Abstract
We sought to investigate the role of aldosterone as a mediator of disease and its relationship with the counter-regulatory natriuretic peptide (NP) system. We measured plasma aldosterone (n=1674; age ≥45 years old) in a random sample of the general population from Olmsted County, MN. In a multivariate logistic regression model, aldosterone analyzed as a continuous variable was associated with hypertension (HTN) (OR=1.75, 95%CI= 1.57,1.96; p<0.0001), obesity (OR=1.34, 95%CI= 1.21,1.48; p<0.0001), chronic kidney disease (CKD) (OR=1.39, 95%CI= 1.22,1.60; p<0.0001), central obesity (OR=1.47, 95%CI=1.32,1.63; p<0.0001), metabolic syndrome (MetS) (OR=1.41, 95%CI= 1.26,1.58; p<0.0001), high triglycerides (OR=1.23, 95%CI=1.11,1.36; p<0.0001), concentric left ventricular hypertrophy (cLVH) (OR=1.22, 95%CI= 1.09,1.38; p=0.0007) and atrial fibrillation (OR=1.24, 95%CI= 1.01,1.53; p=0.04), after adjusting for age and sex. The associations with HTN, central obesity, MetS, triglycerides and cLVH remained significant after further adjustment for BMI, NPs, and renal function. Furthermore, aldosterone in the highest tertile correlated with lower NP levels and increased mortality. Importantly, most of these associations remained significant even after excluding subjects with aldosterone levels above the normal range. In conclusion, we report that aldosterone is associated with HTN, CKD, obesity, MetS, cLVH, and lower NPs in the general community. Our data suggests that aldosterone, even within the normal range, may be a biomarker of cardiorenal and metabolic disease. Further studies are warranted to evaluate a therapeutic and preventive strategy to delay the onset and/or progression of disease, using mineralocorticoid antagonists or chronic NP administration in high risk subjects identified by plasma aldosterone.
Keywords: Aldosterone, general community, cardiorenal disease, obesity, biomarker, natriuretic peptides
INTRODUCTION
Aldosterone is a hormone that is synthesized by the adrenals and plays a key role in body fluid and blood pressure (BP) homeostasis1. Notably, aldosterone and mineralocorticoid receptor activation exert important biological actions that may lead to injury in various target organs, including the heart and kidneys2–4. Studies have reported that high levels of plasma aldosterone were associated with metabolic syndrome (MetS) which may, in part, be explained by the production of aldosterone and the presence of mineralocorticoid receptors in adipocytes5–9. While pathological levels have implicated aldosterone in heart failure and resistant hypertension10, the role of aldosterone as a potential mediator of disease in the general community continues to evolve. In a four-year follow-up analysis, in subjects without hypertension, Vasan et al. observed that, higher plasma aldosterone is associated with development of future hypertension (HTN)11. Additionally, Bochud et al. showed that aldosterone was independently associated with MetS and was significantly higher in subjects with MetS, in a community-based cohort with East African ancestries12.
In the current study, our goal was to better define the relationship between circulating aldosterone and cardiovascular, renal and metabolic diseases as well as myocardial structure and function in the general population. We also evaluated the modulating action of anti-hypertensive medication on circulating aldosterone given the high prevalence of hypertension and its link to aldosterone. Furthermore, recognizing the counter-regulatory relationship between aldosterone and the NP levels, we sought to investigate the association between plasma aldosterone and circulating levels of atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP). Finally, we also determined whether aldosterone could potentially identify subjects at higher risk for mortality.
We hypothesized that, in the general community, the highest levels of circulating aldosterone even within the normal range would be associated with cardiorenal and metabolic disease. We also hypothesized that this strong relationship would remain significant even after adjusting for the use of anti-hypertensive medications. Moreover, despite the known biomarker role for elevated natriuretic peptides (NPs) in predicting future adverse outcomes, we hypothesized that higher plasma aldosterone would be associated with paradoxically lower NP levels. Finally, we hypothesized that the subjects with higher aldosterone levels were at higher risk for increased mortality in a long-term follow-up. To accomplish our goals, we utilized a well-characterized, randomly selected, adult community-based cohort from Olmsted County, MN.
METHODS
Methods are available in the online-only Data Supplement.
RESULTS
Baseline characteristics of the study subjects
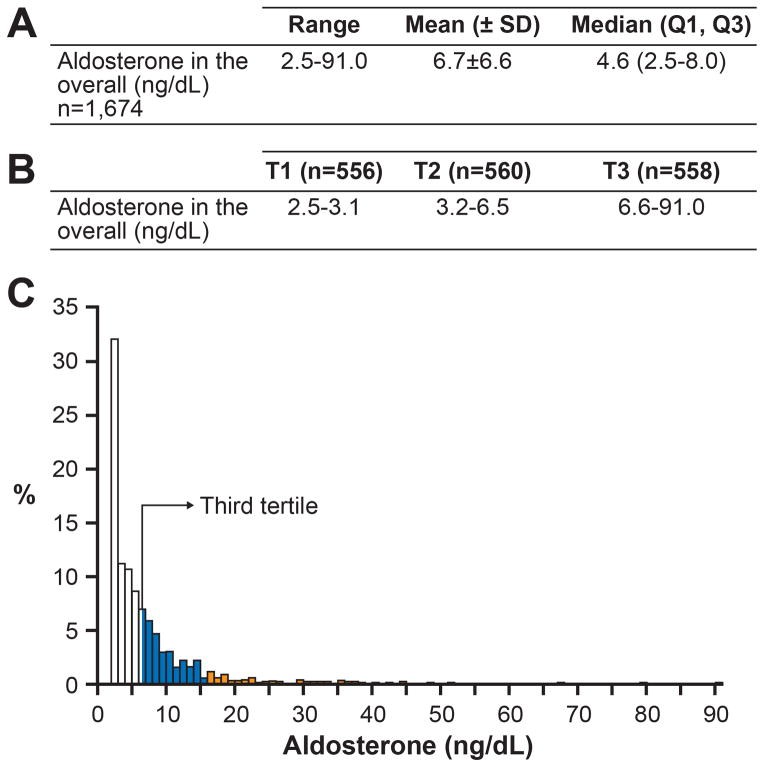
The baseline characteristics of our cohort are presented in Table 1. Plasma aldosterone (n=1674) ranged from 2.5 to 91 ng/dL and the median (Q1, Q3) and mean ± SD values were 4.6 (2.5, 8.0) and 6.7 ± 6.6 ng/dL, respectively (Figure 1-a). There was no difference in aldosterone levels between men and women and age did not influence levels. In the healthy sub-group (n=80) the normal range of aldosterone was from 2.5 to 16.2 ng/dL and here, the median and mean values were 4.2 (2.5, 5.6) and 5.2 ± 4.1 ng/dL, respectively. It should be noted that the normal range from our healthy sub-group is similar to the range values showed by Meyes et al.13 In this previous paper, the authors reported that normal aldosterone levels were 3–16 ng/dL in healthy subjects with sampling in supine position, with unrestricted sodium intakes.
Table 1.
Baseline Characteristics of the Study Population.
| Characteristics | Overall (n=1674) |
|---|---|
| Age, years | 63.09 ± 10.62 |
| Sex F, n (%) | 922 (55%) |
| BMI of Subjects, kg/m2 | 28.40 ± 5.39 |
| Medication Use, n (%) | 1544 (92%) |
| BMI> 30 kg/m2, n (%), | 535 (32%) |
| Waist circumference > 102 cm M, > 88 cm F, n (%), | 554 (33%) |
| Aldosterone ng/dl, median (Q1, Q3) | 4.60 (2.50, 8.00) |
| NT-proANP, pg/ml, median (Q1, Q3) | 2280.0 (1449.5, 3396.0) |
| NT-proBNP, pg/ml, median (Q1, Q3) | 71.94 (29.50, 150.90) |
| BNP pg/ml, median (Q1, Q3) | 14.90 (5.80, 32.40) |
| ANP pg/ml, median (Q1, Q3) | 11.90 (7.60, 16.60) |
| Current/Former Smoker, n (%) | 821 (49%) |
| Diabetes, n (%) | 130 (8%) |
| Verified Hypertension, n (%) | 478 (29%) |
| Atrial Fibrillation/Flutter, n (%) | 85 (5%) |
| Coronary Artery Disease, n (%) | 205 (12%) |
| Chronic Heart Failure at baseline, n (%) | 41 (2%) |
| Myocardial Infarction at baseline, n (%) | 78 (5%) |
| Stroke at baseline, n (%) | 31 (2%) |
| Systolic Blood Pressure, mmHg | 132.87 ± 21.59 |
| Diastolic Blood Pressure, mmHg | 73.30 ± 10.28 |
| Total Cholesterol, mg/dl | 203.33 ± 36.21 |
| HDL Cholesterol, mg/dl | 46.35 ± 14.65 |
| HDL< 40 mg/dl M, < 50 mg/dl F, n (%) | 883 (53%) |
| LDL Cholesterol, mg/dl | 127.79 ± 32.38 |
| LDL> 160 mg/dl, n (%) | 246 (15%) |
| Triglycerides, mg/dl | 145.90 ± 85.27 |
| Triglycerides> 150 mg/dl, n (%) | 596 (36%) |
| Antilipemic Therapy, n (%) | 281 (18%) |
| ACEI, ARBs, BBs, Diuretics, n (%) | 526 (34%) |
| Creatinine mg/dl, median (Q1, Q3), | 0.80 (0.70, 1.00) |
| Calculated GFR ml/min (MDRD formula) | 80.34 ± 18.25 |
| GFR< 60 ml/min, n (%) | 243 (15%) |
| Insulin μU/mL, median (Q1, Q3) | 5.20 (3.60, 7.90) |
| Serum Glucose mg/dl, median (Q1, Q3) | 94.00 (88.00, 101.00) |
| Metabolic Syndrome (at least 3 of 5 criteria), n (%) | 352 (21%) |
| Echocardiographic Parameters | |
| Ejection Fraction < 40, n (%) | 24 (1%) |
| Mild/Moderate/Severe Diastolic Dysfunction, n (%) | 454 (30%) |
| Left Ventricular Hypertrophy, n (%) | 440 (33%) |
| Concentric Left Ventricular Hypertrophy, n (%) | 251 (19%) |
BMI= body mass index. HDL= high-density lipoprotein. LDL= low-density lipoprotein. ACEI= angiotensin converting enzyme inhibitors; ARBs= angiotensin receptor blockers; BBs= beta-blockers; Diuretics= Thiazides, Thiazides-like, Loop Diuretics, Potassium-Sparing and Aldosterone Antagonists. GFR= glomerular filtration rate; MDRD = Modification of Diet in Renal Disease.
Figure 1. Aldosterone Levels in the General Population.
A) Aldosterone range in our entire cohort (overall); B) aldosterone according to tertiles; C) aldosterone distribution. x-axis= aldosterone levels, y-axis= % of subjects. The white bars indicate subjects with aldosterone levels in the 1st and 2nd tertiles; the blue bars indicate individuals in the 3rd tertile; the orange bars indicate subjects in the 3rd tertile with aldosterone levels above the normal range (n=95). The continuous line/arrow indicates the beginning of the 3rd tertile.
For our analysis in the overall population, we also divided aldosterone into tertiles where the first tertile (T1: n=556) ranged from 2.5 to 3.1 ng/dL, the second tertile (T2; n=560) ranged from 3.2 to 6.5 ng/dL and the third tertile (T3: n=558) ranged from 6.6 to 91 ng/dL (Figure 1-b). Importantly 463 subjects (83%) in the third tertile had normal aldosterone levels, while 95 subjects (17%) had aldosterone above 16.2 ng/dL. The distribution of aldosterone in the general population is shown in Figure 1-c and approximately 6% of subjects had aldosterone above the normal range (95 out of 1674).
Aldosterone and cardiovascular, renal and metabolic morbidity in the general community
Plasma aldosterone was associated with HTN (OR=1.75, p<0.0001), obesity (OR=1.34, p<0.0001), chronic kidney disease (CKD) (OR=1.39, p<0.0001), central obesity (OR=1.47, p<0.0001), MetS (OR=1.41, p<0.0001), high triglyceride levels (OR=1.23, p<0.0001), concentric left ventricular hypertrophy (cLVH) (OR=1.25, p=0.001) and atrial fibrillation (AF) (OR=1.24, p=0.04) when analyzed as a continuous variable and adjusted for age and sex. Table 2 illustrates the positive and significant associations between aldosterone levels and HTN, obesity, CKD, central obesity, MetS, higher triglycerides and cLVH after further adjustment for BMI, ANP, NT-proANP, BNP, NT-proBNP and GFR. In a multivariate model including age, sex, BMI, systolic and diastolic BP, aldosterone was correlated with CKD, cLVH and AF.
Table 2.
Associations Between Aldosterone and Different Comorbidities in a Multivariate Logistic Regression Model.
| Comorbidity | OR (95% CI) Per 1 SD increase in log Aldosterone | p-value | OR (95% CI) Top Tertile of Aldosterone | p-value |
|---|---|---|---|---|
| Hypertension (N=478) | ||||
| Base (age, sex, BMI adj.) | 1.68 (1.50,1.89) | <.0001 | 2.45 (1.95,3.09) | <.0001 |
| Base+ ANP adj. | 1.71 (1.53,1.92) | <.0001 | 2.56 (2.02,3.24) | <.0001 |
| Base+ NT-proANP adj. | 1.74 (1.54,1.95) | <.0001 | 2.56 (2.02,3.25) | <.0001 |
| Base+ NT-proBNP adj. | 1.74 (1.55,1.96) | <.0001 | 2.60 (2.05,3.30) | <.0001 |
| Base+ BNP adj. | 1.70 (1.52,1.91) | <.0001 | 2.51 (1.99,3.17) | <.0001 |
| Base+ GFR adj. | 1.66 (1.48,1.87) | <.0001 | 2.41 (1.90,3.05) | <.0001 |
| Obesity (N=535) | ||||
| Base (age, sex adj.) | 1.34 (1.21,1.48) | <.0001 | 1.69 (1.36,2.10) | <.0001 |
| Base+ ANP adj. | 1.31 (1.18,1.45) | <.0001 | 1.64 (1.32,2.04) | <.0001 |
| Base+ NT-proANP adj. | 1.30 (1.17,1.45) | <.0001 | 1.59 (1.28,1.99) | <.0001 |
| Base+ NT-proBNP adj. | 1.35 (1.22,1.50) | <.0001 | 1.71 (1.37,2.12) | <.0001 |
| Base+ BNP adj. | 1.34 (1.21,1.48) | <.0001 | 1.69 (1.36,2.10) | <.0001 |
| Base+ GFR adj. | 1.35 (1.22,1.50) | <.0001 | 1.73 (1.38,2.15) | <.0001 |
| CKD (N=243) | ||||
| Base (age, sex, BMI adj.) | 1.37 (1.20,1.58) | <.0001 | 1.75 (1.30,2.35) | 0.0002 |
| Base+ ANP adj. | 1.38 (1.20,1.59) | <.0001 | 1.76 (1.30,2.38) | 0.0002 |
| Base+ NT-proANP adj. | 1.39 (1.21,1.60) | <.0001 | 1.77 (1.31,2.41) | 0.0002 |
| Base+ NT-proBNP adj. | 1.40 (1.22,1.62) | <.0001 | 1.86 (1.37,2.52) | <.0001 |
| Base+ BNP adj. | 1.41 (1.22,1.62) | <.0001 | 1.86 (1.37,2.50) | <.0001 |
| Base+ GFR adj. | NA* | NA* | ||
| Central Obesity (N=554) | ||||
| Base (age, sex, BMI adj.) | 1.41 (1.20,1.65) | <.0001 | 1.61 (1.17,2.22) | 0.004 |
| Base+ ANP adj. | 1.38 (1.18,1.62) | <.0001 | 1.58 (1.14,2.18) | 0.006 |
| Base+ NT-proANP adj. | 1.38 (1.17,1.62) | 0.0001 | 1.55 (1.11,2.15) | 0.009 |
| Base+ NT-proBNP adj. | 1.40 (1.19,1.65) | <.0001 | 1.60 (1.15,2.23) | 0.005 |
| Base+ BNP adj. | 1.41 (1.20,1.65) | <.0001 | 1.61 (1.17,2.22) | 0.004 |
| Base+ GFR adj. | 1.45 (1.23,1.71) | <.0001 | 1.70 (1.22,2.37) | 0.002 |
| Met Syndrome (N=352) | ||||
| Base (age, sex, BMI adj.) | 1.27 (1.12,1.45) | 0.0002 | 1.53 (1.17,2.00) | 0.002 |
| Base+ ANP adj. | 1.26 (1.11,1.43) | 0.0005 | 1.48 (1.13,1.95) | 0.005 |
| Base+ NT-proANP adj. | 1.26 (1.10,1.43) | 0.0007 | 1.49 (1.13,1.96) | 0.005 |
| Base+ NT-proBNP adj. | 1.25 (1.09,1.42) | 0.0009 | 1.49 (1.13,1.96) | 0.004 |
| Base+ BNP adj. | 1.27 (1.11,1.44) | 0.0003 | 1.51 (1.16,1.99) | 0.003 |
| Base+ GFR adj. | 1.25 (1.10,1.43) | 0.001 | 1.53 (1.16,2.02) | 0.003 |
| High Triglycerides (N=596) | ||||
| Base (age, sex, BMI adj.) | 1.17 (1.06,1.30) | 0.002 | 1.24 (1.00,1.54) | 0.05 |
| Base+ ANP adj. | 1.17 (1.06,1.30) | 0.002 | 1.24 (0.99,1.54) | 0.06 |
| Base+ NT-proANP adj. | 1.17 (1.06,1.30) | 0.003 | 1.22 (0.98,1.52) | 0.08 |
| Base+ NT-proBNP adj. | 1.15 (1.03,1.27) | 0.01 | 1.20 (0.97,1.50) | 0.10 |
| Base+ BNP adj. | 1.16 (1.05,1.29) | 0.004 | 1.22 (0.98,1.52) | 0.07 |
| Base+ GFR adj. | 1.15 (1.03,1.27) | 0.01 | 1.22 (0.97,1.52) | 0.08 |
| Concentric LVH (N=251) | ||||
| Base (age, sex, BMI adj.) | 1.18 (1.02,1.36) | 0.02 | 1.31 (0.96,1.77) | 0.09 |
| Base+ ANP adj. | 1.20 (1.04,1.38) | 0.01 | 1.33 (0.98,1.81) | 0.07 |
| Base+ NT-proANP adj. | 1.19 (1.03,1.38) | 0.02 | 1.33 (0.98,1.81) | 0.07 |
| Base+ NT-proBNP adj. | 1.19 (1.03,1.37) | 0.02 | 1.29 (0.94,1.75) | 0.11 |
| Base+ BNP adj. | 1.19 (1.03,1.37) | 0.02 | 1.33 (0.98,1.81) | 0.07 |
| Base+ GFR adj. | 1.23 (1.06,1.42) | 0.007 | 1.38 (1.01,1.89) | 0.04 |
| Atrial Fibrillation (N=85) | ||||
| Base (age, sex, BMI adj.) | 1.23 (1.00,1.52) | 0.05 | 1.04 (0.65,1.68) | 0.86 |
| Base+ ANP adj. | 1.34 (1.08,1.66) | 0.009 | 1.27 (0.77,2.08) | 0.35 |
| Base+ NT-proANP adj. | 1.33 (1.07,1.65) | 0.01 | 1.31 (0.80,2.17) | 0.28 |
| Base+ NT-proBNP adj. | 1.15 (0.92,1.45) | 0.21 | 1.05 (0.63,1.76) | 0.86 |
| Base+ BNP adj. | 1.27 (1.03,1.58) | 0.03 | 1.16 (0.71,1.88) | 0.56 |
| Base+ GFR adj. | 1.20 (0.97,1.49) | 0.09 | 0.99 (0.61,1.61) | 0.96 |
Correlations with morbidities when aldosterone as continuous variable (2nd column) and when aldosterone in the third tertile (4th column). Aldosterone top tertile compared to the two bottom tertiles combined. CKD= chronic kidney disease. OR= odds ratio; CI= confidence interval; SD= standard deviation. ANP = Atrial Natriuretic Peptide; NT-proANP = N-Terminal pro-Atrial Natriuretic Peptide; NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide; BNP = B-type Natriuretic Peptide. GFR = Glomerular Filtration Rate.
GFR used in the definition of the outcome. p-value significant when < 0.05.
When aldosterone divided into tertiles, the highest tertile was positively and significantly correlated with obesity after accounting for age, sex, NT-proANP, ANP, NT-proBNP, BNP and GFR. Correlation between aldosterone T3 and HTN, CKD, waist circumference and MetS was still valid after further adjustment for BMI (Table 2).
Importantly, we also performed additional multivariate model analyses after excluding subjects who could be potentially affected by primary aldosteronism (PA) and have aldosterone levels above the normal range (>16.2 ng/dL). Here, the associations between aldosterone and HTN, obesity, CKD, central obesity, MetS and high triglycerides were significant when aldosterone was analyzed as continuous variable and in the top tertile. However, we lost the association between aldosterone and cLVH and AF.
As antihypertensive medications could influence aldosterone levels, we divided the entire cohort between subjects taking antihypertensive therapy (n=526) and subjects who did not (n=1024). First, we found aldosterone significantly higher in subjects taking antihypertensive medications [medians (Q1, Q3): 6.20 (3.00, 12.00) vs 4.20 (2.50, 6.80) ng/dL, p<0.001]. Importantly, in the analysis of the entire cohort (n=1674), the associations between aldosterone and obesity (OR=1.21, 95%CI= 1.09,1.35, p=.0005), CKD (OR=1.30, 95%CI= 1.13,1.50, p=.0003), central obesity (OR=1.35, 95%CI= 1.14,1.60, p=.0005), MetS (OR=1.24, 95%CI= 1.08,1.42, p=.002) and high triglycerides (OR=1.14, 95%CI= 1.02,1.27, p=.02) remained significant after adjustment for age, sex, BMI and antihypertensive therapy when aldosterone was analyzed as continuous variable. Moreover, aldosterone third tertile was still significantly associated with obesity (OR=1.47, 95%CI= 1.16,1.85, p=.001), CKD (OR=1.61, 95%CI= 1.18,2.19, p=.003), central obesity (OR=1.45, 95%CI= 1.03,2.04, p=.03) and MetS (OR=1.42, 95%CI= 1.07,1.90, p=.02) after adjustment for age, sex, BMI and antihypertensive therapy.
Relationship between aldosterone and natriuretic peptides in the general population
We observed a highly significant inverse relationships between aldosterone T3 and NT-proBNP (p=0.0003), NT-proANP (p=0.0009) and BNP (p=0.007). An inverse trend was also present between aldosterone and ANP (p=0.07) (Table 3-a). Performing the same analysis distinguishing between subjects taking and not taking antihypertensive treatment, we found that in the treated group, aldosterone still had a significant inverse relationship with NT-proBNP, BNP and ANP (Table 3-b). However, the inverse relationship was attenuated with NT-proANP (p=0.28). In the group without antihypertensive drugs the inverse relationship was still preserved with NT-proBNP and NT-proANP and was attenuated with BNP and ANP (Table 3-c).
Table 3.
Inverse Relationship Between Aldosterone 3rd Tertile and Natriuretic Peptides.
| a) Overall Distribution (n= 1674)
| ||||
|---|---|---|---|---|
| NPs* (pg/mL) | Aldo T1 | Aldo T2 | Aldo T3 | p-value † |
| NT-proBNP | 77.1 (70.5, 84.3) | 64.3 (58.8, 70.2) | 61.0 (55.8, 66.7) | 0.0003 |
| NT-proANP | 2392 (2276, 2514) | 2119 (2018, 2224) | 2132 (2031, 2237) | 0.0009 |
| BNP | 16.8 (15.5, 18.2) | 15.3 (14.1, 16.6) | 14.4 (13.3, 15.6) | 0.007 |
| ANP | 11.5 (10.9, 12.1) | 11.3 (10.7, 11.9) | 10.7 (10.1, 11.3) | 0.07 |
| b) Subjects Taking Anti-hypertensive Therapy (n= 526)
| ||||
|---|---|---|---|---|
| NPs* (pg/mL) | Aldo T1 | Aldo T2 | Aldo T3 | p-value † |
| NT-proBNP | 150.5 (124.6,181.9) | 143.3 (118.6, 173.1) | 100.6 (87.1, 116.1) | 0.001 |
| NT-proANP | 2974 (2683, 3296) | 2942 (2658, 3256) | 2773 (2568, 2994) | 0.28 |
| BNP | 26.7 (22.5, 31.6) | 28.4 (23.9, 33.8) | 19.0 (16.7, 21.7) | 0.002 |
| ANP | 14.9 (13.5, 16.5) | 15.2 (13.7, 16.8) | 12.3 (11.4, 13.2) | 0.003 |
| c) Subjects Not-taking Anti-hypertensive Therapy (n= 1024)
| ||||
|---|---|---|---|---|
| NPs* (pg/mL) | Aldo T1 | Aldo T2 | Aldo T3 | p-value † |
| NT-proBNP | 62.1 (56.1, 68.7) | 48.6 (44.0, 53.8) | 49.3 (43.8, 55.6) | 0.004 |
| NT-proANP | 2192 (2071, 2320) | 1870 (1770, 1976) | 1834 (1717, 1958) | <.0001 |
| BNP | 14.2 (12.9, 15.5) | 12.2 (11.2, 13.4) | 12.6 (11.3, 14.0) | 0.10 |
| ANP | 10.5 (9.9, 11.2) | 10.2 (9.5, 10.8) | 9.8 (9.1, 10.6) | 0.17 |
a) in the overall distribution (n=1674); b) in subjects taking antihypertensive treatment (n=526); c) in subjects without antihypertensive treatment (n=1024). Highest aldosterone levels correlate with lowest natriuretic peptide levels. Models adjusted for age, sex and BMI. p-value significant when < 0.05.
Natriuretic Peptides as continuous variable [adjusted mean (95%CI)].
Aldosterone third tertile vs Aldosterone first tertile. NPs = natriuretic peptides; NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide; NT-proANP = N-Terminal pro-Atrial Natriuretic Peptide; BNP = B-type Natriuretic Peptide; ANP = Atrial Natriuretic Peptide.
Importantly, this analysis was also conducted after excluding subjects with aldosterone levels >16.2 ng/dL and the inverse relationship between aldosterone in the highest tertile and NPs remained unchanged throughout subgroups.
Aldosterone and the incidence of all-cause mortality in the general community
When analyzed as a continuous variable, log-aldosterone was associated with higher risk of mortality after adjusting for age, sex and BMI [HR=1.14 (95% CI: 1.03, 1.26) p=0.012)]. The c-statistic for the baseline model for mortality (including age, sex and BMI) was 0.811 (0.786, 0.836). When log-aldosterone was included in the baseline model there was little effect on the c-statistic [c = 0.813 (0.788, 0837)]. Between the NPs, NT-proBNP was the strongest predictor of mortality [baseline model + NT-proBNP, c-statistic = 0.834 (0.809, 0.859)]. When aldosterone and NT-proBNP were simultaneously included in the model, both remained significantly associated with death (aldosterone p= 0.03, NT-proBNP p<0.001). However, aldosterone did not have important effect in this model with NT-proBNP (c=0.834).
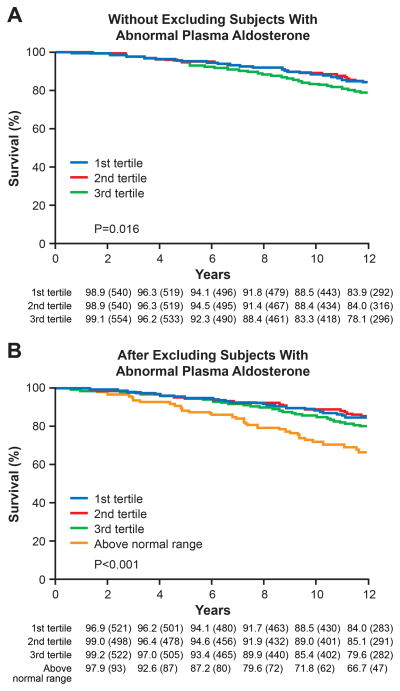
The higher occurrence of death characterized the subjects with plasma aldosterone in third tertile (p=0.016) (Figure 2-a). Importantly, when we excluded the 95 subjects with aldosterone levels > 16.2 ng/dL, the association between aldosterone in the third tertile and all cause-death was no longer present (p=0.07, figure not shown). Nonetheless, there was a very strong association between aldosterone above the normal range and all cause-death in the 12-year follow-up (p<0.001, Figure 2-b).
Figure 2. Kaplan-Meier Curve for Cumulative Incidence of All Cause-Death in the General Population.
A) Aldosterone levels according to tertiles in the total population n=1674 (without excluding subjects with abnormal plasma aldosterone). 1st tertile (blue line) and 2nd tertile (red line) do not significantly correlate with an increased incidence of mortality. Aldosterone 3rd tertile (green line) is associated with an increased incidence of mortality (p=0.016). B) Aldosterone levels according to tertiles after excluding subjects with abnormal plasma aldosterone. Blue line (1st tertile), red line (2nd tertile), and green line (3rd tertile) do not significantly correlate with an increased incidence of mortality. Importantly, aldosterone abnormal levels (>16.2 ng/dL), orange line, were associated with a significant reduction in survival in the general community (p<0.001). Age, gender, BMI adjusted HR compared to 1st tertile: T2: 0.95 (0.70, 1.30), p=0.74; T3: 1.20 (0.90, 1.60), p=0.21; above normal range: 1.54 (1.03, 2.30), p=0.03. Follow-up results are truncated after 12-year. P-value (unadjusted log-rank test) significant when < 0.05.
DISCUSSION
Our study is the first to report the associations between plasma aldosterone levels and hypertension, obesity, high triglycerides, waist circumference, MetS, CKD, AF and cLVH in a randomly selected sample of the general community. Specifically, we found that elevated plasma aldosterone, within the normal range, was strongly associated with HTN, obesity, CKD, central obesity, MetS and hypertriglyceridemia. We also demonstrated that plasma aldosterone levels in individuals treated with antihypertensives were higher compared to subjects without treatment. Importantly, we observed a highly significant inverse relationship between aldosterone and NP levels, and notably this trend remained preserved even after adjustment for antihypertensive drugs. Finally, in a twelve-year follow-up, we found that higher plasma aldosterone, particularly above the normal range, predicted an increase in mortality.
Several previous studies have investigated the associations between aldosterone and cardiovascular and metabolic diseases in selected cohorts of volunteers5, 11, 12, 14–17 without excluding subjects with possible hyperaldosteronism. Indeed, in non-hypertensive subjects free of renal failure, cardiovascular disease and medications, Vasan et al. reported an association between aldosterone levels and the development of new onset hypertension11. Furthermore, these authors observed a trend for increased risk of developing an elevation in BP or HTN across aldosterone quartiles, and reported significant association between aldosterone fourth quartile and future onset of HTN. However, the highest quartile may have included subjects with abnormal aldosterone levels. Moreover, in another cohort without cardiovascular disease and renal dysfunction, aldosterone correlated with future development of MetS5, whereas an association between aldosterone and incidence of CKD was shown in a cohort of subjects with normal renal function15. Studies conducted in other selected cohorts revealed that aldosterone was associated with cLHV14, BMI16, waist circumference, triglycerides17 and both cardiovascular and all-cause mortality18.
The association between aldosterone and hypertension found in our study as well as in other epidemiological studies is supported by the biological effect of the hormone, which includes sodium and water retention1. The inappropriate activation of mineralocorticoid receptors by aldosterone as well as cortisol also induces renal organ damage, cardiac hypertrophy and arrhythmias, and pro-fibrotic and pro-inflammatory actions2, 19–22. Indeed, the mineralocorticoid receptor can be activated also by glucocorticoids, and circulating cortisol levels are physiologically higher than aldosterone23. Moreover, salt intake plays an important role in the aldosterone-mediated organ damage. Common in U.S. society as well as in Minnesota24, high salt diet can potentiate the negative effects of aldosterone via activation of the Rho family member Rac125. Importantly, in Yanomamo Indians, very low salt intake is characterized by high plasma renin and aldosterone levels without the presence of CV disease26.
Our finding of higher aldosterone levels in subjects taking antihypertensive drugs warrants further considerations. Medications such as diuretics could have stimulated aldosterone release, while others such as ACEIs and ARBs may have reduced these levels. A possible mechanistic explanation for this finding may be related to the phenomenon of the “aldosterone breakthrough”, where the cleavage of angiotensin I to angiotensin II through enzymes different from ACE can increase aldosterone levels despite therapy27–29. Further, one could speculate that aldosterone also represents a causative mechanism for both hypertension and organ damage.
In the current study, we observed an inverse relationship between aldosterone and NPs. ANP and BNP have emerged as inhibitors of aldosterone through GC-A receptor activation in the adrenals30–33. This finding of reduced NP levels with higher circulating aldosterone is consistent with the pathophysiology and the roles played by fat, aldosterone and NPs. Importantly, the strong association between aldosterone and metabolic disease can be explained by the aldosterone’s ability to mediate adipocyte activation and lipogenesis7, 9, 34. Indeed, higher aldosterone levels have been found in metabolic syndrome and obesity35, 36, both conditions characterized by increased fat tissue and reduced NP levels. Specifically, the visceral white adipose tissue largely expresses NP clearance receptors which then may be increased in metabolic syndrome and obesity8, reducing NP levels and possibly permitting activation of aldosterone. Further, adipose tissue possesses its own renin-angiotensin-aldosterone system7, 36 and more adipocytes could increase aldosterone production. Interestingly, it has been shown that the expression in the kidney of mineralocorticoid receptors was increased in mice fed a high fat diet37 which could also contribute to sodium retention and increased blood pressure. Further, aldosterone may be involved in the modulation of insulin secretion and signaling2, 38 contributing to the development of insulin resistance and diabetes. In addition, mineralocorticoid receptor antagonism has beneficial effects on LV function, structure and fibrosis in subjects with metabolic syndrome39. Thus, there is strong evidence for interaction in metabolic syndrome and obesity between aldosterone and NP system. Considering that our cohort had a mean BMI higher than 28 kg/m2, with more than a third having central obesity, it is plausible that an increased metabolism of NPs can be present with an enhanced aldosterone production. As reported in Table 3, circulating NPs and their changes by increasing tertiles of aldosterone are similar in the overall distribution (n=1674) and those subjects not taking antihypertensive therapy (n=1024). Specifically, in these two cohorts, levels of NPs are similar in tertile 2 and 3 of aldosterone, and in these two tertiles NPs are lower compared to tertile 1 of aldosterone. In contrast, in those subjects taking antihypertensives (n=526), NPs are similar in tertile 1 and 2 of aldosterone, and both tertiles are higher compared to tertile 3 of aldosterone. The significance of this differential reduction in NP levels by increasing tertiles of aldosterone in the three groups remains unknown and requires further investigation. However, we speculate that the pattern of NP levels according to aldosterone tertiles is similar in the overall distribution and subjects not taking antihypertensives because these two cohorts mostly overlap. By stratifying our study population for antihypertensive drugs we accounted for an important confounding factor that might be related to the presence of myocardial remodeling and stretch, which significantly influence NPs production.
When we evaluated the predicted value of aldosterone in our cohort, we found aldosterone in the third tertile to be prognostic of mortality over the 12-year follow-up. When PA-likely subjects were excluded (aldosterone levels > 16.2 ng/dl, approximately 6% of the whole cohort) this association was no longer present. We did however observe that abnormal aldosterone levels were associated with the reduction in survival that began at approximately three years after measurement. Therefore, it seems that PA-likely subjects were the primary driver of the observed association between aldosterone in the third tertile and all cause-death in our entire cohort. It should also be noted that when we excluded those individuals with abnormal aldosterone levels, we lost the associations between aldosterone and cLVH and AF. However, these associations were not among the strongest ones as, when we adjusted for antihypertensives use, we lost the same associations as well. Taken together the current studies suggest that there is a strong and robust association between aldosterone and cardiovascular, renal and metabolic disease, even with aldosterone in the normal range, but this is not associated with increased mortality which occurs only in the setting of elevated levels of aldosterone. While our data does not support the hypothesis that higher, yet normal, aldosterone levels are associated with higher incidence of death, to our knowledge, this is the first general community study to demonstrate an increased incidence of all cause-death when aldosterone levels are above the normal range, regardless of the cause of the elevated levels.
Despite the availability of a normal range of plasma aldosterone which was established in the paper by Mayes et al. 13 as well as in the commercial radioimmunoassay we employed (3–16 ng/dL and 1–16 ng/dL respectively, in healthy subjects in supine position without restricted salt intake), we redefined the range in our cohort of the general population utilizing rigorous criteria. In our investigation, subjects were carefully analyzed clinically, biochemically and echocardiographically permitting identification of truly healthy individuals to define normal aldosterone values in the general community with subjects 45 years old and older. By using our normal range (2.5–16.2 ng/dL), which is almost identical to the one reported in the paper by Mayes et al. as well as in the assay kit, 94% of the population had aldosterone levels within the normal values. Thus, this provides reassurance for our defined normal values.
When considering therapeutic or preventive treatments for cardiorenal and metabolic diseases in the general community, the use of plasma aldosterone to identify a high risk group could optimize strategies. Also, one could speculate that postponing anti-aldosterone therapy until overt disease occurs may not be optimal. The finding of higher aldosterone levels despite antihypertensive therapy also underscores the need to antagonize the negative effects of aldosterone in the setting of hypertension in the general community. Recognizing the inverse relationship between elevated aldosterone and decreased NP levels, the addition of chronic GC-A agonists with aldosterone antagonism, may be warranted and needs further studies. Indeed, a co-therapeutic strategy to compensate the imbalance between these two counter-regulatory systems is worthy of further investigation40, 41
The current study has several strengths. First, our cohort consisted of a large and randomly selected group of subjects and not volunteers. Secondly, our subjects were well-characterized, having had in-depth echocardiographic data and a long follow-up. Lastly, our cohort consisted of 45 years and older subjects, characterizing a sample of individuals in the general community at increased cardiorenal and metabolic risk. This study also has limitations. Specifically, we were unable to obtain data of known regulators of aldosterone, such as plasma renin activity, dietary sodium, volume depletion, serum potassium, stress and diurnal rhythms. We also do not have any data on urinary sodium excretion and plasma cortisol levels. Further, the majority of subjects were Caucasian and it may not be completely possible to extend our finding to other ethnic groups. Finally, our investigation is a cross-sectional and longitudinal study therefore we cannot establish causality for the associations observed but we can only speculate regarding the possible biological mechanisms that might have caused them.
Perspectives
Our results confirm and extend previous findings regarding positive associations between aldosterone and cardiorenal and metabolic disease. Specifically we observed that higher, normal range, aldosterone levels were strongly associated with HTN, obesity, high triglycerides, waist circumference, MetS and CKD in the general community. Importantly, the majority of these remarkable associations remained significant after adjusting for anti-hypertensive medications. Interestingly, we observed aldosterone levels higher in subjects taking antihypertensive drugs, thus indicating that current antihypertensive therapies may not be effective in decreasing aldosterone levels. Further, aldosterone had a significant inverse relationship with NPs and, when above the normal range, aldosterone was strongly associated with increased mortality. Together, these findings suggest that aldosterone may be a mediator and biomarker of cardiorenal and metabolic disease and importantly support the possibility of a co-therapeutic and preventative strategy using GC-A agonists together with mineralocorticoid receptor antagonists in high-risk subjects within the general population.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
In a randomly select sample of the general community, elevated plasma aldosterone, even within the normal range, is strongly associated with hypertension, obesity, high triglycerides, waist circumference, MetS, and CKD.
Aldosterone above the normal range can significantly predict reduced survival beginning three years after measurement.
Higher aldosterone levels correlate with lower natriuretic peptide levels in our cohort.
What is relevant?
Aldosterone may be a mediator and biomarker of cardiorenal and metabolic disease.
Aldosterone has an inverse relationship with the natriuretic peptides and this supports the relative deficiency of natriuretic peptides shown in dysmetabolic states, such as obesity and MetS, in which increased aldosterone levels have also been documented.
When above the normal range, aldosterone is strongly associated with increased incidence of all cause-mortality.
Treating these high-risk subjects with natriuretic peptides in addition to mineralocorticoid receptor antagonists, to inhibit aldosterone and compensate the NPs deficiency, may be considered.
Summary
Our results confirm and extend previous findings regarding positive associations between higher aldosterone levels and cardiorenal and metabolic diseases. Furthermore, our findings suggest that elevated aldosterone levels, even within the normal values, may identify high risk subjects in the general population. However, additional studies are needed to confirm our data and the potential use of GC-A agonists and aldosterone antagonists as a co-therapeutic strategy to prevent and treat cardiorenal and metabolic diseases in the general community.
Acknowledgments
Source of Funding: NIH PO1 HL76611 grant.
Footnotes
Conflicts of Interest/Disclosures: none.
Author Contributions:
Design and conduct of the study: AB, CGS, KRB, RJR, JCB.
Acquisition of data: DMH, RJR, JCB.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: AB, VC, AC, SJS, RS, PDF, JCB.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: CGS, KRB.
Obtained funding: RJR, JCB.
Administrative, technical, or material support: DMH, JCB.
Study supervision: AB, JCB.
References
- 1.Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. 2012;350:151–162. doi: 10.1016/j.mce.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sowers JR, Whaley-Connell A, Epstein M. Narrative review: The emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Int Med. 2009;150:776–783. doi: 10.7326/0003-4819-150-11-200906020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9:459–469. doi: 10.1038/nrneph.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992;120:893–901. [PubMed] [Google Scholar]
- 5.Ingelsson E, Pencina MJ, Tofler GH, Benjamin EJ, Lanier KJ, Jacques PF, Fox CS, Meigs JB, Levy D, Larson MG, Selhub J, D’Agostino RB, Sr, Wang TJ, Vasan RS. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: The framingham offspring study. Circulation. 2007;116:984–992. doi: 10.1161/CIRCULATIONAHA.107.708537. [DOI] [PubMed] [Google Scholar]
- 6.Musani SK, Vasan RS, Bidulescu A, Liu J, Xanthakis V, Sims M, Gawalapu RK, Samdarshi TE, Steffes M, Taylor HA, Fox ER. Aldosterone, c-reactive protein, and plasma b-type natriuretic peptide are associated with the development of metabolic syndrome and longitudinal changes in metabolic syndrome components: Findings from the jackson heart study. Diabetes Care. 2013;36:3084–3092. doi: 10.2337/dc12-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boscaro M, Giacchetti G, Ronconi V. Visceral adipose tissue: Emerging role of gluco- and mineralocorticoid hormones in the setting of cardiometabolic alterations. Ann N Y Acad Sci. 2012;1264:87–102. doi: 10.1111/j.1749-6632.2012.06597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarzani R, Salvi F, Dessi-Fulgheri P, Rappelli A. Renin-angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: An integrated view in humans. J Hypertens. 2008;26:831–843. doi: 10.1097/HJH.0b013e3282f624a0. [DOI] [PubMed] [Google Scholar]
- 9.Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Correa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, Touyz RM. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: Implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59:1069–1078. doi: 10.1161/HYPERTENSIONAHA.111.190223. [DOI] [PubMed] [Google Scholar]
- 10.Calhoun DA. Aldosteronism and hypertension. Clin J Am Soc Nephrol. 2006;1:1039–1045. doi: 10.2215/CJN.01060306. [DOI] [PubMed] [Google Scholar]
- 11.Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, Benjamin EJ, Levy D. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. doi: 10.1056/NEJMoa033263. [DOI] [PubMed] [Google Scholar]
- 12.Bochud M, Nussberger J, Bovet P, Maillard MR, Elston RC, Paccaud F, Shamlaye C, Burnier M. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48:239–245. doi: 10.1161/01.HYP.0000231338.41548.fc. [DOI] [PubMed] [Google Scholar]
- 13.Mayes D, Furuyama S, Kem DC, Nugent CA. A radioimmunoassay for plasma aldosterone. J Clin Endocrinol Metab. 1970;30:682–685. doi: 10.1210/jcem-30-5-682. [DOI] [PubMed] [Google Scholar]
- 14.Velagaleti RS, Gona P, Levy D, Aragam J, Larson MG, Tofler GH, Lieb W, Wang TJ, Benjamin EJ, Vasan RS. Relations of biomarkers representing distinct biological pathways to left ventricular geometry. Circulation. 2008;118:2252–2258. doi: 10.1161/CIRCULATIONAHA.108.817411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox CS, Gona P, Larson MG, Selhub J, Tofler G, Hwang SJ, Meigs JB, Levy D, Wang TJ, Jacques PF, Benjamin EJ, Vasan RS. A multi-marker approach to predict incident ckd and microalbuminuria. J Am Soc Nephrol. 2010;21:2143–2149. doi: 10.1681/ASN.2010010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi GP, Belfiore A, Bernini G, Fabris B, Caridi G, Ferri C, Giacchetti G, Letizia C, Maccario M, Mannelli M, Palumbo G, Patalano A, Rizzoni D, Rossi E, Pessina AC, Mantero F. Body mass index predicts plasma aldosterone concentrations in overweight-obese primary hypertensive patients. J Clin Endocrinol Metab. 2008;93:2566–2571. doi: 10.1210/jc.2008-0251. [DOI] [PubMed] [Google Scholar]
- 17.Kidambi S, Kotchen JM, Grim CE, Raff H, Mao J, Singh RJ, Kotchen TA. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension. 2007;49:704–711. doi: 10.1161/01.HYP.0000253258.36141.c7. [DOI] [PubMed] [Google Scholar]
- 18.Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, Marz W. Plasma aldosterone levels are associated with increased cardiovascular mortality: The ludwigshafen risk and cardiovascular health (luric) study. Eur Heart J. 2010;31:1237–1247. doi: 10.1093/eurheartj/ehq019. [DOI] [PubMed] [Google Scholar]
- 19.Whaley-Connell A, Johnson MS, Sowers JR. Aldosterone: Role in the cardiometabolic syndrome and resistant hypertension. Progr Cardiovasc Dis. 2010;52:401–409. doi: 10.1016/j.pcad.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocha R, Stier CT, Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: A mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–3878. doi: 10.1210/endo.141.10.7711. [DOI] [PubMed] [Google Scholar]
- 21.Mayyas F, Alzoubi KH, Van Wagoner DR. Impact of aldosterone antagonists on the substrate for atrial fibrillation: Aldosterone promotes oxidative stress and atrial structural/electrical remodeling. Int J Cardiol. 2013;168:5135–5142. doi: 10.1016/j.ijcard.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi G, Boscaro M, Ronconi V, Funder JW. Aldosterone as a cardiovascular risk factor. Trends Endocrinol Metab. 2005;16:104–107. doi: 10.1016/j.tem.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Funder JW. Minireview: Aldosterone and mineralocorticoid receptors: Past, present, and future. Endocrinology. 2010;151:5098–5102. doi: 10.1210/en.2010-0465. [DOI] [PubMed] [Google Scholar]
- 24.Meyer KA, Harnack LJ, Luepker RV, Zhou X, Jacobs DR, Steffen LM. Twenty-two-year population trends in sodium and potassium consumption: The minnesota heart survey. J Am Heart Assoc. 2013;2:e000478. doi: 10.1161/JAHA.113.000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, Takai Y, Ishikawa A, Shimosawa T, Ando K, Nagase M, Fujita T. Rac1 gtpase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121:3233–3243. doi: 10.1172/JCI43124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the yanomamo indians, a “no-salt” culture. Circulation. 1975;52:146–151. doi: 10.1161/01.cir.52.1.146. [DOI] [PubMed] [Google Scholar]
- 27.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–492. doi: 10.1038/ncpneph0575. [DOI] [PubMed] [Google Scholar]
- 28.Sarzani R, Guerra F, Mancinelli L, Buglioni A, Franchi E, Dessi-Fulgheri P. Plasma aldosterone is increased in class 2 and 3 obese essential hypertensive patients despite drug treatment. AmJ Hypertens. 2012;25:818–826. doi: 10.1038/ajh.2012.47. [DOI] [PubMed] [Google Scholar]
- 29.Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the randomized aldactone evaluation study [rales]) AmJ Cardiol. 1996;78:902–907. doi: 10.1016/s0002-9149(96)00465-1. [DOI] [PubMed] [Google Scholar]
- 30.Richards AM. The renin-angiotensin-aldosterone system and the cardiac natriuretic peptides. Heart. 1996;76:36–44. doi: 10.1136/hrt.76.3_suppl_3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 32.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 33.Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med. 2001;79:21–29. doi: 10.1007/s001090000144. [DOI] [PubMed] [Google Scholar]
- 34.Caprio M, Antelmi A, Chetrite G, Muscat A, Mammi C, Marzolla V, Fabbri A, Zennaro MC, Feve B. Antiadipogenic effects of the mineralocorticoid receptor antagonist drospirenone: Potential implications for the treatment of metabolic syndrome. Endocrinology. 2011;152:113–125. doi: 10.1210/en.2010-0674. [DOI] [PubMed] [Google Scholar]
- 35.Calhoun DA, Sharma K. The role of aldosteronism in causing obesity-related cardiovascular risk. Cardiol Clin. 2010;28:517–527. doi: 10.1016/j.ccl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briet M, Schiffrin EL. The role of aldosterone in the metabolic syndrome. Curr Hypertens Rep. 2011;13:163–172. doi: 10.1007/s11906-011-0182-2. [DOI] [PubMed] [Google Scholar]
- 37.Tokuyama H, Wakino S, Hara Y, Washida N, Fujimura K, Hosoya K, Yoshioka K, Hasegawa K, Minakuchi H, Homma K, Hayashi K, Itoh H. Role of mineralocorticoid receptor/rho/rho-kinase pathway in obesity-related renal injury. Int J Obes (Lond) 2012;36:1062–1071. doi: 10.1038/ijo.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luther JM, Luo P, Kreger MT, Brissova M, Dai C, Whitfield TT, Kim HS, Wasserman DH, Powers AC, Brown NJ. Aldosterone decreases glucose-stimulated insulin secretion in vivo in mice and in murine islets. Diabetologia. 2011;54:2152–2163. doi: 10.1007/s00125-011-2158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, O’Moore-Sullivan T, Marwick TH. A randomized study of the beneficial effects of aldosterone antagonism on lv function, structure, and fibrosis markers in metabolic syndrome. J Am Coll Cardiol Img. 2011;4:1239–1249. doi: 10.1016/j.jcmg.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, Burnett JC., Jr Novel protein therapeutics for systolic heart failure: Chronic subcutaneous b-type natriuretic peptide. J Am Coll Cardiol. 2012;60:2305–2312. doi: 10.1016/j.jacc.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 mapk to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.