Abstract
β2-adrenergic agonists have been shown to regulate Na,K-ATPase in the alveolar epithelium by recruiting Na,K-ATPase-containing vesicles to the plasma membrane of alveolar epithelial cells (AEC). Here, we provide evidence that β2-agonists induce store-operated calcium entry (SOCE) in AECs. This calcium entry is necessary for β2-agonist-induced recruitment of Na,K-ATPase to the plasma membrane of AECs. Specifically, we show that β2-agonists induce SOCE via stromal interaction molecule 1 (STIM1)-associated calcium release-activated calcium (CRAC) channels. We also demonstrate that the magnitude of SOCE affects the abundance of Na,K-ATPase at the plasma membrane of AECs.
Keywords: Calcium channels; calcium signaling; store-operated calcium entry; epithelial cell; Na,KATPase
INTRODUCTION
In patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), there is fluid accumulation in the alveoli and impaired gas exchange, in part due to the decreased ability of the lungs to clear edema (1, 2). It is well known that alveolar fluid reabsorption occurs mainly by active transport of sodium ions out of the alveolar spaces with water following the osmotic gradient (3). Sodium transport across the epithelium is regulated in part by the basolateral Na,K-ATPase in addition to apical sodium channels and possibly chloride channels (4, 5). Previous studies in animal models of ARDS demonstrated enhanced alveolar fluid clearance (AFC) following treatment with β-agonists (6–9). The increase in AFC is mediated through the β2-receptor and is due in large part to recruitment of the Na,K-ATPase to the plasma membrane and consequent increased Na,K-ATPase activity (7, 10–13).
Calcium is a second messenger that is important in regulating vesicle fusion and exocytosis in many cell types (14), and β2-agonists have been shown to increase intracellular calcium levels in cardiac myocytes (15, 16). One of the primary modes of calcium entry in non-excitable cells is store-operated calcium entry (SOCE). SOCE consists of two phases: release of Ca2+ from intracellular stores (mainly the endoplasmic reticulum), which then leads to a second phase of sustained Ca2+ entry across the plasma membrane through store-operated channels (17, 18). The most common and well described mechanism of SOCE occurs via calcium release-activated calcium (CRAC) channels (19). CRAC channels have two key components which are the calcium-sensing ER transmembrane protein STIM1 and the plasma membrane pore forming Orai proteins (20).
In this work, we found that β2-agonists elicit SOCE via STIM1-associated CRAC channels in alveolar epithelial cells (AEC). In addition, we show that β2-agonist induced calcium entry is necessary for the β2-agonist-induced recruitment of Na,K-ATPase to the plasma membrane of AECs. Finally, we demonstrate that the magnitude of calcium entry in AECs impacts the abundance of Na,K-ATPase at the plasma membrane.
MATERIALS AND METHODS
Reagents
All cell culture reagents were from Corning Life Sciences. Albuterol sulfate (0.083%) vials from Nephron Pharmaceuticals Corporation were purchased through the Northwestern Memorial Hospital pharmacy store. 1,2-Bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid-acetoxymethyl ester (BAPTA-AM), thapsigargin (TG), and lanthanum (III) chloride were from Sigma-Aldrich. Forskolin was obtained from Ascent Scientific. ICI-118,551 and SQ-22536 were from EMD-Millipore. Rat STIM1 small interfering RNA (siRNA) modified with 3’-AlexaFluor546 was purchased from Qiagen. Non-silencing siRNA and Lipofectamine RNAiMAX were from Life Technologies. EZ-Link N-hydroxysuccinimide-SS-biotin and streptavidin-agarose beads were purchased from Thermo Scientific Pierce Protein Biology. All other chemicals were from Sigma-Aldrich and were the highest grade available.
Cell Lines and Culture
Alveolar type II (ATII) cells were isolated from the lungs of pathogen-free adult male Sprague-Dawley rats (200 – 225 g), as described previously (21). Cells were used on days 2 and 3 after the isolation. Rat lung epithelial (RLE-6TN) cells (ATCC CRL-2300) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin, and 20 µM HEPES.
Transfections
RLE cells were transfected with rat STIM1 siRNA duplexes (100 pmol) by using Lipofectamine RNAiMAX according to the manufacturer’s recommended protocol, and experiments were performed 48–72 h later. A nonsilencing siRNA was used as a control.
Measurement of intracellular calcium
ATII or RLE cells plated on 40-mm coverslips were loaded with fura-2-acetoxymethyl ester (Fura2-AM) (Life Technologies) for 30 min at room temperature in standard buffer solution (150 mM NaCl, 5 mM KCl, 1mM MgCl2, 10 mM glucose, 25 mM sodium bicarbonate, and either 2.5 mM CaCl2 or 0.25 mM EGTA pH 7.4) in the dark, washed with PBS, and further incubated for 30 min at room temperature to complete deesterification of the dye. Fura2 dye was excited through 340-nm and 380-nm interference filters housed in a computer-controlled wheel. The fluorescence emitted was collected at 510 nm. The data acquisition of Fura2 video imaging was obtained using a Nikon TE2000 (Nikon Instruments Inc.) equipped with an environmental control system chamber (FCS2 system; Bioptechs Inc.) and a Plan Super Fluor 40X oil objective (Nikon Instruments Inc.). Images were collected with a Cascade electron-multiplying charge-coupled device (EMCCD) camera TC285 with on-chip multiplication gain (Photometrics) drien by MetaFluor software (Molecular Devices Corp.). Changes in calcium concentration were obtained from the F340/F380 ratio and expressed as nM concentrations. To convert Fura2 fluorescence measurements, a calcium imaging calibration kit (Life Technologies) was used to generate a titration standard curve. Drugs were perfused to the cells using a pumping system with tubes equipped with stopcocks.
Biotinylation of cell surface proteins
Cells were labeled for 20 min at 4°C using 1 mg/ml EZ-Link N-hydroxysuccinimide-SS-biotin and lysed in cell lysis buffer from Cell Signaling as previously described (22, 23). Surface proteins were pulled down with streptavidin-agarose beads and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
Cell lysis and Western blot analysis
After treatment, ATII and RLE cells were washed in ice-cold phosphate-buffered saline (PBS) and solubilized in cell lysis buffer (Cell Signaling). The lysates were cleared by centrifugation for 10 min at 14,000 × g. Protein concentrations were determined by the Bradford assay using a commercial dye reagent (Bio-Rad), and samples containing equal amounts of protein were separated by SDS-PAGE and transferred onto nitrocellulose membranes (GE Healthcare Life Sciences) by using a Trans-Blot Turbo (Bio-Rad) transfer system. The following commercially available antibodies and dilutions were used for Western blotting: mouse anti-Na,K-ATPase subunit α1 (clone 464.6; 1:10,000) was from EMD Millipore; rat anti-STIM1 (1:1000) was from Cell Signaling Technology. Primary antibodies were detected with horseradish peroxidase-conjugated secondary goat anti-mouse antibodies (1:10,000; Bio-Rad) or goat anti-rabbit antibodies (1:2,000; Cell Signaling Technology) by using a chemiluminescence detection kit (Perkin-Elmer Life Sciences). Quantification of protein levels was performed by densitometric scanning with ImageJ 1.29X (NIH).
Statistics
Data are presented as means ± standard errors of the means (SEM) and were statistically analyzed using unpaired t-test or one-way analysis of variance (ANOVA) followed by a multiple comparison (Dunnett) test. P values of less than 0.05 were considered statistically significant.
RESULTS
β2-adrenergic stimulation elicits a rapid increase in intracellular calcium in alveolar epithelial cells
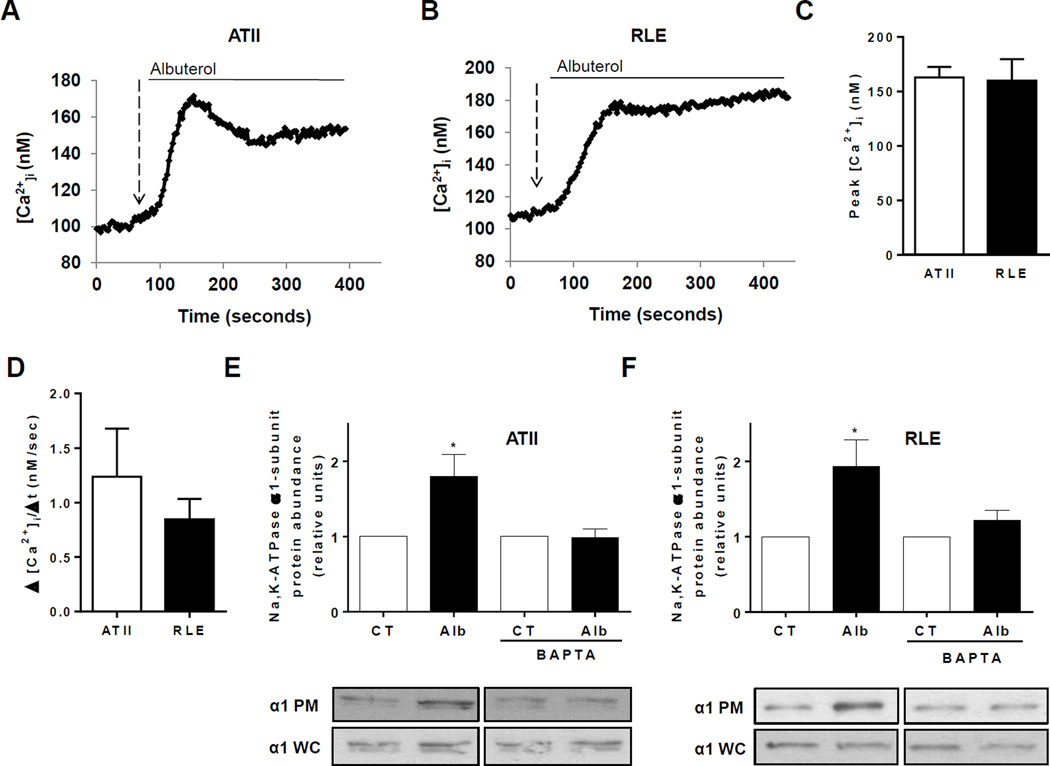
To determine if β2-adrenergic receptor activation results in calcium entry in alveolar epithelial cells, we stimulated ATII epithelial cells and RLE cells with albuterol, a selective β2-receptor agonist. Measurements of calcium concentrations in both cell types revealed a rapid increase in intracellular calcium immediately following the application of albuterol to the media (Figure 1, A and B). The peak [Ca2+]i (Figure 1C) as well as the Ca2+ influx (represented as Δ [Ca2+]/ Δt, (24, 25)) was very similar in both cell types (Figure 1D).
FIGURE 1. β2-adrenergic stimulation elicits a rapid increase in intracellular calcium that is required for the recruitment of the Na,K-ATPase to the plasma membrane in alveolar epithelial cells.
Measurement of intracellular calcium concentration in ATII (A) and RLE (B) cells at baseline and following the addition of albuterol (5 µM) to the media. Peak [Ca2+]i (C) and rate of Ca2+ entry (D) were measured in ATII and RLE cells after addition of 5µM albuterol. Plasma membrane abundance of Na,K-ATPase was measured via biotinylation technique in ATII (E) and RLE (F) cells after treatment with albuterol (5 µM, 15 min) in control conditions and in cells pretreated with BAPTA (50 µM, 5 min pretreatment). Whole-cell lysate Na,K-ATPase was used as a loading control. Results are for 5 experiments with 10–20 cells each.* p < 0.05.
Calcium is necessary for the β2-agonist-induced recruitment of Na,K-ATPase to the plasma membrane of alveolar epithelial cells
Our previous work demonstrated that β2-adrenergic receptor activation leads to increased abundance of Na,K-ATPase at the plasma membrane of AECs (7, 10). To determine if calcium was necessary for the β2-agonist-induced recruitment of Na,K-ATPase to the plasma membrane of AECs, we pretreated both ATII epithelial cells and RLE cells with BAPTA, a chelator of intracellular and extracellular calcium, prior to stimulation with albuterol. As shown in Figure 1, E and F, BAPTA prevented the β2-agonist-induced recruitment of Na,K-ATPase to the plasma membrane of both cell types.
Alveolar epithelial cells exhibit store-operated calcium entry (SOCE)
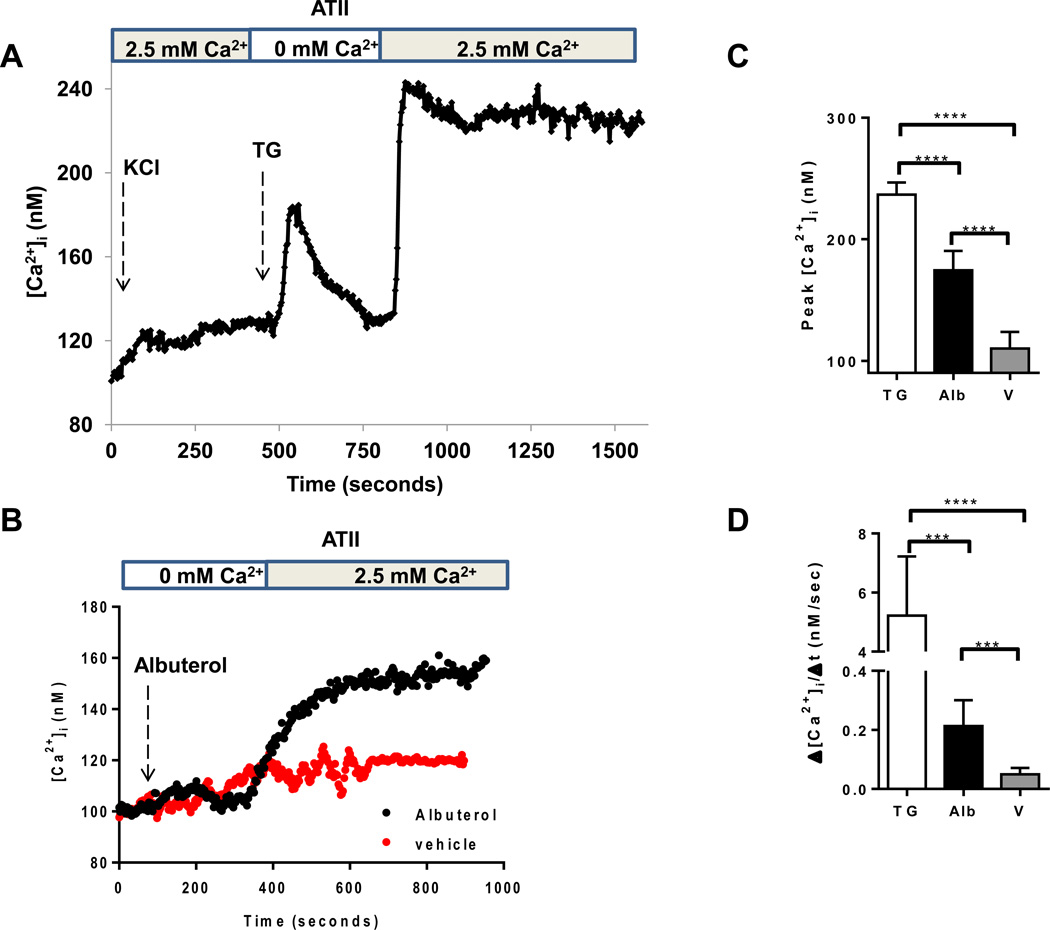
In non-excitable cells, the most common route of calcium signal generation results from activation of cell surface receptors which leads to emptying of intracellular calcium stores, followed by calcium entry into the cell via SOCE channels (17, 18). Other common mode of calcium influx involves voltage-gated calcium channels that respond to depolarization and play a significant role in calcium signaling in excitable cells (16). We observed that ATII epithelial cells lack significant voltage-gated calcium channel activity when stimulated with a depolarizing concentration of potassium chloride, but elicit a robust response to thapsigargin, a non-competitive inhibitor of sarco/endoplasmic reticulum Ca2+ ATPase and a powerful stimulus of SOCE (Figure 2A). In a similar fashion, we found that albuterol triggered the release of intracellular calcium stores when calcium levels were measured in media lacking calcium, and that SOCE occurred when calcium was returned to the perfusing media (Figure 2B). Noteworthy, albuterol caused a milder influx of Ca2+ in alveolar epithelial cells than thapsigargin reflected in the peak [Ca2+]i (Figure 2C) and the rate of Ca2+ influx (Figure 2D) (26).
FIGURE 2. Alveolar epithelial cells lack measurable voltage-gated calcium channel activity, but demonstrate store-operated calcium entry in response to thapsigargin and β2-agonists.
(A) Measurement of intracellular calcium concentration in ATII cells during the addition of a depolarizing concentration of KCl (65 mM), followed by the addition of thapsigargin (1 µM) in media lacking calcium. 2.5 mM calcium was then re-instituted into the media as indicated. (B) Levels of intracellular calcium in ATII cells during the addition of albuterol (5 µM) to calcium-free media, followed by the return of calcium to the perfusing media. Results are for 5 experiments with 10–20 cells each. (C) Peak [Ca2+]i and (D) rate of Ca2+ entry were measured in the cells after re-institution of 2.5 mM calcium to the media. Results are for 3 experiments with 10–20 cells each. ****p<0.0001; *** p < 0.001. TG: Thapsigargin. Alb: Albuterol. V: vehicle.
STIM1-associated CRAC cha nnels mediate albuterol-induced SOCE and recruitment of Na,K-ATPase to the plasma membrane
STIM1 is a transmembrane protein that functions as a calcium sensor in the ER that is responsible for communicating depletion of ER calcium stores to Orai channels in the plasma membrane (18, 19). Calcium entry via CRAC channels can be inhibited various ways, including binding of the channel with lanthanum chloride (LaCl3). We showed that pre-treating ATII epithelial cells with LaCl3 blunted the degree of calcium entry seen after albuterol is applied to the cells (Figure 3A), as well as the peak [Ca2+]i (Figure 3C) and the rate of Ca2+ influx (Figure 3D). We also demonstrated that CRAC channel inhibition with LaCl3 abrogated the increase in Na,K-ATPase protein abundance at the plasma membrane following stimulation with albuterol (Figure 3E). Transfecting RLE cells with siRNA targeting STIM1 tagged with a fluorescent dye (AlexaFluor546) allowed for selection of transfected cells during calcium measurements. We showed that calcium entry in response to albuterol was blocked or blunted in cells transfected with siRNA targeting STIM1 compared to those cells that were not transfected (Figure 3B; ~80% transfection efficiency), as well as the peak [Ca2+]i (Figure 3C) and the rate of Ca2+ influx (Figure 3D). In addition, silencing STIM1 via siRNA prevented the albuterol-induced recruitment of Na,K-ATPase to the plasma membrane (Figure 3F).
FIGURE 3. STIM1-associated CRAC channels mediate the β2 agonist-induced increase in intracellular calcium and recruitment of Na,K-ATPase to the plasma membrane of alveolar epithelial cells.
(A) Measurement of calcium levels following albuterol administration in ATII cells pretreated with LaCl3 (5 µM, 5 min preincubation). (B) Measurement of calcium in RLE cells transfected with fluorescently labeled siRNA against STIM1 during exposure to albuterol (5 µM), with representative images of Fura-2 and AlexaFluor-546 fluorescence. (C) Peak [Ca2+]i and (D) rate of Ca2+ entry were measured in the cells after re-institution of 2.5 mM calcium to the media in cells pre-incubated with LaCl3 (5 µM, 5 min preincubation) or transfected with siRNA against STIM1 previous addition of albuterol (5 µM). (E) Na,K-ATPase abundance in ATII cells in response to albuterol (5 µM, 15 min treatment) in control conditions and following a 5 min pretreatment with LaCl3 (5 µM). (F) Plasma membrane abundance of Na,K-ATPase in response to albuterol (5 µM, 15min) in RLE cells transfected with scramble siRNA or siRNA targeting STIM1. Whole-cell lysate Na,K-ATPase was used as a loading control. Results are for 3 experiments with 10–20 cells each. * p < 0.05, ** p < 0.01, *** p < 0.001.
Activation of adenylyl cyclase results in SOCE
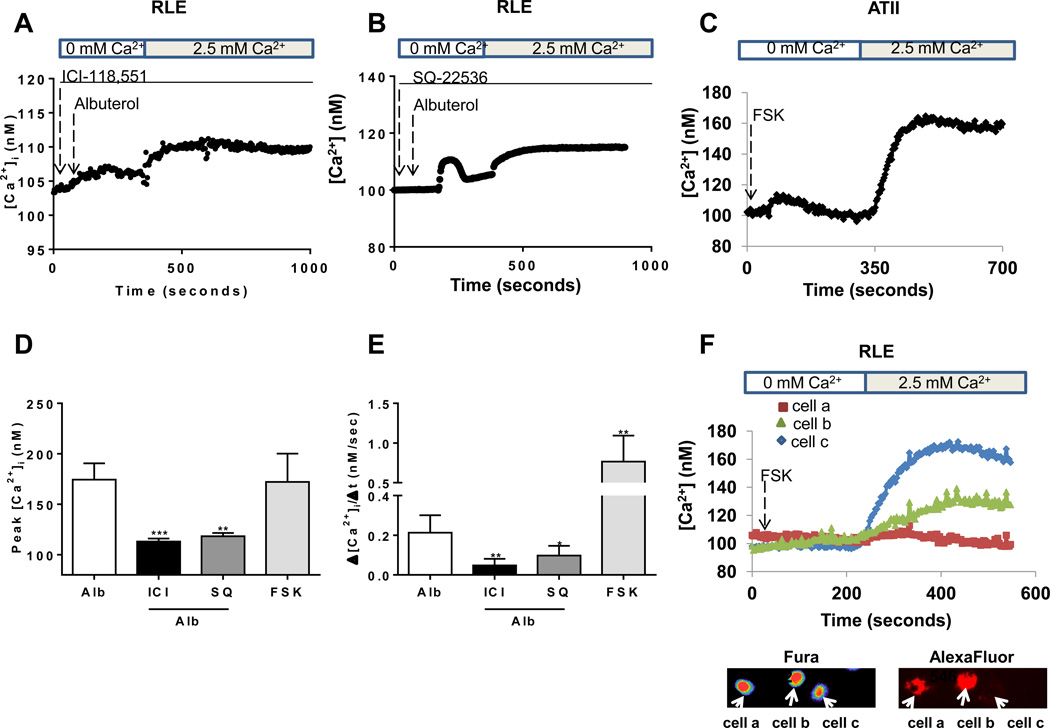
Binding of an agonist to the β2-adrenergic receptor results in dissociation of the Gsα subunit of the G protein and activation of adenylyl cyclase with subsequent increase in cAMP levels and downstream signaling (27). We first confirmed the specificity of albuterol as a β2-agonist by inhibiting the effect of albuterol in calcium entry by pre-incubation with 1 µM of the β2-antagonist ICI-118,551 (Figure 4A, 4D and 4E). The role of cAMP on SOCE was determined by preventing the albuterol-induced calcium entry by pre-incubation with 10 µM of the adenylyl cyclase inhibitor SQ-22536 (Figure 4B, 4D and 4E). We also found that direct activation of adenylyl cyclase by forskolin resulted in SOCE with similar magnitude of peak calcium but with an increased rate of calcium entry as that seen with albuterol (Figure 4C, 4D and 4E). In addition, we showed that forskolin-induced calcium entry is inhibited in RLE cells transfected with siRNA targeting STIM1 and tagged with a fluorescent dye (AlexaFluor546) (Figure 4F).
FIGURE 4. Activation of adenylyl cyclase is required for the β2 agonist-induced store-operated calcium entry in alveolar epithelial cells.
(A) Measurement of calcium levels following albuterol administration in RLE cells pretreated with ICI-118,551 (1 µM, 30 min preincubation). (B) Measurement of calcium levels following albuterol administration in RLE cells pretreated with SQ-22536 (10 µM, 30 min preincubation). (C) Measurement of intracellular calcium levels in ATII cells treated with forskolin (50 µM). Perfusion was started in calcium-free media and switched to 2.5 mM calcium as indicated. (D) Peak [Ca2+]i and (E) rate of Ca2+ entry were measured in the cells after re-institution of 2.5 mM calcium to the media in cells incubated with forskolin (50 µM) or pre-incubated with ICI-118,551 (1 µM, 30 min preincubation) or SQ-22536 (10 µM, 30 min preincubation) previous addition of albuterol (5 µm). (F) Calcium concentration in RLE cells transfected with fluorescently labeled siRNA targeting STIM1 during treatment with forskolin (50 µM) along with Fura-2 and AlexaFluor5–46 fluorescence images. Results are for 3 experiments with 10–20 cells each. * p < 0.05, ** p < 0.01, *** p < 0.001.
The magnitude of SOCE affects the abundance of Na,K-ATPase at the plasma membrane of AECs
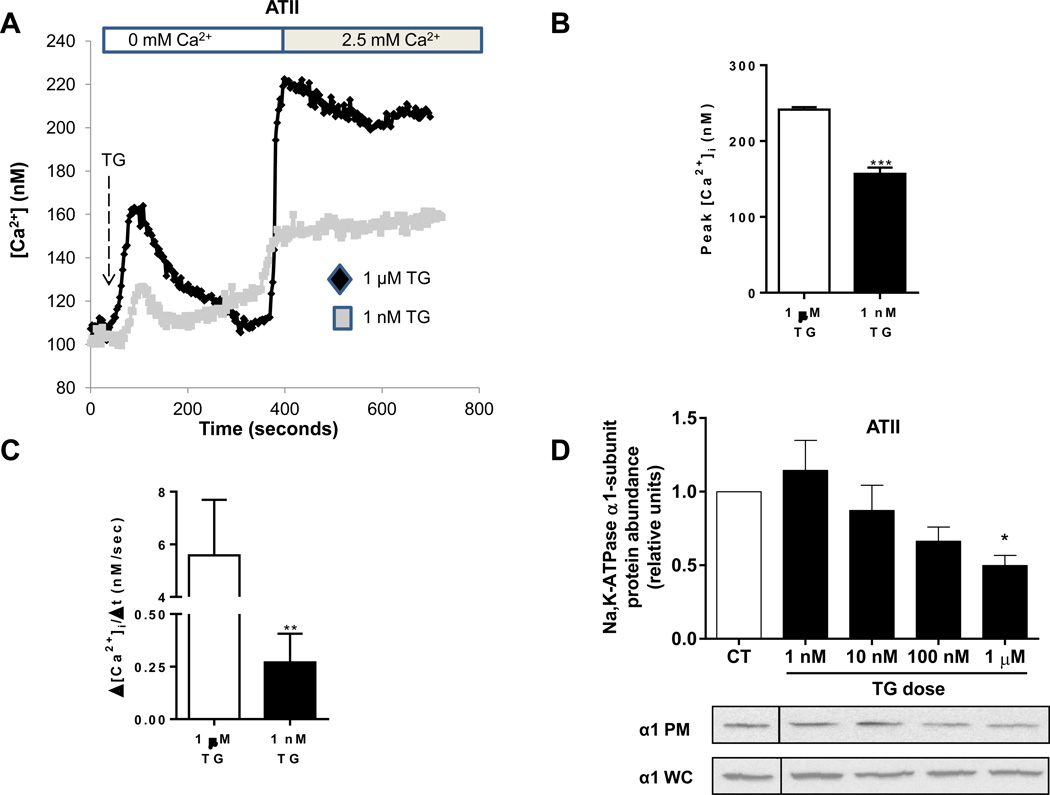
Previous work in our lab has demonstrated that treatment of A549 cells with 1 µM thapsigargin results in significant endocytosis of Na,K-ATPase (28). In attempt to explain why a different stimulus, albuterol, that also elicits SOCE results in recruitment of Na,K-ATPase to the plasma membrane, we compared the effect of treatment with different concentrations of thapsigargin on plasma membrane Na,K-ATPase abundance. We showed that a concentration of 1 nM thapsigargin results in SOCE of similar magnitude as albuterol (Figure 5A) as well as similar peak [Ca2+]i (Figure 5B) and rate of Ca2+ influx (Figure 5C). We also found that only high concentrations of thapsigargin result in endocytosis of the Na,K-ATPase, were as lower concentrations have no significant effect on the abundance of Na,K-ATPase at the plasma membrane (Figure 5D). These results suggest that both calcium and cAMP are necessary for the β2-agonist induced recruitment of Na,K-ATPase to the plasma membrane in AECs.
FIGURE 5. The magnitude of activation of store-operated calcium entry affects the abundance of Na,K-ATPase at the plasma membrane.
(A) Measurement of intracellular calcium in ATII cells exposed to two doses of thapsigargin (1 nM and 1 µM). Perfusion was initially in calcium-free media and then switched to 2.5 mM calcium as indicated. (B) Peak [Ca2+]i and (C) rate of Ca2+ entry were measured in the cells after re-institution of 2.5 mM calcium to the media in cells incubated with 1 µM or 1 nM Thapsigargin (TG). (D) Amount of plasma membrane Na,K-ATPase in ATII cells measured via biotinylation technique after treatment with different doses of thapsigargin (20 min). Whole-cell lysate Na,K-ATPase was used as a loading control.. Results are for 3 experiments with 10–20 cells each. * p < 0.05, ** p < 0.01, *** p < 0.001.
DISCUSSION
Previous in vitro and animal studies have shown that β-agonists enhance alveolar fluid clearance, and this effect is mediated in part by increased abundance of Na,K-ATPase at the plasma membrane of AECs (6–11, 28). In this work, we demonstrated that β2-agonists elicit store-operated calcium entry (SOCE) in AECs, and this calcium entry is necessary for the β2-agonist induced upregulation of Na,K-ATPase at the plasma membrane. Specifically, we showed that SOCE via STIM1-associated CRAC channels is involved in the response. We demonstrated that calcium entry is necessary, but not sufficient for the β2-agonist-induced recruitment of Na,K-ATPase to the plasma membrane of alveolar epithelial cells. Finally, we showed that the magnitude of SOCE independently affected the abundance of Na,K-ATPase at the plasma membrane of AECs.
SOCE has been reported in AECs (29–31), but this is the first report of β2-agonists eliciting SOCE in AECs. Calcium is an important component of vesicle fusion and exocytosis in neurons as well as endocrine cells and T-cells (14). Both cAMP and calcium are recognized to play important independent as well as cooperative roles in exocytosis in non-neuronal cells (32). Within alveolar cells, cAMP promotes the recruitment of Na,K-ATPase from intracellular vesicle pools into the plasma membrane (33, 34). By chelating calcium with BAPTA, we showed that calcium was also necessary for the β2-agonist-induced recruitment of Na,K-ATPase to the plasma membrane.
STIM1 is the primary calcium sensing protein in the ER and a major component of CRAC channels along with the Orai family of proteins (20). Through pharmacologic and siRNA inhibition of CRAC channels and STIM1, we showed that the β2-agonist induced SOCE is mediated by STIM1-containing CRAC channels. In addition to its role in CRAC channels, STIM1 has been shown to activate adenylyl cyclase and increase cAMP following calcium store depletion in a process which is independent of cytosolic calcium (35). This process of store-operated cAMP signaling may also be contributing in a regulatory mechanism to the β2-agonist induced SOCE and recruitment of Na,K-ATPase to the plasma membrane of alveolar epithelial cells. STIM1 has also been shown to bind to Na,K-ATPases through a STIM1-POST (partner of STIM1) complex that is triggered by intracellular store depletion (36). This raises the possibility that STIM1 may have a direct stabilization effect on Na,K-ATPase abundance at the plasma membrane and contribute directly to the β2-agonist induced response.
Activation of β2-agonist receptors leads to dissociation of the Gsα subunit of the G protein and activation of adenylyl cyclase with subsequent increase in cAMP levels. In this work, we show that forskolin elicits SOCE in AECs of similar magnitude as albuterol, indicating that the Gsα subunit/adenylyl cyclase and increased cAMP mediate the β2-agonist induced SOCE; and we confirmed this observation by preventing SOCE in cells treated with albuterol in the presence of the adenylyl cyclase inhibitor SQ22536. We hypothesize that cAMP by a still not determined mechanism stimulate the increase in intracellular calcium, a phenomenon that might be cell specific as cAMP does not have a direct effect on increasing intracellular calcium in kidney cells (37). A similar interaction between cAMP and calcium entry has been shown to regulate prostaglandin E2-mediated chloride secretion in mouse inner medullary collecting duct cells (38). Inhibiting STIM1 with a fluorescently labeled siRNA confirms the role of STIM1-associated CRAC channels in the cAMP-mediated SOCE.
We have previously demonstrated that robust SOCE elicited by 1µM thapsigargin results in endocytosis of the Na,K-ATPase in alveolar epithelial cells (28). However, albuterol causes SOCE of significantly lower magnitude and similar to that of a lower dose (1 nM) of thapsigargin. We showed that low magnitude SOCE does not have significant effect on the abundance of Na,K-ATPase at the plasma membrane and supports the statement that calcium is necessary but not sufficient for the β2-agonist induced recruitment of Na,K-ATPase. We have previously demonstrated that PKA is also necessary for the β2-adrenergic-induced recruitment of the Na,K-ATPase to the plasma membrane (12, 13); therefore, we hypothesize that cAMP by increasing intracellular Ca2+ and activating PKA leads to the recruitment of the Na,K-ATPase in alveolar epithelial cells. There are numerous potential sites of crosstalk between cAMP and calcium signaling within non-excitable cells that may impact the spatio-temporal pattern of calcium signaling (39). We hypothesize that compartmentalized cAMP signaling in concert with mild increases in calcium levels, likely in spatially related microdomains, work together to augment the recruitment of Na,K-ATPase from intracellular vesicles to the plasma membrane of alveolar epithelial cells.
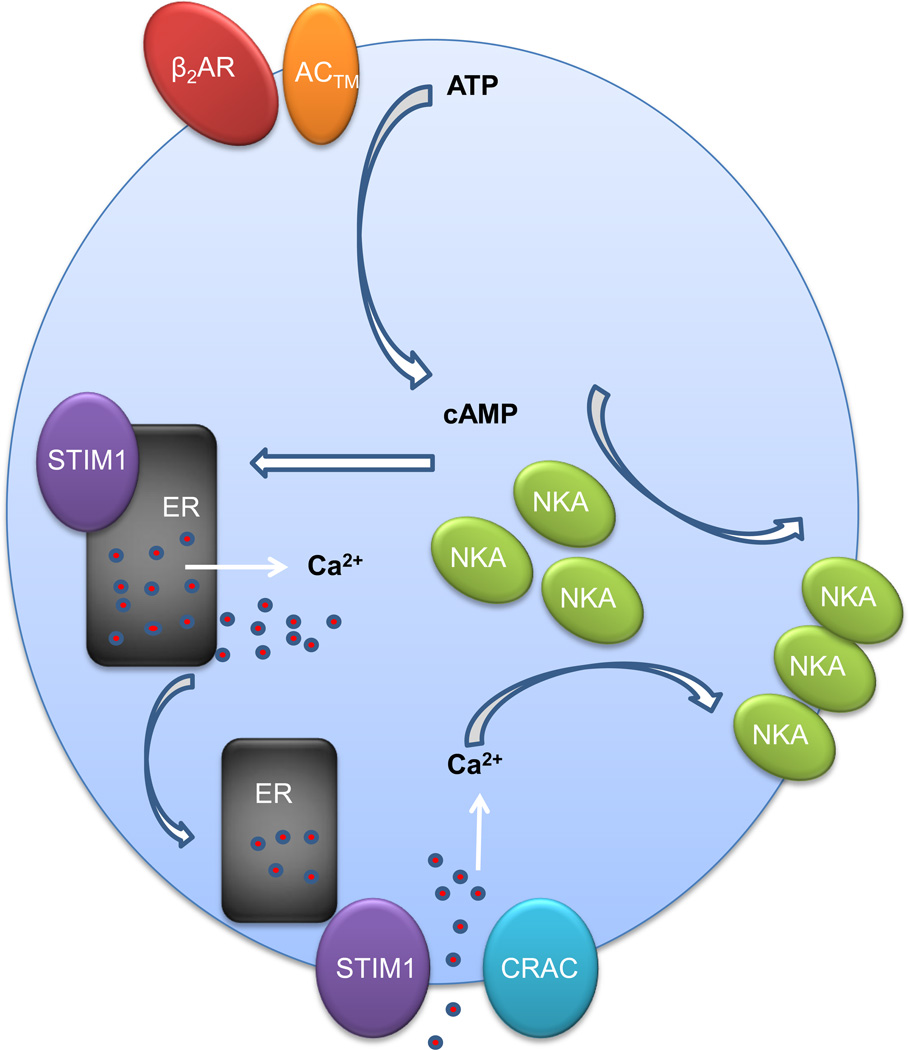
In summary, as shown schematically in Figure 6, we have shown that calcium entry via STIM1-associated CRAC channels is necessary for the recruitment of Na,K-ATPase to the plasma membrane of ATII epithelial cells following treatment with albuterol and the subsequent increase in cAMP. These findings may allow for further investigations into therapeutic options to enhance alveolar fluid clearance in patients with ARDS.
FIGURE 6. Schematic representation of β2 agonist-induced SOCE and recruitment of Na,K-ATPase vesicles to the plasma membrane of alveolar epithelial cells.
β2AR (β2-adrenergic receptor), AC™ (transmembrane adenylyl cyclase), NKA (Na,K-ATPase).
HIGHLIGHTS.
β2-agonists elicit store-operated Ca2+ entry (SOCE) in alveolar epithelial cells.
Ca2+ entry is necessary for the β2-agonist-induced upregulation of Na,K-ATPase.
STIM1-associated CRAC channels mediate the β2-agonist-induced SOCE.
The magnitude of Ca2+ entry affects the abundance of plasma membrane Na,K-ATPase.
ACKNOWLEDGEMENTS
We would like to thank Lynn Welch, BS for assistance in the manuscript preparation as well as Aparna Sundaram, MD and Nimrod Deiss-Yehiely, BS for technical support. This study was funded by HL48129, HL-76139, and HL-71643.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Sznajder JI. Alveolar Edema Must Be Cleared for the Acute Respiratory Distress Syndrome Patient to Survive. Am. J. Respir. Crit. Care Med. 2001;163:1293–1294. doi: 10.1164/ajrccm.163.6.ed1801d. [DOI] [PubMed] [Google Scholar]
- 3.Mutlu GM, Sznajder JI. Mechanisms of pulmonary edema clearance. American Journal of Physiology - Lung Cellular & Molecular Physiology. 2005;289:L685–L695. doi: 10.1152/ajplung.00247.2005. [DOI] [PubMed] [Google Scholar]
- 4.Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-{zeta} J. Clin. Invest. 2003;111:1057–1064. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vadasz I, Raviv S, Sznajder JI. Alveolar epithelium and Na,K-ATPase in acute lung injury. Intensive Care Med. 2007;33:1243–1251. doi: 10.1007/s00134-007-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med. 2004;32:1470–1476. doi: 10.1097/01.ccm.0000129489.34416.0e. [DOI] [PubMed] [Google Scholar]
- 7.Saldias F, Lecuona E, Friedman E, Barnard ML, Ridge KM, Sznajder JI. Modulation of lung liquid clearance by isoproterenol in rat lungs. Am J Physiol. 1998;274:L694–L701. doi: 10.1152/ajplung.1998.274.5.L694. [DOI] [PubMed] [Google Scholar]
- 8.Saldias FJ, Comellas A, Ridge KM, Lecuona E, Sznajder JI. Isoproterenol improves ability of lung to clear edema in rats exposed to hyperoxia. J Appl Physiol. 1999;87:30–35. doi: 10.1152/jappl.1999.87.1.30. [DOI] [PubMed] [Google Scholar]
- 9.Saldias FJ, Lecuona E, Comellas AP, Ridge KM, Rutschman DH, Sznajder JI. beta -Adrenergic Stimulation Restores Rat Lung Ability to Clear Edema in Ventilator-associated Lung Injury. Am. J. Respir. Crit. Care Med. 2000;162:282–287. doi: 10.1164/ajrccm.162.1.9809058. [DOI] [PubMed] [Google Scholar]
- 10.Dumasius V, Sznajder JI, Azzam ZS, Boja J, Mutlu GM, Maron MB, Factor P. {beta}2-Adrenergic Receptor Overexpression Increases Alveolar Fluid Clearance and Responsiveness to Endogenous Catecholamines in Rats. Circ Res. 2001;89:907–914. doi: 10.1161/hh2201.100204. [DOI] [PubMed] [Google Scholar]
- 11.Mutlu GM, Koch WJ, Factor P. Alveolar epithelial beta 2-adrenergic receptors: their role in regulation of alveolar active sodium transport. Am J Respir Crit Care Med. 2004;170:1270–1275. doi: 10.1164/rccm.200404-470CP. [DOI] [PubMed] [Google Scholar]
- 12.Lecuona E, Ridge K, Pesce L, Battle D, Sznajder JI. The GTP-binding protein RhoA mediates Na,K-ATPase exocytosis in alveolar epithelial cells. Mol Biol Cell. 2003;14:3888–3897. doi: 10.1091/mbc.E02-12-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertorello AM, Ridge KM, Chibalin AV, Katz AI, Sznajder JI. Isoproterenol increases Na,K-ATPase activity by membrane insertion of alpha-subunits in lung alveolar cells. Am J Physiol. 1999;276:L20–L27. doi: 10.1152/ajplung.1999.276.1.L20. [DOI] [PubMed] [Google Scholar]
- 14.Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogrodnik J, Niggli E. Increased Ca(2+) leak and spatiotemporal coherence of Ca(2+) release in cardiomyocytes during beta-adrenergic stimulation. J Physiol. 2010;588:225–242. doi: 10.1113/jphysiol.2009.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou P, Zhao YT, Guo YB, Xu SM, Bai SH, Lakatta EG, Cheng H, Hao XM, Wang SQ. Beta-adrenergic signaling accelerates and synchronizes cardiac ryanodine receptor response to a single L-type Ca2+ channel. Proc Natl Acad Sci U S A. 2009;106:18028–18033. doi: 10.1073/pnas.0906560106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luik RM, Lewis RS. New insights into the molecular mechanisms of store-operated Ca2+ signaling in T cells. Trends Mol Med. 2007;13:103–107. doi: 10.1016/j.molmed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Smyth JT, Hwang SY, Tomita T, DeHaven WI, Mercer JC, Putney JW. Activation and regulation of store-operated calcium entry. J Cell Mol Med. 2010;14:2337–2349. doi: 10.1111/j.1582-4934.2010.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parekh AB. Functional consequences of activating store-operated CRAC channels. Cell Calcium. 2007;42:111–121. doi: 10.1016/j.ceca.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Derler I, Schindl R, Fritsch R, Romanin C. Gating and permeation of Orai channels. Front Biosci (Landmark Ed) 2012;17:1304–1322. doi: 10.2741/3988. [DOI] [PubMed] [Google Scholar]
- 21.Ridge KM, Olivera WG, Saldias F, Azzam Z, Horowitz S, Rutschman DH, Dumasius V, Factor P, Sznajder JI. Alveolar Type 1 Cells Express the {alpha}2 Na,K-ATPase, Which Contributes to Lung Liquid Clearance. Circ Res. 2003;92:453–460. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- 22.Lecuona E, Sun H, Vohwinkel C, Ciechanover A, Sznajder JI. Ubiquitination participates in the lysosomal degradation of Na,K-ATPase in steady-state conditions. Am J Respir Cell Mol Biol. 2009;41:671–679. doi: 10.1165/rcmb.2008-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vadasz I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Toth PT, Lecuona E, Witters LA, Schumacker PT, et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Invest. 2008;118:752–762. doi: 10.1172/JCI29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 25.Somasundaram A, Shum AK, McBride HJ, Kessler JA, Feske S, Miller RJ, Prakriya M. Store-operated CRAC channels regulate gene expression and proliferation in neural progenitor cells. J Neurosci. 2014;34:9107–9123. doi: 10.1523/JNEUROSCI.0263-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutlu GM, Factor P. Alveolar epithelial beta(2)-adrenergic receptors. American Journal of Respiratory Cell and Molecular Biology. 2008;38:127–134. doi: 10.1165/rcmb.2007-0198TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gusarova GA, Trejo HE, Dada LA, Briva A, Welch LC, Hamanaka RB, Mutlu GM, Chandel NS, Prakriya M, Sznajder JI. Hypoxia leads to Na,K-ATPase downregulation via Ca(2+) release-activated Ca(2+) channels and AMPK activation. Mol Cell Biol. 2011;31:3546–3556. doi: 10.1128/MCB.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balghi H, Robert R, Rappaz B, Zhang X, Wohlhuter-Haddad A, Evagelidis A, Luo Y, Goepp J, Ferraro P, Romeo P, et al. Enhanced Ca2+ entry due to Orai1 plasma membrane insertion increases IL-8 secretion by cystic fibrosis airways. FASEB J. 2011;25:4274–4291. doi: 10.1096/fj.11-187682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dietl P, Haller T, Frick M. Spatio-temporal aspects, pathways and actions of Ca(2+) in surfactant secreting pulmonary alveolar type II pneumocytes. Cell Calcium. 2012;52:296–302. doi: 10.1016/j.ceca.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Usmani SM, von Einem J, Frick M, Miklavc P, Mayenburg M, Husmann M, Dietl P, Wittekindt OH. Molecular basis of early epithelial response to streptococcal exotoxin: role of STIM1 and Orai1 proteins. Cell Microbiol. 2012;14:299–315. doi: 10.1111/j.1462-5822.2011.01724.x. [DOI] [PubMed] [Google Scholar]
- 32.Szaszak M, Christian F, Rosenthal W, Klussmann E. Compartmentalized cAMP signalling in regulated exocytic processes in non-neuronal cells. Cell Signal. 2008;20:590–601. doi: 10.1016/j.cellsig.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Bertorello AM, Ridge KM, Chibalin AV, Katz AI, Sznajder JI. Isoproterenol increases Na+-K+-ATPase activity by membrane insertion of alpha-subunits in lung alveolar cells. Am. J. Physiol. 1999;276:L20–L27. doi: 10.1152/ajplung.1999.276.1.L20. [DOI] [PubMed] [Google Scholar]
- 34.Lecuona E, Ridge K, Pesce L, Batlle D, Sznajder JI. The GTP-binding protein RhoA mediates Na,K-ATPase exocytosis in alveolar epithelial cells. Molecular Biology of the Cell. 2003;14:3888–3897. doi: 10.1091/mbc.E02-12-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefkimmiatis K, Moyer MP, Curci S, Hofer AM. "cAMP sponge": a buffer for cyclic adenosine 3', 5'-monophosphate. PLoS One. 2009;4:e7649. doi: 10.1371/journal.pone.0007649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krapivinsky G, Krapivinsky L, Stotz SC, Manasian Y, Clapham DE. POST, partner of stromal interaction molecule 1 (STIM1), targets STIM1 to multiple transporters. Proc Natl Acad Sci U S A. 2011;108:19234–19239. doi: 10.1073/pnas.1117231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tovey SC, Dedos SG, Rahman T, Taylor EJ, Pantazaka E, Taylor CW. Regulation of inositol 1,4,5-trisphosphate receptors by cAMP independent of cAMP-dependent protein kinase. J Biol Chem. 2010;285:12979–12989. doi: 10.1074/jbc.M109.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajagopal M, Thomas SV, Kathpalia PP, Chen Y, Pao AC. Prostaglandin E2 induces chloride secretion through crosstalk between cAMP and calcium signaling in mouse inner medullary collecting duct cells. Am J Physiol Cell Physiol. 2014;306:C263–C278. doi: 10.1152/ajpcell.00381.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruce JI, Straub SV, Yule DI. Crosstalk between cAMP and Ca2+ signaling in nonexcitable cells. Cell Calcium. 2003;34:431–444. doi: 10.1016/s0143-4160(03)00150-7. [DOI] [PubMed] [Google Scholar]