Abstract
Cyclin dependent kinase 5 (Cdk5), a proline-directed serine/threonine kinase, requires p39 for its enzymatic activity, and is implicated in cytoskeletal organization and contraction in numerous cell types. The C-terminus of p39 binds muskelin, a multi-domain scaffolding protein known to affect cytoskeletal organization, but the mechanism(s) by which muskelin affects cytoskeletal organization remain unclear. The present study sought to determine whether p39 might serve as an adaptor protein that links muskelin to stress fibers and to investigate the possible biological relevance of such an interaction. Double immunoprecipitation showed that muskelin, p39, and myosin II are components of a single intracellular complex, and suppressing p39 abrogated the interaction between muskelin and the myosin subunits, demonstrating that p39 is required to link muskelin to myosin II. Muskelin is colocalized with myosin regulatory light chain (MRLC) and on stress fibers. The suppression of muskelin reduced Rho-GTP, MRLC phosphorylation, disrupted stress fiber organization, and promoted cell migration, all of which closely mimics the effect of Cdk5 inhibition. Moreover, suppressing muskelin and inhibiting Cdk5 together has no additional effect, indicating that muskelin plays an important role in Cdk5-dependent signaling. P39 is necessary and sufficient for Cdk5-dependent regulation of MRLC phosphorylation, as suppression of p39, but not p35, reduced MRLC phosphorylation. Together, these results demonstrate that p39 specifically links muskelin to myosin II and consequently, to stress fibers and reveal a novel role for muskelin in regulating myosin phosphorylation and cytoskeletal organization.
Keywords: Cdk5, p39, muskelin, myosin, stress fibers, cytoskeleton, cell migration, lens
INTRODUCTION
Muskelin is a multi-domain protein expressed in brain [1–3], the ocular lens [2], and a variety of other tissues [4] throughout life. Although little is known about the in vivo functions of muskelin, its structure suggests it may act as a scaffold protein [4–6]. It does not bind directly to either monomeric or polymerized actin or tubulin [6], but has been functionally linked to cytoskeletal regulation in a number of cell types [4, 7], and is recruited to cortical actin in epithelial cells transfected with the Cdk5-activating protein, p39 [2]. Recent work has shown that muskelin binds directly to the GABAA receptor during endocytotic transport on both actin and microtubules [8]. Although mice with a muskelin loss-of-function retroviral insertion are viable, they are defective in endocytosis of the GABAA receptor, providing a clear demonstration of muskelin function in vivo [8]. The diluted coat color of these mice indicates that the physiological importance of muskelin is not limited to the brain [8]. The expression of muskelin is upregulated during physiological stress including ischemia [1] and hyperosmolarity [9], suggesting that muskelin may be a clinically relevant protein in a variety of cell types.
Cyclin Dependent Kinase 5 (Cdk5), a predominantly cytoplasmic proline-directed serine/threonine kinase, is an important regulator of cytoskeletal organization and contraction in several cell types [10–12]. In particular, we have shown that Cdk5 regulates Rho-dependent myosin phosphorylation, stress fiber formation, and cytoskeletal contraction in spreading lens epithelial cells [12]. Stress fibers, which are contractile bundles of actin filaments and non-muscle myosin II cross-linked by α-actinin and other actin bundling proteins, are essential for cell adhesion, migration, and maintenance of cell shape [13]. Myosin II, a critical component of stress fibers, is a hexamer containing one pair each of non-muscle myosin heavy chains (MHC II), myosin essential light chains (MLC), and myosin regulatory light chains (MRLC) [14]. Phosphorylation of MRLC at Thr18/Ser19 is required for the organization, contraction, and stability of stress fibers [15], contributing to several cellular functions such as adhesion, spreading, migration, and mitosis.
Since Cdk5 has numerous potential substrates, its cellular functions may depend in large measure on its proper subcellular localization. In many situations, Cdk5 and its two known activating proteins, p39 and p35, colocalize with actin and actin-associated cytoskeletal proteins [12, 16–18]. The Cdk5 activating proteins may be responsible for recruiting Cdk5 to these sites. For example, p39 and p35 both bind directly to α-actinin [16], an actin cross-linking protein involved in stress fiber formation and in the joining of adhesion complexes to the actin cytoskeleton [19]. P39, but not p35, has two additional binding partners, myosin essential light chain (MLC) and muskelin, both of which are associated with cytoskeleton function. MLC, a 17 kDa myosin subunit essential for structural stability of myosin II [20], binds to the N-terminus of p39 [21], whereas muskelin binds to a unique sequence in the C-terminus of p39 [2]. Since muskelin and MLC bind opposite ends of the p39 protein, the present study seek to determine whether they bind simultaneously, with p39 forming a bridge to join muskelin to myosin II and thus to the actin cytoskeleton and to explore the role of muskelin in cytoskeletal regulation.
MATERIALS AND METHODS
Cell culture and transfection
Human lens epithelial cells FHL124 were cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2 in medium consisting of one part Keratinocyte Growth Medium (KGM) (Lonza Biologics Inc., Portsmouth, NH) to four parts M199 (Invitrogen-GIBCO, Carlsbad, CA), 50 µg/ml gentamicin (Quality Biological, Inc., Gaithersburg, Maryland), and 10% fetal bovine serum. Where indicated, cells were transiently transfected using Lipofectamine (Invitrogen) according to the manufacturer's instructions, and cultured for 48 hrs before use. To suppress muskelin, p35, or p39 expression, cells were transfected with 160 nM of the appropriate antisense oligonucleotides or with control siRNA, and were harvested after 48 hrs of transfection. The suppression of protein expression was confirmed with two different siRNAs in each case. Negative control siRNAs (Control siRNA 1, Cat. # 1027280; Control siRNA 2, Cat. # 1027310), muskelin siRNAs (Muskelin siRNA 1, Cat. # SI05147058; Muskelin siRNA 2, Cat. # SI04263364) and p39 siRNAs (p39 siRNA 1, Cat. # SI02223424; p39 siRNA 2, Cat. # SI02223431) were procured from Qiagen, Valencia, CA. p35 siRNA 1 (Cat. # L-008988-00-0005) and p35 siRNA 2 (Cat. # sc-36153) were procured from Dharmacon RNAi Technology, Chicago, IL and Santa Cruz Biotechnology, Santa Cruz, CA respectively.
Antibodies and fluorescent probes
Two antibodies each for p39 and muskelin from different sources were used in this study to confirm the findings. We generated one set of antibodies for p39 and muskelin in our laboratory. Peptides corresponding to the N-terminus of p39 (KGRRPGGLPEE) and the C-terminus of muskelin (GNLVDLITL) were used for polyclonal antibody production in rabbits. The IgG fraction was purified from the resulting antisera using protein A-agarose beads (Pierce Biotechnology, Inc., Rockford, IL), and the p39 antibody was further purified by affinity chromatography against the antigenic peptide covalently coupled to agarose beads as a secondary amine using the AminoLink™ kit and AminoLink coupling gel (Pierce). Commercially available antibodies for p39 (3275S) and muskelin (ab56135-100) were purchased from Cell Signaling Technology Inc., Danvers, MA and Abcam Inc., Cambridge, MA respectively. Myosin essential light chain (MLC) (ab680-100) and myosin regulatory light chain (MRLC) (ab11082-100) monoclonal antibodies were purchased from Abcam Inc. Phosphorylated MRLC (pMRLC) (sc-12896) and polyclonal antibody against phsphorylated Thr18/Ser19 pMRLC (sc-3674S) were purchased from Santa Cruz Biotechnology Inc., Santa Cruz, CA, and Cell Signaling Technology, respectively. The specificity of the pMRLC antibody was confirmed with a blocking peptide. GAPDH (14C10) and hemagglutinin (HA-Tag) (#2367) antibodies were purchased from Cell Signaling Technology. GFP polyclonal antibody was purchased from Clontech Laboratory Inc., Mountain View, CA. Cdk5 monoclonal (sc-6247) and MHC II polyclonal antibodies were procured from Santa Cruz Biotechnology and Covance Research Products Inc., Princeton, New Jersey respectively. Anti-rabbit and anti-mouse IgG horseradish peroxidase-linked secondary antibodies were from Amersham Bioscience, Piscataway, NJ. Alexa 488-donkey anti-mouse IgG, Alexa 568-goat anti-rabbit IgG, Alexa 488-phalloidin, Alexa 568-phalloidin, Alexa 647-phalloidin, and 4',6-diamidino-2-phenylindole (DAPI) were from Invitrogen.
Biochemical analysis
Cells plated on culture dishes were collected by scraping, pelleted, and lysed with PBSTDS buffer, which contains 1× phosphate-buffered saline [PBS], 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], complete protease inhibitor (Roche Diagnostics Corporation, Indianapolis, IN), and protein tyrosine and serine/threonine phosphatase inhibitors (Chemicon/Upstate USA, Inc., Lake Placid, NY). After sonication for 5 seconds, lysates were centrifuged at 10000 rpm for 20 min at 4°C and supernatants were used for immunoprecipitation and immunoblotting as previously described [2].
Double Immunoprecipitation
This protocol made it possible to isolate intact protein complex containing p39, muskelin and myosin II, by using a previously described method with minor modification [22]. Antibody for p39 or p35 was immobilized on column using the co-immunoprecipitation kit from Pierce (Rockford, IL), which allows for the isolation of intact, native protein complex from lysates. Control co-IP was performed by immobilizing non-immune rabbit IgG. Antibody immobilization was performed by coupling antibody to Aminolink Plus coupling gel as instructed by the manufacturer. The immobilized antibody was used to isolate protein complex from lens and brain tissues, and from lens epithelial cells. Tissues or cells were lysed with IP lysis buffer (Pierce), and pre-cleared lysates were incubated with immobilized antibody at 4°C for 4 hours. Complexes were eluted from the immobilized column using a non-reducing elution buffer provided by the manufacturer. Isolated, intact p39 or p35 protein complexes were then subjected to a second immunoprecipitation using muskelin antibody following a previously described immunoprecipitation protocol [2].
In vitro Cdk5 kinase assay
Lysates from FHL124 human lens epithelial cells were pre-cleared with protein G sepharose beads and immunoprecipitated with muskelin antibody. The immunopellets were sequentially washed once with high-salt HNTG buffer (20 mM Hepes, 500 mM NaCl, 0.1% Triton-X-100, 10% Glycerol), twice with low-salt HNTG buffer (20 mM Hepes, 150 mM NaCl, 0.1% Triton-X-100, 10% glycerol), and once with kinase reaction buffer (35 mM Hepes pH 7.4, 10 mM MgCl2, 1 mM EGTA, 1% Tween 20, 0.1 mM sodium vanadate, 1 mM DTT). The kinase reaction was carried out in 30 µL of reaction buffer containing 15 µM cold ATP, 2.5 µCi 32P-γ-ATP, and 100 ng of recombinant active Cdk5 (Millipore) at 30°C for 45 min. The reaction was stopped by adding 10 µL of 4× Laemmli sample buffer and heating at 95°C for 5 min. Proteins was separated by gel electrophoresis and autoradiographed to detect 32P incorporation.
Rho-GTP (Rhotekin-RBD pull-down) assay
GTP-bound Rho (Rho-GTP) was measured by Rho activation assay kit (Millipore, Lake Placid, NY), according to the manufacturer's instructions. Briefly, cells were lysed in ice-cold lysis buffer (50 mM Tris, pH 7.2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2) containing a protease inhibitor mixture (Roche Diagnostics Corporation). Equal amounts (500 ug) of each cell lysates were incubated with 30 µg of GST-Rhotekin Rho binding domain coupled to glutathione-agarose beads on a rocker platform at 4°C for 45 min. After incubation, beads were washed three times with the washing buffer (50 mM Tris·HCl pH 7.2, 150 mM NaCl, 10 mM MgCl2, 0.1 mM phenylmethylsulfonyl fluoride, and 1% Triton X-100). GTP bound Rho was eluted with Laemmli's SDS-sample buffer (Boston Bio products; BP-110R) containing 1mM dithiothreitol and boiled for 5 min. The samples were subjected to 4–12% SDS-PAGE, transferred onto nitrocellulose membranes (Invitrogen), and detected by immunoblotting, using Rho antibody (clone 55; catalog no. 05–778; Upstate).
Transwell cell migration and scratch wound assay
Transwell cell migration (Boyden) assays were performed with 6.5-mm-diameter Falcon cell culture inserts (8-µm pore size; Becton Dickinson) precoated with 0.01% gelatin in 24-well cell culture plates. Cells were co-transfected with GFP-expressing plasmids and muskelin siRNA or control siRNA, the transfectants were incubated for 48 hours, and sorted by FACS. Purified populations of GFP co-transfected cells from both control or muskelin siRNA were then resuspended in serum-free media, and transferred to the upper chamber (1.0 × 105 cells in 350 µL). 900 µL media containing 10% FBS were added to the lower chamber. After an incubation for 24 hours, the cells remaining on the upper surface of the filter were removed with a cotton swab; cells that had migrated to the lower surface were fixed with methanol for 10 min at room temperature, stained with 1% crystal violet (Sigma-Aldrich, Saint Louis, MO) in methanol for 30 min, distained with deionized water, visualized microscopically, and photographed. For quantification, migrated cells were solubilized with 1% Triton X-100 (Triton). The collected lysates were quantified by colorimetric analysis in a spectrophotometer at O.D. 590 nm.
For cell migration in scratch wounds, 1.0×106 cells/ 60 mm dish were evenly plated, grown for 24 hours to become confluent, and multiple scratch wounds were made with standard pipette tips. After the suspended cells were washed away, the cultures were refed with fresh medium with or without the indicated inhibitors, and incubated for 12 hours. The migrated cells within the scratch wound area were observed and photographed at indicated times under microscope equipped with a digital camera (Carl Zeiss, Thornwood, NY). The extent of wound closure was quantified by ImageProPlus software and defined as the ratio of the wound area remaining at 12 hours to the original wound area (0 hour) (n = 4).
Immunofluorescence staining
After indicated incubation, cells were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.25% Triton X-100 in PBS and blocked with 3% BSA in PBS. The cells were incubated with a 1:250 dilution of the indicated primary antibodies in PBS at 4°C overnight. After being thoroughly washed in PBS, the cells were incubated in 1:500 Alexa-conjugated appropriate secondary antibodies for 1 hour. To visualize actin or nuclei, cells were incubated with phalloidin (1:50) or DAPI (1:2500) for 1 hour. After being stained, cells were thoroughly washed with PBS and mounted with gel mounting solution (Biomeda Corporation, Foster City, CA).
Confocal microscopy
Fluorescence-labeled cells were viewed using a Zeiss LSM 780 confocal microscope with an excitation wavelength of 488 nm to detect transfected GFP or GFP-tagged proteins. Fluorescent Alexa probes were viewed with excitation wavelengths of 488 nm (Alexa 488), 568 nm (Alexa 568), 647 nm (Alexa 647), and co-localization was assessed by Zeiss ZEN image analysis software. Images were made using a 63× objective with a 2× magnifier to produce a 126× magnification. We also performed single staining for each color (not shown) to confirm that the co-localization did not results from “bleed through” between channels, and adjusted the gain in a similar way for both channels to eliminate spill-over between channels.
Data analysis
Immunoblots were quantified by densitometry scanning using ImageQuant (GE Healthcare, Piscataway, NJ) image analysis software. Results are expressed as mean densities ± standard error mean (s.e.m.) from three or four independent experiments. The relative density for the protein of interest was normalized to β-tubulin or GAPDH. For statistical analysis, Student's t test was performed using SigmaPlot software (Systat, San Jose, CA), and p<0.05 was considered statistically significant.
RESULTS
Myosin, p39, and muskelin form a protein complex in vivo
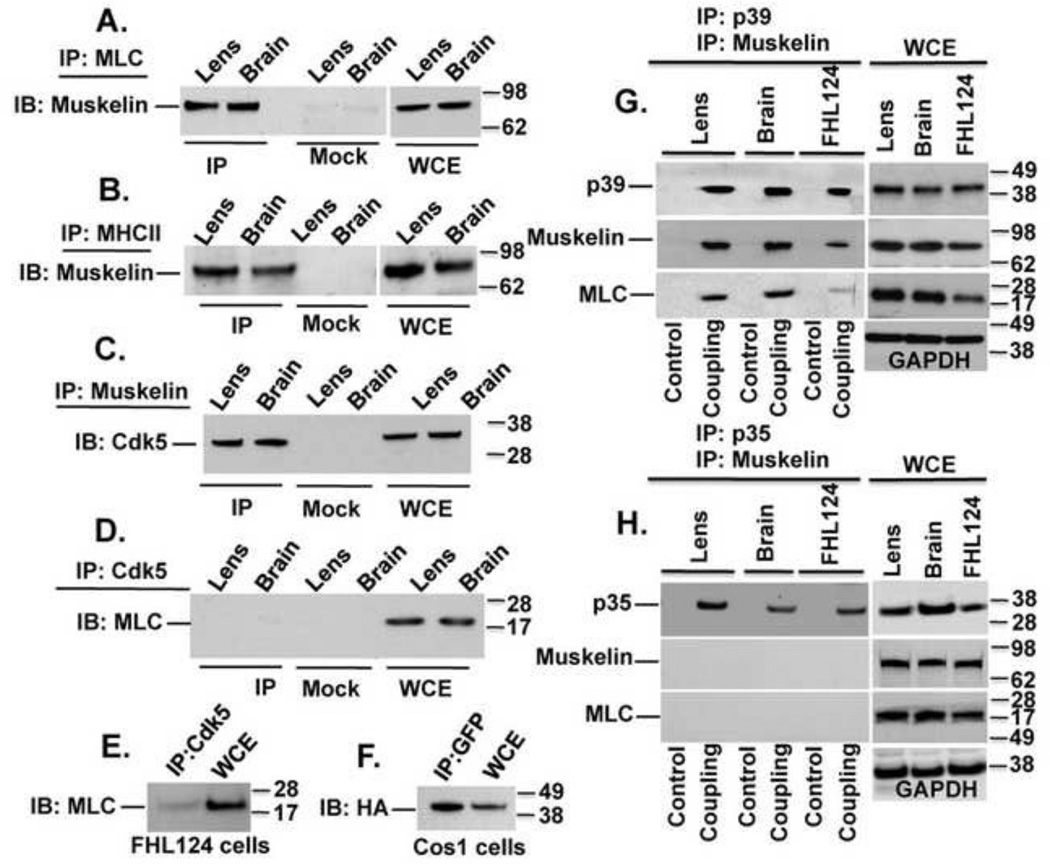
To determine whether a protein complex containing p39, myosin, and muskelin exists in vivo, we first performed a series of co-immunoprecipitation experiments. Protein extracts from rat lens and brain tissues were immunoprecipitated (IP) with MLC antibody and immunoblotted (IB) for muskelin. The co-IP assay showed that MLC and muskelin do form an endogenous protein complex in both tissues (Fig. 1A). No muskelin was detected in a ‘Mock’ IP control containing non-immune IgG. Since MLC is constitutively associated with myosin heavy chain (MHC II) as part of non-muscle myosin II, we carried out another IP assay using MHC II antibody followed by IB for muskelin. The results showed that muskelin forms an endogenous protein complex with MHC II in both tissues (Fig. 1B). We confirmed the interaction of p39 and muskelin from lens epithelial cells, rat lens, and brain tissues, as previously seen [2]. Cdk5 was also part of the complex (Fig. 1C), as positive results were obtained when the extracts from lens and brain tissues were IP with muskelin followed by IB with Cdk5 antibodies. Although we were not able to detect an immune complex of endogenous Cdk5 with MLC (Fig.1D and 1E) in lens, brain, or FHL124 cells, co-IP of HA-Cdk5 and GFP-MLC was seen in Cos1 cells expressing these constructs (Fig. 1F). While the studies presented above demonstrated that p39 and Cdk5 were linked to muskelin and myosin II, it was not clear whether muskelin, p39, and myosin were part of a single protein complex in vivo. Therefore, we used a double sequential IP approach to first isolate a native intact protein complex. P39 antibody (or antibody against p35, as a negative control) was immobilized on a column (Co-IP kit, Pierce) and was used to isolate the intact p39 (or p35) protein complexes. These p39- or p35-containing protein complexes were isolated by a non-reducing elution buffer and were therefore free of any associated antibody. This procedure allowed us to perform a second IP reaction for another member of the complex, muskelin. The linkage of MLC to isolated p39 and muskelin complexes, and the expression of each of these proteins were determined by western blot analysis using the appropriate antibodies (Fig. 1G). The specificity of the linkage of myosin and muskelin to p39 complex was verified using immobilized non-immune rabbit IgG (control). These results demonstrate that p39, muskelin, and myosin are part of the same protein complex in vivo, in contrast to the p35 negative control (Fig. 1H).
FIGURE 1. Myosin, p39, and muskelin form an endogenous protein complex in rat tissues.
(A) Lysates from rat lens and brain tissues were IP with MLC antibody followed by IB with muskelin antibody. Muskelin was detected in immunoprecipitates from both tissues but was not present in ‘Mock IP’ control. WCE = whole cell extract. (B) Immune complex between MHC II and muskelin was confirmed by experiments analogous to those in A. (C) Immune complex between muskelin and Cdk5. (D–E) No immune complex between MLC and Cdk5 in rats tissues and in FHL24 lens cells. (F) Immune complex between GFP-MLC and HA-Cdk5 in Cos 1 cells. (G) A double sequential IP (described in detail in the Material and Methods) was used to demonstrate that a protein complex containing p39, muskelin, and myosin does exist in rat lens, brain, and FHL24 lens cells. Immunoblots of WCE with indicated antibodies detect the expression of p39, muskelin, MLC and GAPDH (Right panels). (H) Experimental conditions were similar as shown in G except p35 antibody was immobilized on column as a negative control.
Interaction of muskelin with myosin requires p39
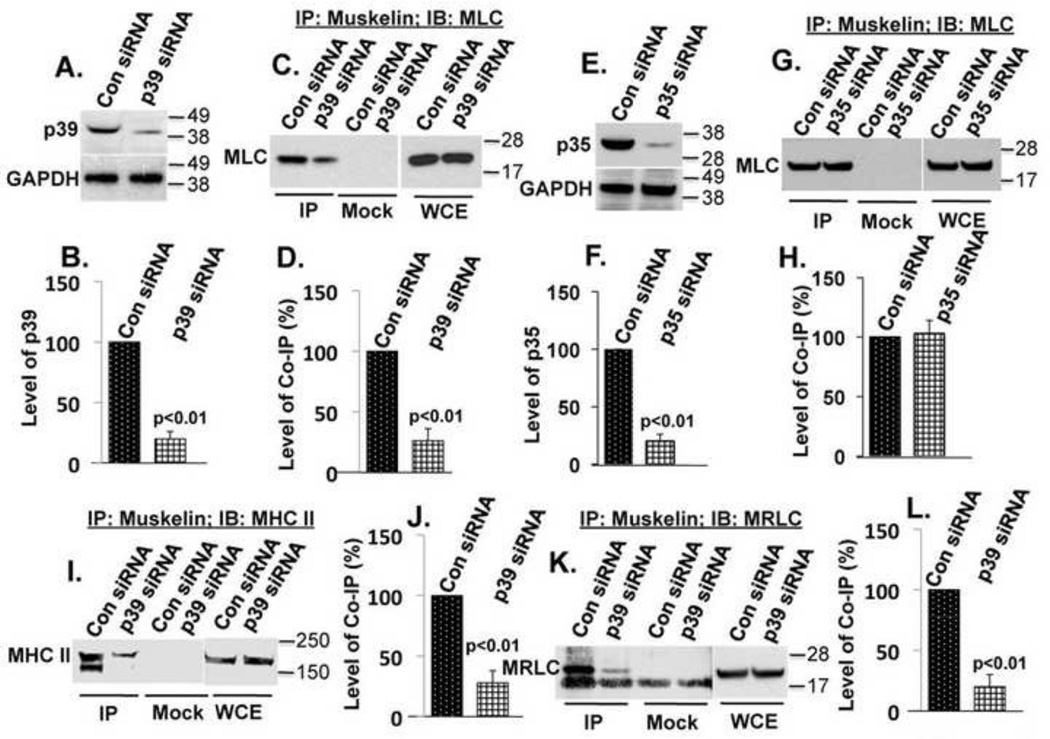
If p39 directly links muskelin to myosin, the absence of p39 should prevent co-IP of muskelin and myosin subunits MLC, MRLC and MHC II. To test this possibility, FHL124 cells were transfected simultaneously with two different p39 siRNAs to suppress p39 expression. Transfection efficiency of cells, as judged by green fluorescence (derived from co-transfection with a GFP-expressing plasmid), was consistently about 70–75%, and p39 expression was suppressed to a similar degree (78%), as shown by immunoblotting (Fig.2A and 2B). Suppression of p39 expression by siRNAs correlates fairly well with the reduction in co-IP of muskelin with myosin subunits MLC (Fig.2C and 2D), MHC II (Fig.2I and 2J), and MRLC (Fig.2K and 2L), demonstrating that p39 is required for efficient complex formation between muskelin and myosin II. However, suppression of p35 expression (Fig.2E and 2F) did not affect the binding of muskelin to MLC (Fig.2G and 2H).
FIGURE 2. p39 specifically links muskelin to myosin.
(A) Suppression of p39 by siRNAs (upper panel), GAPDH as a loading control (lower panel). (B) The results of three experiments shown in A were quantified by densitometry and normalized to GAPDH. The graph represents mean ± s.e.m. p<0.01. (C) Experimental conditions were similar as those in A. Suppression of p39 reduced co-IP of muskelin and MLC. WCE showed equal protein expression in siRNAs transfected cells. (D) The results of three experiments as shown in C were quantified by densitometry. The graph represents mean ± s.e.m. p<0.01. (E) Suppression of p35 by siRNAs (upper panel) and GAPDH as a loading control (lower panel). (F) The results of three experiments shown in E were quantified by densitometry and normalized to GAPDH. The graph represents mean ± s.e.m. p<0.01 (G) Experimental conditions were similar, as those in E. Suppression of p35 did not alter the co-IP of muskelin and MLC. (H) The results of three experiments as shown in G were quantified by densitometry. The graph represents mean ± s.e.m. (I) Suppression of p39 reduced MHC II and muskelin co-IP. (J) The results of three experiments as shown in I were quantified by densitometry. The graph represents mean ± s.e.m. p<0.01 (K) Suppression of p39 reduced MRLC and muskelin co-IP. A nonspecific band of approximately 15 kDa was seen in both IP and Mock IP control. (L) The results of three experiments as shown in K were quantified by densitometry. The graph represents mean ± s.e.m. p<0.01.
Localization of muskelin on stress fibers
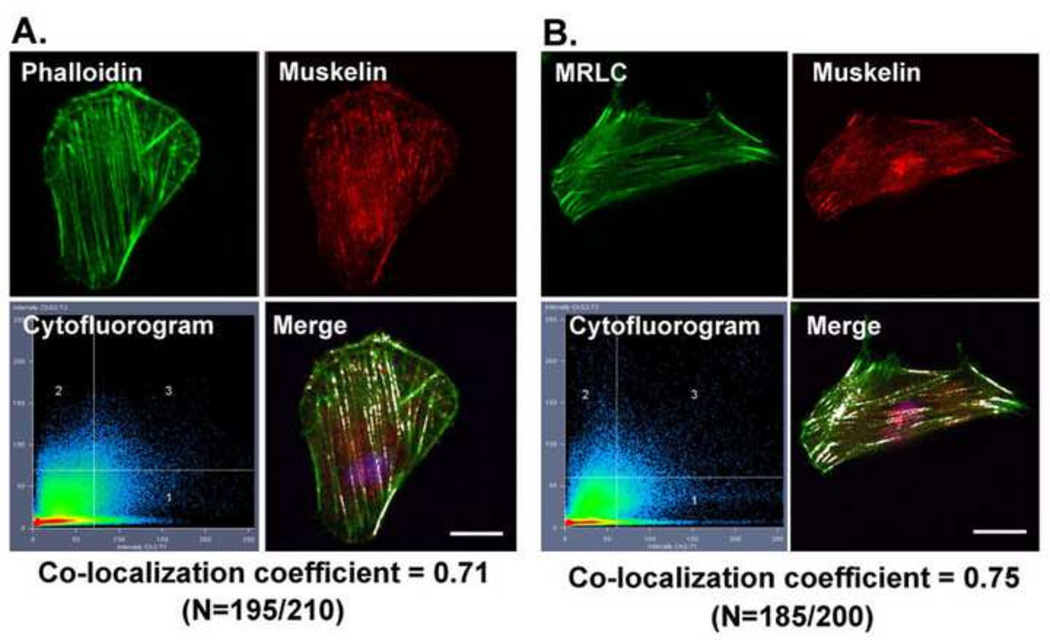
Since Cdk5 kinase activity is associated with stress fiber organization and contraction [12], we tested whether muskelin, which is a binding partner of the Cdk5 activating protein p39, co-localized with actin and myosin on stress fibers. Confocal and quantitative analysis showed co-localization of muskelin with actin, with co-localization coefficients of approximately 0.70 (Fig. 3A), consistent with a functional role for muskelin in stress fiber organization. As an additional test of the localization of muskelin on stress fibers, cells were doubly immunostained with muskelin and MRLC antibodies, which confirmed the co-localization of muskelin and MRLC on stress fibers, with co-localization coefficients of approximately 0.75 (Fig. 3B).
FIGURE 3. Muskelin co-localize with MRLC and on stress fibers in spreading lens epithelial cells.
(A) Co-localization of endogenous muskelin (red) with actin (green). The cytofluorogram of the confocal image shows the distribution of red and green pixels and co-localization of muskelin and actin are highlighted in white color in the merged image with the co-localization coefficient 0.71. The images are representative of the majority of the cells (N=195/210), and scale bars = 20 µm. (B) Co-localization of endogenous muskelin (red) and MRLC (green). The co-localization of muskelin and MRLC are highlighted in white color in the merged image with the co-localization coefficient 0.75. The images are representative of the majority of the cells (N=185/200), and scale bars = 20 µm.
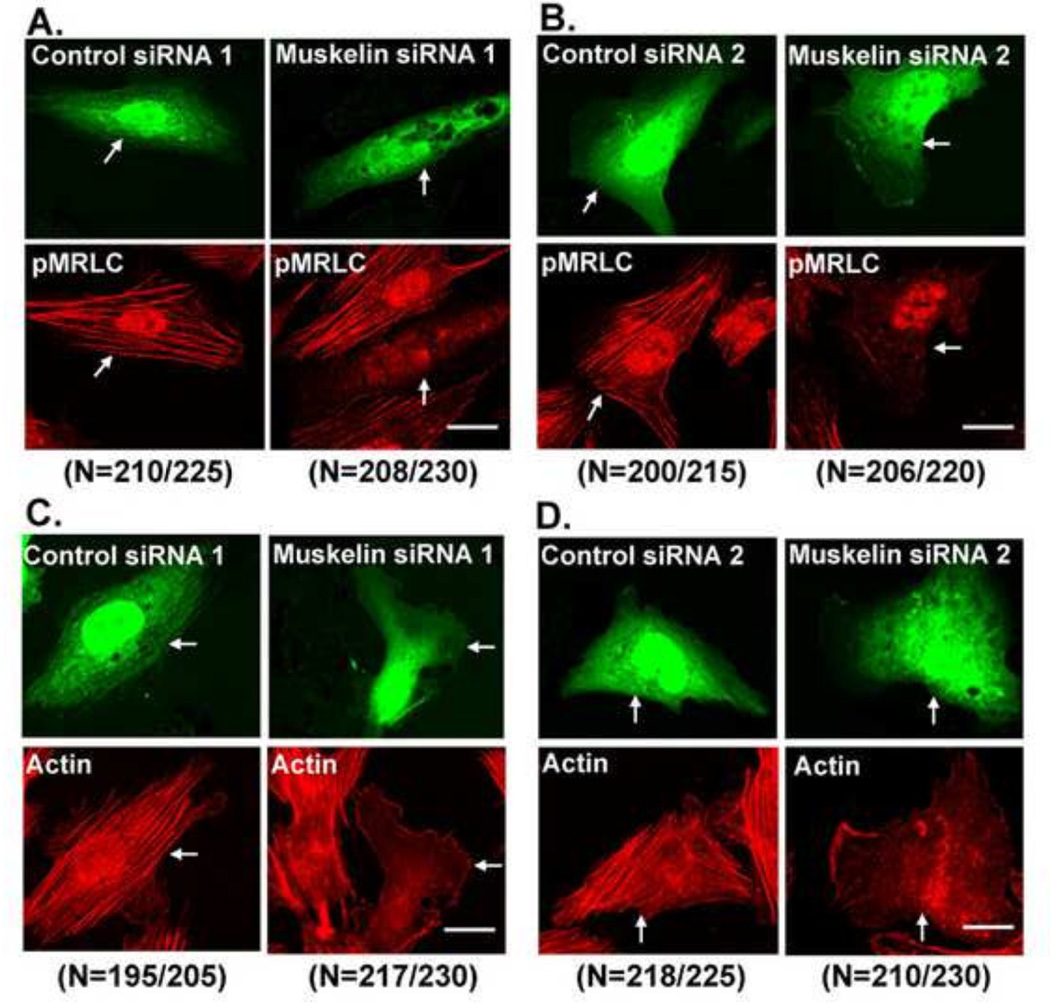
Suppression of muskelin expression reduces MRLC phosphorylation and disrupts stress fiber organization
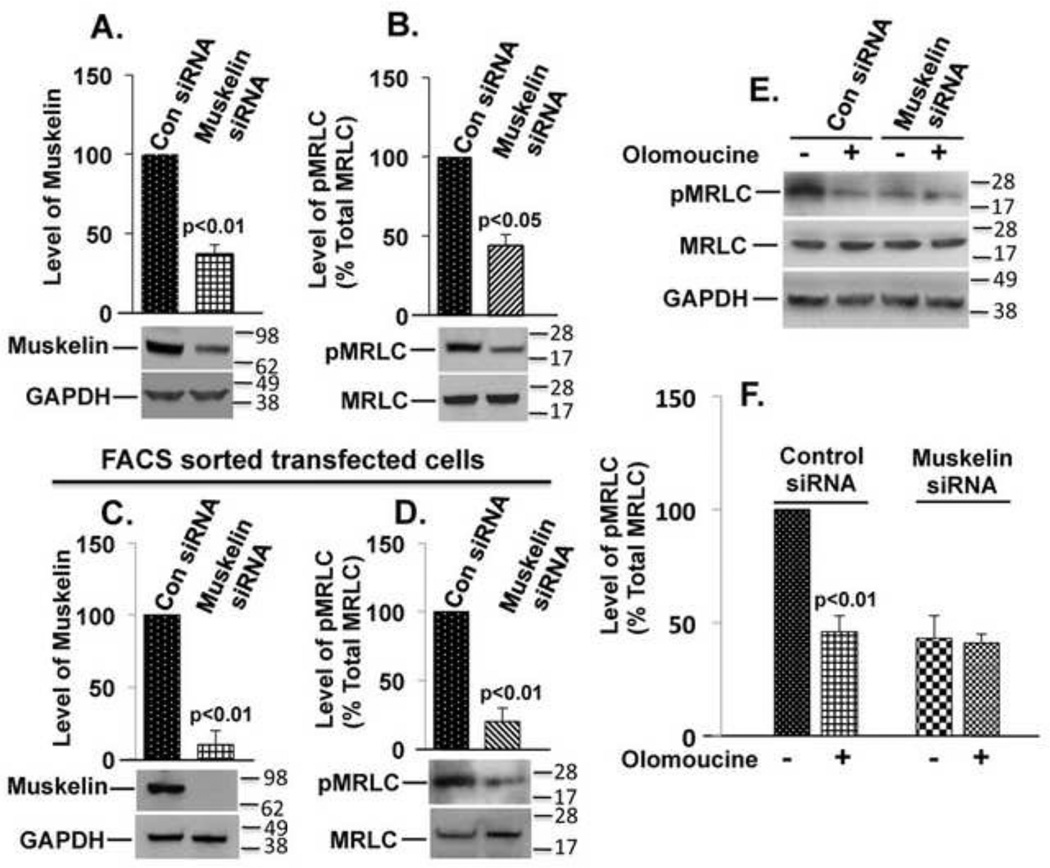
To determine the possible physiological significance of the co-localization of muskelin with actin and MRLC, we suppressed muskelin expression and examined the effect on MRLC phosphorylation and stress fiber organization. Cells were transfected with either of two different muskelin siRNAs that recognize dissimilar target sites or with two non-targeting siRNAs as negative controls. By 48 hours after transfection, we observed 63% reduction in muskelin expression by muskelin siRNA 1 (Fig. 4A) and 61% reduction by muskelin siRNA 2 (not shown) compared to control siRNAs transfected cells. Suppression of muskelin expression reduced 56% (p<0.05) phosphorylated MRLC (pMRLC) level by muskelin siRNA 1 (Fig. 4B) and 53% (p<0.05) by muskelin siRNA 2 (not shown) respectively, compared to non-targeting siRNA controls. The suppression of muskelin expression correlates fairly well with the reduction in MRLC phosphorylation, which closely corresponds to the transfection efficiency of muskelin siRNA, as judged by green fluorescence (derived from co-transfection with a GFP-expressing plasmid). These results demonstrate the role of muskelin in efficient MRLC phosphorylation in lens epithelial cells. To investigate whether the remainder of MRLC phosphorylation in the muskelin siRNA transfected cells was a result of cells that were not-transfected or residual muskelin expression in siRNA transfected cells, cells were co-transfected with muskelin siRNA and GFP-expressing plasmids, the transfectants were incubated for 48 hours, and sorted by FACS. Purified populations of GFP co-transfected cells from both control or muskelin siRNA were then harvested. Approximately 90% reduction in muskelin expression (Fig. 4C) was accompanied by 80% (p<0.01) reduction in MRLC phosphorylation (Fig. 4D) in the purified populations of cells transfected with muskelin siRNA compared to control siRNA. By confocal microscopy, more than 90% of the cells transfected with muskelin siRNAs displayed less pMRLC immunofluorescence and appeared less contracted, having only thin and faintly stained fibrils at the cell periphery. In contrast, cells transfected with non-targeting control siRNAs appeared to be similar to untransfected cells and showed no reduction in pMRLC immunofluorescence (Fig.5A and 5B). Next we examined the effects of muskelin suppression on stress fiber organization and architecture by two different muskelin siRNAs. Cells transfected with a non-targeting siRNAs showed no change in either parameter compared to untransfected cells. In contrast, cells transfected with a muskelin siRNAs contained no visible stress fibers, and had only a few stained actin fibrils at the cell periphery (Fig.5C and 5D). As with the inhibition of Cdk5 kinase activity, suppression of muskelin expression reduced MRLC phosphorylation and consequently dissociation of stress fibers. To assess the effects of muskelin knockdown on MRLC phosphorylation and actin stress fiber formation in the same cell, we performed triple co-localization of GFP (to identify transfected cells), pMRLC, and actin. Cells transfected with non-targeting siRNAs appear very similar to untransfected cells (Suppl. Fig. S1A). In contrast, cells transfected with muskelin siRNAs showed reduced pMRLC immunofluorescence, had only a few stained actin fibrils at the cell periphery, but no apparent center stress fibers (Suppl. Fig. S1B). Since Cdk5 is important regulator for stabilizing focal adhesions during cell migration [23, 24], we next examined the effects of muskelin knockdown on focal adhesions. Cells transfected with muskelin siRNAs showed no visible change in focal adhesions and appear similar to cells transfected with control siRNAs (Suppl. Fig. S2A and S2B), indicating that apparent loss of stress fibers by suppressing muskelin is not due to a loss of focal adhesions.
FIGURE 4. Muskelin siRNA reduces MRLC phosphorylation and blocks the effect of Cdk5.
(A) Graph represents the results of three experiments shown in immunoblots (below) for suppression of muskelin expression by siRNA, quantified by densitometry and normalized to GAPDH. p<0.01. (B) The experimental conditions were similar those in A. Suppression of muskelin decreases Thr18/Ser19 phosphorylated MRLC (pMRLC) but not total MRLC. Graph represents the ratio of pMRLC to total MRLC. N=3, p<0.05 (C) Graph represents the suppression of muskelin expression by siRNA in the purified populations (sorted by FACS) of transfected cells. N=3, p<0.01. (D) The experimental conditions were similar those in C. The graph represents mean ± s.e.m. of pMRLC to total MRLC in purified cells transfected with control siRNA or muskelin siRNA. N=3, p<0.01. (E) Muskelin siRNA or control siRNA-transfected cells were incubated without or with the Cdk5 inhibitor Olomoucine. immunoblotted for pMRLC (upper panel), total MRLC (middle panel), and GAPDH (lower panel). (F) The results of three independent experiments as shown in E were quantified by densitometry and normalized. The graph represents mean ± s.e.m. of pMRLC to total MRLC. The level of pMRLC in the cells treated with Olomoucine or transfected with muskelin siRNA was statistically different (p<0.01) from the untreated control cells, but there was no difference between Olomoucine-treated and untreated cells transfected with muskelin siRNA.
FIGURE 5. Suppression of muskelin expression by siRNAs significantly affects MRLC phosphorylation and stress fiber organization in lens cells.
(A) Cells were co-transfected with GFP and control siRNA 1 or GFP and muskelin siRNA 1, replated after 48 hours. GFP fluorescence identifies transfected cells. Cells transfected with control siRNA 1 showed intense staining of pMRLC (lower left panel) with well-formed stress fibers throughout the entire cell, which were similar to untransfected cells. In contrast, cells transfected with muskelin siRNA 1 showed significantly less pMRLC immunofluorescence (lower right panel), few if any concave boundaries, consistent with reduced contraction. Scale bar, 20 µm. (B) The experimental conditions were similar as in A except cells were transfected with muskelin siRNA 2 or control siRNA 2. GFP fluorescence identifies transfected cells. Cells transfected with control siRNA 2 showed intense staining of pMRLC (lower left panel), with well-formed stress fibers throughout the entire cell as observed in control siRNA 1 transfected cells. Cells transfected with muskelin siRNA 2 (lower right panel) appear very similar to cells transfected with muskelin siRNA 1. Scale bar, 20 µm. (C) The experimental conditions were similar as in A except cells were immunostained with actin (red). Cells transfected with control siRNA 1 showed well-formed stress fibers throughout the cell, had concave boundaries, and appear similar to untransfected cells (lower left panel). In contrast, cells transfected with muskelin siRNA 1 (lower right panel of C) showed a significant loss of center stress fibers, with only fewer stress fibers at cell periphery. Scale bar, 20 µm. (D) The experimental conditions were similar as in C except cells were transfected with muskelin siRNA 2 or control siRNA 2. Cells transfected with control siRNA 2 (lower left panel) had concave boundaries with well-formed stress fibers throughout the cell. In contrast, cells transfected with muskelin siRNA 2 (lower right panel) showed a significant loss of center stress fibers, with only fewer stress fibers at cell periphery. Scale bar, 20 µm. The images presented in each panels are representative of the majority of the cells. We have counted more than 200 cells from several fields (Number of cells counted for each condition are presented in parenthesis below each panel). More than 90% of muskelin siRNAs-transfected cells showed less pMRLC immunofluorescence and lost center stress fibers, with only fewer stress fibers at cell periphery.
Silencing muskelin blocks the effects of Cdk5 on MRLC phosphorylation
The above findings suggest that muskelin may contribute to the previously observed effects of Cdk5 on myosin phosphorylation. To test this possibility, we compared the effects of muskelin suppression and Cdk5 inhibition on MRLC phosphorylation by transfecting the cells with control or muskelin siRNAs, followed by treatment with Cdk5 inhibitor Olomoucine. Olomoucine reduced MRLC phosphorylation by 54% (p<0.01) in the cells transfected with control siRNAs compared to untreated controls (Fig.4E and 4F). Suppression of muskelin with siRNAs had an almost identical effect (57%; p<0.01) on MRLC phosphorylation. Moreover, the addition of Olomoucine to the cells transfected with muskelin siRNAs produced no significant additional effect (59%) on MRLC phosphorylation (Fig.4E and 4F), implying that Cdk5 and muskelin affect the same pathway.
Suppression of muskelin decreases Rho activity
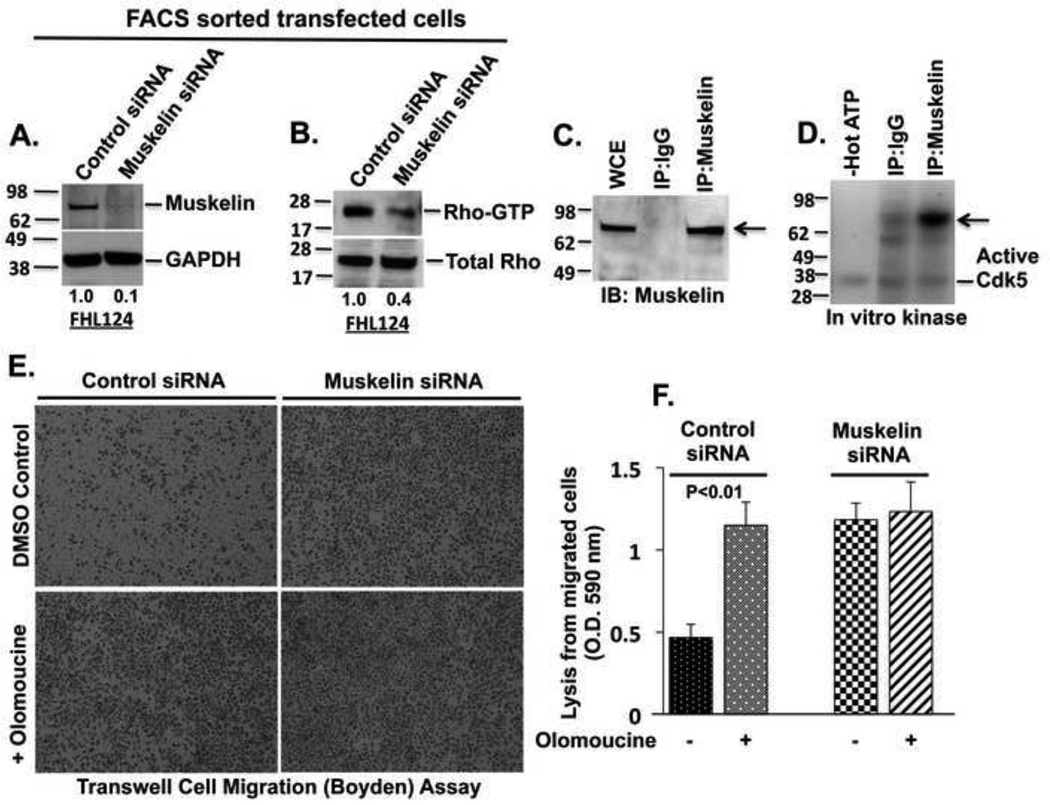
The Cdk5-dependent Rho-ROCK pathway is the major regulator of MRLC phosphorylation in lens epithelial cells (12). Since both Cdk5 and muskelin affect the same signaling pathway, we examined the effect of muskelin suppression on Rho activity. The efficient siRNA-induced suppression of muskelin (Fig. 6A) was accompanied by an approximately 60% (p<0.01) reduction in the Rho-GTP level (Fig. 6B), demonstrating that muskelin regulates the Rho activation upstream of MRLC phosphorylation.
FIGURE 6. Muskelin is a substrate for Cdk5, muskelin-dependent regulation of Rho signaling affects cell migration rate.
(A) Suppression of muskelin expression by siRNAs (upper panel) and GAPDH as a loading control (lower panel). (B) Suppression of muskelin decreases Rho-GTP but not total Rho. (C) Immunoblot from IP reaction with muskelin antibody to verify the purified muskelin protein from lens cells. (D) IP purified muskelin is strongly phosphorylated in vitro (arrow) by recombinant Cdk5 kinase, as detected with 32P autoradiography. (E) Migration in transwell. 1 × 105 cells from purified populations of control or muskelin siRNA transfected cells (purified by FACS) were resuspended in serum-free media in the absence or presence of Olomoucine (15 µM) or the indicated combinations, and seeded in a transwell dish, and cultured for 24 hours. The migrated cells stained with 1.0% crystal violet and photographed. Images represent the migrated cells through transwell dish in indicated conditions. (F) Migrated cells were solubilized with 1% Triton X-100, and the lysates were quantified by colorimetric analysis in a spectrophotometer at O.D. 590 nm. Graph represents average of four individual experiments ± S.D. p < 0.01. All treatment groups were statistically different (p< 0.01) from the control, but there was no significant difference among treatments with Olomoucine, muskelin siRNA, or Olomoucine plus muskelin siRNA.
Muskelin is a Cdk5 substrate
To determine whether muskelin is a direct substrate for Cdk5 kinase, muskelin was immunoprecipitated from FHL124 lens cells (Fig. 6C), and examined for phosphorylation by recombinant Cdk5 in an in-vitro kinase assay. The results indicated that immunoprecipitated muskelin was highly phosphorylated, whereas IgG control showed no phosphorylation signal (Fig. 6D), indicating that muskelin is a direct substrate for Cdk5 kinase.
Impact of muskelin suppression on cell migration rate
Since myosin phosphorylation-dependent cytoskeletal contraction plays an important role in cell migration, regulation of myosin phosphorylation by muskelin may contribute to control cell migration rate. To test this possibility, we compared the effects of muskelin suppression and Cdk5 inhibition on transwell cell migration and scratch wound closure. Cells were co-transfected with GFP expressing plasmids with muskelin siRNAs or control siRNAs for 48 hours, sorted by FACS, and the purified populations of transfected cells were used for these bioassays. Cells transfected with control siRNA were migrated significantly slower compared to muskelin siRNA transfected cells (Fig.6E and 6F), and only 32% of the scratch wound area was healed after 12 hours in untreated cells transfected with control siRNA (Suppl. Fig. S3A and S3B). Olomoucine treatment significantly increased transwell cell migration rate (Fig.6E and 6F), and scratch wound healing to 74% (Suppl. Fig S3A and S3B). The suppression of muskelin showed an almost identical effect on transwell cell migration and on scratch wound closure (76% healed). Moreover, the addition of Olomoucine to muskelin siRNA-transfected cells did not yield any significant additional effect on transwell cell migration (Fig.6E and 6F) and on scratch wound closure (Suppl. Fig. S3A and S3B), indicating that Cdk5 and muskelin may affect the same signaling pathway.
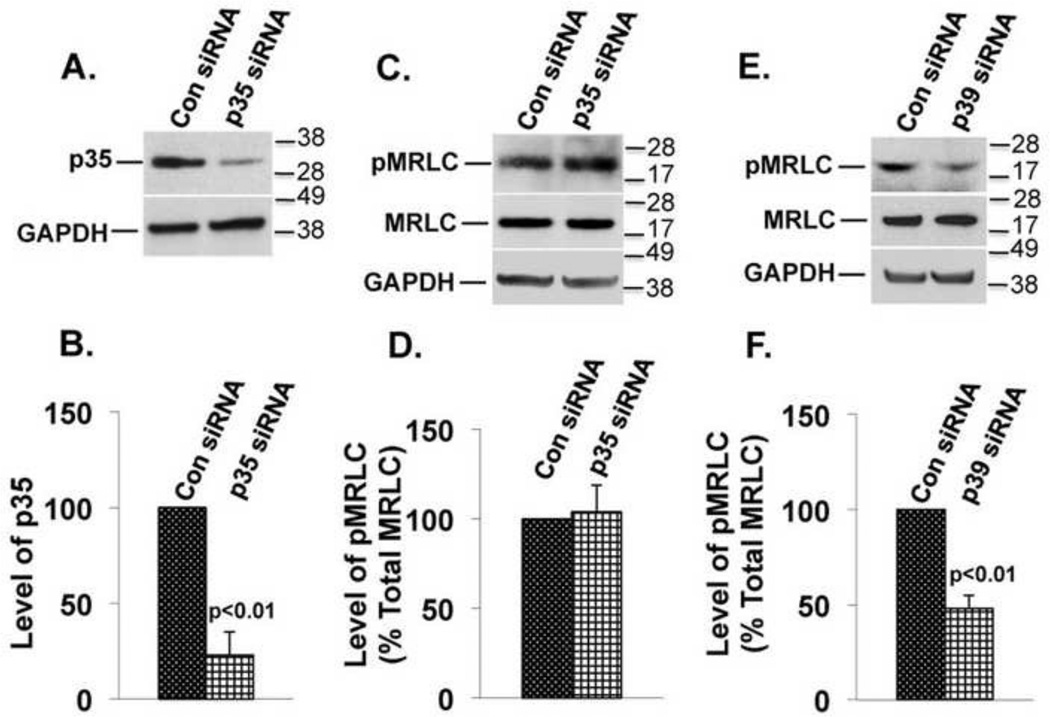
Suppressing p39 but not p35 inhibits MRLC phosphorylation
To determine whether Cdk5 is activated by p35, p39, or both during Cdk5-dependent regulation of MRLC phosphorylation, we tested the effects of suppressing these activators on MRLC phosphorylation. Cells were transfected with p35 or p39 siRNAs or with control siRNAs and harvested 48 hours later. Suppression of p35 (Fig.7A and 7B) had no effect on MRLC phosphorylation (Fig.7C and 7D). In contrast, suppression of p39 (see Fig.2A and 2B) was accompanied by a 55% (p<0.01) reduction in MRLC phosphorylation (Fig.7E and 7F), indicating that p39, but not p35, is necessary and sufficient for Cdk5-dependent regulation of myosin phosphorylation. Almost identical effects on MRLC phosphorylation were previously seen when Cdk5 expression was suppressed or Cdk5 kinase activity was inhibited (12), indicating that p39 is the relevant Cdk5 activator for regulation of myosin phosphorylation.
FIGURE 7. p39 siRNA but not p35 siRNA reduces MRLC phosphorylation.
(A) Suppression of p35 protein expression by p35 siRNAs (upper panel) and GAPDH was used as a loading control (lower panel). (B) The results of three independent experiments as in A were quantified by densitometry and normalized to GAPDH. The graph represents mean ± s.e.m. (C) The experimental conditions were similar to those in A. Immunoblots for pMRLC (upper panel), total MRLC (middle panel), and GAPDH (lower panel). (D) Results of three independent experiments as shown in C were quantified by densitometry, and the ratio of pMRLC to total MRLC was plotted. The values represent mean ± s.e.m. Suppression of p35 did not alter the level of pMRLC. (E) The experimental conditions were similar to those in A except p39 siRNAs was transfected. Immunoblots for pMRLC (upper panel), total MRLC (middle panel), and GAPDH (lower panel) are shown. (F) Results of three independent experiments as shown in E were quantified by densitometry, and the ratio of pMRLC to total MRLC was plotted. The values represent mean ± s.e.m. The level of pMRLC was reduced (p<0.01) in cells transfected with p39 siRNAs as compared to cells transfected with control siRNA.
DISCUSSION
The present study demonstrates that muskelin regulates myosin II motor function and stress fiber formation when linked to myosin essential light chain (MLC) through p39. In addition, muskelin colocalizes with MRLC and actin in stress fibers, and is necessary for efficient Rho-dependent MRLC phosphorylation and stress fibers formation. The data further show that muskelin, MLC, and p39 form a ternary complex in human lens epithelial cells in vitro and in brain and lens in vivo. Since Cdk5 immunoprecipitates with muskelin and MLC, it may be associated with this complex. Furthermore, suppressing muskelin mimics the effects of inhibiting or suppressing Cdk5 on MRLC phosphorylation and cell migration [12], and suppressing muskelin and inhibiting Cdk5 simultaneously produces no additional effect, indicating that Cdk5 and muskelin act in the same signaling pathway. Binding of Cdk5 to the muskelin-p39-myosin complex is also consistent with the previous demonstration that Cdk5 binds p39 at a site distinct from the binding sites for muskelin and MLC [2, 21]. Binding of p39 to Cdk5 activates the kinase activity of Cdk5, which regulates the Rho-ROCK pathway of MRLC phosphorylation by controlling Src-dependent phosphorylation of the upstream inhibitor of Rho, p190RhoGAP [12, 24, 25]. This Cdk5-dependent Rho-ROCK pathway is by far the major regulator of MRLC phosphorylation in the lens, with little or no contribution from myosin light chain kinase (MLCK) [12]. Other kinases known to regulate MRLC phosphorylation, including PAK-1 [26], citron kinase [27], zipper interacting protein (ZIP) kinase [28], and calcium-calmodulin regulated (CaM) kinase [29], have not yet been examined in this cell type, and might be responsible for the low residual levels of MRLC phosphorylation seen when p39 and/or muskelin are suppressed. Although p39 has long been known as an activator of Cdk5, the present findings reveal that it also has an important role as an adaptor protein linking muskelin to the MLC subunit of myosin II. Moreover, since the residual co-immunoprecipitation of muskelin and MLC seen when p39 expression was suppressed was quantitatively proportional to the residual expression of p39, the data suggest that p39 may be the only cellular protein able to fulfill this role. Certainly, this function is not shared by the other activator of Cdk5, p35, as demonstrated by the lack of effect of p35 suppression on muskelin-MLC co-immunoprecipitation or MRLC phosphorylation. Furthermore, muskelin and p39 are colocalized in certain regions of the brain [3], raising the possibility that the adaptor role of p39 in linking muskelin to the cytoskeleton may be important in other cell types where both proteins are expressed. Thus, p39 has a dual role in cytoskeletal regulation: on the one hand, it is an integral subunit of Cdk5 kinase; on the other, it acts as an adaptor protein that recruits muskelin and other muskelin-associated proteins to stress fibers.
In addition to its role in cytoskeletal regulation reported here, p39 has other specific functions for which p35 cannot compensate. P39 is the principal Cdk5 activator in oligodendroglia and is required for myelin repair and oligodendroglial differentiation [30]. Cdk5/p39 is also responsible for Munc18-1 phosphorylation during Ca++-induced insulin exocytosis [31] and phosphorylates the microtubule protein tau in the developing mouse brain [31]. The in vivo specificity of Cdk5/p39 versus Cdk5/p35 may be controlled by spatial and temporal regulation of their expression [32, 33] or by specific subcellular localization mediated by particular binding partners of p35 and p39 [34].
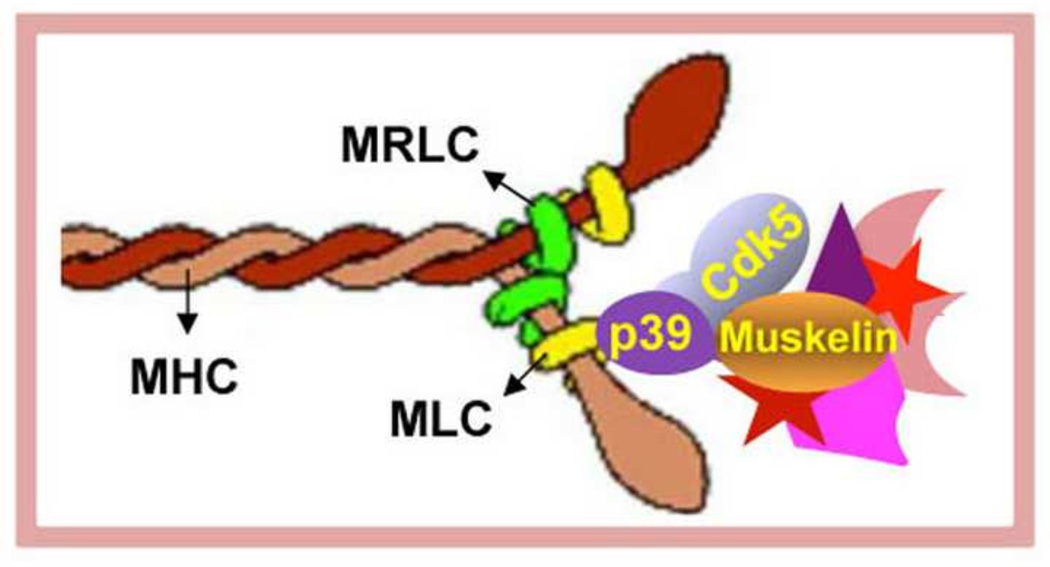
Multi-domain structure of muskelin [4, 6] suggests that muskelin may be acting as a scaffolding protein in the muskelin-p39-MLC complex. In support of this possibility, muskelin has been shown to have a number of binding partners in addition to MLC, p39, and Cdk5. These include RanBP9 [7], the transcription factor TBX20 (35), and the GABA receptor, GABAAR α1 [8]. Interestingly, when bound to muskelin, each of these proteins regulates some aspect of cytoskeletal function: TBX20 governs transcription of several cytoskeletal and myofibrillar proteins [35, 36]; RanBP9 affects focal adhesion signaling, β1-integrin endocytosis, and cell morphology [7, 36]; the muskelin-GABAAR α1complex is required for endocytosis and transport of the GABA receptor [8]; and, as shown here, the MLC-p39-muskelin complex regulates stress fiber formation and contraction. Possibly, the scaffolding function of muskelin may bring together signaling components involved in myosin-dependent cytoskeletal contraction, as shown in the model depicted in Fig. 8. These may include known regulators of the cytoskeleton such as Src kinase family members, RhoGEFs, and RhoGAPs. Our findings support this model. First, the Cdk5 is part of the complex containing muskelin-p39-myosin proteins. Second, muskelin is a direct substrate for Cdk5 kinase. Phosphorylation of muskelin by Cdk5 may facilitate the binding of other proteins involved cytoskeletal regulation. Remarkably, suppression of muskelin reduces Rho-GTP, MRLC phosphorylation, disrupted stress fiber, and promoted cell migration, just as pharmacological or genetic suppression of Cdk5 does [12]. Moreover, suppressing muskelin and inhibiting Cdk5 together has no additional effect, suggesting that Cdk5 may exert its effect through muskelin. Interestingly, the muskelin binding protein RanBP9 has been shown to bind directly to obscurin, a RhoGEF involved in myofibril assembly [37], although the possibility that the RanBP9-obscurin binary complex may be part of a larger complex that includes muskelin or MLC-p39-muskelin has not yet been explored.
FIGURE 8.
Schematic diagram shows Cdk5 activating protein p39 directly links muskelin to myosin II and thus to stress fibers, and indicates that p39 has a dual role in cytoskeletal regulation. It is an integral subunit of Cdk5 kinase as well as acting as an adaptor protein to recruit muskelin and other muskelin-associated cytoskeletal proteins, which may regulate MRLC phosphorylation, stress fibers formation, and cytoskeletal contraction.
The finding that p39 links muskelin to myosin II may be of particular importance in tissues such as lens and brain where both p39 and muskelin are highly expressed [2]. Since the formation of stress fibers in lens epithelial cells is Rho-dependent [38, 39], it seems likely that the in vivo role of the muskelin-p39-myosin complex in the lens epithelium may be to facilitate stress fiber formation. As lens epithelial cells differentiate and elongate to form lens fiber cells, actin is reorganized into cortical actin filaments [38]. Since muskelin shows a similar peripheral localization in elongating lens fiber cells, which requires p39 [2], p39 may continue to link muskelin to the actin cytoskeleton in differentiating fiber cells.
In summary, the results of this study not only reveal a novel adaptor function for p39 and provide a mechanism for linking muskelin to stress fibers, but also suggest that muskelin may affect multiple signaling pathways of cytoskeletal regulation.
Supplementary Material
HIGHLIGHTS.
This study reveals a novel adaptor function for the Cdk5 activator p39.
p39 is essential for recruiting scaffolding protein muskelin to stress fibers.
Muskelin regulates myosin phosphorylation and cytoskeletal organization in lens
A muskelin-p39-myosin protein complex forms in cells and regulates stress fiber formation and cell migration.
ACKNOWLEDGMENTS
We thank Dr. John Reddan, Oakland University, for providing the human lens epithelial cells (FHL124), the NIH Imaging Core Facility for confocal microscopy, and the FACS Core facility for isolating the GFP-positive transfected cells. This work is supported by the National Institutes of Health, Intramural Research Program Z01-EY000238-20.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: None
REFERENCES
- 1.Dhodda VK, Sailor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J. Neurochem. 2004;89:73–89. doi: 10.1111/j.1471-4159.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- 2.Ledee DR, Gao CY, Seth R, Fariss RN, Tripathi BK, Zelenka PS. A specific interaction between muskelin and the cyclin-dependent kinase 5 activator p39 promotes peripheral localization of muskelin. J. Biol. Chem. 2005;280:21376–21383. doi: 10.1074/jbc.M501215200. [DOI] [PubMed] [Google Scholar]
- 3.Tagnaouti N, Loebrich S, Heisler F, Pechmann Y, Fehr S, De Arcangelis A, Georges-Labouesse E, Adams JC, Kneussel M. Neuronal expression of muskelin in the rodent central nervous system. BMC Neurosci. 2007;8:28–39. doi: 10.1186/1471-2202-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams JC, Seed B, Lawler J. Muskelin, a novel intracellular mediator of cell adhesive and cytoskeletal responses to thrombospondin-1. The EMBO J. 1998;17:4964–4974. doi: 10.1093/emboj/17.17.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emes RD, Ponting CP. A new sequence motif linking lissencephaly, Treacher Collins and oral-facial-digital type 1 syndromes, microtubule dynamics and cell migration. Hum. Mol. Genet. 2001;10:2813–2820. doi: 10.1093/hmg/10.24.2813. [DOI] [PubMed] [Google Scholar]
- 6.Prag S, Collett GD, Adams JC. Molecular analysis of muskelin identifies a conserved discoidin-like domain that contributes to protein self-association. Biochem. J. 2004;381:547–559. doi: 10.1042/BJ20040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valiyaveettil M, Bentley AA, Gursahaney P, Hussien R, Chakravarti R, Kureishy N, Prag S, Adams JC. Novel role of the muskelin-RanBP9 complex as a nucleocytoplasmic mediator of cell morphology regulation. J. Cell. Biol. 2008;182:727–739. doi: 10.1083/jcb.200801133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heisler FF, Loebrich S, Pechmann Y, Maier N, Zivkovic AR, Tokito M, Hausrat TJ, Schweizer M, Bähring R, Holzbaur EL, Schmitz D, Kneussel M. Muskelin regulates actin filament- and microtubule-based GABA(A) receptor transport in neurons. Neuron. 2011;70:66–81. doi: 10.1016/j.neuron.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyd LM, Richardson WJ, Chen J, Kraus VB, Tewari A, Setton LA. Osmolarity regulates gene expression in intervertebral disc cells determined by gene array and real-time quantitative RT-PCR. Ann. Biomed. Eng. 2005;33:1071–1077. doi: 10.1007/s10439-005-5775-y. [DOI] [PubMed] [Google Scholar]
- 10.Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat. Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- 11.Liebl J, Weitensteiner SB, Vereb G, Takacs L, Fuerst R, Vollmar AM, Zahler S. Cyclin-dependent kinase 5 regulates endothelial cell migration and angiogenesis. J. Biol. Chem. 2010;285:35932–35943. doi: 10.1074/jbc.M110.126177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripathi BK, Zelenka PS. Cdk5-dependent regulation of Rho activity, cytoskeletal contraction, and epithelial cell migration via suppression of Src and p190RhoGAP. Mol. Cell. Biol. 2009;29:6488–6499. doi: 10.1128/MCB.01098-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellegrin S, Mellor H. Actin stress fibres. J. Cell. Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- 14.Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fibre assembly. J. Microsc. 2008;231:446–454. doi: 10.1111/j.1365-2818.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki T, Murata-Hori M, Ishitobi S, Hosoya H. Diphosphorylated MRLC is required for organization of stress fibers in interphase cells and the contractile ring in dividing cells. Cell. Struct. Funct. 2001;26:677–683. doi: 10.1247/csf.26.677. [DOI] [PubMed] [Google Scholar]
- 16.Dhavan R, Greer PL, Morabito MA, Orlando LR, Tsai LH. The cyclin-dependent kinase 5 activators p35 and p39 interact with the alpha-subunit of Ca2+/calmodulin-dependent protein kinase II and alpha-actinin-1 in a calcium-dependent manner. J. Neurosci. 2002;22:7879–7891. doi: 10.1523/JNEUROSCI.22-18-07879.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humbert S, Dhavan R, Tsai LH. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J. Cell. Sci. 2000;113:975–983. doi: 10.1242/jcs.113.6.975. [DOI] [PubMed] [Google Scholar]
- 18.Qiao F, Gao CY, Tripathi BK, Zelenka PS. Distinct functions of Cdk5(Y15) phosphorylation and Cdk5 activity in stress fiber formation and organization. Exp. Cell Res. 2008;314:3542–3550. doi: 10.1016/j.yexcr.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez OM, Jones M, Guzman G, Szczesna-Cordary D. Myosin essential light chain in health and disease. Am. J. Physiol. Heart. Circ. Physiol. 2007;292:H1643–H1654. doi: 10.1152/ajpheart.00931.2006. [DOI] [PubMed] [Google Scholar]
- 21.Ledee DR, Tripathi BK, Zelenka PS. The CDK5 activator, p39, binds specifically to myosin essential light chain. Biochem. Biophys. Res. Commun. 2007;354:1034–1039. doi: 10.1016/j.bbrc.2007.01.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard M, Chan Y, Menko AS. Identification of a novel intermediate filament-linked N-Cadherin/y-catenin complex involved in the establishment of the cytoarchitecture of differentiated lens fiber cells. Develop. Bio. 2008;319:298–308. doi: 10.1016/j.ydbio.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Rajfur Z, Yousefi N, Chen Z, Jacobson K, Ginsberg MH. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat. Cell Biol. 2009;11:624–630. doi: 10.1038/ncb1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tripathi BK, Zelenka PS. Cdk5: A regulator of epithelial cell adhesion and migration. Cell. Adh. Migr. 2010;4:333–336. doi: 10.4161/cam.4.3.11131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Q, Qiao F, Gao C, Norman B, Optican L, Zelenka PS. Cdk5 targets active Src for ubiquitin-dependent degradation by phosphorylating Src(S75) Cell. Mol. Life. Sci. 2011;68:3425–3436. doi: 10.1007/s00018-011-0638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK) J. Muscle. Res. Cell. Motil. 1998;19:839–854. doi: 10.1023/a:1005417926585. [DOI] [PubMed] [Google Scholar]
- 27.Yamashiro S, Totsukawa G, Yamakita Y, Sasaki Y, Madaule P, Ishizaki T, Narumiya S, Matsumura F. Citron kinase, a Rho-dependent kinase, induces di-phosphorylation of regulatory light chain of myosin II. Mol. Biol. Cell. 2003;14:1745–1756. doi: 10.1091/mbc.E02-07-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murata-Hori M, Fukuta Y, Ueda K, Iwasaki T, Hosoya H. HeLa ZIP kinase induces diphosphorylation of myosin II regulatory light chain and reorganization of actin filaments in nonmuscle cells. Oncogene. 2001;20:8175–8183. doi: 10.1038/sj.onc.1205055. [DOI] [PubMed] [Google Scholar]
- 29.Suizu F, Fukuta Y, Ueda K, Iwasaki T, Tokumistu H, Hosoya H. Characterization of Ca2+/calmodulin-dependent protein kinase I as a myosin II regulatory light chain kinase in vitro and in vivo. Biochem. J. 2002;367:335–345. doi: 10.1042/BJ20020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bankston AN, Li W, Zhang H, Ku L, Liu G, Papa F, Zhao L, Bibb JA, Cambi F, Tiwari-Woodruff SK, Feng Y. p39, the primary activator for cyclin-dependent kinase 5 (Cdk5) in oligodendroglia, is essential for oligodendroglia differentiation and myelin repair. J. Biol. Chem. 2013;288:18047–18057. doi: 10.1074/jbc.M113.453688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lilja L, Johansson JU, Gromada J, Mandic SA, Fried G, Berggren PO, Bark C. Cyclin-dependent kinase 5 associated with p39 promotes Munc18-1 phosphorylation and Ca(2+)-dependent exocytosis. J. Biol. Chem. 2004;279:29534–29541. doi: 10.1074/jbc.M312711200. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi S, Saito T, Hisanaga S, Pant HC, Kulkarni AB. Tau phosphorylation by cyclin-dependent kinase 5/p39 during brain development reduces its affinity for microtubules. J. Biol. Chem. 2003;278:10506–10515. doi: 10.1074/jbc.M211964200. [DOI] [PubMed] [Google Scholar]
- 33.Valin A, Cook JD, Ross S, Saklad CL, Gill G. Sp1 and Sp3 regulate transcription of the cyclin-dependent kinase 5 regulatory subunit 2 (p39) promoter in neuronal cells. Biochim. Biophy. Acta. 2009;1789:204–211. doi: 10.1016/j.bbagrm.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim AC, Qu D, Qi RZ. Protein-protein interactions in Cdk5 regulation and function. Neurosignals. 2003;12:230–238. doi: 10.1159/000074625. [DOI] [PubMed] [Google Scholar]
- 35.Debenedittis P, Harmelink C, Chen Y, Wang Q, Jiao K. Characterization of the novel interaction between muskelin and TBX20, a critical cardiogenic transcription factor. Biochem. Biophys. Res. Commun. 2011;409:338–343. doi: 10.1016/j.bbrc.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo JA, Roh SE, Lakshmana MK, Kang DE. Pivotal role of RanBP9 in integrin-dependent focal adhesion signaling and assembly. FASEB J. 2012;26:1672–1681. doi: 10.1096/fj.11-194423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowman AL, Catino DH, Strong JC, Randall WR, Kontrogianni-Konstantopoulos A, Bloch RJ. The rho-guanine nucleotide exchange factor domain of obscurin regulates assembly of titin at the Z-disk through interactions with Ran binding protein 9. Mol. Biol. Cell. 2008;19:3782–3792. doi: 10.1091/mbc.E08-03-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber GF, Menko AS. Actin filament organization regulates the induction of lens cell differentiation and survival. Dev. Bio. 2006;295:714–729. doi: 10.1016/j.ydbio.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 39.Liou W, Rafferty NS. Actin filament patterns in mouse lens epithelium: a study of the effects of aging, injury, and genetics. Cell Motil. Cytoskel. 1988;9:17–29. doi: 10.1002/cm.970090104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.