Abstract
The zebrafish olfactory system is a valuable model for examining neural regeneration after damage due to the remarkable plasticity of this sensory system and of fish species. We applied detergent to the olfactory organ and examined the effects on both morphology and function of the olfactory system in adult zebrafish. Olfactory organs were treated once with Triton X-100 unilaterally to study glomerular innervation patterns or bilaterally to study odor detection. Fish were allowed to recover for 4–10 days and were compared to untreated control fish. Axonal projections were analyzed using whole mount immunocytochemistry with anti-keyhole limpet hemocyanin, a marker of olfactory axons in teleosts. Chemical lesioning of the olfactory organ with a single dose of Triton X-100 had profound effects on glomerular distribution in the olfactory bulb at 4 days after treatment, with the most significant effects in the medial region of the bulb. Glomeruli had returned by 7 days post-treatment. Analysis of the ability of the fish to detect cocktails of amino acids or bile salts consisted of counting the number of turns the fish made before and after odorant delivery. Control fish turned more after exposure to both odorants. Fish tested 4 and 7 days after chemical lesioning made more turns in response to amino acids but did not respond to bile salts. At 10 days post-lesion, these fish had regained the ability to detect bile salts. Thus, the changes seen in bulbar innervation patterns correlated to odorant-mediated behavior. We show that the adult zebrafish brain has the capacity to recover rapidly from detergent damage of the olfactory epithelium, with both glomerular distribution and odorant-mediated behavior returning in 10 days.
Keywords: Olfactory sensory neuron, Chemical lesion, Triton X-100, Anti-keyhole limpet hemocyanin, Teleost, Plasticity
1
The olfactory system is a useful model for studies on neuroplasticity because of its ability to recover from lesion, in part due to the inherent neuronal turnover seen in the olfactory organ. Various methods of chemical lesioning have been used to examine the mechanisms by which the olfactory system responds to damage. Exposure of the olfactory epithelium to a variety of chemicals can eliminate the sensory input to the olfactory bulb by destroying the olfactory sensory neurons (OSNs). The olfactory epithelium can replenish itself, reinnervate the olfactory bulb, and restore function (Schwob et al., 1995; Herzog and Otto, 1999; Schwob et al., 1999; Paskin and Byrd-Jacobs, 2012). While a number of toxic chemicals have been used, Triton X-100 application is a common technique in studies examining the degeneration and regeneration of the olfactory system. Application of the detergent to the nasal cavity destroys OSNs, which temporarily reduces afferent input to the olfactory bulb (Nadi et al., 1981; Baker et al., 1983; Cummings et al., 2000).
A number of studies have examined the effects of chemicals on the fish olfactory system, due to concerns about pollution and toxins in the aquatic environment (Tierney et al., 2010). Application of Triton X-100 to the olfactory organ of catfish damages the olfactory epithelium to various extents, depending on the concentration (Cancalon, 1982; 1983). Low doses of the detergent affect only the superficial portions of the cells of the olfactory epithelium, while high doses destroy both sensory and non-sensory regions of the olfactory organ. In zebrafish, intranasal infusion of Triton X-100 causes immediate disruption of the olfactory epithelium (Iqbal and Byrd-Jacobs, 2010). One day post-lesion the olfactory epithelium is significantly thinner and has an apparent loss of most OSNs. The thickness of the epithelium progresses with return of epithelial depth and density of OSNs by five days post-lesion, and rosette morphology returns to near control levels within seven days. This time course is more rapid than in mammals (Verhaagen et al., 1990; Cummings et al., 2000) and larger fish (Cancalon, 1983). Chronic treatment with Triton X-100 severely disrupts rosette morphology and removes most of the OSNs, although some subsets of OSNs appear more affected than others (Paskin et al., 2011; Paskin and Byrd-Jacobs, 2012).
Zebrafish possess three physiologically distinct OSNs, which are dispersed throughout the olfactory epithelium (Hansen and Zieske, 1998). In general, ciliated OSNs detect bile salts and pheromones (Koide et al., 2009), microvillous OSNs detect amino acids and nucelotides (Lipschitz and Michel, 2002), and crypt OSNs appear to detect pheromones, although these cells are much less understood (Germana et al., 2004; Hamdani et al., 2008). Interestingly, chronic Triton X-100 exposure appears to affect ciliated OSNs primarily, while some microvillous and crypt neurons survive the treatment (Paskin et al., 2011; Paskin and Byrd-Jacobs, 2012).
The axons of the OSNs project to the olfactory bulb in the brain, where they relay sensory information to projection neurons and interneurons in discrete glomeruli. Adult zebrafish have approximately 140 glomeruli per olfactory bulb, a subset of which are highly stereotyped and distinguishable (Baier and Korsching, 1994; Braubach et al., 2012). Glomeruli in the olfactory bulb contain the axonal projections of a single OSN subtype and group in functional zones (Li et al., 2005; Sato et al., 2007; Yaksi et al., 2007). Ciliated OSNs project to the dorsal and medial regions of the olfactory bulb, microvillous OSNs project to the lateral and ventrolateral regions of the bulb (Friedrich and Korsching, 1997; Sato et al., 2005), and crypt neurons project to a single glomerulus in the dorsomedial group (Ahuja et al., 2013). Consequently, the medial bulb regions process social and reproductive odors (Li et al., 2005; Yaksi et al., 2007) whereas the lateral region of the olfactory bulb tend to process feeding behavior (Li et al., 2005; Yaksi et al., 2007; Koide et al., 2009). Thus, the three zebrafish OSN subtypes are distinct in anatomy, physiology, and behavior.
The olfactory bulb is also affected by detergent application to the peripheral olfactory organ, since its afferent input is reduced. Following chronic application of Triton X-100 over three weeks, there is a reduction in olfactory bulb volume (Paskin et al., 2011). The glomeruli in the olfactory bulb that receive innervation from ciliated OSNs are lost, while those containing the axons of the other OSN subtypes show less damage (Paskin and Byrd-Jacobs, 2012). These fish lose the ability to detect bile salts but retain the ability to perceive amino acids. It is unclear if these results are due to accumulated damage from repeated exposure to the detergent.
In the current study, we examined whether a single intranasal infusion with Triton X-100 would yield results similar to the chronic exposure to the detergent. We hypothesized that the axonal projections of ciliated OSNs, and the glomeruli they innervate, would be most affected by detergent exposure, with degradation and recovery within a week. We also wanted to see if regeneration of the OSNs would result in regeneration of glomeruli in the same place and with the same morphology. From this, we also hypothesized that if chemical lesioning causes glomerular disruption, olfactory-mediated behavior would also be affected, with responses to odors mediated by ciliated OSNs most affected. Our work provides a model for rapid degeneration and regeneration of olfactory innervation patterns and of odorant-mediated behavior in a vertebrate.
2 Experimental Procedures
Adult zebrafish of both sexes were generously donated by R. Warga and D. Kane. Fish were maintained in 10–15 gallon aquaria and fed flake food twice daily. All experimental procedures were approved by the WMU Institutional Animal Care and Use Committee.
2.1 Chemical Lesioning of the Olfactory Epithelium
Adult zebrafish were anesthetized in tricaine (0.03% MS222, Sigma, St. Louis, MO, USA), a barrier of petroleum jelly was placed between the two nasal cavities to prevent crossover of the chemical to the contralateral organ, and the olfactory organ was exposed to a solution of 0.7% Triton X-100 and 0.005% methylene blue in phosphate buffered saline (PBS) for two minutes. For glomerular analysis, the detergent solution was applied to the right nasal cavity, while the left nasal cavity was not treated to serve as an internal control. For the behavior study, both nasal cavities were exposed to the detergent solution. Treated fish were allowed to survive for either 4, 7, or 10 days. Untreated, control fish were also processed. Fish were euthanized with an overdose of tricaine, perfused transcardially with PBS, and incubated in 2% paraformaldehyde for 24 hours at 4 °C.
2.2 Whole Mount Immunocytochemistry
Dissected brains were labeled with an antibody to keyhole limpet hemocyanin (KLH) to identify the projections of olfactory sensory neuron axons in the olfactory bulb, using a method similar to Braubach and colleagues (Braubach et al., 2012). Briefly, whole brains were washed in PBS, put in a PBS blocking solution (PBS-T; 0.25% Triton X-100, 2% dimethyl sulfoxide, 1% bovine serum albumin, and 1% goat serum in PBS), and incubated in anti-KLH (Sigma; 1:200 in PBS-T) for 14 days at 4 °C. Brains were then washed in PBS-T, soaked in Alexa Flour 594-conjugated anti-rabbit secondary antibody (Invitrogen, Carlsbad, CA, USA; 1:200 in PBS-T) for 3–14 days, and rinsed thoroughly in PBS before being stored in a 3:1 glycerol-Tris solution containing 2% propyl gallate until viewing.
2.3 Glomerular Analysis
Antibody-labeled brains were mounted in 1.5% agar between two coverslips and imaged using a Zeiss LSM 510 laser scanning confocal microscope. Z-stacks of 50–150 µm were collected from dorsal and ventral views and examined as single slices or as image projections. The olfactory axon component of glomeruli was identified from previously established anatomical and spatial glomerular maps in zebrafish (Baier and Korsching, 1994; Braubach et al., 2012). All glomeruli were individually examined in multiple views and at multiple magnifications from control (n=3), 4 day post-treatment (n=6), and 7 day post-treatment (n=6) fish. Data analysis entailed rating glomeruli based on presence and completeness. The glomerular nomenclature of Braubach et al. (2012) was used.
2.4 Behavioral Assay
Fish were food deprived for 48 hours before testing their olfactory ability. Control and treated fish were individually transferred to an experimental tank consisting of a five-gallon bucket with a center drainpipe and surgical tubes mounted to the inside wall on opposite sides, as previously described (Paskin and Byrd-Jacobs, 2012). A video camera was mounted above the tank to record fish behavior before and after odor exposure. Fish were allowed to acclimate for 90 minutes. An odorant mixture of either amino acids (alanine, cysteine, histidine, methionine, and valine; all from Sigma; 100 µM each) or bile salts (taurocholic acid, taurodeoxycholic acid, taurochenodeoxycholate, lithocholic acid, glycocholic acid, and glycochenodeoxycholate; all from Sigma; 100 µM each) was delivered via one tube while another tube simultaneously delivered water to the opposite side of the tank. The side of the tank receiving the odor mixture and the type of odor given in a trial was randomly assigned. The number of turns the fish made 30 seconds prior to and 30 seconds after odorant delivery was then compared. An anosmic control group consisted of fish that had both nasal cavities occluded with vetbond tissue adhesive (3M, Maplewood, MN, USA) and fish that had both olfactory organs ablated with a small cautery iron. Sample sizes for each test scenario ranged from 5–17 trials from a minimum of 5 fish per treatment group. Pre-odor turning behavior was compared between groups with analysis of variance followed by Tukey’s multiple comparison test. Comparisons of pre-odor and odor trial behavior between groups were made using a two-way repeated measures analysis of variance with a Bonferroni posttest. A significance level of 0.05 was used.
3 Results
3.1 Time course of the effects of detergent ablation of the olfactory epithelium on glomeruli in the olfactory bulb
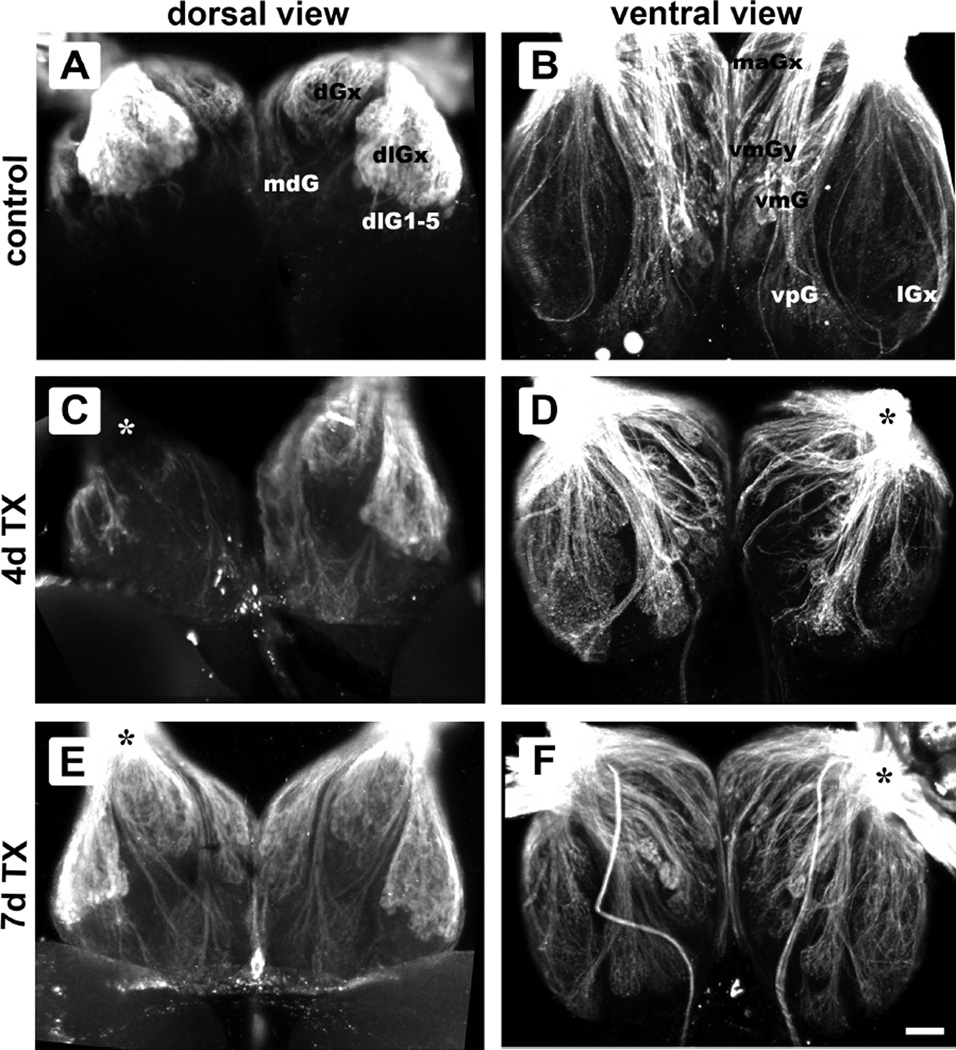
Anti-KLH allowed visualization of most, if not all, OSN axons from the olfactory rosette as they spread over the surface of the bulb and terminated in the glomerular layer. Only the axonal component of the glomerulus was considered in this study. In untreated control fish, each glomerulus consisted of a distinct axonal bundle terminating in a spheroidal structure made of axonal fibers (Fig. 1A, B). The size, shape, and location of specific glomeruli appeared to be the same in the left and right olfactory bulbs, as previously reported (Baier and Korsching, 1994; Braubach et al., 2012). Multiple views of the whole-mounted brain of fish that survived 4 days after Triton X-100 application to the olfactory epithelium showed that the olfactory bulb on the treated side had gross morphological disruption of axonal projections, particularly in the medial region (Fig. 1C, D). The untreated, internal control bulb had glomerular patterns similar to untreated fish and seemed to possess the full complement of glomeruli (Fig. 1C, D). By 7 days post-lesion, glomerular patterns appeared to have largely returned, with no obvious differences between treated and internal control bulbs (Fig. 1E, F). Also observed were KLH-immunoreactive extrabulbar primary olfactory projections coursing over the surface of the bulb to the telencephalon (Fig. 1F) as described by Gayoso and colleagues (Gayoso et al., 2011).
Figure 1.
Confocal images of anti-KLH immunoreactivity in the olfactory bulb following intranasal irrigation with Triton X-100 showing glomerular innervation patterns in dorsal (A, C, E) and ventral (B, D, F) views. In untreated control fish, glomerular patterns were similar between bulbs, when observed from either the dorsal (A) or ventral (B) aspect. A subset of glomerular groups are labeled. The olfactory bulbs of fish 4 days post-lesion (C, D) had axonal projections that were less organized and appeared disrupted in the lesioned side (asterisk), while the internal control bulb seemed to possess the full complement of glomeruli. Seven days after detergent treatment (E, F), the glomerular patterns of the bulb on the treated side (asterisk) seemed to have largely returned to control morphology. Sometimes visible are extrabulbar primary olfactory projections coursing over the ventral surface of the olfactory bulb directly to the telencephalon (F). Scale bar = 50 µm for all.
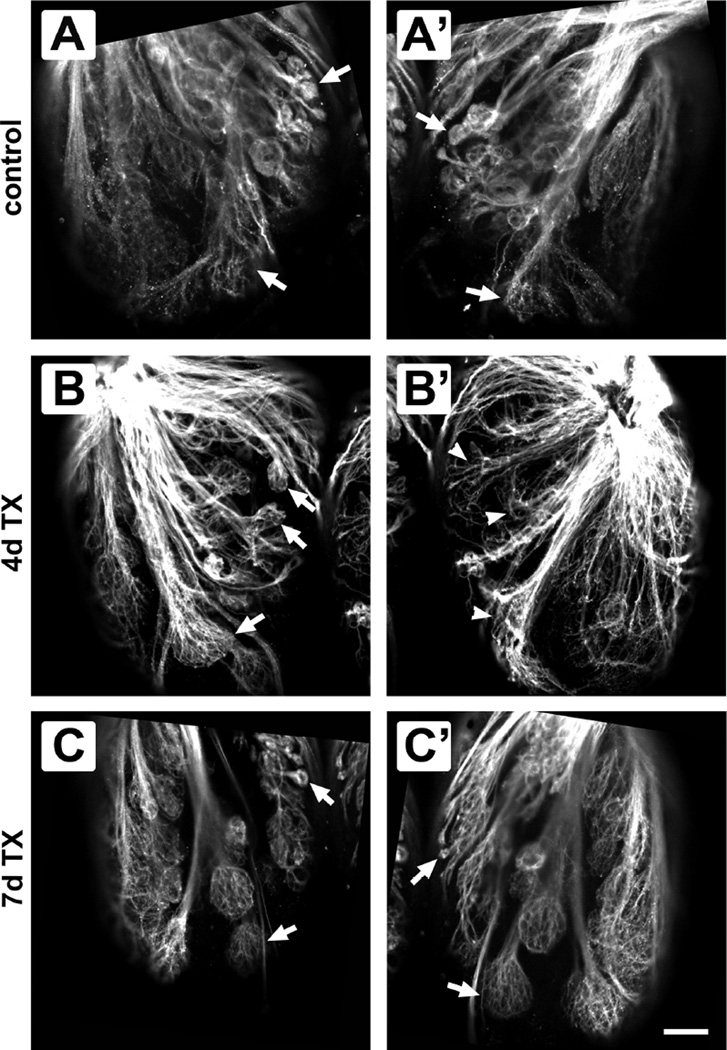
Closer examination of the axonal projections within the medial region of olfactory bulbs at higher magnification allowed further examination of individual glomeruli. In control fish, ventral views allowed direct comparison of specific glomeruli, and no noticeable differences were noted between bulbs (Fig. 2A, A’). Glomerular labeling did not appear equivalent between the olfactory bulb on the lesioned side and the internal control bulb 4 days after detergent treatment (Fig. 2B, B’). Glomeruli in the olfactory bulb on the treated side appeared less organized than those in the internal control bulb, with an apparent loss of fibers and absence of spreading into a spheroidal shape at their axonal termini. In fish that were allowed to recover for 7 days after detergent ablation of the olfactory epithelium, the glomeruli seemed to have regained their normal morphology and were similar between internal control and treated-side olfactory bulbs (Fig. 2C, C’).
Figure 2.
Comparison of ventral glomerular patterns between olfactory bulbs of control and Triton X-100-treated fish. In untreated control fish, glomeruli (arrows) appeared the same between the left (A) and right (A’) olfactory bulbs. Four days after detergent ablation of the olfactory epithelium, glomeruli in the treated bulb (B’) seemed disrupted when compared to the internal control bulb (B). Even with this disruption, the same glomeruli in the untreated bulb (arrows in B) could be identified in the experimental bulb (arrowheads in B’). Seven days after treatment, glomeruli in the internal control side (C) and the treated side (C’) had similar morphology, and the same glomeruli were seen in both olfactory bulbs (arrows). Scale bar = 50 µm for all.
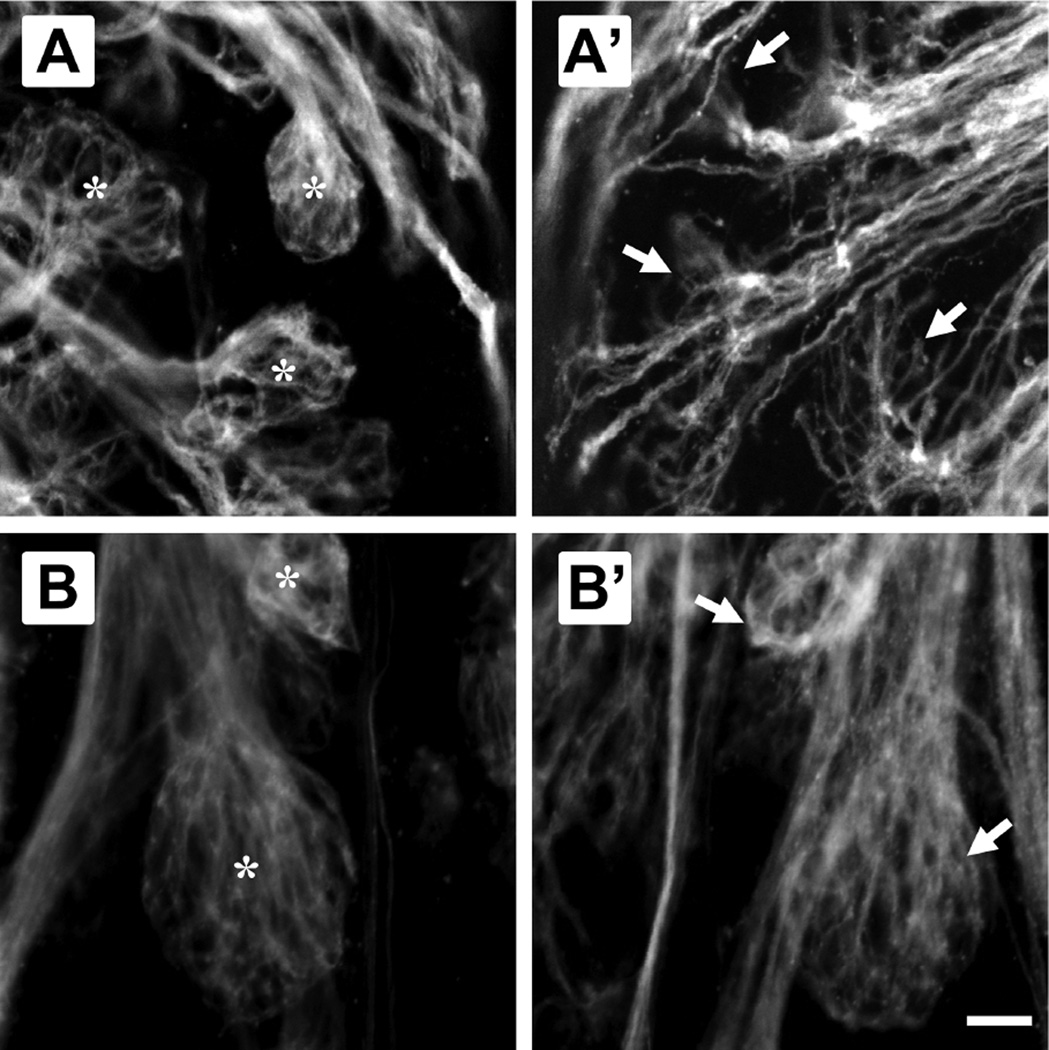
Higher magnification confocal images allowed further analysis of the shape of each glomerulus or glomerular cluster. In control fish and in the internal control side of lesioned fish, glomeruli had a spheroidal shape, were uniform, and were bounded by olfactory axon fibers that formed a distinct stalk entering each glomerulus (Fig. 3A, B). In the olfactory bulb on the treated side of fish allowed to survive for 4 days post-treatment, many glomeruli were morphologically disrupted, although some remnant of the structure was present (Fig. 3A’). These glomeruli were no longer spheroidal, had an apparent lack of olfactory axon fibers, and showed defasciculation of the axonal bundle, although they did accurately retain their general location. Recovery of glomerular morphology was seen at 7 days after the detergent treatment, when the olfactory bulb on the lesioned side appeared no different from the internal control side in size, shape, or location (Fig. 3B’).
Figure 3.
Higher magnification views of specific glomeruli allowed comparison between detergent-treated and internal control side olfactory bulbs of fish 4 and 7 days post-treatment. Glomeruli in the internal control bulb (A) 4 days after detergent ablation of the olfactory epithelium were spheroidal and compact, and each had a distinct axonal stalk (*); the maG1 glomerulus and glomeruli in the vmGy cluster are shown. Glomeruli in the olfactory bulb on the lesioned side of the same animal (A’) showed an incomplete morphology, with apparent defasciculation of the axon bundle and spreading of the axonal fibers (arrows). The disrupted glomeruli still appeared to be in the same general location. The internal control bulb of fish 7 days after detergent ablation (B) continued to show the typical, spherical glomerular morphology (*); the vpG1 glomerulus and one glomerulus of the vmGx cluster are shown. The treated side bulb at this time point (B’) also exhibited a spherical, complete morphology, and glomeruli were not distinguishable from controls (arrows) in size, shape, or location. Scale bar = 20 µm for all.
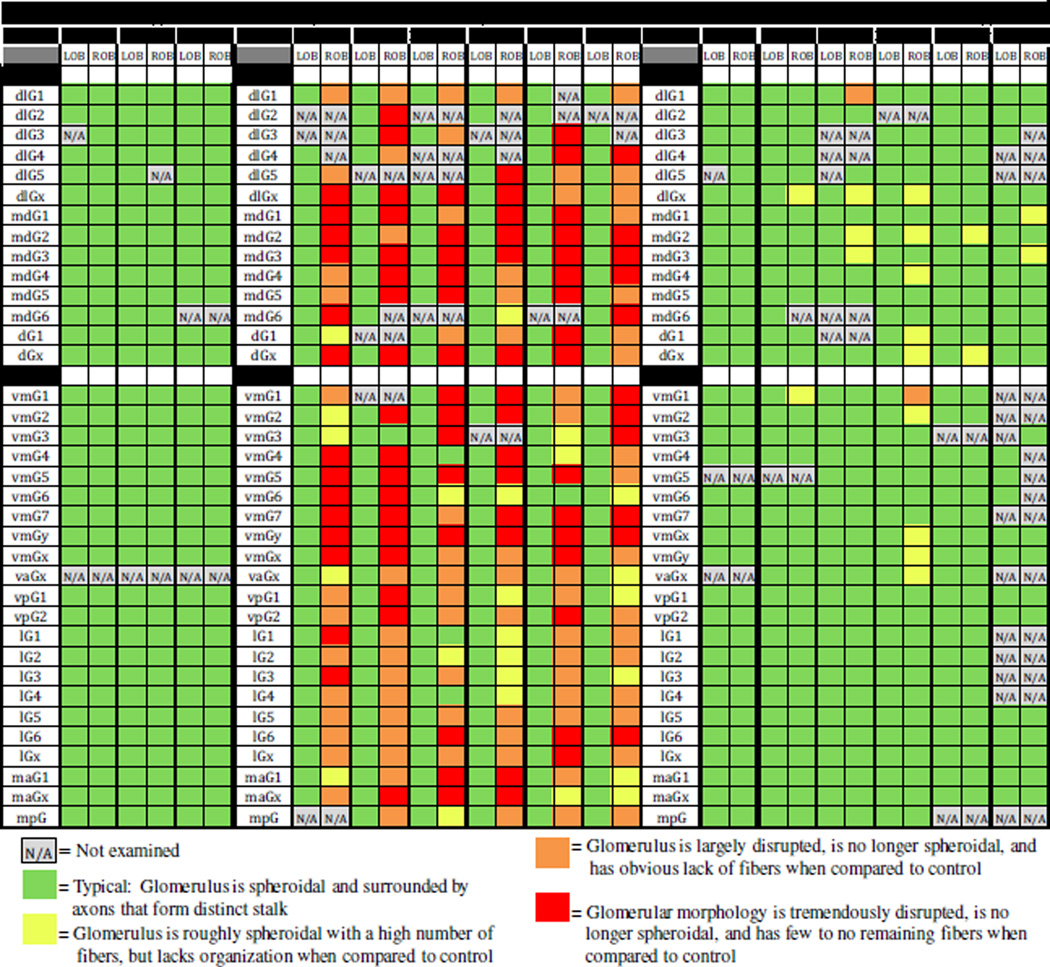
In order to determine the extent of the effect of peripheral damage to the olfactory epithelium on glomeruli in the olfactory bulb, all glomeruli were identified and evaluated for completeness. Some glomeruli were not observed in each specimen due to variability in mounting of the specimens; these were designated as N/A. All identifiable glomeruli or glomerular clusters were rated as either displaying a normal morphology, showing some lack of organization, being largely disrupted, or having significant loss of structure. Individually staging every glomerulus in each fish throughout the treatment time course illustrated some trends (Fig. 4). Almost all glomeruli in control fish could be identified, and those that were identified always had a typical morphology. The same was true for the internal control bulb of the 4-day and 7-day treatment groups. In the treated-side olfactory bulb of 4 day post-treatment fish, almost all glomeruli had some extent of morphological disruption. Glomeruli in the medial regions of the olfactory bulb of these fish tended to show a higher degree of damage than those in the lateral region of the olfactory bulb. In the treated-side bulb of 7 day post-treatment fish, most glomeruli appeared similar to control, although a few in the medial region still exhibited minor damage.
Figure 4.
Analysis of glomerular distribution in the olfactory bulb after a single dose of Triton X-100 to the olfactory organ. The glomerular map was used to identify individual glomeruli and glomerular clusters, and each glomerulus was given a color rating based on presence and completeness, using multiple views. Glomeruli that could not be identified in a given specimen were not examined (N/A). In untreated control fish, all identified glomeruli in the left (LOB) and right (ROB) olfactory bulbs consisted of a bundle of axons forming a distinct stalk and dispersing into a spheroid. Fish examined 4 days after detergent treatment had glomeruli with varying degrees of morphological disruption on the treated side (ROB); the internal control side (LOB) had glomerular morphology consistent with untreated control fish. The treated side (ROB) of fish examined 7 days after detergent treatment was more similar to untreated control fish, although a few glomeruli still displayed some lack of organization; the internal control side (LOB) had glomeruli with a normal appearance.
3.2 Effects of Triton X-100 ablation of the olfactory epithelium on odorant-mediated behavior
Behavior experiments were conducted on untreated control, bilaterally detergent-treated, or anosmic fish to determine their olfactory ability. In the following description, terms from the Zebrafish Behavior Catalog (ZBC; Kalueff et al., 2013) are used. Initially, all fish displayed erratic movement (ZBC term 1.51) and darting behavior (ZBC term 1.41) when introduced into the experimental tank, but they generally settled down within 30–90 minutes. There were some trials when the fish appeared agitated throughout the acclimation period, likely due to background noise and vibrations; these trials were eliminated from the study. After the acclimation period, pre-test swimming behavior for fish from all groups was random and generally consisted of rotating around the bucket in either direction and making few turns, typical of exploratory activity (ZBC term 1.54).
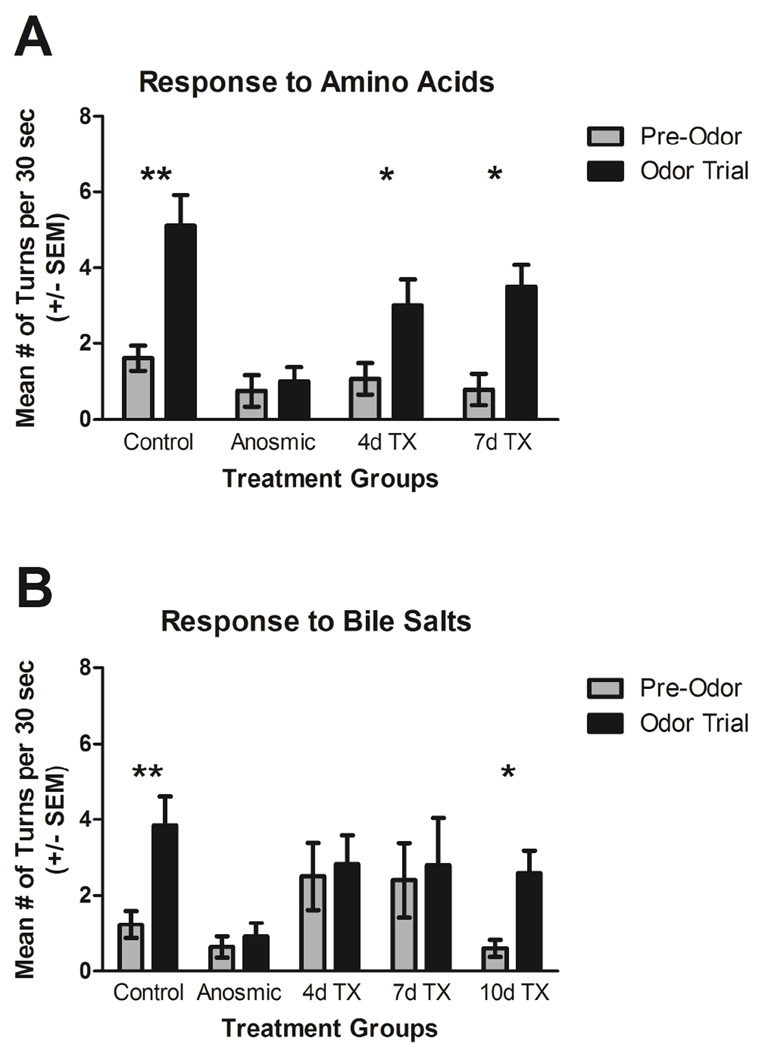
Control fish showed obvious alterations in their behavior after an amino acid mixture was added to the tank, including swimming more quickly, making large turns, and exploring more of the tank. These movements are typical of appetitive behavior (ZBC term 1.7). Quantification of their behavioral response revealed that they made significantly more turns after addition of the odor to the tank (P<0.001; Fig. 5A). In the 30 seconds before addition of the odor mixture, control fish made an average of 1.61±1.12 turns. After exposure to the amino acid mixture, control fish made an average of 5.11±1.49 turns in 30 seconds. Fish that had been rendered anosmic appeared to explore less of the tank even before addition of the odor mixture and made few turns (0.75±0.41). These fish did not appear to react to the addition of the amino acid mixture, with no difference in the number of turns after amino acid delivery (1.00±0.38; P>0.05; Fig. 5A). At 4 and 7 days following detergent ablation of the olfactory epithelium, fish responded to the amino acid odor similar to control fish. At 4 days post-lesion fish made an average of 1.07±0.41 turns in 30 seconds pre-odor and 2.86±0.74 turns in 30 seconds during the odor trial, which was a significant increase (P<0.05; Fig. 5A). At 7 days post-lesion fish made significantly more turns after exposure to the odor (P<0.05), with an average of 0.79±0.41 turns per 30 seconds before and 3.50±0.58 turns per 30 seconds after amino acid delivery (Fig. 5A). The pre-odor turning behavior was not significantly different between treatment groups (P>0.05).
Figure 5.
Behavioral responses to amino acids and bile salts following Triton X-100 exposure. A) Control fish made significantly more turns in the 30 seconds after an amino acid mixture was added to the tank (odor trial) than in the 30 seconds before odorant delivery (pre-trial). Fish that had been rendered anosmic by ablating or occluding the olfactory organs made approximately the same number of turns per 30 seconds before and after odorant delivery. Fish 4 and 7 days post-lesion made significantly more turns after turns after amino acid delivery. B) Control fish made significantly more turns in the 30 seconds after a bile salt mixture was added to the tank (odor trial) than in the 30 seconds before odorant delivery (pre-trial). Anosmic fish and fish 4 and 7 days post-lesion did not alter turning behavior after bile salt delivery. At 10 days after detergent treatment, fish again made significantly more turns in response to the bile salt mixture. **=P<0.001; *=P<0.05.
Control fish altered their behavior noticeably after the addition of the bile salt mixture: they came to the surface of the water more often and spent more time in the area of the bucket near the odorant-dispending tube. Some fish appeared to exhibit a kin recognition response (ZBC term 1.89) to their reflection in the side of the bucket and showed preference for swimming near the reflection. They significantly increased the number of turns after the bile salt mixture was added to the tank (P<0.001), from 1.23±0.36 turns per 30 seconds before to 3.85±0.76 turns per 30 seconds after (Fig. 5B). Anosmic fish did not seem to react to the bile salts, and they did not make more turns after exposure to the odorant (P>0.05), with an average of 0.64±0.28 turns per 30 seconds pre-odor and 0.91±0.37 turns per 30 seconds during the odor trial. Detergent-treated fish at 4 and 7 days post-lesion did not alter their turning behavior after bile salt delivery (P>0.05 for both; Fig. 5B) and did not appear to show any behavioral response to the bile salt mixture. At 4 days post-lesion, they made an average of 2.50±0.89 turns in 30 seconds before and 2.83±0.75 turns in 30 seconds after odorant delivery. At 7 days post-lesion, the fish made 2.40±0.98 turns in 30 seconds before and 2.80±1.24 turns in 30 seconds after odorant delivery. By 10 days after detergent ablation of the olfactory epithelium, fish regained their ability to sense the bile salt mixture and made significantly more turns after odor exposure (P<0.05). These fish made 0.60±0.22 turns in 30 seconds pre-odor and 2.60±0.58 turns in 30 seconds during the odor trial. The pre-odor turning behavior was not significantly different between treatment groups (P>0.05).
4 Discussion
Results of this study show that chemical lesioning of the olfactory organ in adult zebrafish with Triton X-100 results in significant, but temporary, damage to the olfactory bulb followed by rapid regeneration of structure and function. Confocal analysis of labeling with an antibody to KLH allowed us to perform a detailed investigation into structural alterations in OSN projections following detergent application to the olfactory organ. Anti-KLH binds an unknown epitope associated with OSN axons in teleosts (Riddle and Oakley, 1992). This antibody has been used extensively to label OSN axons in the zebrafish olfactory bulb and appears to label the projections of all three OSN subtypes (Fuller et al., 2006; Gayoso et al., 2011; Braubach et al., 2012).
We found a number of morphological changes in the glomeruli of chemically deafferented fish that reversed with recovery time. Four days after exposure of the olfactory organ to Triton X-100, glomeruli were less organized and incomplete, predominantly in the medial olfactory bulb. There was defasciculation of the axon bundles entering the glomeruli, and in general the glomeruli appeared diffuse. Glomerular development in zebrafish embryos relies on the expression of selective adhesion molecules and axon guidance cues for bundling olfactory axons as they target the appropriate glomeruli, and disruption of these factors results in mistargeted projections and defasciculation of the olfactory nerve (Miyasaka et al., 2005; Yanicostas et a., 2009; Lakhina et al., 2012). It is possible in our study that the axons present at 4 days are injured ones that are being eliminated and are no longer tightly bundled because they have lost the adhesion molecules that bound them together. An alternative possibility is that these are newly formed olfactory axons that are targeting their final glomerular location and have not yet bundled tightly. In addition, the olfactory system displays well-defined topography: the axons of OSNs expressing the same odorant receptor converge on a few glomeruli in conserved positions of the olfactory bulb (Ressler et al., 1994; Vassar et al., 1994) using a variety of molecular cues (St. John et al., 2002). In mammals, the olfactory epithelium-olfactory bulb topography is maintained even after chemical ablation of mature OSNs, with restoration of the pattern of new OSN axonal projections to the same specific glomeruli in the olfactory bulb (Cummings et al., 2000). Similarly, we found the regenerated glomeruli in the same general location as the partner glomerulus in the contralateral, unlesioned olfactory bulb.
Seven days after detergent destruction of the olfactory epithelium, most glomeruli had recovered and appeared to be the same size, shape, and location as untreated glomeruli. Glomerular map analysis revealed that only a few glomeruli, located mostly in the medial and dorsal regions of the bulb, showed some lack of organization and the vast majority of glomeruli were morphologically similar to unlesioned controls. The time course of degeneration and regeneration is faster than that seen in mammals and larger fish. Recovery from olfactory nerve transection takes about 2 weeks in the garfish (Cancalon, 1987) and about 6 weeks in the rat (Graziadei and Monti Graziadei, 1980). In rats exposed to methyl bromide, olfactory axons reach the olfactory bulb between 1–2 weeks after the damage, form glomeruli between 2–3 weeks after the lesion, but are not stable until 8 weeks following injury (Schwob et al., 1999). Rats and mice subjected to intranasal irrigation with Triton X-100 take up to 6–8 weeks to recover glomerular innervation that resembles unlesioned mice (Verhaagen et al., 1990; Cummings et al., 2000). The small size of the zebrafish likely contributes to its rapid recovery.
A detailed examination of the zebrafish glomerular map revealed additional effects. The nomenclature and glomerular identification proposed by Braubach and colleagues (2012) was used. This map is similar to the original zebrafish glomerular map described by Baier and Korsching (1994), although more glomeruli were identified and the names were simplified. All glomeruli in Braubach’s map were observed and examined in control and lesioned fish (although not in every specimen, due to variations in viewing orientation). At 4 days after treatment, most glomeruli ipsilateral to the Triton X-100 application had some level of morphological disruption, although to varying degrees. Those in the medial bulb were most severely affected. In general, glomeruli in the lateral region of the bulb appeared less disrupted than glomeruli in the dorsal-medial or ventral-medial bulb. The internal control side was not affected, suggesting that the effects are due directly to alterations in OSN axonal projections following damage to the olfactory organ. The differential effect of Triton X-100 application to the olfactory organ on glomeruli in the medial and lateral regions of the olfactory bulb suggests that OSN subtypes may respond differently to chemical exposure, either in their ability to endure or recover from treatment. Interestingly, a similar phenomenon is seen when detergent treatment is chronically repeated every 2–3 days for 3 weeks (Paskin et al., 2011; Paskin and Byrd-Jacobs, 2012). During this time, the olfactory bulb atrophies, although its volume and afferent activity return to control levels within 3 weeks of the cessation of treatment (Paskin et al., 2011). This suggests the effect of Triton X-100 treatment on OSNs and their axonal projections is similar regardless of whether the detergent is applied once or chronically, and that the bulbar atrophy is likely due to the prolonged reduction in afferent input achieved in chronic treatment.
Similar studies in other fish have shown that OSN subtypes respond differently to damage. In the trout, ciliated OSNs degenerate more rapidly than microvillous OSNs following nerve transection and reappear sooner in the regenerating epithelium (Zielinski and Hara, 1992). In the goldfish, transection of the olfactory nerve eliminates responses to amino acids (Zippel et al., 1993). Exposure of goldfish to copper affects detection of bile salts, prostaglandins, and steroids more than detection of amino acids (Kolmakov et al., 2009). Thus a number of studies show selective retention or elimination of specific OSN subtypes following injury.
The time course of glomerular atrophy and recovery lags behind that of the damage to the olfactory epithelium following detergent treatment, where the greatest severity of damage was seen at 1 day post treatment and the neuronal component of the epithelium was largely recovered by 5 days (Iqbal and Byrd-Jacobs, 2010). Our current study showed the greatest damage to bulbar structure and function at 4 days post-lesion, with morphology returning by 7 days and functional recovery apparent at 10 days. These time courses work well together, as it is plausible that the population of basal cells and neuronal precursors would repopulate the olfactory epithelium first while their axons take a longer duration to find their synaptic targets within the olfactory bulb.
Analysis of odorant-mediated behavior showed that treated fish lost the ability to detect bile salts and retained the ability to respond to amino acids; they regained the perception of bile salts after bulb recovery was complete. The analysis of the glomerular map was predictive of the findings of the behavior study. The glomeruli in the lateral regions of the olfactory bulb that better withstood treatment correlate to microvillous OSNs and the detection of amino acids, while glomeruli in the medial regions of the olfactory bulb that were more severely damaged by treatment correlate to ciliated OSNs and the detection of bile salts. Our behavioral analysis involved counting the number of turns before and after exposure to an odorant mixture consisting of either amino acids or bile salts. Both odorant mixtures significantly affected the number of turns post-exposure in control fish. Control fish reacted immediately to the odorants by turning sharply and exploring the tank. Fish that had been rendered anosmic did not respond to either odor, indicating that increased turning behavior after odorant delivery in the control and detergent-treated fish was based on olfaction. In general, the anosmic fish appeared less interested in exploring the tank even in the pretrial period, likely due to the loss of this important sensory system.
The behavior study indicated that there are differences in odorant detection ability following chemical lesioning of the peripheral olfactory system. At both 4 and 7 days after detergent ablation of the olfactory epithelium, the fish could still react to amino acids. Amino acids are detected by microvillous OSNs (Lipschitz and Michel, 2002) that project primarily to the lateral region of the olfactory bulb (Sato et al., 2005). The mild to moderate glomerular disruption seen in the lateral bulb did not appear to affect the perception of this category of odorant. The detection of bile salts, however, was affected to a greater extent and took more than a week to recover. At both 4 and 7 days post-treatment, fish did not appear to detect the bile salt mixture, as they exhibited no change in their turning behavior during the odor trial. Bile salts are detected by ciliated OSNs (Koide et al., 2009) that project primarily to the medial olfactory bulb region (Sato et al., 2005). Chronic application of detergent to the olfactory organ of adult zebrafish appears to diminish selectively the ciliated OSNs of the olfactory epithelium and affects bile salt detection (Paskin et al., 2011; Paskin and Byrd-Jacobs, 2012). Since the medial glomeruli were noticeably more affected by the treatment, the lack of behavioral response at 4 days is expected. The fish did not respond to bile salts even at 7 days post-treatment, although the glomeruli appear to return to normal morphology at this time point. It is likely that, although the axonal projections display control-like structure, there may still be lack of connections with the dendrites of bulbar neurons. By 10 days after treatment, fish regained the ability to detect bile salts, showing that behavioral responses appear to lag behind the morphology.
The results of this study demonstrate the plasticity of zebrafish glomeruli and reaffirm chemical lesioning as a form of reversible deafferentation: glomeruli can be largely destroyed yet return to unlesioned morphology in one week. The regions of the olfactory bulb that appear to be more affected correlate to the functional zones that processes social behavior, while regions that were less affected correlate to areas that process feeding behavior. This suggests that there is a difference in the ability of OSN subtypes to withstand or remodel after chemical ablation.
Highlights.
Detergent ablation of the olfactory organ disrupts glomeruli in the olfactory bulb.
In general, glomeruli in the medial region of the bulb are the most affected.
By 7 days most glomeruli are morphologically similar to unlesioned controls.
Fish retain the ability to detect amino acids after Triton X-100 treatment.
Fish lose detection of bile salts, but they regain olfactory acuity by 10 days.
Acknowledgements
This work was supported by the National Institutes of Health-NIDCD grant #011137 (CBJ), Western Michigan University Lee Honors College research award (EJW), and the National Science Foundation REU DBI-1062883 grant to WMU (SKK). We are grateful to Taylor Paskin for technical advice.
Abbreviations
- OSN
Olfactory Sensory Neuron
- KLH
Keyhole limpet hemocyanin
- PBS
Phosphate buffered saline
- PBS-T
Phosphate buffered saline blocking solution
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Evan J. White, Email: evan.j.white@wmich.edu.
Savannah K. Kounelis, Email: skouneli@nd.edu.
Christine A. Byrd-Jacobs, Email: christine.byrd@wmich.edu.
References
- Ahuja G, Ivandic I, Salturk M, Oka Y, Nadler W, Korsching SI. Zebrafish crypt neurons project to a single, identified mediodorsal glomerulus. Sci Rep. 2013 doi: 10.1038/srep02063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier H, Korsching S. Olfactory glomeruli in the zebrafish form an invariant pattern and are identifiable across animals. J Neurosci. 1994;14:219–230. doi: 10.1523/JNEUROSCI.14-01-00219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Kawano T, Margolis FL, Joh TH. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci. 1983;3:69–78. doi: 10.1523/JNEUROSCI.03-01-00069.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braubach O, Fine A, Croll R. Distribution and functional organization of glomeruli in the olfactory bulbs of zebrafish (Danio rerio) J Comp Neurol. 2012;520:2317–2339. doi: 10.1002/cne.23075. [DOI] [PubMed] [Google Scholar]
- Cancalon P. Degeneration and regeneration of olfactory cells induced by ZnSO4 and other chemicals. Tissue Cell. 1982;14:717–733. doi: 10.1016/0040-8166(82)90061-1. [DOI] [PubMed] [Google Scholar]
- Cancalon P. Influence of a detergent on the catfish olfactory mucosa. Tissue Cell. 1983;15:245–258. doi: 10.1016/0040-8166(83)90020-4. [DOI] [PubMed] [Google Scholar]
- Cancalon P. Survival and subsequent regeneration of olfactory neurons after a distal axonal lesion. J Neurocytol. 1987;16:829–841. doi: 10.1007/BF01611989. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Emge DK, Small SL, Margolis FL. Pattern of olfactory bulb innervation returns after recovery from reversible peripheral deafferentation. J Comp Neurol. 2000;421:362–373. [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Fuller CL, Yettaw HK, Byrd CA. Mitral cells in the olfactory bulb of adult zebrafish (Danio rerio): morphology and distribution. J Comp Neurol. 2006;499:218–230. doi: 10.1002/cne.21091. [DOI] [PubMed] [Google Scholar]
- Gayoso JA, Castro A, Anadon R, Manso MJ. Differential bulbar and extrabulbar projections of diverse olfactory receptor neuron populations in the adult zebrafish (Danio rerio) J Comp Neurol. 2011;519:247–276. doi: 10.1002/cne.22518. [DOI] [PubMed] [Google Scholar]
- Germana A, Montalbano G, Laura R, Ciriaco E, del Valle ME, Vega JA. S100 protein-like immunoreactivity in the crypt olfactory neurons of the adult zebrafish. Neurosci Lett. 2004;71:196–198. doi: 10.1016/j.neulet.2004.08.077. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Monti Graziadei Neurogenesis and neuron regeneration in the olfactory system of mammals. III. Deafferentation and reinnervation of the olfactory bulb following section of the fila olfactoria in rat. J Neurocytol. 1980;9:145–162. doi: 10.1007/BF01205155. [DOI] [PubMed] [Google Scholar]
- Hamdani EH, Lastein S, Gregersen F, Doving KB. Seasonal variations in olfactory sensory neurons-fish sensitivity to sex pheromones explained. Chem Senses. 2008;33:119–123. doi: 10.1093/chemse/bjm072. [DOI] [PubMed] [Google Scholar]
- Hansen A, Zeiske E. The peripheral olfactory organ of the zebrafish, Danio rerio: an ultrastructural study. Chem Senses. 1998;23:39–48. doi: 10.1093/chemse/23.1.39. [DOI] [PubMed] [Google Scholar]
- Herzog C, Otto T. Regeneration of olfactory receptor neurons following chemical lesion: time course and enhancement with growth factor administration. Brain Res. 1999;849:155–161. doi: 10.1016/s0006-8993(99)02075-2. [DOI] [PubMed] [Google Scholar]
- Iqbal T, Byrd-Jacobs CA. Rapid degeneration and regeneration of the zebrafish olfactory epithelium after Triton X-100 application. Chem Senses. 2010;35:351–361. doi: 10.1093/chemse/bjq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS, Craddock C, Kyzar EJ, Roth A, Landsman S, Gaikwad S, Robinson K, Baatrup E, Tierney K, Shamchuk A, Norton W, Miller N, Nicolson T, Braubach O, Gilman CP, Pittman J, Rosemberg DB, Gerlai R, Echevarria D, Lamb E, Neuhauss SC, Weng W, Bally-Cuif L, Schneider H Zebrafish Neuroscience Research Consortium. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish. 2013;10:70–86. doi: 10.1089/zeb.2012.0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide T, Miyasaka N, Morimoto K, Asakawa K, Urasaki A, Kawakami K, Yoshiara Y. Olfactory neural circuitry for attraction to amino acids revealed by transposon-mediated gene trap approach in zebrafish. Proc Nat Acad Sci USA. 2009;106:9884–9889. doi: 10.1073/pnas.0900470106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmakov NN, Hubbard PC, Lopes O, Canario AVM. Effect of acute copper sulfate exposure on olfactory responses to amino acids and pheromones in goldfish (Carassius auratus) Environ Sci Technol. 2009;43:8393–8399. doi: 10.1021/es901166m. [DOI] [PubMed] [Google Scholar]
- Lakhina V, Marcaccio CL, Shao X, Lush ME, Jain RA, Fujimoto E, Bonkowsky JL, Granato M, Raper JA. Netrin/DCC signaling guides olfactory sensory axons to their correct location in the olfactory bulb. J Neurosci. 2012;32:4440–4456. doi: 10.1523/JNEUROSCI.4442-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Mack JA, Souren M, Yaksi E, Higashijima S, Mione M, Fetcho JR, Friedrich RW. Early development of functional spatial maps in the zebrafish olfactory bulb. J Neurosci. 2005;25:5784–5795. doi: 10.1523/JNEUROSCI.0922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipschitz DL, Michel WC. Amino acid odorants stimulate microvillar sensory neurons. Chem Senses. 2002;27:277–286. doi: 10.1093/chemse/27.3.277. [DOI] [PubMed] [Google Scholar]
- Miyasaka N, Sato Y, Yeo S-Y, Hutson LD, Chien C-B, Okamoto H, Yoshiara Y. Robo2 is required for establishment of a precise glomerular map in the zebrafish olfactory system. Development. 2005;132:1283–1293. doi: 10.1242/dev.01698. [DOI] [PubMed] [Google Scholar]
- Nadi NS, Head R, Grillo M, Hempstead J, Grannot-Reisfeld N, Margolis F. Chemical deafferentation of the olfactory bulb: plasticity of the levels of tyrosine hydroxylase, dopamine and norepinephrine. Brain Res. 1981;213:365–371. doi: 10.1016/0006-8993(81)90241-9. [DOI] [PubMed] [Google Scholar]
- Paskin TR, Byrd-Jacobs CA. Reversible deafferentation of the adult zebrafish olfactory bulb affects glomerular distribution and olfactory-mediated behavior. Behav Brain Res. 2012;235:293–301. doi: 10.1016/j.bbr.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskin TR, Iqbal TR, Byrd-Jacobs CA. Olfactory bulb recovery following reversible deafferentation with repeated detergent application in the adult zebrafish. Neurosci. 2011;196:276–284. doi: 10.1016/j.neuroscience.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A molecular dissection of spatial patterning in the olfactory system. Curr Opin Neurobiol. 1994;4:588–596. doi: 10.1016/0959-4388(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Riddle DR, Oakley B. Immunocytochemical identification of primary olfactory afferents in rainbow trout. J Comp Neurol. 1992;324:575–589. doi: 10.1002/cne.903240410. [DOI] [PubMed] [Google Scholar]
- Sato Y, Miyasaka N, Yoshihara Y. Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. J Neurosci. 2005;25:4889–4897. doi: 10.1523/JNEUROSCI.0679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Miyasaka N, Yoshihara Y. Hierarchical regulation of odorant receptor gene choice and subsequent axonal projection of olfactory sensory neurons in zebrafish. J Neurosci. 2007;27:1606–1615. doi: 10.1523/JNEUROSCI.4218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Mezza RC. Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J Comp Neurol. 1995;359:15–37. doi: 10.1002/cne.903590103. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Ring G, Iwema CL, Mezza RC. Reinnervation of the rat olfactory bulb after methyl bromide-induced lesion: Timing and extent of reinnervation. J Comp Neurol. 1999;412:439–457. doi: 10.1002/(sici)1096-9861(19990927)412:3<439::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- St. John JA, Clarris HJ, Key B. Multiple axon guidance cues establish the olfactory topographic map: how do these cues interact? Int J Dev Biol. 2002;46:639–647. [PubMed] [Google Scholar]
- Tierney KB, Baldwin DH, Hara TJ, Ross PS, Scholz NL, Kennedy CJ. Olfactory toxicity in fishes. Aquat Toxicol. 2010;96:2–26. doi: 10.1016/j.aquatox.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Verhaagen J, Oestreicher AB, Grillo M, Khew-Goodall Y-S, Gispen WH, Margolis FL. Neuroplasticity in the olfactory system: Differential effects of central and peripheral lesions of the primary olfactory pathway on the expression of B-50/GAP43 and the olfactory marker protein. J Neurosci Res. 1990;26:31–44. doi: 10.1002/jnr.490260105. [DOI] [PubMed] [Google Scholar]
- Yaksi E, Judkewitz B, Friedrich RW. Topological reorganization of odor representations in the olfactory bulb. PLoS Biol. 2007;5:e178. doi: 10.1371/journal.pbio.0050178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanicostas C, Herbomel E, Dipietromaria A, Soussi-Yanicostas N. Anosmin-1a is required for fasciculation and terminal targeting of olfactory sensory neuron axons in the zebrafish olfactory system. Mol Cell Endocrinol. 2009;312:53–60. doi: 10.1016/j.mce.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Zielinski B, Hara TJ. Ciliated and microvillar receptor cells degenerate and then differentiate in the olfactory epithelium of rainbow trout following olfactory nerve section. Microsc Res Tech. 1992;23:22–27. doi: 10.1002/jemt.1070230103. [DOI] [PubMed] [Google Scholar]
- Zippel HP, Lago-Schaaf T, Caprio J. Ciliated olfactory receptor neurons in goldfish (Carassius auratus) partially survive nerve axotomy, rapidly regenerate and respond to amino acids. J Comp Physiol A. 1993;173:537–547. [Google Scholar]