater knee flexion angles at heel contact due to reduced knee extension range of motion and the patients’ inability to fully extend their knees [19]. Limited knee extension range of motion can also lead to increased knee flexion angles and greater knee flexion moments during the single-leg stance phase of gait when the knee should be fully extended to reduce the need for quadriceps muscle activity. As a flexed knee joint during the single-leg stance phase of gait creates an external flexion moment about the knee and requires increased quadriceps force to provide lower limb stability, limitations of knee extension range of motion will likely lead to an overall increase in patellofemoral joint loading during this phase of gait.
The purpose of this study was to elucidate whether severity of coexisting PFOA in patients with TFOA is associated with altered sagittal-plane gait biomechanics and increased knee-specific impairments. It was hypothesized that compared to patients with no PFOA, those with increased severity of PFOA will demonstrate decreased loading response knee flexion excursions and increased single-leg stance peak external knee flexion moments. It was also hypothesized that the alterations in knee joint biomechanics during gait will be associated with quadriceps muscle weakness and limited knee extension range of motion.
2. METHODS
2.1. Subjects
Biomechanical data from a subsample of 106 participants recruited as part of a randomized clinical trial of exercise therapy for knee OA [20] were utilized in this study. Individuals were included in the current study if they met the 1986 American College of Rheumatology clinical criteria for knee OA [21] and had primarily medial compartment disease of grade II or greater on the Kellgren and Lawrence (KL) radiographic disease severity scale [22]. All data reported in this study were collected at baseline and prior to the participants receiving any intervention. All subjects provided written informed consent approved by the institutional review board.
2.2. Radiographic views and scoring
Radiographs of the painful knee in those with unilateral symptoms and the most painful knee in patients with bilateral involvement were analyzed. Three radiographic views of the index knee were obtained for each subject as follows: 1) a semiflexed, weight-bearing, anteroposterior view, 2) a lateral view, and 3) a skyline view. The KL radiographic OA grades for each knee compartment were obtained based on the following criteria: grade 0 = no osteophytes; grade I = doubtful osteophytes (1 mm); grade II = minimal osteophytes, possibly with joint space narrowing, cysts, and sclerosis; grade III = moderate or definite osteophytes and/or moderate joint space narrowing; and grade IV = large osteophytes and/or severe joint space narrowing. TFOA was defined by a KL score of grade ≥ II on the anteroposterior view. Subjects with TFOA were then stratified into three groups of no PFOA (KL grade ≤ I), mild PFOA (KL grade = II), or severe PFOA (KL grade ≥ III) based on the severity of their PF disease assessed on the skyline and/or the lateral radiographic views. To determine whether reliable radiographic data could be obtained, scoring was repeated for a subset of 15 knees on 2 different days at least 7 days apart. Unweighted kappa coefficients and exact percentage agreement were calculated. Intrarater reliability scores were excellent for the medial and lateral TF compartments (κ= 0.86, agreement=93.3%) and the PF compartment (κ=0.80, agreement=86.7%).
2.3. Quadriceps Strength & Range of Motion
The maximum voluntary isometric torque output for the quadriceps was measured using a Biodex System 3 dynamometer. All tests were performed with the subject seated and the knee flexed to 60 degrees. A minimum of three trials and a maximum of six trials were performed. After three trials, when a trial had a maximum torque output less than the previous trial, the strength testing was concluded. The highest maximum torque output from all trials was recorded as the quadriceps strength score and was normalized to the subject’s body weight. To verify the reliability of quadriceps strength measurements, testing was repeated for 40 subjects on two different days which yielded a reliability intraclass correlation coefficient (ICC2,3) of 0.97. Due to its relevance to gait biomechanics, knee extension range of motion was also measured in degrees using standard goniometric procedures. Excellent ICCs of between 0.84 and 0.93 have been reported for goniometric measurements of knee joint range of motion [23].
2.4. Self-reported symptoms and functional status
The 24-item Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) was used to gather knee-specific information on symptoms and functional limitations. The WOMAC is a valid and reliable disease-specific measure of pain, stiffness, and physical function in patients with knee OA [24, 25]. Higher scores on the WOMAC subscales indicate increased severity of symptoms or functional limitations.
2.5. Gait Analysis
Subjects walked along an 8.5m long vinyl-tiled walkway at a self-selected pace. An eight-camera Vicon® 612 motion measurement system (Vicon Peak-UK) was used to capture three-dimensional motion data at a sampling rate of 120 Hz using a Plug-In-Gait marker set. Two Bertec® force platforms (Bertec Corporation, OH, USA) were used to obtain ground reaction forces at a rate of 1080Hz, which were then synchronized with the motion data. Five gait trials were collected for each subject and averaged for analysis. Gait data was processed using a custom-written code (MatLab TM version7.0 The Mathworks, Inc, Natick, MA, USA). For each trial, loading response was defined as the time period between initial contact to the peak knee flexion angle during the mid-stance phase of gait and the single-leg stance as the time period starting with maximum knee flexion angle in mid-stance to the peak knee extension angle during the terminal stance phase of gait. Joint angle trajectories from the reflective markers and ground reaction forces were low-pass filtered (Butterworth fourth order, zero phase lag) at 6 and 40 Hz, respectively. The joint trajectory data combined with the ground reaction forces were used to calculate the peak external net knee joint moments using inverse dynamics equations and were normalized by body mass.
2.6. Statistical Analysis
Analysis of variance (ANOVA) and chi-square tests were used to determine group differences in demographics and radiographic knee OA severity. Comparisons of the group differences in WOMAC scores, knee-specific impairments, and gait characteristics were performed using Analysis of Covariance (ANCOVA), adjusting for gender, body mass index (BMI) and radiographic TFOA disease severity. ANCOVA analyses of the knee joint kinematics and kinetic variables were also adjusted for the effects of gait velocity. When differences were detected, post-hoc t-tests with Tukey corrections were performed to compare group differences. Additionally, multiple regression analyses were performed to evaluate the strength of associations between knee-specific impairments and gait biomechanics for the entire cohort after adjusting for age, gender, BMI, gait speed, and radiographic TFOA and PFOA disease severity.
3. RESULTS
Patients with severe PFOA had significantly greater body weight (p=0.04) and BMI (p=0.01) compared to the no PFOA group (Table 1). The severe PFOA group also had the highest proportion of patients with grade IV TFOA, while the mild PFOA group had the highest proportion of patients with grade III TFOA, and the no PFOA group had the highest proportion of patients with grade II TFOA (Table 1; p=0.03). Additionally, severe PFOA was associated with lower quadriceps strength compared to no PFOA and mild PFOA groups (p<0.001; Table 2).
Table 1.
Mean subject demographics and frequency of tibiofemoral compartmental radiographic score.
| Demographics | No PFOA (N = 24) | Mild PFOA (N = 38) | Severe PFOA (N = 44) |
|---|---|---|---|
| Age (years) | 61.4 (7.1) | 64.3 (10.2) | 65.3 (8.6) |
| Female (%) | 15 (62.5) | 20 (52.6) | 34 (77.3) |
| Height (cm) | 169.0 (8.7) | 170.0 (9.7) | 167.7 (7.5) |
| Weight (kg) | 78.6 (13.0) | 89.1 (21.3) | 90.3 (18.2)* |
| BMI (kg/m2) | 27.5 (4.3) | 30.9 (7.2) | 32.1 (6.5)* |
| Tibiofemoral Joint Radiographic Severity1 | |||
| Grade II (%) | 8 (33.3) | 5 (13.2) | 4 (9.1) |
| Grade III (%) | 13 (54.2) | 23 (60.5) | 21 (47.7) |
| Grade IV (%) | 3 (12.5) | 10 (26.3) | 19 (43.2) |
PFOA = Patellofemoral osteoarthritis; BMI = body mass index; Values are mean (SD) or N (%).
All patients had to have at least a grade II tibiofemoral OA to be included in the study.
Significantly different than “No PFOA” group
Table 2.
Mean Western Ontario and McMaster Universities (WOMAC) OA Index scores, knee-specific impairments, and gait characteristics.
| No PFOA (N = 24) | Mild PFOA (N = 38) | Severe PFOA (N = 44) | |
|---|---|---|---|
| WOMAC Total Score | 26.5 (15.0) | 30.3 (15.2) | 32.5 (18.4) |
| WOMAC Physical Function Score | 17.8 (11.3) | 21.7 (11.5) | 22.3 (13.5) |
| WOMAC Pain Score | 5.6 (2.9) | 5.6 (3.2) | 6.8 (4.2) |
| WOMAC Stiffness Score | 3.1 (1.7) | 3.1 (1.4) | 3.4 (1.8) |
| Quadriceps Strength (Nm/kg) | 1.9 (0.4) | 1.7 (0.5) | 1.4 (0.4)*† |
| Knee Extension Range of Motion (degrees) | −2.1 (5.0) | −5.4 (6.1) | −5.9 (5.8) |
| Gait Velocity (m/s) | 1.3 (0.1) | 1.3 (0.2) | 1.2 (0.2) |
| Step Length (m) | 0.70 (0.08) | 0.68 (0.08) | 0.65 (0.08) |
PFOA = Patellofemoral Osteoarthritis; Values are mean (SD).
All analyses were adjusted for gender, BMI and radiographic tibiofemoral joint disease severity.
Significantly different than “No PFOA” group
Significantly different than “Mild PFOA” group
Peak loading response knee flexion angle was significantly reduced for the severe PFOA group compared to the mild PFOA group (p=0.045; Table 3; Figure 1). Knee flexion excursion during the loading response phase of gait was also significantly reduced for the severe PFOA group compared to the no PFOA group (p=0.002; Table 3; Figure 1). Additionally, a significant difference in the peak external knee moment was observed during the single-leg stance phase of gait, with the severe PFOA group exhibiting a peak flexion moment, as opposed to the no PFOA group who exhibited an external knee extension moment (p= 0.046; Table 3; Figure 1).
Table 3.
Gait characteristics for groups during walking.
| No PFOA (N = 24) | Mild PFOA (N = 38) | Severe PFOA (N = 44) | |
|---|---|---|---|
| Knee flexion angle at heel contact (degrees) | 3.5 (5.8) | 6.1 (6.5) | 5.0 (6.6) |
| Peak loading response knee flexion angle (degrees) | 17.5 (6.0) | 18.3 (6.9) | 14.9 (5.6)† |
| Loading response knee flexion excursion (degrees) | 14.0 (4.7) | 12.2 (5.3) | 10.0 (4.8)* |
| Peak single leg stance knee flexion (+)/extension (−) angle (degrees) | 4.0 (5.9) | 7.4 (8.0) | 5.8 (5.7) |
| Peak loading response external knee flexion (+)/extension (−) moment (Nm/kg) | 0.6 (0.4) | 0.6 (0.4) | 0.6 (0.4) |
| Peak single leg stance external knee flexion (+)/extension (−) moment (Nm/kg) | −0.06 (0.4) | 0.08 (0.3) | 0.1 (0.3)* |
PFOA = Patellofemoral Osteoarthritis; Values are mean (SD).
All analyses were adjusted for gender, BMI and radiographic tibiofemoral joint disease severity.
Significantly different than “No PFOA” group
Significantly different than “Mild PFOA” group
FIGURE 1.
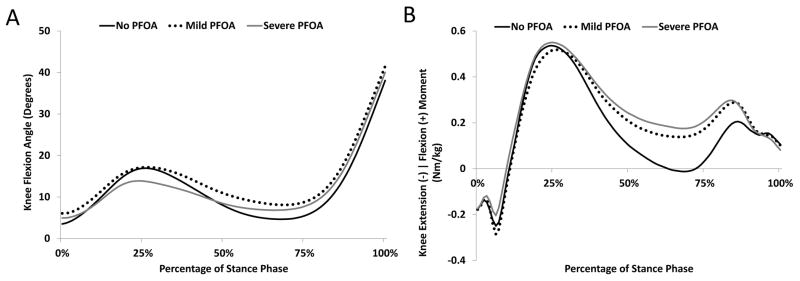
Ensemble average knee flexion angle (A) and external knee moment (B) profiles of patients with no patellofemoral osteoarthritis (PFOA), mild PFOA, and severe PFOA during the stance phase of gait.
Regression analyses further revealed that for every 1 Nm/kg increase in quadriceps strength there was a 2.89° increase in loading response knee flexion excursion, a 0.32 Nm/kg increase in peak loading response external knee flexion moment, and a 0.18 Nm/kg increase in the peak single-leg stance external knee flexion moment after adjusting for age, gender, BMI, gait speed, TFOA and PFOA severity (Table 4). Additionally, for every one degree reduction in knee extension range of motion there was a 0.44° increase in knee flexion angle at heel contact, a 0.34° increase in peak loading response knee flexion angle, a 0.66° increase in peak single-leg stance knee flexion angle, and a 0.13 Nm/kg increase in peak single-leg stance external knee flexion moment, after adjusting for age, gender, BMI, gait speed, TFOA and PFOA severity (Table 4).
Table 4.
Strength of association between quadriceps strength and knee extension range of motion with sagittal plane knee joint biomechanics during gait in patients with knee osteoarthritis.
| Quadriceps Strength
|
Knee Extension Range of Motion
|
|||
|---|---|---|---|---|
| β Coefficient | p-value | β Coefficient | p-value | |
| Knee flexion angle at heel contact (degrees) | −0.58 | 0.75 | −0.44 | <0.001* |
| Peak loading response knee flexion angle (degrees) | 2.32 | 0.19 | −0.34 | 0.002* |
| Loading response knee flexion excursion (degrees) | 2.89 | 0.03* | 0.10 | 0.24 |
| Peak single leg stance knee flexion (+)/extension (−) angle (degrees) | −1.35 | 0.47 | −0.66 | <0.001* |
| Peak loading response external knee flexion (+)/extension (−) moment (Nm/kg) | 0.32 | 0.002* | −0.002 | 0.70 |
| Peak single leg stance external knee flexion (+)/extension (−) moment (Nm/kg) | 0.18 | 0.03* | −0.13 | 0.01* |
All multiple regression analyses were adjusted for age, gender, BMI, radiographic tibiofemoral and patellofemoral joint disease severity and gait speed.
Denotes significant association (P < 0.05) between variables within the multiple regression model.
4. DISCUSSION
The hypothesis that patients with TFOA and more severe coexisting PFOA exhibit reduced loading response knee flexion angles compared to those with less severe PFOA was supported by the data and is consistent with previous findings in younger patients with PF pain [11, 12]. Given that greater loading response knee flexion angles have been associated with higher PF joint compressive forces [15], the observed reduction in knee flexion angle may be a compensatory strategy to limit the deleterious effects of increased PF joint loading and pain during gait in patients with more severe PFOA. This notion is supported by previous reports of increased loading response knee flexion angles during gait in younger patients with PF pain immediately after pain alleviating strategies such as patellar taping [26] or bracing [27].
It is also plausible that reduced loading response knee flexion angles in patients with more severe PFOA may be partially due to knee-specific impairments of quadriceps weakness and limited knee extension range of motion [19]. To this end, the results of our regression analysis revealed that quadriceps weakness was independently associated with reduced loading response knee flexion excursion. Additionally, reduced knee flexion angle at heel contact, which can lead to reduced knee flexion excursion during loading response, was also associated with limited knee extension range of motion. Therefore, it stands to reason that strategies to improve quadriceps strength and knee extension range of motion may be beneficial in mitigating the observed loading response gait deviations in knee OA patients with more severe PFOA.
Despite having similar peak external knee flexion moments during the loading response phase of gait, patients with more severe PFOA exhibited greater external knee flexion moments during the single-leg stance phase of gait compared to patients with no PFOA. During the single-leg stance phase of normal gait, as the center of mass moves forward and the ground reaction force passes anterior to the knee center of rotation, the knee extends passively which diminishes the need for quadriceps activity and minimizes PF joint loading. Whereas this was the case for our patients with no PFOA, patients with more severe PFOA walked with much higher levels of external knee flexion moment throughout the single-leg stance phase of gait. Increased external knee flexion moments, in turn, require greater quadriceps force production which elevates the PF joint compressive forces. Considering that the PF joint contact area is reduced when the knee is near full extension during the single-leg stance phase of gait, the greater external knee flexion moments and the resulting PF joint compressive forces during this phase of gait will most likely lead to significantly higher PF joint compressive stresses in patients with more severe PFOA.
The results of our regression analysis further revealed that higher peak external knee flexion moments during the single-leg stance phase of gait may be partially due to limited knee extension range of motion. The inability of the patients to fully extend their knees leads to the need for increased quadriceps force production for stabilizing the knee joint during weightbearing, which in turn, increases the PF joint reaction forces and stress. Therefore, strategies to improve knee extension range of motion may be indicated for reducing the external knee flexion moments during the single-leg stance phase of gait in knee OA patients with more severe PFOA.
An important implication of the findings of this study is that the specific gait deviations observed in knee OA patients with more severe PFOA may increase the risk of disease progression for both PF and TF joints. For instance, reduced loading response knee flexion excursion can limit the contact areas over which the large sagittal-plane loads of the gait cycle could be distributed for both TF and PF joints, potentially leading to more focal areas of contact stress and increased cartilage damage. To this end, reduced loading response knee flexion angles have been previously associated with lower patellar cartilage volume in healthy women [28]. Reduced loading response knee flexion angles in both healthy subjects and patients with TFOA have also been linked with a more rapid increase in the ground reaction forces and greater rates of lower limb loading, which make the TF joint especially susceptible to disease progression through increased axial compression and impulse loading [16, 29, 30]. Additionally, the increased single-leg stance peak external knee flexion moment can also increase compressive stresses of PF [15] and TF [31] joints, which can increase the risk of disease progression.
The findings of our study should be interpreted with a number of limitations in mind. The cross-sectional nature of this study precludes predicting if progression of PF joint disease is associated with alteration in gait biomechanics or changes in knee-specific impairments. Additionally, whether severity of PFOA is associated with altered frontal or transverse plane knee biomechanics was not examined in our study and should be evaluated in future research. As disorders of the patellofemoral joint in younger patients have been associated with impairments of the hip, foot and ankle joints, the influence of other lower extremity joints on alterations in gait biomechanics in patients with coexisting PFOA and TFOA should also be evaluated in future research.
In summary, patients with TFOA and more severe PFOA exhibited reduced loading response knee flexion angles and greater single-leg stance external knee flexion moments during gait compared to their counterparts with less severe PFOA. Additionally, the observed alteration in sagittal-plane gait biomechanics appeared to be partially related to knee-specific impairments of quadriceps weakness and limited knee extension range of motion. Future research is warranted to elucidate whether mitigation of knee-specific impairments will lead to improved gait biomechanics in patients with TFOA and co-existing PFOA.
Highlights.
Gait analysis was completed in 106 patients with knee osteoarthritis (OA).
Severe patellofemoral (PF) OA was associated with reduced knee shock absorption.
Severity of PFOA was also associated with higher single-leg stance knee joint loads.
Altered gait was related to quadriceps weakness and limited knee extension motion.
Acknowledgments
The project described was supported by the National Institutes of Health through Grant Numbers 1-R01-AR048760 and K12 HD055931 and the Pittsburgh Claude D. Pepper Older Americans Independence Center through Grant number P30 AG024827.
ROLE OF THE FUNDING SOURCE
The funding sources had no role in the study design, data collection, analysis or writing of this manuscript.
Footnotes
CONFLICT OF INTEREST STATEMENT
No authors had any financial or personal relationships with other people or organizations that could have influenced this study.
Conflict of interest
None of the authors of this manuscript “Altered Gait Biomechanics and Increased Knee-Specific Impairments in Patients with Coexisting Tibiofemoral and Patellofemoral Osteoarthritis” have any conflicts of interest. In addition, the study sponsors had no role in the study design, data collection, analysis or writing of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan RC, Hay EM, Saklatvala J, Croft PR. Prevalence of radiographic osteoarthritis--it all depends on your point of view. Rheumatology (Oxford) 2006;45:757–60. doi: 10.1093/rheumatology/kei270. [DOI] [PubMed] [Google Scholar]
- 4.Hinman RS, Lentzos J, Vicenzino B, Crossley KM. Patellofemoral osteoarthritis is common in middle-aged people with chronic patellofemoral pain. Arthritis Care Res (Hoboken) 2013 doi: 10.1002/acr.22274. [DOI] [PubMed] [Google Scholar]
- 5.Duncan R, Peat G, Thomas E, Wood L, Hay E, Croft P. Does isolated patellofemoral osteoarthritis matter? Osteoarthritis Cartilage. 2009;17:1151–5. doi: 10.1016/j.joca.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Farrokhi S, Piva SR, Gil AB, Oddis CV, Brooks MM, Fitzgerald GK. Association of severity of coexisting patellofemoral disease with increased impairments and functional limitations in patients with knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65:544–51. doi: 10.1002/acr.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51:844–9. doi: 10.1136/ard.51.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crossley KM. Is patellofemoral osteoarthritis a common sequela of patellofemoral pain? Br J Sports Med. 2014;48:409–10. doi: 10.1136/bjsports-2014-093445. [DOI] [PubMed] [Google Scholar]
- 9.Utting MR, Davies G, Newman JH. Is anterior knee pain a predisposing factor to patellofemoral osteoarthritis? Knee. 2005;12:362–5. doi: 10.1016/j.knee.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Pohl MB, Patel C, Wiley JP, Ferber R. Gait biomechanics and hip muscular strength in patients with patellofemoral osteoarthritis. Gait Posture. 2013;37:440–4. doi: 10.1016/j.gaitpost.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Dillon PZ, Updyke WF, Allen WC. Gait analysis with reference to chondromalacia patellae. J Orthop Sports Phys Ther. 1983;5:127–31. doi: 10.2519/jospt.1983.5.3.127. [DOI] [PubMed] [Google Scholar]
- 12.Nadeau S, Gravel D, Arsenault AB, Bourbonnais D, Goyette M. Dynamometric assessment of the plantarflexors in hemiparetic subjects: relations between muscular, gait and clinical parameters. Scand J Rehabil Med. 1997;29:137–46. [PubMed] [Google Scholar]
- 13.Powers CM, Heino JG, Rao S, Perry J. The influence of patellofemoral pain on lower limb loading during gait. Clin Biomech (Bristol, Avon) 1999;14:722–8. doi: 10.1016/s0268-0033(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 14.Kuster M, Sakurai S, Wood GA. Kinematic and kinetic comparison of downhill and level walking. Clin Biomech (Bristol, Avon) 1995;10:79–84. doi: 10.1016/0268-0033(95)92043-l. [DOI] [PubMed] [Google Scholar]
- 15.Ho KY, Blanchette MG, Powers CM. The influence of heel height on patellofemoral joint kinetics during walking. Gait Posture. 2012;36:271–5. doi: 10.1016/j.gaitpost.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Radin EL, Yang KH, Riegger C, Kish VL, O’Connor JJ. Relationship between lower limb dynamics and knee joint pain. J Orthop Res. 1991;9:398–405. doi: 10.1002/jor.1100090312. [DOI] [PubMed] [Google Scholar]
- 17.Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2002;17:56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 18.Mizner RL, Snyder-Mackler L. Altered loading during walking and sit-to-stand is affected by quadriceps weakness after total knee arthroplasty. J Orthop Res. 2005;23:1083–90. doi: 10.1016/j.orthres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Childs JD, Sparto PJ, Fitzgerald GK, Bizzini M, Irrgang JJ. Alterations in lower extremity movement and muscle activation patterns in individuals with knee osteoarthritis. Clin Biomech (Bristol, Avon) 2004;19:44–9. doi: 10.1016/j.clinbiomech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald GK, Piva SR, Gil AB, Wisniewski SR, Oddis CV, Irrgang JJ. Agility and perturbation training techniques in exercise therapy for reducing pain and improving function in people with knee osteoarthritis: a randomized clinical trial. Phys Ther. 2011;91:452–69. doi: 10.2522/ptj.20100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 22.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gnat R, Kuszewski M, Koczar R, Dziewonska A. Reliability of the passive knee flexion and extension tests in healthy subjects. J Manipulative Physiol Ther. 2010;33:659–65. doi: 10.1016/j.jmpt.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of rheumatology. 1988;15:1833–40. [PubMed] [Google Scholar]
- 25.Stucki G, Sangha O, Stucki S, Michel BA, Tyndall A, Dick W, et al. Comparison of the WOMAC (Western Ontario and McMaster Universities) osteoarthritis index and a self-report format of the self-administered Lequesne-Algofunctional index in patients with knee and hip osteoarthritis. Osteoarthritis Cartilage. 1998;6:79–86. doi: 10.1053/joca.1997.0097. [DOI] [PubMed] [Google Scholar]
- 26.Powers CM, Landel R, Sosnick T, Kirby J, Mengel K, Cheney A, et al. The effects of patellar taping on stride characteristics and joint motion in subjects with patellofemoral pain. J Orthop Sports Phys Ther. 1997;26:286–91. doi: 10.2519/jospt.1997.26.6.286. [DOI] [PubMed] [Google Scholar]
- 27.Powers CM, Doubleday KL, Escudero C. Influence of patellofemoral bracing on pain, knee extensor torque, and gait function in females with patellofemoral pain. Physiother Theory Pract. 2008;24:143–50. doi: 10.1080/09593980701665793. [DOI] [PubMed] [Google Scholar]
- 28.Teichtahl AJ, Jackson BD, Morris ME, Wluka AE, Baker R, Davis SR, et al. Sagittal plane movement at the tibiofemoral joint influences patellofemoral joint structure in healthy adult women. Osteoarthritis Cartilage. 2006;14:331–6. doi: 10.1016/j.joca.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Mundermann A, Dyrby CO, Andriacchi TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum. 2005;52:2835–44. doi: 10.1002/art.21262. [DOI] [PubMed] [Google Scholar]
- 30.Cook TM, Farrell KP, Carey IA, Gibbs JM, Wiger GE. Effects of restricted knee flexion and walking speed on the vertical ground reaction force during gait. J Orthop Sports Phys Ther. 1997;25:236–44. doi: 10.2519/jospt.1997.25.4.236. [DOI] [PubMed] [Google Scholar]
- 31.Walter JP, D’Lima DD, Colwell CW, Jr, Fregly BJ. Decreased knee adduction moment does not guarantee decreased medial contact force during gait. J Orthop Res. 2010;28:1348–54. doi: 10.1002/jor.21142. [DOI] [PMC free article] [PubMed] [Google Scholar]


