Abstract
Members of the NOD-like receptor (NLR) family mediate the innate immune response to a wide range of pathogens, tissue damage and other cellular stresses. They achieve modulation of these signals by forming oligomeric signaling platforms, which in analogy to the apoptosome are predicted to adopt a defined oligomeric architecture and will here be referred to as NLR oligomers. Once formed, oligomers of the NLR proteins NLRP3 or NLRC4 “recruit” the adaptor protein ASC and the effector caspase-1, whereby NLRC4 can also directly interact with caspase-1. This results in large multi-protein assemblies, termed inflammasomes. Ultimately, the formation of these inflammasomes leads to the activation of caspase-1, which then processes the cytokines IL-1β and IL-18 triggering the immune response. Here we review new insights into NLR structure and implications on NLR oligomer formation as well as the nature of multi-protein inflammasomes. Of note, so dubbed “canonical inflammasomes” [1] can also be triggered by the NLR NLRP1b and the non-NLR protein AIM2, however the most detailed mechanistic information at hand pertains to NLRC4 while NLRP3 represents the quintessential inflammasome trigger. Thus these two NLRs are mainly used as examples in this article.
Introduction
In humans the NLR protein family comprises 22 members [2–4], which are capable of sensing a plethora of pathogen- or damage-associated molecular patterns (DAMPs or PAMPs) [1,5,6]. Sensing these patterns ultimately leads to the oligomerization of initially auto-inhibited monomeric NLRs to form defined NLR oligomers [7] (Figure 1a). Of note, we specifically use the term “NLR oligomer” in the following, since commonly the term inflammasome refers to large multi protein assemblies also containing ASC and caspase-1 next to NLRs. This is as such different from “apoptosomes” (see below), which commonly refer to the oligomeric form of the NLR relative Apaf-1 and its homologues such as CED4 [8,9].
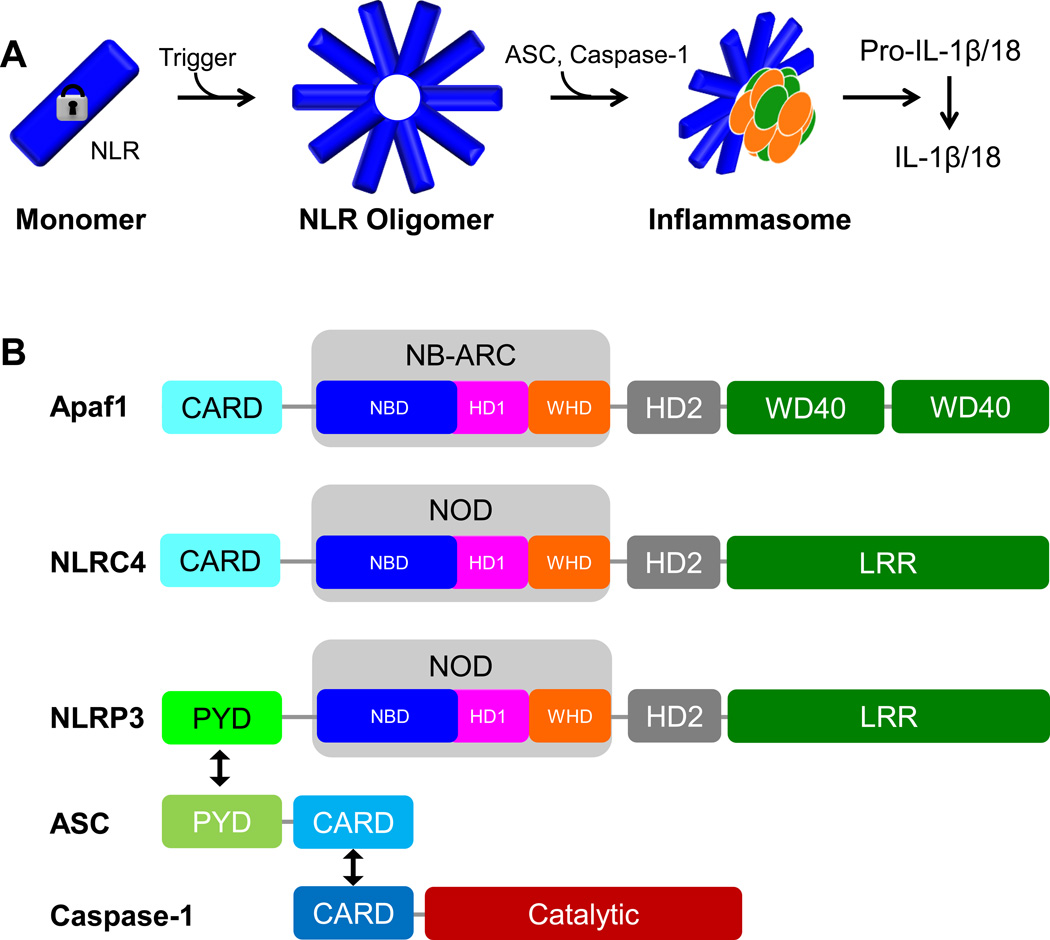
Figure 1. Inflammasome signaling.
A. Illustration of inflammasome formation. Upon a trigger a monomeric autoinhibited NLR (blue) becomes activated and oligomerizes to form a defined oligomer. Recruitment of ASC (orange) and caspase-1 (green) results in formation of a large signaling particle (inflammasome) which leads to the activation of caspase-1 and processing of Pro-IL1β or IL-18. B. Domain organization of Apaf-1, NLRC4, NLRP3, ASC and caspase-1. Black arrows symbolize homotypical PYD/PYD and CARD/CARD interactions. Abbreviations: Apaf-1: Apoptotic protease-activating factor 1; NLRC4: NLR family CARD domain-containing protein 4; NLRP3: Nucleotide-binding domain and leucine-rich repeat pyrin domain 3; ASC: Apoptosis-associated speck-like protein containing a CARD; CARD: Caspase activation and recruitment domain; PYD: Pyrin domain; NOD: Nucleotide-binding oligomerization domain; NB-ARC: nucleotide-binding, APAF-1, R proteins, and CED-4; NBD: Nucleotide-binding domain; HD: Helical domain; WHD: winged-helix domain; WD40: WD40 domain; LRR: Leucine-rich repeat domain.
Importantly, once formed, these NLR oligomers recruit or seed ASC and caspase-1 [10,11] and as such represent the key regulatory trigger of inflammasome formation. The term (canonical) inflammasome commonly refers to multi-protein assemblies formed by the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) and effector caspase-1 and the NLRs NLRP3 and NLRC4 as well as NLRP1b and the non-NLR protein AIM2 [1]. Hereby the NLR NLRC4 can also directly interact with and activate caspase-1, yet ASC leads to a stark amplification of this signal, which is outlined in later part of the manuscript.
In any case, NLR oligomerization is the key factor in triggering inflammasome formation. NLR oligomerization however is dependent on the transition of a monomeric NLR to an architecturally defined NLR oligomer. Thus, obtaining structural and mechanistic insight into this event is arguably the most crucial point to understand the regulation of inflammasome formation and for example to depict structural features for drug development leveraging this transition to combat aberrant inflammasome signaling in disease.
Although no high-resolution structure is available for such an NLR oligomer, a defined ring-like structure can be implied for NLR inflammasomes based on homologies to a closely related oligomer, the apoptosome [8,9,12,13]. In analogy to the apoptosome the NLR monomer-to-oligomer transition is strictly dependent on a conformational change of the monomeric NLR [14–17]. This change occurs when the NLR senses PAMPs or DAMPs leading to an opening of the NLR that includes the reorientation of multiple domains and subsequently allows the NLR to oligomerize [1]. The recent structure of a monomeric NLR, NLRC4 [15] allowed the first detailed insights into NLR regulatory features as well as their implications on NLR oligomerization and is therefore at the heart of this review. The nature of the subsequent larger multi-protein inflammasome assemblies is less clear and will be discussed in the end of this review.
NLR domain organization
The ability of NLRs to translate pathogen and danger signals into conformational changes and oligomerization and thus inflammasome formation lies in the unique domain architecture of NLRs [1]. While exceptions exist, the common architecture is exemplified by the inflammasome forming NLRs NLRP3 and NLRC4 (Figure 1b). NLRs commonly possess a pyrin domain (PYD) or caspase activation and recruitment domain (CARD) – both are members of the death domain superfamily - in their N-terminal regions (Figure 1b). This domain is followed by a nucleotide binding and oligomerization domain (NOD). The NOD is highly similar to the NB-ARC domain of Apaf-1 proteins and is the defining hallmark of NLRs, making them members of the STAND clan of the “extended ATPase associated with diverse cellular activities” (AAA+) superfamily [18,19]. The NOD is also often referred to as NACHT domain based on proteins sharing this domain (NAIP, CIITA, HET-E and TP1) [2]. The NOD itself consists of an ATPase nucleotide binding domain (NBD), a helical domain (HD1) and a winged helix domain (WHD). C-terminal to the NOD resides helical domain 2 (HD2), followed by a leucine-rich repeat (LRR) domain. Functionally, the LRR domain is responsible for sensing PAMPs or DAMPs, while the NOD mediates NLR oligomerization. Upon oligomerization, the N-terminal PYD (NLRP3) or CARD (NLRC4) domains seed inflammasomes via the PYD/CARD domains of ASC and caspase-1 [1].
Auto-inhibited NLRC4 – the first insight into an NLR at atomic resolution
Over more than a decade, the structural information available on NLR proteins was in essence restricted to constructs only containing one or two domains and importantly lacking the NOD [7,20]. However recently, a major breakthrough in understanding the detailed architecture of an NLR and implications on NLR inflammasome regulation was provided by the crystal structure of monomeric mouse NLRC4 [15]. Chai and co-workers solved the 3.2Å structure of NLRC4, covering the entire NLR with the exception of the N-terminal CARD domain. The structure shows that, like Apaf-1 [14,17], monomeric NLRC4 exists in an autoinhibited/closed form with a bound ADP molecule residing in the center of the closed NOD domain (Figure 2a). The sensory LRR domain further “locks” the closed form by spatially bridging NBD and HD2. Thus, the NLR monomer [15] appears overall analogous to the Apaf-1 monomer [14,17] (Figure 2a), however a closer look reveals that NLRC4 does exhibit marked distinctions that may have specifically evolved as adaption to the regulation of inflammatory responses and might be shared amongst NLRs.
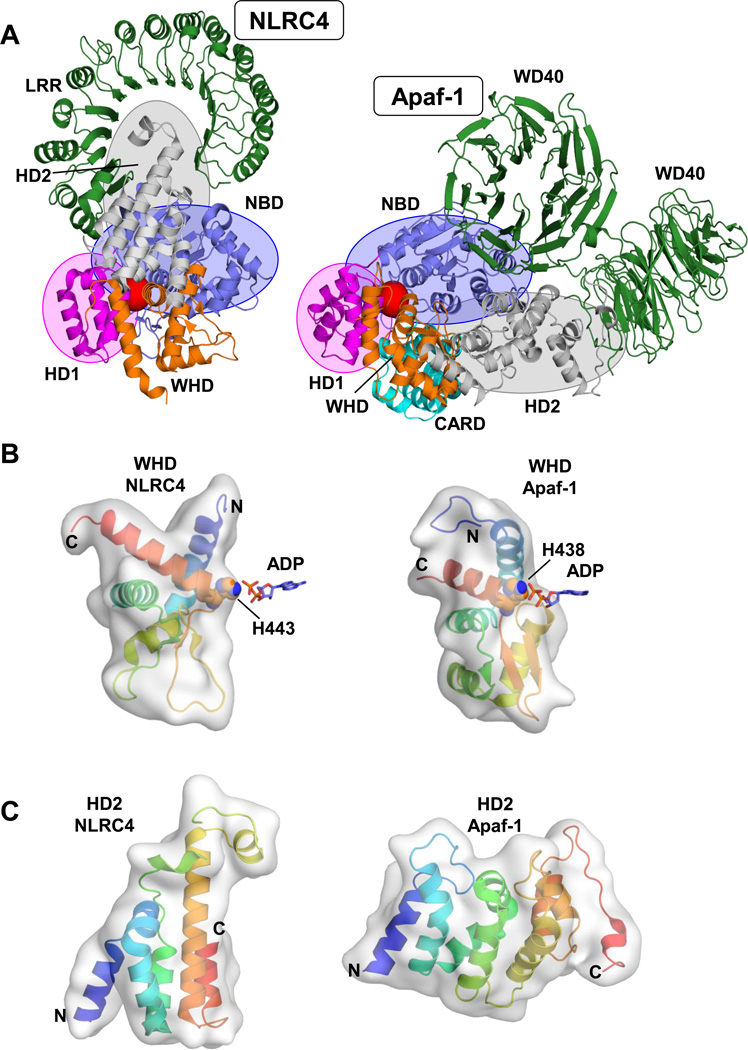
Figure 2. Auto-inhibited NLRC4 and Apaf-1.
A. Structures of monomeric NLRC4 and Apaf-1. Displayed are the crystal structures of mouse NLRC4 (delta CARD; PDBid: 4KXF [15]; left) and mouse Apaf-1 (PDBid: 3SFZ [17]; with CARD modeled based on the structure of human Apaf-1ΔWD40, PDBid: 1Z6T [14]) in similar orientation (overlay of NBD and HD1). Domains are colored analogous to Figure 1b with the relative positions of NBD, HD1 and HD2 emphasized by blue, magenta and grey spheroids respectively. The position of the bound ADP molecule is indicated by a red sphere. B. Winged helix domains of NLRC4 and Apaf-1 exhibit different shapes. Close-up onto the respective WHDs with the critical histidines coordinating the phosphate moieties of the bound ADP depicted (colored “rainbow” - N to C-terminus: blue to red). C. HD2 of NCLRC4 and Apaf-1 adopt different tertiary fold. Display analogous to panel B depicting the different shapes of the respective HD2 domains.
Topology of a closed NLR: The winged helix domain and HD2
While the principle of autoinhibition in NLRC4 is similar to that of Apaf-1 the overall domain arrangement is different [14,15,17] (Figure 2a). As in the NB-ARC domain of Apaf-1, the NOD of NLRC4 contains a winged helix domain (Figures 1 and 2). This domain is central to the auto-inhibited conformation of both NLRC4 and Apaf-1 where it utilizes a crucial histidine residue (His443 in NLRC4) to directly coordinate the β-phosphate of the bound ADP molecule (Figure 2b). As in Apaf-1, this interaction positions the winged helix domain proximal to the NBD (Figure 2a). However, in NLRC4 the orientation of the WHD is markedly changed and the WHD even adopts a different tertiary fold/shape than that of its NB-ARC relative (Figure 2b). In consequence, HD2, which also adopts a different fold (Figure 2c), is significantly repositioned to interact with the sensory LRR domain (Figure 2a). Thus, these changes represent most likely an inherent adaption of NLRs to position the LRR region as a lock in the absence of a suitable trigger, but also as a sensor to relieve this lock when pathogen and danger related signals are present. The actual sensing of these signals by the LRRs, and NLR opening and oligomerization are structurally still elusive. However as mentioned before, hints on the oligomeric NLR arrangement can be drawn from structures of apoptosomes [8,9,13]. These structurally well-defined architectural oligomers are formed by two ring arrangements in which the NBD domains of each protein form one central ring and the HD1 domains form a characteristic second with the WHD domains of the ring neighbor (see also Figure 4b). Thus, the deviations in the WHD shape observed for NLRC4 hints also to distinct differences in the shape of NLR inflammasomes.
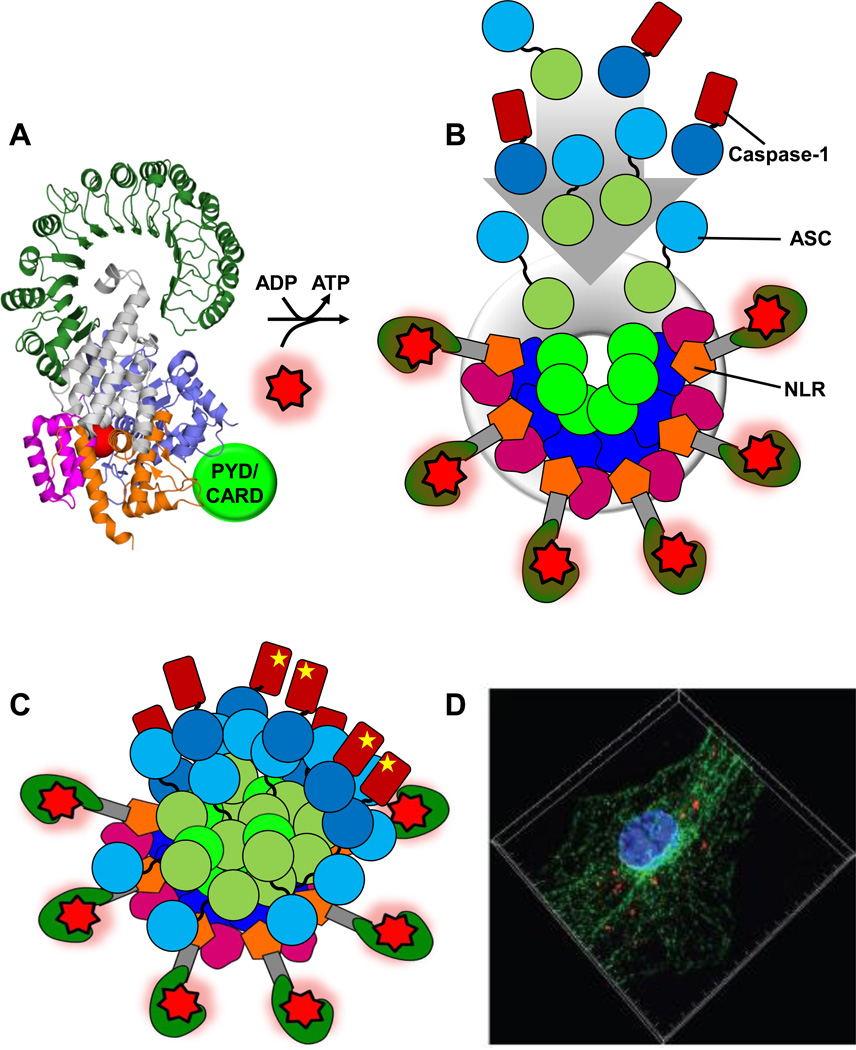
Figure 4. Model of NLRC4/NLRP3 activation and inflammasome formation – endogenous NLRP3 inflammasomes.
A. Autoinhibited NLRC4/NLRP3. Prior sensing of a trigger NLRC4/NLRP3 exist as autoinhibited monomers. Displayed is the structure of NLRC4 (PDBid: 4KXF [15]) with the PYD (NLRP3) or CARD (NLRC4) schematically depicted (light green). B. Schematic of formed NLRP3 oligomer poised for inflammasome formation. Binding of PAMPs/DAMPs (red star) and exchange of bound ADP with ATP leads to the open NLR conformation and formation of the NLR oligomer. A NLRP3 oligomer is depicted schematically (based on the architecture of apoptosomes) with domain color code analogous to Figure 1b. The oligomer is formed through a ring-like interaction of neighboring NBD domains and a secondary ring formed by HD1 and WHD interactions (six molecules are displayed, yet the exact number of molecules in the oligomers is not unambiguously known – numbers of 5–11 have been proposed for NLR oligomers [23,53]). The NLRP3 PYD is oligomerized and poised to “recruit and seed” ASC and caspase-1 molecules (dark green: ASC PYD, light blue: ASC CARD, dark blue: Caspase-1 CARD with catalytic domain indicated in brown). C. Schematic of the NLRP3/ASC/caspase-1 inflammasome. The NLR oligomers have seeded an ASC/Caspase-1 network that is structurally formed through both PYD and CARD interactions leading to the formation of a large multi-protein particle. Illustrated is a potentially heterogeneous nature of the particle that resembles more a signaling entity than a highly defined structural entity. Ultimately caspase-1 catalytic domains are brought into close proximity leading to their activation (illustrated by a yellow star). D. Endogenous NLRP3/ASC inflammasomes. Image depicting endogenous inflammasomes (red) reproduced with generous permission from Akira et al. [49] (Copyright 2013 John Wiley & Sons Ltd). As outlined in Akira et al. [49], the image depicts the assembly of ASC and NLRP3 on microtubules after stimulation with nigericin. Mouse bone marrow-derived macrophages were stimulated with nigericin and fixed. Samples were subjected to proximity ligation assay followed by fluorescence staining with Alexa488-labelled anti-α-tubulin antibody (green) and Hoechst dye (DNA, blue). Endogenous proximity of NLRP3 with ASC (red) on microtubules was observed by super resolution structured illumination microscopy. Highlights: We discuss recent advances in understanding inflammasome formation and regulation. We compare the recent structure of NLRC4 to Apaf-1 and apoptosomes. NLRC4 autoinhibition and NLR oligomerization are discussed. The nature of NLRC4- and NLRP3/ASC/Caspase-1 inflammasome assemblies is discussed. We discuss role and nature of PYD and CARD domains in inflammasomes.
NLR(C4) opening – the LRR lock and the phospho-serine 533 conundrum
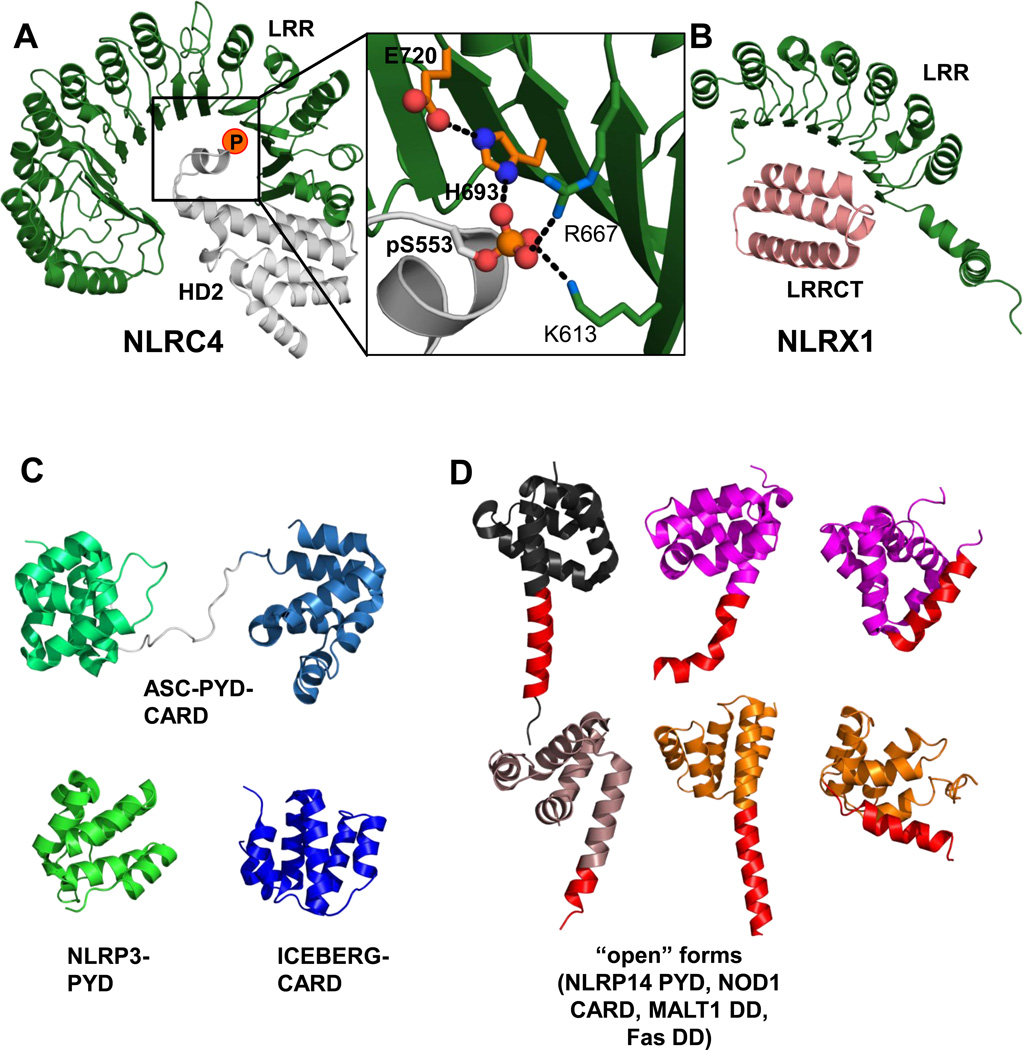
As outlined, events leading to the opening of an NLR and inflammasome formation remain to be structurally explored further. However, the NLRC4 monomer already bears a conundrum in this regard. The HD2-LRR interaction, which locks the closed structure, is centered around a phosphorylated serine (Ser533) on HD2 (Figure 3a). Yet, elegant previous work identified the phosphorylation of NLRC4 Ser533 as a key event to foster caspase-1 activation in response to Salmonella Typhimurium infection [21]. This is therefore at odds with the stabilizing function of phospho-Ser533 in auto-inhibited NLRC4. Closer inspection of the interaction pattern surrounding phospho-serine 533 reveals that the closest coordinating residue is a histidine (His693) from the LRR (Figure 3a). Intriguingly, His693 is in turn coordinated by the neighboring Glu720, resulting in an arrangement that is reminiscent of His-Asp arrangements found in catalytic triads. Thus, much like a titration of a histidine-dependent enzyme, a change in the protonation state of His693 could alter the interaction between the LRR and the phospho-moiety on HD2 and propagate opening of the LRR, offering a possible solution to the conundrum.
Figure 3. The LRR lock – internal LRR domain interactions; PYD/CARD structures.
A. The NLRC4 LRR lock. Interaction of NLRC4 LRR (green) with HD2 (grey, position of phospho-Ser553 is indicated). Close up on the interaction pattern of HD2 pSer553 with LRR residues. His693 and Glu720 from the LRR are colored in orange (PDBid: 4KXF [15]). B. NLRX1: LRR-LRRCT interaction. In NLRX1 the LRR is internally bound by a helix domain C-terminal to the LRR domain (LRRCT) endemic to NLRX1. LRRCT interacts with the LRR similar to HD2 in NLRC4 thus overriding the internal LRR lock in NLRX1 (PDBid: 3UN9 [22]). C/D. Structures of inflammasome PYD/CARD domains - potential conformational flexibility. C. Structures of NLRP3 PYD (PDBid: 3QF2 [40]), ASC full length (PDBid: 2KN6 [37]) and ICEBERG (PDBid: 1DGN [39]; CARD-only relative of caspase-1 CARD for which no structure is available) as indicated. D. Possible conformational flexibility in the death domain fold – “Open conformations”. NLRP14 PYD (grey, PDBid: 4N1J [44]), NOD1 CARD (magenta, from a domain swapped dimer in the crystal structure; PDBid: 2NZ7 [43]). Two examples for death domains showing an open conformation due to a helix-5/helix-6 fusion captured in non-swapped crystal structures (grey: MALT1 death domain (DD), PDBid: 2G7R; orange: Fas DD, PDBid 3EZQ [51]). Helix 6 is indicated in red. For NOD1 CARD and Fas DD the closed forms are also displayed (analogous color code, PDBids: 2B1W [52] and 1DDF [41], respectively); no structures of closed NLRP14 PYD, MALT1 DD exist.
An additional spin on LRR domain interactions within NLRs comes from the structure of NLRX1 [22]. Here, Wilson and colleagues revealed that the LRR region from NLRX1 has an additional helical bundle C-terminal to the LRR (LRRCT). LRRCT packs against the inside of the LRR in a similar position to HD2 of NLRC4 (Figure 3b). In this case the LRR cannot partake in autoinhibition and allows for an oligomeric arrangement of the NLRX1 fragment used in this study. This summarizes the complexity of LRR interactions. The actual sensing of PAMPs and DAMPs is structurally even less understood and undoubtedly will bear new unexpected mechanisms. As a final layer of complexity so called mixed or combinatorial NLR oligomers have also been described, most notably of NLRC4 and NAIP (Neuronal Apoptosis Inhibitory Protein) proteins [23,24] and future high resolution insight into these arrangements may reveal further intriguing features underlying NLR signaling.
Inflammasomes – NLR/ASC/Caspase-1 platforms
In any case, activation and oligomerization of inflammasome-triggering NLRs such as NLRP3 or NLRC4 naturally lead to the oligomerization of their N-terminal PYD/CARD domains (Figure 4b). This oligomerization in turn leads to recruitment of other PYD/CARD containing proteins via homotypical interactions. This means that the PYD of NLRP3 recruits the PYD of ASC, which in turn via its CARD recruits caspase-1 CARD (Figure 1b). NLRC4 contains a CARD and can in principle directly recruit caspase-1 [25,26]. Yet, a line of studies [25,27–29] have observed that NLRC4 signaling can be significantly more efficient in the presence of ASC which has led to the “bridge model”. In this model NLRC4 CARD recruits ASC CARD, which in turn interacts via its PYD with another PYD of ASC, whose CARD then recruits caspase-1. However, mechanistically the use of the terms “recruits and interacts” is merely descriptive, since inflammasome formation deviates from a classical simple 1:1 complex formation between individual PYD or CARD domains – no single KD of these extraordinarily weak and complex interactions has been reported. It seems that the process is better viewed as a set of nucleation events which ultimately lead to the formation of a structurally rather heterogeneous signaling particle [10,11] (Figure 4b/c). Thus, inflammasome formation occurs through an interactive agglomeration of the PYD or CARD domains of the NLR, ASC and even the effector caspase-1 [10,28] (Figure 4c). ASC is frequently considered the central mediator for inflammasome assembly [10,28]. It was initially found to form dot like perinuclear “specks” upon over-expression which are visible by light microscopy [28,30]. However, the exact architecture of these “specks” and of endogenous NLR/ASC/caspase-1 inflammasomes is elusive. As outlined ASC specks are most commonly punctuate [28,30,31]. However, recent studies using overexpression and re-constitution approaches have described highly ordered elongated filamentous arrangements built through helical assemblies of globular ASC domains which are initially seeded by NLR oligomers [32] and have been discussed elsewhere [11]. These ASC filaments are very appealing due to their striking order and their immediate reminiscence to filamentous scaffolding assemblies such as the actin or tubulin cytoskeleton fibers. However, although this study contains several technically very elegant experiments, at this point, it is not entirely clear if/how such highly structured filamentous arrangements relate to functional inflammasomes in the cell.
A main point for caution in this context is that proteins can have tendencies to self-assemble upon overexpression, under in vitro and reconstitution settings or when expressed as fragments of their full-length protein. In fact the formation of protein crystals is one example of a highly ordered multi-dimensional self-assembly. Filaments constitute another example, resulting from a one-dimensional periodic assembly. In the case of ASC for instance it was originally observed that its PYD forms filaments when overexpressed [33], however the full-length protein forms distinct “specks” within cells [28,30].
Of note, it has to be emphasized that bona fide “fibrous arrangements” of inflammasome components indeed could result from the decoration of existing linear or fibrous elements in the cell. The non-NLR protein AIM2 [1] for example binds with its HIN200 domain to DNA and thus a linear polymeric entity, which could result in linear/fibrous arrangements of AIM2/inflammasomes. Another possibility is that PYD or CARD domains of inflammasome components interact with cellular fibers analogous to other death domains, where for example death effector domains were observed to decorate tubular structures [34].
In any case, despite these concerns, information derived from re-constituted ordered assemblies as well as from crystal structures, including packing of CARD and PYD domains within crystals or filaments, can be of key relevance in disclosing near range interfaces used in actual natural inflammasome particles.
A final layer of complexity – “instability” of CARD and PYD domains
Death domains, including CARD and PYD domains, are often regarded as stable globular entities adopting a strictly defined fold built by six helices in Greek key topology [35]. However at least groups of these domains appear to be prone to conformational instability. Due to the technical challenges inherent to this phenomenon most evidence for this is circumstantial, however undoubtedly present. One clear clue in this regard was provided by the Clark laboratory while investigating the conformational stability of caspase-1 CARD, which the study found to be “marginally stable” [36]. Also an intriguing observation is that structures of ASC, ICEBERG CARD and NLRP3 PYD (Figure 3c) [37–40] could only be obtained at low pH (ASC full-length: pH3.8 ASC PYD: pH3.7; ICEBERG CARD: pH3.8; NLRP3 PYD: pH4.6) which was also used to stabilize the first (globular) death domain structure, the Fas death domain (pH4) [41]. Finally, some NLR CARD/PYD crystal structures show an opening of the death domain in the region of helix 6, resulting in domain swaps (NOD1 CARD) [42,43] or a helix 5 and 6 fusion (NLRP14 PYD) [44] (analogs depicted in Figure 3d).
Thus, it is possible that additional to interactions between globular PYD and CARD domains, conformational changes of helix 6 or other re-arrangements may be integral to inflammasomes. This phenomenon may in fact have contributed to recent observations that attribute a “prionlike” behavior to inflammasome components [45].
Outlook
While the exact structure of these inflammasomes at atomic resolution remains at least in part enigmatic, exciting studies have provided a glimpse on endogenous inflammasomes. Bogyo and colleagues have used fluorescent activity based probes to visualize active caspase-1 as inflammasome indicator, which clearly show punctuate speck-like particles [46,47]. In an alternative approach, Akira and colleagues used a proximity ligation assay (PLA) based system to detect associating NLRP3 and ASC and probe for endogenous inflammasomes using super-resolution structured illumination microscopy [48,49]. This resulted in the striking images of endogenous speck-like NLRP3 inflammasomes displayed in Figure 4d. Additionally, during the last stages of the preparation of this article a study was published by Bryant and colleagues which shows amongst other findings circular/spherical arrangements of ASC surrounding caspase-1 molecules in speck-like bodies, which also contain ASC protrusions [50].
In summary, recent research has provided exciting new insights into mechanisms underlying NLR- and inflammasome signaling. Undoubtedly, the complex nature of NLR inflammasomes and large multi-protein inflammasome particles will require further multi disciplinary investigation and will result in exciting new findings in the future.
Highlights.
We discuss recent advances in understanding inflammasome formation and regulation.
We compare the recent structure of NLRC4 to Apaf-1 and apoptosomes.
NLRC4 autoinhibition and NLR oligomerization are discussed.
The nature of NLRC4- and NLRP3/ASC/Caspase-1 inflammasome assemblies is discussed.
We discuss role and nature of PYD and CARD domains in inflammasomes.
Acknowledgements
This work was supported by the National Institutes of Health (R01AA017238 to S.J.R.) and a EMBO long-term postdoctoral fellowship to B.C.L. We thank Guy Salvesen for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lamkanfi M, Dixit VM, et al. Mechanisms and Functions of Inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, et al. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Kersse K, Bertrand MJ, Lamkanfi M, Vandenabeele P. NOD-like receptors and the innate immune system: coping with danger, damage and death. Cytokine Growth Factor Rev. 2011;22:257–276. doi: 10.1016/j.cytogfr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald JA, Wijekoon CP, Liao KC, Muruve DA. Biochemical and structural aspects of the ATP-binding domain in inflammasome-forming human NLRP proteins. IUBMB Life. 2013;65:851–862. doi: 10.1002/iub.1210. [DOI] [PubMed] [Google Scholar]
- 8. Qi S, Pang Y, Hu Q, Liu Q, Li H, Zhou Y, He T, Liang Q, Liu Y, Yuan X. Crystal structure of the Caenorhabditis elegans apoptosome reveals an octameric assembly of CED-4. Cell. 2010;141:446–457. doi: 10.1016/j.cell.2010.03.017. This study presents the only existing crystal structure of a formed apoptosome to date. Thus it constitutes the highest resolution structure, which can serve as homology template for NLR oligomers.
- 9.Yuan S, Akey CW. Apoptosome structure, assembly, and procaspase activation. Structure. 2013;21:501–515. doi: 10.1016/j.str.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng J, Waite AL, Tkaczyk ER, Ke K, Richards N, Hunt AJ, Gumucio DL, et al. Kinetic properties of ASC protein aggregation in epithelial cells. J Cell Physiol. 2010;222:738–747. doi: 10.1002/jcp.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruland J. Inflammasome: putting the pieces together. Cell. 2014;156:1127–1129. doi: 10.1016/j.cell.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 12.Yuan S, Yu X, Asara JM, Heuser JE, Ludtke SJ, Akey CW. The holo-apoptosome: activation of procaspase-9 and interactions with caspase-3. Structure. 2011;19:1084–1096. doi: 10.1016/j.str.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan S, Yu X, Topf M, Dorstyn L, Kumar S, Ludtke SJ, Akey CW. Structure of the Drosophila apoptosome at 6.9 a resolution. Structure. 2011;19:128–140. doi: 10.1016/j.str.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riedl SJ, Li W, Chao Y, Schwarzenbacher R, Shi Y. Structure of the apoptotic protease-activating factor 1 bound to ADP. Nature. 2005;434:926–933. doi: 10.1038/nature03465. [DOI] [PubMed] [Google Scholar]
- 15. Hu Z, Yan C, Liu P, Huang Z, Ma R, Zhang C, Wang R, Zhang Y, Martinon F, Miao D. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science. 2013;341:172–175. doi: 10.1126/science.1236381. This study presents the first and only available structure of a full length NLR, which besides the CARD domain contains all functional elements of an NLR. The structure depicts details of autoinhibited monomeric NLRC4.
- 16. Yuan S, Topf M, Reubold TF, Eschenburg S, Akey CW. Changes in Apaf-1 conformation that drive apoptosome assembly. Biochemistry. 2013;52:2319–2327. doi: 10.1021/bi301721g. This work includes the highest resolution EM structure of the human apoptosome.
- 17.Reubold TF, Wohlgemuth S, Eschenburg S. Crystal structure of full-length Apaf-1: how the death signal is relayed in the mitochondrial pathway of apoptosis. Structure. 2011;19:1074–1083. doi: 10.1016/j.str.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Leipe DD, Koonin EV, Aravind L. STAND, a class of P-loop NTPases including animal and plant regulators of programmed cell death: multiple, complex domain architectures, unusual phyletic patterns, and evolution by horizontal gene transfer. J Mol Biol. 2004;343:1–28. doi: 10.1016/j.jmb.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Jaroszewski L, Rychlewski L, Reed JC, Godzik A. ATP-activated oligomerization as a mechanism for apoptosis regulation: fold and mechanism prediction for CED-4. Proteins. 2000;39:197–203. doi: 10.1002/(sici)1097-0134(20000515)39:3<197::aid-prot10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.Proell M, Riedl SJ, Fritz JH, Rojas AM, Schwarzenbacher R. The Nod-like receptor (NLR) family: a tale of similarities and differences. PLoS One. 2008;3:e2119. doi: 10.1371/journal.pone.0002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qu Y, Misaghi S, Izrael-Tomasevic A, Newton K, Gilmour LL, Lamkanfi M, Louie S, Kayagaki N, Liu J, Komuves L, et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature. 2012;490:539–542. doi: 10.1038/nature11429. This study reveals a phosphorylation event in NLRC4 that is critical for NLRC4 inflammasome function and is discussed in light of the NLRC4 structure in this review.
- 22.Hong M, Yoon SI, Wilson IA. Structure and functional characterization of the RNA-binding element of the NLRX1 innate immune modulator. Immunity. 2012;36:337–347. doi: 10.1016/j.immuni.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halff EF, Diebolder CA, Versteeg M, Schouten A, Brondijk TH, Huizinga EG, et al. Formation and structure of a NAIP5-NLRC4 inflammasome induced by direct interactions with conserved N- and C-terminal regions of flagellin. J Biol Chem. 2012;287:38460–38472. doi: 10.1074/jbc.M112.393512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenthorey JL, Kofoed EM, Daugherty MD, Malik HS, Vance RE, et al. Molecular basis for specific recognition of bacterial ligands by NAIP/NLRC4 inflammasomes. Mol Cell. 2014;54:17–29. doi: 10.1016/j.molcel.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 27.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 28. Proell M, Gerlic M, Mace PD, Reed JC, Riedl SJ, et al. The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem J. 2013;449:613–621. doi: 10.1042/BJ20121198. This study depicts a crucial architectural role of ASC- and caspase-1 CARD domains in inflammasome signaling.
- 29.Van Opdenbosch N, Gurung P, Vande Walle L, Fossoul A, Kanneganti TD, Lamkanfi M, et al. Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nat Commun. 2014;5:3209. doi: 10.1038/ncomms4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, Niikawa N, Hidaka E, Katsuyama T, Higuchi T, Sagara J. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1999;274:33835–33838. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- 31.De Nardo CM, Latz E. The Inflammasome. Vol. 1040. Humana Press; 2013. [Google Scholar]
- 32. Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schroder GF, Fitzgerald KA, Wu H, Egelman EH, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. Structurally, this study depicts elegant work on reconstituted PYD and CARD arrangements.
- 33.Moriya M, Taniguchi S, Wu P, Liepinsh E, Otting G, Sagara J. Role of charged and hydrophobic residues in the oligomerization of the PYRIN domain of ASC. Biochemistry. 2005;44:575–583. doi: 10.1021/bi048374i. [DOI] [PubMed] [Google Scholar]
- 34.Mielgo A, Torres VA, Clair K, Barbero S, Stupack DG, et al. Paclitaxel promotes a caspase 8-mediated apoptosis through death effector domain association with microtubules. Oncogene. 2009;28:3551–3562. doi: 10.1038/onc.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kersse K, Verspurten J, Vanden Berghe T, Vandenabeele P. The death-fold superfamily of homotypic interaction motifs. Trends Biochem Sci. 2011;36:541–552. doi: 10.1016/j.tibs.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Chen YR, Clark AC. Kinetic traps in the folding/unfolding of procaspase-1 CARD domain. Protein Sci. 2004;13:2196–2206. doi: 10.1110/ps.03521504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Alba E. Structure and interdomain dynamics of apoptosis-associated speck-like protein containing a CARD (ASC) J Biol Chem. 2009;284:32932–32941. doi: 10.1074/jbc.M109.024273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liepinsh E, Barbals R, Dahl E, Sharipo A, Staub E, Otting G. The death-domain fold of the ASC PYRIN domain, presenting a basis for PYRIN/PYRIN recognition. J Mol Biol. 2003;332:1155–1163. doi: 10.1016/j.jmb.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Humke EW, Shriver SK, Starovasnik MA, Fairbrother WJ, Dixit VM. ICEBERG: a novel inhibitor of interleukin-1 beta generation. Cell. 2000;103:99–111. doi: 10.1016/s0092-8674(00)00108-2. [DOI] [PubMed] [Google Scholar]
- 40.Bae JY, Park HH, et al. Crystal structure of NALP3 protein pyrin domain (PYD) and its implications in inflammasome assembly. J Biol Chem. 2011;286:39528–39536. doi: 10.1074/jbc.M111.278812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang B, Eberstadt M, Olejniczak ET, Meadows RP, Fesik SW. NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature. 1996;384:638–641. doi: 10.1038/384638a0. [DOI] [PubMed] [Google Scholar]
- 42.Coussens NP, Mowers JC, McDonald C, Nunez G, Ramaswamy S, et al. Crystal structure of the Nod1 caspase activation and recruitment domain. Biochem Biophys Res Commun. 2007;353:1–5. doi: 10.1016/j.bbrc.2006.11.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srimathi T, Robbins SL, Dubas RL, Hasegawa M, Inohara N, Park YC, et al. Monomer/dimer transition of the caspase-recruitment domain of human Nod1. Biochemistry. 2008;47:1319–1325. doi: 10.1021/bi7016602. [DOI] [PubMed] [Google Scholar]
- 44.Eibl C, Hessenberger M, Wenger J, Brandstetter H, et al. Structures of the NLRP14 pyrin domain reveal a conformational switch mechanism regulating its molecular interactions. Acta Crystallogr D Biol Crystallogr. 2014;70:2007–2018. doi: 10.1107/S1399004714010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cai X, Chen J, Xu H, Liu S, Jiang QX, Halfmann R, Chen ZJ, et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156:1207–1222. doi: 10.1016/j.cell.2014.01.063. This work proposes an interesting hypothesis attributing prion like behavior to ASC.
- 46.Xiao J, Broz P, Puri AW, Deu E, Morell M, Monack DM, Bogyo M, et al. A coupled protein and probe engineering approach for selective inhibition and activity-based probe labeling of the caspases. J Am Chem Soc. 2013;135:9130–9138. doi: 10.1021/ja403521u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Puri AW, Broz P, Shen A, Monack DM, Bogyo M, et al. Caspase-1 activity is required to bypass macrophage apoptosis upon Salmonella infection. Nat Chem Biol. 2012;8:745–747. doi: 10.1038/nchembio.1023. This study visualizes active caspase-1 in endogenous inflammasomes using an activity-based probe.
- 48.Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, Akira S, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 49. Akira S, Misawa T, Satoh T, Saitoh T. Macrophages control innate inflammation. Diabetes Obes Metab. 2013;15(Suppl 3):10–18. doi: 10.1111/dom.12151. This article depicts endogenous inflammasomes (displayed in this review) using a proximity ligation assay (PLA) approach.
- 50. Man SM, Hopkins LJ, Nugent E, Cox S, Gluck IM, Tourlomousis P, Wright JA, Cicuta P, Monie TP, Bryant CE. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc Natl Acad Sci U S A. 2014;111:7403–7408. doi: 10.1073/pnas.1402911111. This study shows very interesting high resolution images of endogenous inflammasomes.
- 51.Scott FL, Stec B, Pop C, Dobaczewska MK, Lee JJ, Monosov E, Robinson H, Salvesen GS, Schwarzenbacher R, Riedl SJ. The Fas-FADD death domain complex structure unravels signalling by receptor clustering. Nature. 2009;457:1019–1022. doi: 10.1038/nature07606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manon F, Favier A, Nunez G, Simorre JP, Cusack S. Solution structure of NOD1 CARD and mutational analysis of its interaction with the CARD of downstream kinase RICK. J Mol Biol. 2007;365:160–174. doi: 10.1016/j.jmb.2006.09.067. [DOI] [PubMed] [Google Scholar]
- 53.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]