Abstract
PlexinsA1–A4 participate in class 3 semaphorin signaling as co-receptors to neuropilin 1 and 2. PlexinA4 is the latest member of the PlexinA subfamily to be identified. In previous studies, we described the expression of PlexinA4 in the brain and spinal cord of the adult rat. Here, antibodies to PlexinA4 were used to reveal immunolabeling in most of the cranial nerve surveyed. Labeling was found in the olfactory, optic, oculomotor, trochlear, trigeminal, abducens, facial, vestibulocochlear, glossopharyngeal, vagus, and hypoglossal nerves. This is the first detailed description of the cellular and subcellular distribution of PlexinA4 in the adult cranial nerves. The findings will set the basis for future studies on the potential role of PlexinA4 in regeneration and repair of the adult central and peripheral nervous system.
Keywords: Semaphorin, axon guidance molecule, cranial nerve, Rattus norvegicus, protein distribution, plexin-a4, plxna4, plexa4
INTRODUCTION
Semaphorins (Sema) are axon guidance molecules involved in diverse cellular processes including axon pruning and repulsion, dendritic attraction and branching, growth cone collapse, regulation of cell migration, and vascular remodelling (Tang, Tanelian et al. 2004; Potiron and Roche 2005; Bussolino, Valdembri et al. 2006; Gross, Mei et al. 2007; Tang, Heron et al. 2007; Waimey, Huang et al. 2008; Giger, Hollis et al. 2010). PlexinA4, is a member of the PlexinA family that mediates the effects of multiple semaphorins (Yazdani and Terman 2006). In Sema3 signalling, PlexinA4 acts as a critical signal transducer by way of its interaction with the ligand-binding class 3 semaphorin co-receptors neuropilin (Nrp) 1 and 2 (Yaron, Huang et al. 2005), that lack intracellular domains and thus the ability to solely transmit an extracellular signal into a cell (Nakamura, Tanaka et al. 1998). Further, PlexinA4 serves as the receptor and signal transducer for membrane-bound Sema6A and 6B, which are particularly important in the development of the corticospinal tract and the layered organization of hippocampal mossy fibers (Suto, Ito et al. 2005; Faulkner, Low et al. 2008; Runker, Little et al. 2008).
Mouse PlexinA4 is a type 1 transmembrane protein consisting of 1890 amino acids (aa) and includes what is likely to be a signal sequence (aa 1-20), a transmembrane domain (aa 1230-1255), and 12 extracellular, N-linked glycosylation sites. There are also domains within the PlexinA4 protein that are common to all members of the PlexinA family: an extracellular Sema domain (aa 36-554), three extracellular “Met-related sequences” (MRS)/cysteine clusters, three extracellular glycine-proline repeats, intracellular spacer (SP) domains, and a putative intracellular tyrosine kinase phosphorylation site (aa 1804-1811) (Suto, Murakami et al. 2003). While the rat PlexinA4 protein has not been characterized in this much detail, a BLAST search with the sequence for rat PlexinA4 obtained from the Treefam database (Treefam ID: ENSRNOG00000013072) confirms that rat and mouse PlexinA4 share 100% sequence similarity.
With few exceptions (Suto, Murakami et al. 2003; Okada, Tominaga et al. 2007; Okada and Tomooka 2012), studies that have addressed PlexinA4 localization have focused on the distribution of its mRNA (Perala, Immonen et al. 2005; Spinelli, McPhail et al. 2007; Faulkner, Low et al. 2008; Low, Liu et al. 2008; Runker, Little et al. 2008; Schwarz, Waimey et al. 2008). We recently described the protein expression of PlexinA4 in the brain and spinal cord of the adult rat (Gutekunst, Stewart et al. 2010; Gutekunst, Stewart et al. 2012). PlexinA4 is known to participate in the development of various cranial nerves. For example, in mice the combined loss of PlexinA3 and PlexinA4 impaired facial branchiomotor axon guidance (Schwarz et al, 2008). However, little is known about the distribution, expression and role of PlexinA4 in the adult. In the present study we describe the expression of PlexinA4 in the adult rat cranial nerves and their nuclei.
MATERIALS AND METHODS
2.1 Animals
Adult (two months old) Sprague–Dawley rats (200–250g) were obtained from Charles River (Wilmington, MA). All animals were maintained in a 12/12 light/dark cycle with ad libitum access to food and water. All protocols involving animals were approved by the Emory University Institutional Animal Care and Use Committee (IACUC) and conform to NIH guidelines.
2.2 Immunohistochemistry
Adult male Sprague Dawley rats (n = 8) were used for light microscopic immunohistochemistry. Each adult rat was deeply anesthetized with a lethal dose of Euthasol (130 mg/kg), injected intraperitoneally, and then perfused intracardially with 0.9% NaCl, followed by 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.2 (PB) for 15 min at a rate of 20 ml per min. Spinal cords were removed and cryoprotected in 30% sucrose at 4°C, sectioned in coronal and sagittal planes at 50 μm thickness using a freezing microtome, collected in PB, and rinsed in 0.1 M phosphate-buffered saline (PBS), pH 7.2.
Immunohistochemistry was performed as described previously (Gutekunst, Levey et al. 1995; Gutekunst, Li et al. 1998; Gutekunst, Stewart et al. 2010). Free-floating sections were incubated in 0.1% TritonX-100 and 3% hydrogen peroxide to eliminate endogenous peroxidase, rinsed in PBS, and preblocked in 4% normal goat serum (NGS) in PBS for 30 min at room temperature (RT). Rabbit polyclonal antibodies specific for PlexinA4 were used at 1:500 (ab39350-200; Abcam, Cambridge, MA). Sections were incubated in PlexinA4 antibodies in PBS containing 2% NGS at 4°C for 48 hr, then rinsed and incubated for 1 hr at RT in biotinylated anti-rabbit antibody (ABC Elite; Vector Laboratories, Burlingame, CA) in PBS containing 2% NGS. After several rinses in PBS, the sections were incubated in avidin-biotin complex (ABC Elite; Vector) for 90 min at 4°C. Immunoreactivity was visualized by incubation in 0.05% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma, St. Louis, MO) and 0.01% hydrogen peroxide in PBS, until a dark brown reaction product was evident (5–10 min). Sections were rinsed and mounted on gelatin coated glass slides, air dried and coverslipped. Controls included the omission of primary antibody and preabsorption of antibodies with excess PlexinA4 peptide (ab39349; Abcam) for 1hr at room temperature prior to use. Sections were visualized using either a Nikon eclipse E400 microscope and images captured using a color digital camera (Nikon Instruments Inc, Melville, NY). The identification of the various cranial nerves (designated using lowercase n) and motor nuclei (designated using uppercase N) was based on the Rat Brain Atlas from Paxinos and Watson (Paxinos and Watson 1998).
RESULTS
3.1 Specificity of antibodies
The specificity of the antibodies used in this study has been extensively described in our previous studies (Gutekunst, Stewart et al. 2010; Gutekunst, Stewart et al. 2012). PlexinA4 is detected using a rabbit polyclonal antibody that was raised against a synthetic 16 amino acid peptide derived from within residues 500–600 of mouse PlexinA4, identical to that of rat PlexinA4. We previously demonstrated that PlexinA4 antibodies detect a protein band with an approximate molecular mass of 210 kDa on immunoblots of rat and mouse brain and spinal cord tissue (Gutekunst, Stewart et al. 2010). Immunoreactivity was abolished when the antibodies were first preabsorbed with the PlexinA4 peptide or when the primary antibody was omitted. Based on a BLAST search it is unlikely that the PlexinA4 antibody cross reacts with other members of the PlexinA family or other Plexin families. The 16 amino acid peptide sequence used to generate the PlexinA4 antibody showed no homology to rat PlexinA2 or 3, and, using HEK293 cells transfected with PlexinA1 or PlexinA4 expressing plasmids, we have further confirmed the specificity of the antibodies to PlexinA4.
3.2 PlexinA4 expression in the olfactory, optic, and oculomotor nerves
The olfactory nerve (1n) carries sensory information from the olfactory mucosa through the olfactory tract to the olfactory cortex and amygdala. We showed previously that PleinxA4 is present in the olfactory bulb of the adult rat where it is expressed by the mitral cells and can be seen in axons of the lateral olfactory tract (Gutekunst, Stewart et al. 2012). The optic nerves (2n), consisting of axons from the retinal ganglion cells, converge in the optic chiasm at the base of the brain where information from the nasal half of each retina crosses over (Fig. 1A–C). Although PlexinA4 is not found in retinal ganglion cells during embryogenesis (Kuwajima, Yoshida et al. 2012), PlexinA4 immunolabelling is visible in adult axons coursing through the optic chiasm (Fig. 1B–C). The oculomotor nerve (3n) is a motor nerve arising from the oculomotor nuclei located in the midbrain which contains neurons expressing PlexinA4 (as we have previously shown, Fig. 1D). PlexinA4 labeled axons of the oculomotor nerve are visible as they make their way ventrally from the oculomotor nucleus passing through the red nucleus (Fig. 1D) and the substantia nigra (Fig. 1E) as well as at their exit point in the nerve root (Fig. 1F).
Figure 1.
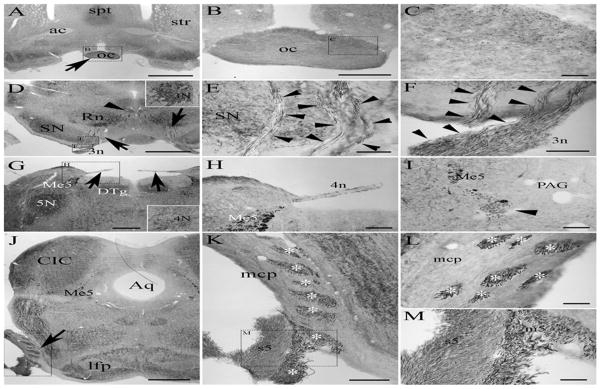
Micrographs of coronal sections through the adult rat brain showing PlexinA4 immunolabeling in the optic (A–C), the oculomotor (D–F), the trochlear (G–I) and the trigmenial nerve (J–M). PlexinA4 is visible in the optic nerve at the optic chiasm (arrow in A), in the oculomotor nucleus (D, arrowhead and insert) as well as in its motor roots (arrows and arrowheads in D and E respectively). PlexinA4 is also present in the trochlear nerve (arrows and arrowhead in G and I respectively) and in the trigeminal nerve (arrow in J). Both sensory (s5) and motor (asterix in K and L and m5 in M) roots of trigeminal nerve express PlexinA4. Figures B, C, E, F, H, K and M are higher magnifications of the dashed insets as indicated. Abbrev: ac: anterior commissure, oc: optic chiasm, spt: septum, str: striatum, Rn: red nucleus, SN: substantia nigra, 3n: nerve 3, DTg: dorsal tegmentum, Me5: mesencephalic trigeminal nucleus, 4n: nerve 4, PAG: periaqueductal grey, Aq: aqueduct, CIC: central nucleus of the inferior colliculus, lfp: longitudinal fasciculus pons, mcp: middle cerebellar peduncle, m5: motor root of the trigeminal nerve, s5: sensory root of the trigeminal nerve. Scale bars: A: 2 mm, B and K: 500 μm, C, H, I and L: 100 μm, E-F: 200 μm, G and J: 1 mm.
3.3 PlexinA4 expression in the trochlear, trigeminal, abducens nerves
The trochlear nerve (4n) is a motor nerve that innervates the superior oblique muscle of the eye. PlexinA4 labeled axons of the trochlear nerve are visible in the roots exiting the brain dorsal to the mesencephalic trigeminal nucleus (Me5) (Fig. 1G–H). Further rostrally the labeled nerve is seen ventromedial to the plexinA4 labeled Me5 neurons near the periaqueductal grey (Fig. 1I). The trigeminal nerve (5n) is a mixed nerve carrying both sensory and motor information. The motor nerve fibers of 5n originate in the motor nucleus (5N) of the trigeminal nerve located near the main mesencephalic trigeminal nucleus in the pons (Fig. 5G). PlexinA4 labeled large motor axons are visible as they traverse through the middle cerebellar peduncle and in the root of the trigeminal nerve lateral to the brachium pontis (Fig. 1J–M). The smaller size sensory axons are also plexinA4 positive (Fig. 1K and M). The abducens nerve (6n) is a motor nerve that originates in the abducens nucleus located in the pons immediately posterior to the trochlear nucleus and medioventral to the genu of the facial nerve (Fig. 2D). PlexinA4 labeled axons of 6n can be seen in the pyramids ventral to the gigantocellularis nucleus (Fig 2. A–C).
Figure 2.
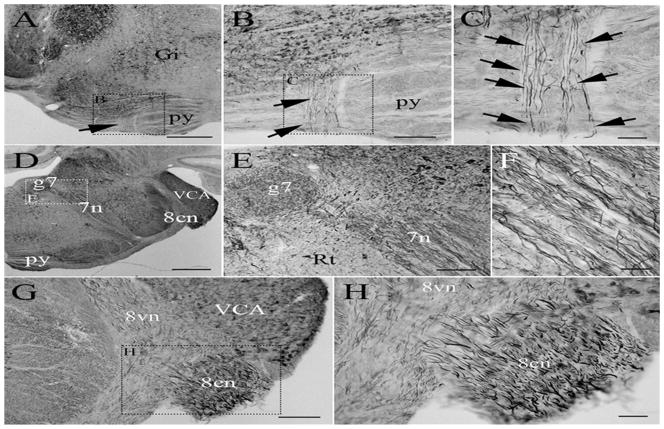
Micrographs of coronal sections through the adult rat brain showing PlexinA4 immunolabeling in the abducens (A–C), the facial (D–F), and the vestibulocochlear nerve (G–H). Plexin A4 is visible in axons of the abducens nerve coursing through the pyramids (arrows in AC) as well as in axons in the facial and auditory nerves. Figures B, C, E and H are higher magnifications of the dashed insets as indicated. Abbrev: Gi: gigantocellularis nucleus, py: pyramids, g7: genu of facial nerve, 7n: nerve 7, 8cn: cochlear root of the 8th nerve, 8vn: vestibular root of the 8th nerve, Rt: reticular nucleus, VCA: ventral cochlear nucleus anterior.
Scale bars: A and D: 1 mm, B: 200 μm, C, E, F and H: 100 μm, G: 250 μm.
3.4 PlexinA4 expression in the facial and vestibulocochlear nerves
The facial nerve (7n) is a mixed nerve that carries both sensory and motor information. The motor portion of the nerve, which controls facial expression, originates in the facial nucleus located in the ventrolateral area of the reticular formation in the pons. We have previously shown that neurons of the facial nucleus express PlexinA4 (Gutekunst, Stewart et al. 2010). Fine bundles of fibers composed of the axons of the facial nerve nucleus ascend dorsomedially through the reticular formation. The fibers then make a sharp turn above the abducens nucleus where PlexinA4 is visible in the genu of the facial nerve (Fig. 2D–E) and in the fibers as they continue their course ventrolaterally (Fig. 2D–F) to exit the brain, in the caudal pons, lateral to the lateral superior olive. The vestibulocochlear nerve (8n) is a sensory nerve comprising two divisions, the cochlear nerve involved in hearing and the vestibular nerve involved in balance. PlexinA4 immunostaining is present in large fibers of the root of the cochlear nerve (8cn) as they enter the brain at the level of the pons ventral to the ventral cochlear nucleus (Fig. 2G–H). PlexinA4 labeling is also visible in thinner fibers in the vestibular root (8vn) medial to the ventral cochlear nucleus (Fig. 2G).
3.5 PlexinA4 expression in the vagus and hypoglossal nerves
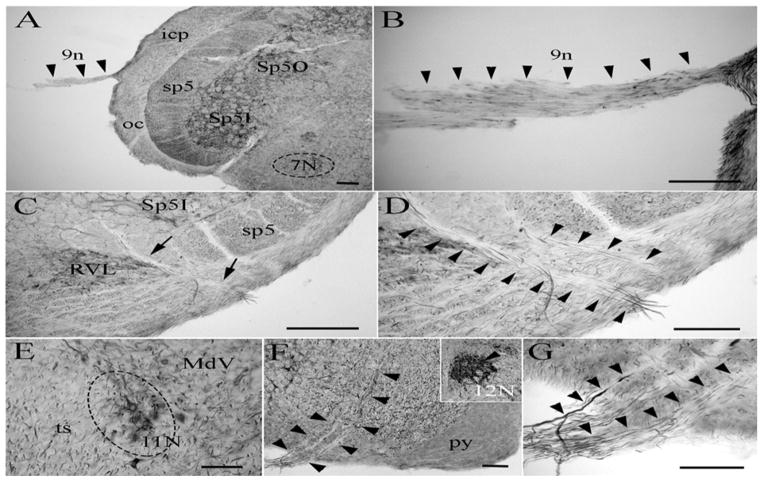
The glossopharyngeal nerve (9n) carries both afferent sensory and efferent motor information. PlexinA4 labeling of the glossopharyngeal nerve is visible as the nerve exits the brain lateral to the inferior cerebellar peduncle (Fig 3A–B). The vagus nerve (10n) conveys both motor and sensory information from various organs throughout the body. The axons carrying the motor output converge from both the vagus nucleus and the nucleus ambiguous. PlexinA4 is visible in the neurons of the dorsal motor nucleus of the vagus nerve (Fig. 3C) and in the root of the vagus axons ventral to the spinal trigeminal tract between the medullary pyramid and the inferior cerebellar peduncle (Fig. 3C–D). Although we were unable to locate the accessory nerve (11n), PlexinA4 expression was present in the accessory nerve nucleus (11N) (Fig. 3E). The hypoglossal nerve (12n) originates in the hypoglossal nucleus (12N) ventrolateral to the central canal in the medulla and emerges between the pyramid and the inferior olive (Fig. 3F). PlexinA4 is expressed by the neurons of the hypoglossal nucleus (Fig. 3F) and is seen in the axons traversing the medulla and in the nerve root (Fig. 3F–G).
Figure 3.
Micrographs of coronal sections through the adult rat brain showing PlexinA4 immunolabeling in the glossopharyngeal nerve 9n (A–B), the dorsal motor nucleus of the vagus nerve (C–D), and the hypoglossal nerve (F–G). Plexin A4 expression is also found in neurons of the accessory nerve nucleus (E) and the hypoglossal nucleus (insert in F). Abbrev: Sp5I: spinal trigeminal nucleus interpolar, Sp5O: spinal trigeminal nucleus oralis, icp: inferior cerebellar peduncle, oc: olivocerebellar tract, sp5: spinal trigeminal tract, 7N: facial nucleus, RVL: rostroventrolateral reticular nucleus, 12N: hypoglossal nucleus, MdV: ventral part of the medullary reticular nucleus, ts: tectospinal tract. Scale bars: A–B, D–F: 100 μm, C: 250 μm.
DISCUSSION
In embryos PlexinA4 mRNA has been found in both the central and peripheral nervous systems and shown to participate in the axon guidance and the development of various circuits. In two previous studies we described the expression of PlexinA4 in the adult brain and spinal cord suggesting a role beyond the early stages of development (Gutekunst, Stewart et al. 2010; Gutekunst, Stewart et al. 2012). Here we provide the first description of PlexinA4 distribution in the adult rat cranial nerves. Our finding of PlexinA4 in all of the cranial nerves surveyed is consistent with previous knockout and localization studies. In PlexinA4 knockout mice embryos, many of the cranial nerves are defasciculated, arboring fanned out terminal fields (Suto, Ito et al. 2005). Defects have been reported in the ophthalmic, maxillary and mandibular branches of the trigeminal nerve, in the facial nerve and in the vagus and glossopharyngeal nerves (Suto, Ito et al. 2005). Plexin A4 is part of a Sema3A/Nrp1/PlexinA4 signaling complex, and the defects seen in PlexinA4 knockout embryos are similar but more moderate than those present in Nrp1 knockouts (Kitsukawa, Shimizu et al. 1997) or Sema3A knockouts (Taniguchi, Yuasa et al. 1997). The Sema3A/Nrp1/PlexinA4 complex is thought to participate in the directional guidance of these nerves during development. Sema3A was also shown to control patterning of sensory axons in late embryonic and in adult stages and it is conceivable that this effect is conveyed in part via PlexinA4 signaling (Dillon, Saldanha et al. 2004). In mice lacking Sema3A, nerve endings at whisker pads are disorganized (Ulupinar, Datwani et al. 1999) and the olfactory nerve is disrupted (Schwarting, Raitcheva et al. 2004). Selective removal of Nrp1 from somatic motor neurons impairs initial fasciculation and assembly of hypoglossal rootlets and leads to reduced numbers of abducens and hypoglossal fibers (Huettl and Huber 2011).
Class 3 semaphorins along with their receptors and co-receptors have been implicated in the development of the visual/oculomotor system. The four cranial nerves that participate in the visual system are the optic nerve, the oculomotor nerve, the trochlear nerve and the ophthalmic branch of the trigeminal nerve. During the development of the cornea, Nrp1 is expressed by the trigeminal ganglion and Sema3A is expressed by the corneal tissue. Furthermore, innervation of the cornea is premature and aberrant in mice with a non-functioning semaphorin binding domain (McKenna, Munjaal et al. 2012). We have previously shown that PlexinA4 proteins are expressed by neurons of the oculomotor nuclei (3N) and the trochlear nuclei (4N) (Gutekunst, Stewart et al. 2010). In the present study we show their expression in the optic nerve, the oculomotor nerve, the trochlear nerve and the trigeminal nerve. Consistent with our findings, optic nerve transection in the adult rat resulted in decrease PlexinA4 mRNA in the superior colliculus both ipsilateral and contralateral to the lesion (Sharma, Pollett et al. 2012). The persistent expression of PlexinA4 in the adult optic nerve suggests that PlexinA4 plays a role beyond the guidance of the tract during development and that it could possibly participate in maintenance and the regenerative properties of the pathway. Interestingly, the oculomotor motor nerve is not affected in PlexinA4 knockout mice embryos. The presence of PlexinA4 in the adult oculomotor nerve raises the possibility that while other proteins are involved in its development, PlexinA4 might participate in its maintenance. Other proteins including (i.e. L1-CAM) have been shown to contribute to visual system development and maintenance.
PlexinA4 mRNA and proteins have been found in facial motor neurons (Spinelli, McPhail et al. 2007; Gutekunst, Stewart et al. 2010). In the adult rat, transection of the facial nerve leads to an increase in PlexinA4 mRNA in the facial nucleus suggesting a potential role of PlexinA4 in facial nerve maintenance and regeneration (Spinelli, McPhail et al. 2007). Sema3A mRNA is expressed by adult motor neurons, including facial motor neurons (Giger, Pasterkamp et al. 1998), and the expression levels are lowered by peripheral nerve transection (Pasterkamp, Giger et al. 1998). Loss of Sema3A/Nrp1 causes defasciculation and ectopic projection of facial nerve axons (Schwarz et al., 2008), which is consistent with our observation that PlexinA4 is present in neurons of the facial nucleus and their projections in the facial nerve. Although this has not been directly tested, it is likely that the defects seen in Nrp1 knockout mice are transduced via Nrp1/PlexinA4 interaction.
Semaphorin family members and their receptors and co-receptors continue to be expressed in the adult CNS. Furthermore, their expression levels are regulated in response to injury, physiological or pharmacological manipulation and in the context of neurological diseases (Holtmaat, De Winter et al. 2002; Barnes, Puranam et al. 2003; Jassen, Yang et al. 2006; Mann, Chauvet et al. 2007). Such findings lead to the suggestion that these molecules contribute to the maintenance and stability of neuronal networks, as well as repair and remodeling of such networks (De Winter, Holtmaat et al. 2002; de Wit and Verhaagen 2003; Pasterkamp and Verhaagen 2006). A more detailed analysis of adult PlexinA4 knockout animals, including histological and behavioral assessment, could further clarify the role of PlexinA4 in the mature CNS. In addition, genetic manipulation of PlexinA4 using lentiviral or adenoviral delivery of PlexinA4 shRNA should provide a deeper understanding of the mechanisms by which PlexinA4 signaling contributes to structural plasticity and regeneration in the adult nervous system.
Highlights.
PlexinA4 specific antibodies were used to immunostain adult rat cranial nerves.
PlexinA4 staining was present in all cranial nerves surveyed.
PlexinA4 was found in both sensory and motor nerves.
The expression of PlexinA4 in adult cranial nerves suggests its participation in maintenance and repair mechanisms.
Acknowledgments
We want to thank Eric Stewart for his assistance with sectioning and immunostaining. This work was supported by a grant from NIH (K08 NS46322-01A1) to REG. CAG is funded in part by NIH (R03 NS58376-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes G, Puranam RS, et al. Temporal specific patterns of semaphorin gene expression in rat brain after kainic acid-induced status epilepticus. Hippocampus. 2003;13(1):1–20. doi: 10.1002/hipo.10041. [DOI] [PubMed] [Google Scholar]
- Bussolino F, Valdembri D, et al. Semaphoring vascular morphogenesis. Endothelium. 2006;13(2):81–91. doi: 10.1080/10623320600698003. [DOI] [PubMed] [Google Scholar]
- De Winter F, Holtmaat AJ, et al. Neuropilin and class 3 semaphorins in nervous system regeneration. Adv Exp Med Biol. 2002;515:115–39. doi: 10.1007/978-1-4615-0119-0_10. [DOI] [PubMed] [Google Scholar]
- de Wit J, Verhaagen J. Role of semaphorins in the adult nervous system. Prog Neurobiol. 2003;71(2–3):249–67. doi: 10.1016/j.pneurobio.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Dillon TE, Saldanha J, et al. Sema3A regulates the timing of target contact by cranial sensory axons. J Comp Neurol. 2004;470(1):13–24. doi: 10.1002/cne.11029. [DOI] [PubMed] [Google Scholar]
- Faulkner RL, Low LK, et al. Dorsal turning of motor corticospinal axons at the pyramidal decussation requires plexin signaling. Neural Dev. 2008;3:21. doi: 10.1186/1749-8104-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger RJ, Hollis ER, 2nd, et al. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2010;2(7):a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger RJ, Pasterkamp RJ, et al. Anatomical distribution of the chemorepellent semaphorin III/collapsin-1 in the adult rat and human brain: predominant expression in structures of the olfactory-hippocampal pathway and the motor system. J Neurosci Res. 1998;52(1):27–42. doi: 10.1002/(SICI)1097-4547(19980401)52:1<27::AID-JNR4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gross RE, Mei Q, et al. The pivotal role of RhoA GTPase in the molecular signaling of axon growth inhibition after CNS injury and targeted therapeutic strategies. Cell Transplant. 2007;16(3):245–62. doi: 10.3727/000000007783464740. [DOI] [PubMed] [Google Scholar]
- Gutekunst CA, Levey AI, et al. Identification and localization of huntingtin in brain and human lymphoblastoid cell lines with anti-fusion protein antibodies. Proc Natl Acad Sci U S A. 1995;92(19):8710–4. doi: 10.1073/pnas.92.19.8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutekunst CA, Li SH, et al. The cellular and subcellular localization of huntingtin-associated protein 1 (HAP1): comparison with huntingtin in rat and human. J Neurosci. 1998;18(19):7674–86. doi: 10.1523/JNEUROSCI.18-19-07674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutekunst CA, Stewart EN, et al. PlexinA4 distribution in the adult rat spinal cord and dorsal root ganglia. J Chem Neuroanat. 2012;44(1):1–13. doi: 10.1016/j.jchemneu.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutekunst CA, Stewart EN, et al. Immunohistochemical distribution of Plexin A4 in the adult rat central nervous system. Front Neuroanat. 2010;4(25):1–17. doi: 10.3389/fnana.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat AJ, De Winter F, et al. Semaphorins: contributors to structural stability of hippocampal networks? Prog Brain Res. 2002;138:17–38. doi: 10.1016/s0079-6123(02)38068-3. [DOI] [PubMed] [Google Scholar]
- Huettl RE, Huber AB. Cranial nerve fasciculation and Schwann cell migration are impaired after loss of Npn-1. Dev Biol. 2011;359(2):230–41. doi: 10.1016/j.ydbio.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Jassen AK, Yang H, et al. Receptor regulation of gene expression of axon guidance molecules: implications for adaptation. Mol Pharmacol. 2006;70(1):71–7. doi: 10.1124/mol.105.021998. [DOI] [PubMed] [Google Scholar]
- Kitsukawa T, Shimizu M, et al. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19(5):995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- Kuwajima T, Yoshida Y, et al. Optic chiasm presentation of Semaphorin6D in the context of Plexin-A1 and Nr-CAM promotes retinal axon midline crossing. Neuron. 2012;74(4):676–90. doi: 10.1016/j.neuron.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low LK, Liu XB, et al. Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proc Natl Acad Sci U S A. 2008;105(23):8136–41. doi: 10.1073/pnas.0803849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann F, Chauvet S, et al. Semaphorins in development and adult brain: Implication for neurological diseases. Prog Neurobiol. 2007;82(2):57–79. doi: 10.1016/j.pneurobio.2007.02.011. [DOI] [PubMed] [Google Scholar]
- McKenna CC, Munjaal RP, et al. Distinct roles for neuropilin1 and neuropilin2 during mouse corneal innervation. PLoS One. 2012;7(5):e37175. doi: 10.1371/journal.pone.0037175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura F, Tanaka M, et al. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron. 1998;21(5):1093–100. doi: 10.1016/s0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- Okada A, Tominaga M, et al. Plexin-A4 is expressed in oligodendrocyte precursor cells and acts as a mediator of semaphorin signals. Biochem Biophys Res Commun. 2007;352(1):158–63. doi: 10.1016/j.bbrc.2006.10.176. [DOI] [PubMed] [Google Scholar]
- Okada A, Tomooka Y. Possible roles of Plexin-A4 in positioning of oligodendrocyte precursor cells in developing cerebral cortex. Neurosci Lett. 2012;516(2):259–64. doi: 10.1016/j.neulet.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ, et al. Regulation of semaphorin III/collapsin-1 gene expression during peripheral nerve regeneration. Exp Neurol. 1998;153(2):313–27. doi: 10.1006/exnr.1998.6886. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Verhaagen J. Semaphorins in axon regeneration: developmental guidance molecules gone wrong? Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1499–511. doi: 10.1098/rstb.2006.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Perala NM, Immonen T, et al. The expression of plexins during mouse embryogenesis. Gene Expr Patterns. 2005;5(3):355–62. doi: 10.1016/j.modgep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Potiron V, Roche J. Class 3 semaphorin signaling: the end of a dogma. Sci STKE. 2005;2005(285):pe24. doi: 10.1126/stke.2852005pe24. [DOI] [PubMed] [Google Scholar]
- Runker AE, Little GE, et al. Semaphorin-6A controls guidance of corticospinal tract axons at multiple choice points. Neural Dev. 2008;3:34. doi: 10.1186/1749-8104-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting GA, Raitcheva D, et al. Semaphorin 3A-mediated axon guidance regulates convergence and targeting of P2 odorant receptor axons. Eur J Neurosci. 2004;19(7):1800–10. doi: 10.1111/j.1460-9568.2004.03304.x. [DOI] [PubMed] [Google Scholar]
- Schwarz Q, Waimey KE, et al. Plexin A3 and plexin A4 convey semaphorin signals during facial nerve development. Dev Biol. 2008;324(1):1–9. doi: 10.1016/j.ydbio.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Pollett MA, et al. Changes in mRNA expression of class 3 semaphorins and their receptors in the adult rat retino-collicular system after unilateral optic nerve injury. Invest Ophthalmol Vis Sci. 2012;53(13):8367–77. doi: 10.1167/iovs.12-10799. [DOI] [PubMed] [Google Scholar]
- Spinelli ED, McPhail LT, et al. Class A plexin expression in axotomized rubrospinal and facial motoneurons. Neuroscience. 2007;144(4):1266–77. doi: 10.1016/j.neuroscience.2006.10.057. [DOI] [PubMed] [Google Scholar]
- Suto F, Ito K, et al. Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J Neurosci. 2005;25(14):3628–37. doi: 10.1523/JNEUROSCI.4480-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto F, Murakami Y, et al. Identification and characterization of a novel mouse plexin, plexin-A4. Mech Dev. 2003;120(3):385–96. doi: 10.1016/s0925-4773(02)00421-5. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Heron P, et al. Targeting sensory axon regeneration in adult spinal cord. J Neurosci. 2007;27(22):6068–78. doi: 10.1523/JNEUROSCI.1442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XQ, Tanelian DL, et al. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci. 2004;24(4):819–27. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Yuasa S, et al. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron. 1997;19(3):519–30. doi: 10.1016/s0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
- Ulupinar E, Datwani A, et al. Role of semaphorin III in the developing rodent trigeminal system. Mol Cell Neurosci. 1999;13(4):281–92. doi: 10.1006/mcne.1999.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waimey KE, Huang PH, et al. Plexin-A3 and plexin-A4 restrict the migration of sympathetic neurons but not their neural crest precursors. Dev Biol. 2008;315(2):448–58. doi: 10.1016/j.ydbio.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron A, Huang PH, et al. Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron. 2005;45(4):513–23. doi: 10.1016/j.neuron.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7(3):211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]