Abstract
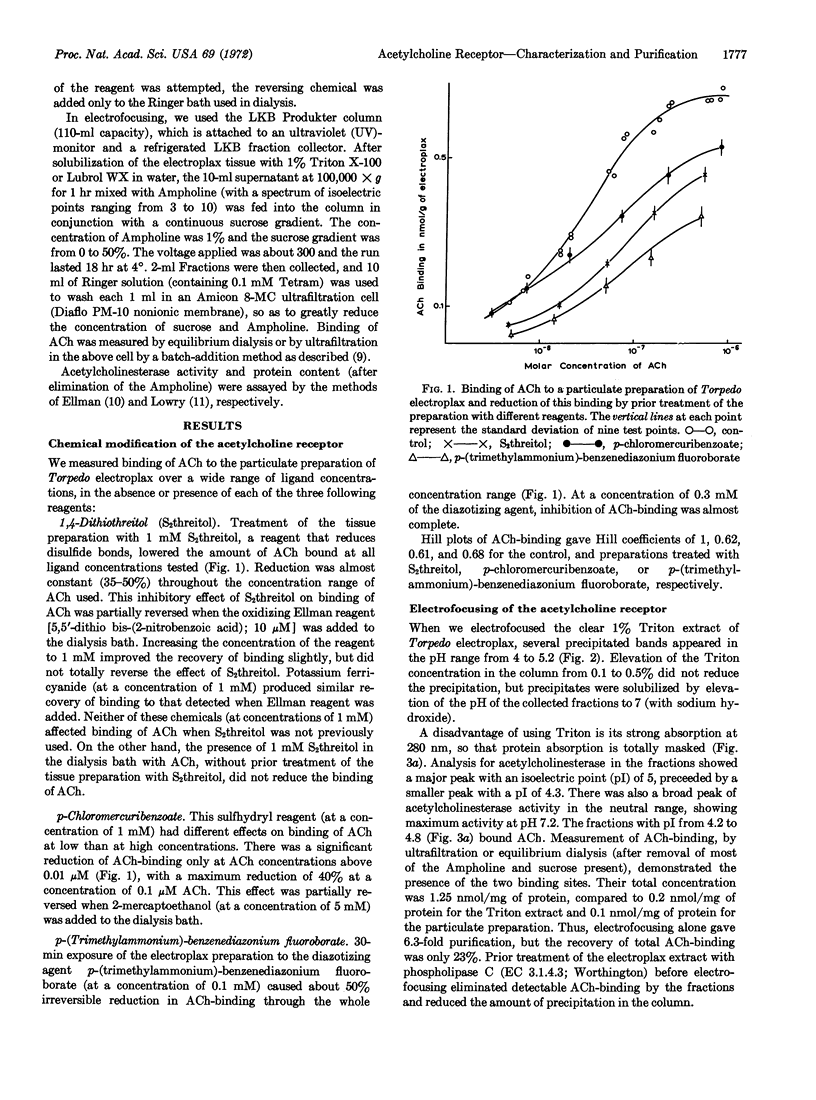
Binding of acetylcholine in the concentration range 1 nM-1 μM was measured by equilibrium dialysis to a particulate preparation of Torpedo electroplax, without or with prior treatment of the tissue with one of three chemical modifying reagents. Significant reduction in binding of acetylcholine resulted after treatment with 1,4-dithiothreitol, p-chloromercuribenzoate, or p-(trimethylammonium)-benzenediazonium fluoroborate. Partial reversal of the reduction in binding occurred when dialysis was performed in the presence of 5,5′-dithiobis-(2-nitrobenzoic acid) or potassium ferricyanide (in the case of treatment with dithiothreitol), and 2-mercaptoethanol (in the case of treatment with p-chloromercuribenzoate). It is concluded that the functional acetylcholine-receptor macromolecule of Torpedo electroplax has disulfide bond(s), sulfhydryl group(s), and one or more of the amino acids vulnerable to diazotization by p-(trimethylammonium)-benzenediazonium fluoroborate. This, plus the effect of phospholipase C (EC 3.1.4.3) in elimination of detectable binding of acetylcholine after electrofocusing, is additional evidence that the functional acetylcholine receptor is a phospholipoprotein or a phospholipid-protein complex, which has a low isoelectric point of 4.5 ± 0.2, yet is denatured by exposure to low pH for 24 hr. Due to this adverse effect, recovery of binding of acetylcholine after electrofocusing, as detected by equilibrium dialysis or ultrafiltration, is only 23% and, so far, only 6.3-fold purification of functional acetylcholine receptors by this technique is possible.
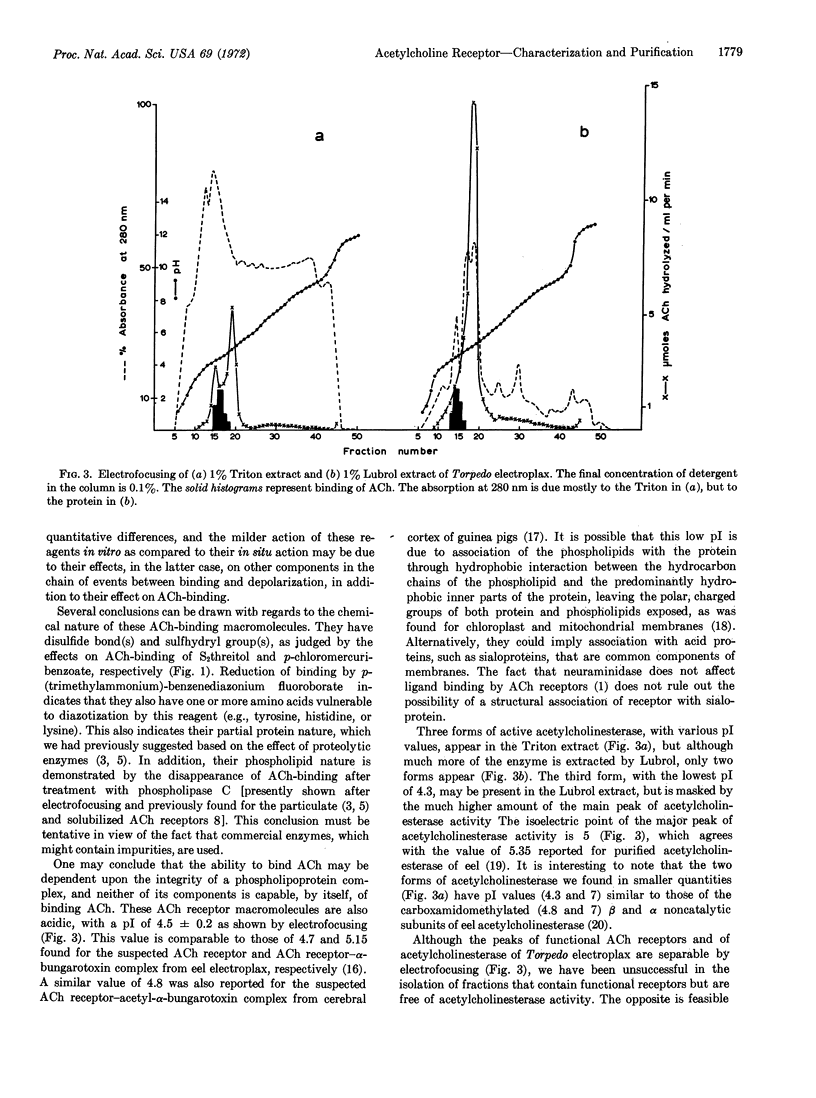
Three or two forms of acetylcholinesterase (EC 3.1.1.7), whose peaks have isoelectric points ranging from 4.3 to 7.2, appear after electrofocusing of Torpedo extracted with 1% Triton X-100 or Lubrol, respectively. The major peak in either preparation has an isoelectric point of 5. Although the peaks of the functional acetylcholine receptors and of acetylcholinesterase of Torpedo electroplax are separable by electrofocusing, it has not been possible to isolate fractions that contain functional receptors but that are free of acetylcholinesterase. The opposite is possible.
Keywords: acetylcholine binding, acetylcholinesterase, chemical modification, electrofocusing
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. V. Comparative physiology: electric organs. Annu Rev Physiol. 1970;32:471–528. doi: 10.1146/annurev.ph.32.030170.002351. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Acetylcholine receptor. I. Identification and biochemical characteristics of a cholinergic receptor of guinea pig cerebral cortex. J Biol Chem. 1972 Jan 10;247(1):130–145. [PubMed] [Google Scholar]
- Changeux J. P., Gerhart J. C., Schachman H. K. Allosteric interactions in aspartate transcarbamylase. I. Binding of specific ligands to the native enzyme and its isolated subunits. Biochemistry. 1968 Feb;7(2):531–538. doi: 10.1021/bi00842a007. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., Britten A. G., Eldefrawi A. T. Acetylcholine binding to Torpedo electroplax: relationship to acetylcholine receptors. Science. 1971 Jul 23;173(3994):338–340. doi: 10.1126/science.173.3994.338. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T., Gilmour L. P., O'Brien R. D. Multiple affinities for binding of cholinergic ligands to a particulate fraction of Torpedo electroplax. Mol Pharmacol. 1971 Jul;7(4):420–428. [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T., O'Brien R. D. Binding of five cholinergic ligands to housefly brain and Torpedo electroplax. Relationship to acetylcholine receptors. Mol Pharmacol. 1971 Jan;7(1):104–110. [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T., O'Brien R. D. Binding sites for cholinergic ligands in a particulate fraction of Electrophorus electroplax. Proc Natl Acad Sci U S A. 1971 May;68(5):1047–1050. doi: 10.1073/pnas.68.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., MARTINS-FERREIRA H. Membrane potentials in the electroplates of the electric eel. J Physiol. 1953 Feb 27;119(2-3):315–351. doi: 10.1113/jphysiol.1953.sp004849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., Bartels E. Effects of blocking sulfhydryl groups and of reducing disulfide bonds on the acetylcholine-activated permeability system of the electroplax. Biochim Biophys Acta. 1966 Nov 8;126(3):525–535. doi: 10.1016/0926-6585(66)90010-0. [DOI] [PubMed] [Google Scholar]
- Karlin A., Winnik M. Reduction and specific alkylation of the receptor for acetylcholine. Proc Natl Acad Sci U S A. 1968 Jun;60(2):668–674. doi: 10.1073/pnas.60.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leuzinger W., Baker A. L., Cauvin E. Acetylcholinesterase. II. Crystallization, absorption spectra, isoionic point. Proc Natl Acad Sci U S A. 1968 Feb;59(2):620–623. doi: 10.1073/pnas.59.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Molinoff P., Potter L. T. Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature. 1971 Feb 19;229(5286):554–557. doi: 10.1038/229554a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. D., Eldefrawi M. E., Eldefrawi A. T. Isolation of acetylcholine receptors. Annu Rev Pharmacol. 1972;12:19–34. doi: 10.1146/annurev.pa.12.040172.000315. [DOI] [PubMed] [Google Scholar]
- O'Brien R. D., Gilmour L. P., Eldefrawi M. E. A muscarone-binding material in electroplax and its relation to the acetylcholine receptor. II. Dialysis assay. Proc Natl Acad Sci U S A. 1970 Feb;65(2):438–445. doi: 10.1073/pnas.65.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M. A., Schmidt J., Clark D. G., Wolcott R. G. Demonstration of a specific -bungarotoxin binding component in electrophorus electricus electroplax membranes. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1622–1629. doi: 10.1016/0006-291x(71)90207-5. [DOI] [PubMed] [Google Scholar]
- Silman I., Karlin A. Acetylcholine receptor: covalent attachment of depolarizing groups at the active site. Science. 1969 Jun 20;164(3886):1420–1421. doi: 10.1126/science.164.3886.1420. [DOI] [PubMed] [Google Scholar]