Abstract
Purpose
First proposed by Dr. David Barker and now supported by numerous epidemiological and experimental studies, the theory of the developmental origins of health and disease hypothesizes that low birth weight (5.5 pounds or less) indicative of poor fetal growth is associated with an increased risk for chronic, non-communicable disease in later life including hypertension, type 2 diabetes and osteoporosis. Whether women are at greater risk than men is not clear. Experimental studies that mimic the cause of slow fetal growth are being used to examine the underlying mechanisms that link a poor fetal environment with later chronic disease and investigate how sex and age impact programmed risk. Thus, the aim of this review is to summarize the current literature related to the impact of low birth weight on women’s health and provide insight into potential mechanisms that program increased risk of chronic disease across the lifespan.
Methods
A search of PubMed was utilized with key words related to low birth weight, women’s health, female and sex differences; additional terms included blood pressure, hypertension, renal, cardiovascular, obesity, glucose intolerance, type 2 diabetes, osteoporosis, bone health, reproductive senescence, menopause and aging.
Findings
The major chronic diseases associated with low birth weight include high blood pressure and cardiovascular disease, impaired glucose homeostasis and Type 2 Diabetes, impaired bone mass and osteoporosis, and early reproductive aging.
Implications
Low birth weight increases the risk for chronic disease in men and women. Low birth weight is also associated with increased risk for early menopause. Further studies are needed to fully address the impact of sex and age on the developmental programming of adult health and disease in women across their lifespan.
Keywords: low birth weight, women’s health, blood pressure, type 2 diabetes, osteoporosis, early menopause
INTRODUCTION
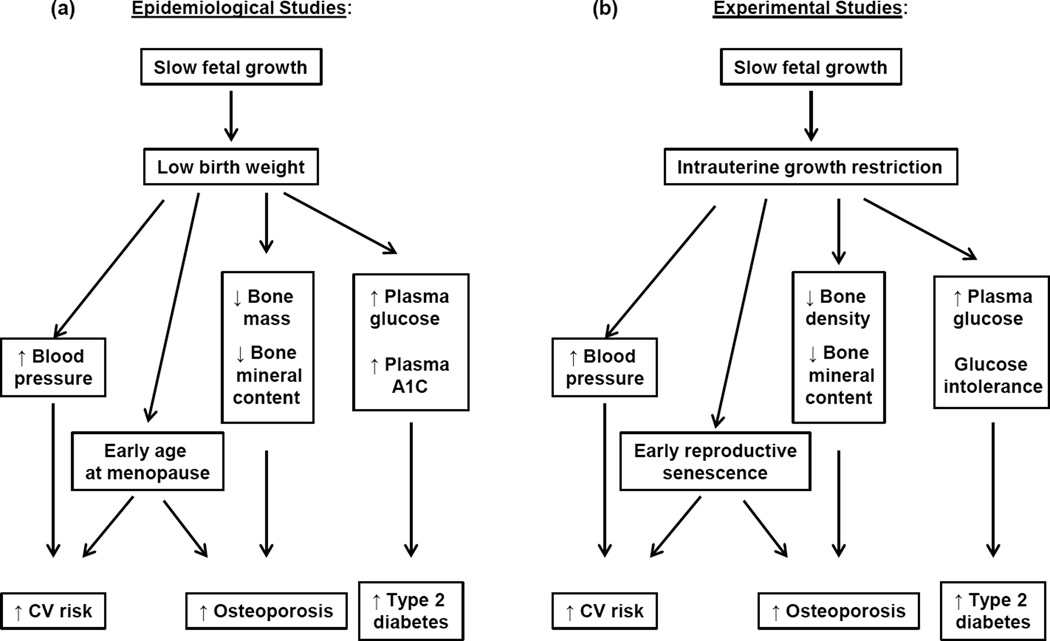
The theory of the developmental origins of adult health and disease (DOHaD) was first proposed by David Barker and colleagues who noted a geographical association between deaths from coronary heart disease and infant mortality (6). Low birth weight indicative of slow growth during fetal life contributes to infant mortality and morbidity. Thus, they proposed that events during fetal life that slow fetal growth may increase cardiovascular (CV) risk in individuals that survive a complicated pregnancy (7). To further their investigation Barker examined the association between high blood pressure, a risk factor for CV disease, and low birth weight (LBW), a marker of poor fetal growth, and noted an inverse relationship between birth weight and blood pressure supporting their original hypothesis (5). Since these initial observations numerous epidemiological studies have investigated the inverse association between birth weight and blood pressure (for systematic review see 50) and substantiated Barker and colleagues findings. Furthermore, additional studies indicate that birth weight is inversely associated with an increased risk for Type 2 Diabetes (T2D) (64, 99) and osteoporosis (58) (Figure); LBW also increases the risk for early menopause (19, 86, 95) (Figure). Experimental models are providing proof of principle and use of experimental models that mimic the pathophysiological and environmental conditions that slow fetal growth are being use to investigate the underlying mechanisms that link fetal life and chronic disease in later life (8, 17,36, 41, 108) (Figure). Thus, the goal of this review is to highlight the current field and explore how LBW and influences that slow growth during fetal life impair chronic health and disease in women.
Figure 1.
Epidemiological studies indicate that slow growth during fetal life programs an increased risk for cardiovascular (CV) disease, Type 2 Diabetes, osteoporosis, and earlier age at menopause in low birth weight women (A). Experimental models that mimic slow fetal growth are providing proof of concept (B) and are also allowing investigation in the mechanisms that link low birth weight with later chronic disease in women.
METHODS
An electronic search was conducted via PubMed related to the impact of low birth weight on chronic health in women and in experimental models of developmental programming of CV disease, T2D, and osteoporosis.
RESULTS
Low birth weight and experimental models of developmental programming
LBW results from a number of different factors including maternal complications such as preeclampsia or diabetes, improper nutrition, poor prenatal care, maternal smoking and age (48, 105). Teenagers and women over 40 are at greater risk for a LBW baby (63). Race also impacts the risk of low birth weight with African American woman having a two-fold increased risk compared to American women of European descent (63). Maternal smoking also increases the risk for a LBW baby by two-fold (90) and maternal infection, placental abruption are also causative factors (48). Numerous studies now indicate that complications during pregnancy increase the risk for chronic disease in later life of the offspring (1, 12,23, 84, 98). Preeclampsia is associated with an increased risk of LBW and the development of hypertension and CV complications in the offspring (23, 84). Children born to pregnancies complicated by diabetes also demonstrate an increased risk for hypertension, CV disease (1, 98) and metabolic disturbances including T2D (12). Experimental models that mimic the etiology of LBW are being used to explore the underlying mechanisms that link a poor fetal environment with later increased chronic disease. Poor fetal nutrition is associated with hypertension in later life (85) and undernutrition during fetal life induced via protein restriction in the rat is a commonly used model to study the mechanisms that program hypertension (103) or impaired glucose homeostasis (72) in the offspring after birth. The initiating event in preeclampsia involves placental insufficiency (35). Placental insufficiency in the rat is another model used to study the developmental programming of hypertension (3) and glucose intolerance (44, 88) in the intrauterine growth restriction (IUGR) offspring. Fetal exposure to maternal glucocorticoids impairs fetal growth (73) and expression of placental 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2), the enzyme that protects the fetus from exposure to maternal glucocorticoids (73), is reduced in pregnancies complicated by preeclampsia (81). Experimental models of fetal exposure to glucocorticoid are being utilized to elucidate the renal mechanism that program hypertension following a developmental insult (71). Prenatal exposure to nicotine is used to mimic the adverse impact of maternal smoking during pregnancy (105). Thus, this model of prenatal insult is being used to mimic the causative factors that impair growth during prenatal life and program increased risk for chronic disease (77). These different models of developmental insults are providing insight into the mechanisms that link complications during pregnancy or impaired fetal growth with later chronic disease. They also demonstrate that the fetal responses to a developmental insult are sex-specific and result in sex-specific outcomes on chronic health. This review will discuss the impact of LBW on chronic health and disease and provide insight into known mechanisms with a special emphasis on women’s health.
Low birth weight and blood pressure: increased cardiovascular risk
Birth weight is inversely related to blood pressure in men (21) and women (20). Age greatly impacts the risk of high blood pressure in LBW women (5) and accelerated weight gain in early life adds to the risk (51). It is well established that within the general population men have a higher blood pressure than women prior to menopause although age abolishes this difference (54). Differences in CV risk factors may contribute to this sex difference in blood pressure in men and women in young adulthood (47). Being born small for gestational age (SGA) is associated with higher LDL and lower HDL cholesterol levels in young adulthood relative to appropriate for gestational age (AGA) counterparts (79). Yet, prenatal undernutrition is associated with an increase in total cholesterol concentration in women at 58 years of age but not men (55). Whether LBW programs sex-specific differences in CV risk that is age-dependent is not yet reported. However, birth weight is inversely associated with coronary heart disease in men (30) and in women after menopause (52, 107). Vos et al. examined the relationship between size at birth and the absolute 10-year CV risk in young adulthood (96). They reported that small size at birth increased overall CV risk in men and women; however, CV risk was greater in LBW men relative to LBW women (96). Thus, birth weight is inversely associated with blood pressure and risk for coronary heart disease in women. However, risk is greater in LBW men relative to LBW women prior to menopause; age may impact this risk and the mechanisms that mediate the sex difference in programmed risk require further investigation.
Numerous experimental studies note a sex difference in blood pressure in offspring exposed to insults during early life (3, 62,71,101, 103,104, 105). Blood pressure is significantly elevated in male offspring exposed to placental insufficiency (3, 101), maternal protein restriction (103), or prenatal exposure to glucocorticoids (71); yet female offspring in these models of developmental insult do not develop hypertension in young adulthood (3, 62, 104). Extensive investigation has centered on the role of the renin angiotensin system (RAS) in the etiology of hypertension programmed by in utero insults. The RAS contributes to the long-term control of blood pressure through its influence on sodium reabsorption, aldosterone secretion and vasoconstriction. Inhibition of the RAS abolishes hypertension in male offspring exposed to prenatal protein restriction (56) and placental insufficiency (69) implicating an important role for the RAS in the etiology of hypertension programmed by a developmental insult. Circulating levels of angiotensin II (Ang II) and ACE activity are elevated in SGA boys but not SGA girls (32) providing support for inappropriate activation of the RAS within a human cohort and a potential mediator of increased CV risk observed in boys relative to girls following a developmental insult. In the experimental rat model of placental insufficiency expression of renal ACE2, a component of the vasodilator arm of the RAS is elevated in female IUGR rats that are normotensive in adulthood (68). Thus, up-regulation of vasoconstrictor arm of the RAS may contribute to the development of increased CV risk in males exposed to a developmental insult whereas up-regulation of the vasodilator arm may be a compensatory mechanism that protects against programmed CV risk in the young female. Oxidative stress is a known contributor to hypertension and CV disease (99). Markers of oxidative stress are elevated in children born SGA (33) and in male rats exposed to maternal protein restriction (87) or placental insufficiency (70). Antioxidants abolish hypertension in these male offspring; yet, female IUGR offspring exposed to placental insufficiency that are normotensive in young adulthood do not exhibit an increase in renal markers of oxidative stress (70). Renal antioxidant expression and activity are up-regulated in the female IUGR rats that are normotensive in young adulthood in this model implicating a compensatory mechanism that may be protective against the generation of reactive oxygen species in the young female IUGR rat. Thus, experimental models suggest that sex specific programming of the RAS and oxidative stress contribute to the sexual dimorphism of blood pressure in experimental models of fetal insult and implicate the RAS and oxidative stress as potential mediators of increased risk in LBW individuals.
Blood pressure increases with age within the general population (54). Recent studies indicate that age may also increase CV risk in female offspring exposed to a developmental insult. Female IUGR offspring in a model of placental insufficiency are normotensive in early adulthood relative to their same-sex control counterparts (3). Yet, a marked increase in blood pressure is observed by 12 months of age relative to control (42) indicating that age serving as a second hit increases CV risk following IUGR in the female rat. Increases in total fat mass and visceral adiposity are noted in conjunction with the age-dependent increase in blood pressure in female IUGR induced via placental insufficiency (42). Whether the increase in adiposity directly contributes to the development of age-dependent hypertension in this model is not clear. However, the increase in adiposity in the female IUGR rats at 12 months of age is associated with an increase in circulating levels of leptin, an adipokine released from adipose tissue (42). Leptin contributes to the development of obesity-related hypertension via activation of the renal sympathetic nerves (38). Renal denervation abolishes age-dependent hypertension in the female IUGR rats at 12 months of age (42) implicating an important role for the renal nerves. The renal nerves also contribute to hypertension in male offspring in young adulthood programmed by fetal exposure to placental insufficiency (4) or glucocorticoids (22) implicating similar pathways contribute to programming of hypertension following a developmental insult but in a manner that is age-dependent. Age also impacts the development of increased CV in offspring exposed to prenatal nicotine (105). Overt hypertension is not observed in all models of developmental insult (105). Male and female offspring exposed to prenatal nicotine are normotensive in young adulthood but male offspring exhibit an enhanced sensitivity to vasoactive factors such as Ang II, a marker of increased CV risk (105). Yet, sensitivity to Ang II is not enhanced in female rats exposed to prenatal nicotine until 22 months of age (91). Thus, these studies indicate that age abolishes protection against the developmental programming of CV risk observed in young female animals and highlights the need for additional studies to determine the sex- and age-dependent mechanisms that programming hypertension and increased CV risk in LBW women.
Low birth weight and adiposity and glucose homeostasis: risk for Type 2 Diabetes
A systematic review of the literature conducted in 2003 noted an inverse relationship between birth weight and several glycemic indices including fasting plasma glucose, fasting plasma insulin and two-hour glucose that is present in men and women (64) suggesting that influences that slow growth during fetal life program impaired glucose homeostasis in later life. The inverse relationship between birth weight and the risk for development of T2D is noted in men (15, 99) and women (24, 74, 99). However, findings from the Australian Diabetes, Obesity and Lifestyle (AUSDIAB) Study report that the association between birth weight and an increased risk for T2D is greater in LBW women relative to LBW men, an association that is present independent of current body mass index (BMI) (2). In this study high fasting plasma glucose and high mean hemoglobin A1c were strongly and inversely associated with birth weight with the proportion for all glycemic abnormalities greater in LBW women relative to LBW men (2). Mogren et al. observed an elevation in fasting plasma glucose and two-hour plasma glucose in LBW women relative to their normal birth weight counterparts that was only noted in LBW men in conjunction with a hereditary background for T2D (67). Both of these studies used cohorts composed of young adults, average age 25 and 32, respectively (1, 67). Studies that examine this association at older ages report that an increased risk for T2D is present in LBW women by 50 years of age (24) with the risk for T2D increased after menopause in African American women (76). LBW is also associated with an increased risk of central visceral adiposity (20) with females more susceptible relative to males (106). LBW when coupled with accelerated weight gain in childhood regardless of sex also increases the risk for the metabolic syndrome in young adulthood (65). Women with LBW are also at increased risk for glucose intolerance during a first pregnancy (91) and the development of gestational diabetes mellitus (GDM) (10, 39, 67). GDM is a strong predictor of later T2D (18). Thus, LBW indicative of poor fetal nutrition and slow growth during fetal life increases the risk for impaired metabolic health during pregnancy and in later life in LBW women and indicates that susceptibility to diabetes is programmed during early development.
Experimental studies are providing insight into the mechanisms that program impaired metabolic health and also highlight that exposure to adverse events during early life program impaired glucose homeostasis in a manner that is sex, age and insult specific. Insulin release in response to a glucose challenge is impaired in both male and female offspring of protein restricted dams but only male offspring exhibit a reduction in beta-cell mass (94) and insulin resistance in young adulthood (89). Yet, placental insufficiency programs a reduction in beta cell mass in male and female IUGR offspring at 3 months of age that is not associated with impaired glucose tolerance but is associated with lower glucose-stimulated insulin release in the female IUGR rats relative to female control (88). Placental insufficiency also programs an increase in mean fasting glucose levels in female but not male IUGR offspring at this age (44). Fasting glucose levels do not differ in either sex by 6 months of age following fetal exposure to placental insufficiency but glucose intolerance is observed in male IUGR offspring (82). Maternal protein restriction programs an increase in fasting glucose levels in male offspring that is associated with glucose intolerance by 21 months of age (72). Although maternal protein restriction is not associated with changes in fasting glucose or glucose intolerance in female offspring at this age (31), fetal exposure to maternal protein restriction programs hyperinsulinemia associated with a reduction in insulin-signaling protein expression in the female offspring by 21 months of age (31). Glucose tolerance is impaired in female IUGR offspring during their pregnancy indicating that pregnancy increases vulnerability to metabolic disturbances following a developmental insult (34). Additionally, hyperglycemia, hyperinsulinemia, and unsuppressed hepatic glucose production are observed in offspring of female rats exposed to nutrient restriction during their gestational life implicating that programming of impaired metabolic health is transmitted to the next generation (93). Thus, further studies are needed to clearly elucidate the impact that poor nutrition and growth during fetal life have on later metabolic health and to determine the exact mechanisms that underlie the sex specific developmental programming of T2D in later life and in response to a physiological challenge such as pregnancy.
Low birth weight and bone health: risk for osteoporosis
Osteoporosis is the most common bone disease. It involves compromised bone strength and results from an imbalance in bone formation and bone reabsorption leading to an increased risk for bone fracture (9). Menopause is associated with an increase in osteoporosis due to changes in bone remodeling mediated by estrogen deficiency (92). Smoking, excessive alcohol and caffeine intake, inadequate calcium/vitamin D intake and lack of weight bearing exercise are all modifiable risk factors for osteoporosis (92). Other factors such as sex and genetics also impact bone health with older women exhibiting a higher prevalence of bone fracture relative to age-matched men (16). A recent systematic review and meta-analysis of the literature noted that birth weight is associated with bone health in childhood implicating that bone density has its origins during fetal life (58). Yet, the impact of birth weight on bone health during adolescence and adulthood is not clear. A systematic review of the literature suggests that low birth weight is associated with low adult bone mass with the association for low birth weight greater for bone mineral content than for bone mineral density, or the ratio of bone mineral content to bone size (80). Yet, individual studies indicate contradictory findings. Jones et al. report that low birth weight is associated with a reduction in bone mass, an indirect marker of fracture risk, at 8 (45) but not at 16 years of age (46) in boys and girls. However, El Hage et al. report that low birth weight is associated with low bone mineral content and bone mineral density in girls at 15 years (27). Callreus et al. note that low birth weight is associated with low bone mineral content in women at 25 years of age (14) and Yarbrough et al. report that low birth weight remains associated with low bone mineral content in women at 70 years of age (107). Dennison and colleagues report that birth weight is a determinant of bone strength in men and women at 70 years of age (26). Yet, Byberg and colleagues report that despite a positive association between birth weight and bone mineral content, low birth weight is not associated with an increased risk of fracture in men and women aged 50–94 years (13).
Experimental studies also differ in regards to the importance of fetal life on later bone health. Lanham et al reported that rat offspring of protein restricted dams exhibit a reduction in bone density and mechanical strength in female offspring (49). Placental insufficiency in the rat also programs a reduction in bone mineral content and bone strength in the offspring with lower bone density noted in the female growth restricted offspring relative to male (78). Additionally, findings from this study noted that supplementation with calcium starting in adolescence does not reverse the bone strength deficient initiated by placental insufficiency in the growth-restricted offspring (77) suggesting that developmental influences that impair bone health are not reversible with post-natal interventions. Engelbregt et al. observed that a reduction in bone mineral content in male and female offspring exposed to prenatal malnutrition or placental insufficiency does not remain significant at 6 months of age after adjustment for total body weight (29); yet Metha et al. reported that a reduction in bone mass occurred with age in offspring exposed to protein restriction during fetal life (60). Therefore, numerous epidemiological and experimental studies indicate that risk of osteoporosis may have its origins in fetal life. Studies thus far indicate that females may be more susceptible to risk for osteoporosis following an insult during fetal life than males. However, the impact of age and transition into menopause on bone health in low birth weight women has not yet been explored and further studies are needed to determine the mechanistic pathways that link fetal life with later bone health in order to develop preventative and therapeutic options.
Low birth weight and menopause: risk for early reproductive senescence
Menopause usually occurs around 52 years of age and indicates the end of reproductive viability (11). Risk factors for early onset menopause include smoking, stress within the African American population, nulliparity, and genetics (11). Early menopause is positively associated with coronary heart disease and stroke independent of other cardiovascular risk factors (97). Recent studies indicate that LBW may be a risk factor for earlier age at menopause (19, 86, 95) and women exposed to famine during late gestation exhibit a greater prevalence of early onset menopause (28, 108). Experimental studies indicate that prenatal exposure to undernutrition in the rat is also associated with markers of early reproductive senescence (8, 36, 17) as indicated by a reduction in ovarian follicle numbers (8, 17) and early onset of estrous acyclicity following maternal undernutrition (17). Thus, LBW indicative of undernutrition and slow growth during fetal life programs early onset reproductive senescence suggesting that accelerated reproductive aging may contribute to the increased risk of chronic diseases such as CV, metabolic, and osteoporosis in LBW women.
Low birth weight and gestation: risk for complications during pregnancy
LBW increases a woman’s risk for complications during pregnancy including hypertension (25, 40), GDM (38) and birth of a LBW baby. Preeclampsia is a disorder associated with significant endothelial dysfunction (59). LBW is also associated with endothelial dysfunction with observations noted in children (57) and young adults (53). Therefore, LBW may program an increased risk for hypertensive complications during pregnancy due to the increase in preexisting cardiovascular risk factors. LBW also increases the risk for GDM (39). Furthermore, complications during pregnancy including the birth of a LBW baby increase the mother’s risk of later T2D (43) and CV disease (75) in her life. Thus, complications during pregnancy in one generation resulting in impaired fetal growth can impact the gestational health of the next generation. These studies indicate that influences during fetal life have long reaching impact on subsequent generations. The exact mechanisms are not known but epigenetic processes are thought to contribute to the transmission of programmed risk from one generation to the next (66) and could conceivably contribute to the risk for a complicated pregnancy in a LBW woman. Thus, LBW as a consequence of a complicated pregnancy increases a women’s own risk to have a complicated pregnancy and also increases her risk for chronic disease in later life. Complicated pregnancies also program an increased CV and metabolic risk in the offspring highlighting the broad impact and severity that slow fetal growth in one generation has on the quality of life and health of the next.
CONCLUSIONS
Slow growth during fetal life exerts an adverse influence on gestational and chronic health in women. Low birth weight indicative of poor fetal growth increases the risk of early onset menopause, cardiovascular and metabolic disease, and osteoporosis in later life. Experimental models are providing insight into the underlying mechanisms that program later increased risk for chronic disease in women. Yet, additional studies are needed to provide insight into preventative and therapeutic options that may intervene and reduce the risk of chronic disease in low birth weight women across their lifespan and improve their gestational health to prevent the transmission of programmed risk to the next generation.
HIGHLIGHTS.
Low birth weight programs an increased risk for chronic disease in men and women. However, women may differ in their susceptibility to chronic disease risk relative to men in a manner that may be impacted by age.
Low birth weight is associated with early menopause. Early menopause is a risk factor for cardiovascular and metabolic health. Early menopause can also impact bone density suggesting that early reproductive senescence programmed in response to slow growth during fetal life may exacerbate risk for chronic disease that is already inherent in low birth weight women.
Low birth weight increases a woman’s risk for complications during pregnancy. Thus, the impact of poor fetal growth in one generation impacts the chronic health of subsequent and future generations highlighting the importance of understanding how slow growth during fetal life impacts a women’s health across her lifespan.
ACKNOWLEDGEMENTS
Dr Alexander is supported by National Institutes of Health grants HL074927, HL51971 and an American Heart Grant GRNT19900004, Dr Intapad is supported by an American Heart Association Post-Doctoral Fellowship Grant, 12POST1198002, and both receive support from the NIH P20GM104357.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
REFERENCES
- 1.Aceti A, Santhakumaran S, Logan KM, Philipps LH, Prior E, Gale C, Hyde MJ, Modi N. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta-analysis. Diabetologia. 2012;55:3114–3127. doi: 10.1007/s00125-012-2689-8. [DOI] [PubMed] [Google Scholar]
- 2.Al Salmi I, Hoy WE, Kondalsamy-Chennakesavan S, Wang Z, Gobe GC, Barr EL, Shaw JE. Disorders of glucose regulation in adults and birth weight: results from the Australian Diabetes, Obesity and Lifestyle (AUSDIAB) Study. Diabetes Care. 2008;31:159–164. doi: 10.2337/dc07-1170. [DOI] [PubMed] [Google Scholar]
- 3.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41:457–462. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 4.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension. 2005;45:754–758. doi: 10.1161/01.HYP.0000153319.20340.2a. [DOI] [PubMed] [Google Scholar]
- 5.Andersson SW, Lapidus L, Niklasson A, Hallberg L, Bengtsson C, Hulthen L. Blood pressure and hypertension in middle-aged women in relation to weight and length at birth: a follow-up study. J Hypertens. 2000;18:1753–1761. doi: 10.1097/00004872-200018120-00008. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ, Osmond c. Low birth weight and hypertension. BMJ. 1988;297:134–135. doi: 10.1136/bmj.297.6641.134-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernal AB, Vickers MH, Hampton MB, Poynton RA, Sloboda DM. Maternal undernutrition significantly impacts ovarian follicle number and increases ovarian oxidative stress in adult rat offspring. PLoS One. 2010;5:e15558. doi: 10.1371/journal.pone.0015558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhutani G, Gupta MC. Emerging therapies for the treatment of osteoporosis. J Midlife Health. 2013;4:147–152. doi: 10.4103/0976-7800.118991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bo S, Marchisio B, Volpiano M, Menato G, Pagano G. Maternal low birth weight and gestational hyperglycemia. Gynecol Endocrinol. 2003;17:133–136. [PubMed] [Google Scholar]
- 11.Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am. J. Epidemiol. 1997;145:124–133. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- 12.Burguet A. Long-term outcome in children of mothers with gestational diabetes. Diabetes Metab. 2010;36:682–694. doi: 10.1016/j.diabet.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Byberg L, Michaëlsson K, Goodman A, Zethelius B, Koupil I. Birth weight is not associated with risk of fracture. Results from two Swedish cohort studies. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2246. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Callréus M, McGuigan F, Åkesson K. Birth weight is more important for peak bone mineral content than for bone density: the PEAK-25 study of 1,061 young adult women. Osteoporos Int. 2013;24:1347–1355. doi: 10.1007/s00198-012-2077-8. [DOI] [PubMed] [Google Scholar]
- 15.Carlsson S, Persson PG, Alvarsson M, Efendic S, Norman A, Svanström L, Ostenson CG, Grill V. Low birth weight, family history of diabetes, and glucose intolerance in Swedish middle-aged men. Diabetes Care. 1999;22:1043–1047. doi: 10.2337/diacare.22.7.1043. [DOI] [PubMed] [Google Scholar]
- 16.Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res. 2011;469:1900–1905. doi: 10.1007/s11999-011-1780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chernoff N, Gage MI, Stoker TE, Cooper RL, Gilbert ME, Rogers EH. Reproductive effects of maternal and pre-weaning undernutrition in rat offspring: age at puberty, onset of female reproductive senescence and intergenerational pup growth and viability. Reprod Toxicol. 2009;28:489–494. doi: 10.1016/j.reprotox.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Coustan DR. Diabetes in America. 2nd ed. Vol. 2. Washington, DC: National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; 1995. Gestational diabetes; pp. 703–717. [Google Scholar]
- 19.Cresswell JL, Egger P, Fall CH, Osmond C, Fraser RB, Barker DJ. Is the age of menopause determined in-utero? Early Hum Dev. 1997;49:143–148. doi: 10.1016/s0378-3782(97)00028-5. [DOI] [PubMed] [Google Scholar]
- 20.Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, Speizer FE, Stampfer MJ. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94:1310–1315. doi: 10.1161/01.cir.94.6.1310. [DOI] [PubMed] [Google Scholar]
- 21.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94:3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 22.Dagan A, Kwon HM, Dwarakanath V, Baum M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am J Physiol Renal Physiol. 2008;295:F29–F34. doi: 10.1152/ajprenal.00123.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, Leeson P. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–e1561. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 24.de Lauzon-Guillain B, Balkau B, Charles MA, Romieu I, Boutron-Ruault MC, Clavel-Chapelon F. Birth weight, body silhouette over the life course, and incident diabetes in 91,453 middle-aged women from the French Etude Epidemiologique de Femmes de la Mutuelle Generale de l'Education Nationale (E3N) Cohort. Diabetes Care. 2010;33:298–303. doi: 10.2337/dc09-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dempsey JC, Williams MA, Luthy DA, Emanuel I, Shy K. Weight at birth and subsequent risk of preeclampsia as an adult. Am J Obstet Gynecol. 2003;189:494–500. doi: 10.1067/s0002-9378(03)00491-5. [DOI] [PubMed] [Google Scholar]
- 26.Dennison EM, Syddall HE, Sayer AA, Gilbody HJ, Cooper C. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the Hertfordshire cohort study. Pediatr Res. 2005;57:582–586. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- 27.El Hage R, Moussa E, Hammoud A, Dandachi G, Jacob C. Birth weight is an independent determinant of whole body bone mineral content and bone mineral density in a group of Lebanese adolescent girls. J Bone Miner Metab. 2010;28:360–363. doi: 10.1007/s00774-010-0165-4. [DOI] [PubMed] [Google Scholar]
- 28.Elias SG, van Noord PA, Peeters PH, den Tonkelaar I, Grobbee DE. Caloric restriction reduces age at menopause: the effect of the 1944–1945 Dutch famine. Menopause. 2003;10:399–405. doi: 10.1097/01.GME.0000059862.93639.C1. [DOI] [PubMed] [Google Scholar]
- 29.Engelbregt MJ, van Weissenbruch MM, Lips P, van Lingen A, Roos JC, Delemarre-van de Waal HA. Body composition and bone measurements in intra-uterine growth retarded and early postnatally undernourished male and female rats at the age of 6 months: comparison with puberty. Bone. 2004;34:180–186. doi: 10.1016/j.bone.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322(7292):949–953. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R368–R373. doi: 10.1152/ajpregu.00206.2004. [DOI] [PubMed] [Google Scholar]
- 32.Franco MC, Casarini DE, Carneiro-Ramos MS, Sawaya AL, Barreto-Chaves ML, Sesso R. Circulating renin-angiotensin system and catecholamines in childhood: is there a role for birthweight? Clin Sci (Lond) 2008;114:375–380. doi: 10.1042/CS20070284. [DOI] [PubMed] [Google Scholar]
- 33.Franco MC, Kawamoto EM, Gorjão R, Rastelli VM, Curi R, Scavone C, Sawaya AL, Fortes ZB, Sesso R. Biomarkers of oxidative stress and antioxidant status in children born small for gestational age: evidence of lipid peroxidation. Pediatr Res. 2007;62:204–208. doi: 10.1203/PDR.0b013e3180986d04. [DOI] [PubMed] [Google Scholar]
- 34.Gallo LA, Tran M, Moritz KM, Mazzuca MQ, Parry LJ, Westcott KT, Jefferies AJ, Cullen-McEwen LA, Wlodek ME. Cardio-renal and metabolic adaptations during pregnancy in female rats born small: implications for maternal health and second generation fetal growth. J. Physiol. 2012;590:617–630. doi: 10.1113/jphysiol.2011.219147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George EM, Granger JP. Linking placental ischemia and hypertension in preeclampsia: role of endothelin 1. Hypertension. 2012;60:507–511. doi: 10.1161/HYPERTENSIONAHA.112.194845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guzmán C, Cabrera R, Cárdenas M, Larrea F, Nathanielsz PW, Zambrano E. Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol. 2006;572:97–108. doi: 10.1113/jphysiol.2005.103903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guzmán C, García-Becerra R, Aguilar-Medina MA, Méndez I, Merchant-Larios H, Zambrano E. Maternal Protein Restriction During Pregnancy and/or Lactation Negatively Affects Follicular Ovarian Development and Steroidogenesis in the Prepubertal Rat Offspring. Arch Med Res. 2014 doi: 10.1016/j.arcmed.2014.05.005. pii: S0188-4409(14)00080-0. [DOI] [PubMed] [Google Scholar]
- 38.Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: role of leptin and sympathetic nervous system. Am J Hypertens. 2001;14:103S–115S. doi: 10.1016/s0895-7061(01)02077-5. [DOI] [PubMed] [Google Scholar]
- 39.Innes KE, Byers TE, Marshall JA, Barón A, Orleans M, Hamman RF. Association of a woman's own birth weight with subsequent risk for gestational diabetes. JAMA. 2002;287:2534–2541. doi: 10.1001/jama.287.19.2534. [DOI] [PubMed] [Google Scholar]
- 40.Innes KE, Byers TE, Marshall JA, Barón A, Orleans M, Hamman RF. Association of a woman's own birth weight with her subsequent risk for pregnancy-induced hypertension. Am J Epidemiol. 2003;158:861–870. doi: 10.1093/aje/kwg211. [DOI] [PubMed] [Google Scholar]
- 41.Intapad S, Ojeda NB, Dasinger JH, Alexander BT. Sex differences in the developmental origins of cardiovascular disease. Physiology (Bethesda) 2014;29:122–132. doi: 10.1152/physiol.00045.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Intapad S, Tull FL, Brown AD, Dasinger JH, Ojeda NB, Fahling JM, Alexander BT. Renal denervation abolishes the age-dependent increase in blood pressure in female intrauterine growth-restricted rats at 12 months of age. Hypertension. 2013;61:828–834. doi: 10.1161/HYPERTENSIONAHA.111.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James-Todd TM, Karumanchi SA, Hibert EL, Mason SM, Vadnais MA, Hu FB, Rich-Edwards JW. Gestational age, infant birth weight, and subsequent risk of type 2 diabetes in mothers: Nurses' Health Study II. Prev Chronic Dis. 2013;10:E156. doi: 10.5888/pcd10.120336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jansson T, Lambert GW. Effect of intrauterine growth restriction on blood pressure, glucose tolerance and sympathetic nervous system activity in the rat at 3–4 months of age. J Hypertens. 1999;17:1239–1248. doi: 10.1097/00004872-199917090-00002. [DOI] [PubMed] [Google Scholar]
- 45.Jones G, Dwyer T. Birth weight, birth length, and bone density in prepubertal children: evidence for an association that may be mediated by genetic factors. Calcif Tissue Int. 2000;67:304–308. doi: 10.1007/s002230001148. [DOI] [PubMed] [Google Scholar]
- 46.Jones G, Hynes KL, Dwyer T. The association between breastfeeding, maternal smoking in utero, and birth weight with bone mass and fractures in adolescents: a 16-year longitudinal study. Osteoporos Int. 2013;24:1605–1611. doi: 10.1007/s00198-012-2207-3. [DOI] [PubMed] [Google Scholar]
- 47.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. 1999;99:1165–1172. doi: 10.1161/01.cir.99.9.1165. [DOI] [PubMed] [Google Scholar]
- 48.Kramer MS. The epidemiology of low birthweight. N. estle Nutr Inst Workshop Ser. 2013;74:1–10. doi: 10.1159/000348382. [DOI] [PubMed] [Google Scholar]
- 49.Lanham SA, Roberts C, Perry MJ, Cooper C, Oreffo RO. Intrauterine programming of bone. Part 2: alteration of skeletal structure. Osteoporos Int. 2008;19:157–167. doi: 10.1007/s00198-007-0448-3. [DOI] [PubMed] [Google Scholar]
- 50.Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14:935–941. [PubMed] [Google Scholar]
- 51.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, Martyn CN, de Swiet M. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105:1088–1092. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- 52.Lawlor DA, Davey Smith G, Ebrahim S. Birth weight is inversely associated with coronary heart disease in post-menopausal women: findings from the British women's heart and health study. J Epidemiol Community Health. 2004;58:120–125. doi: 10.1136/jech.58.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leeson CP, Kattenhorn M, Morley R, Lucas A, Deanfield JE. Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation. 2001;103:1264–1268. doi: 10.1161/01.cir.103.9.1264. [DOI] [PubMed] [Google Scholar]
- 54.Li Z, Snieder H, Su S, Harshfield GA, Treiber FA, Wang X. A longitudinal study of blood pressure variability in African-American and European American youth. J Hypertens. 2010;28:715–722. doi: 10.1097/HJH.0b013e328336ed5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lumey LH, Stein AD, Kahn HS, Romijn JA. Lipid profiles in middle-aged men and women after famine exposure during gestation: the Dutch Hunger Winter Families Study. Am J Clin Nutr. 2009;89:1737–1743. doi: 10.3945/ajcn.2008.27038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manning J, Vehaskari VM. Postnatal modulation of prenatally programmed hypertension by dietary Na and ACE inhibition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R80–R84. doi: 10.1152/ajpregu.00309.2004. [DOI] [PubMed] [Google Scholar]
- 57.Martin H, Hu J, Gennser G, Norman M. Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birthweight. Circulation. 2000;102:2739–2744. doi: 10.1161/01.cir.102.22.2739. [DOI] [PubMed] [Google Scholar]
- 58.Martínez-Mesa J, Restrepo-Méndez MC, González DA, Wehrmeister FC, Horta BL, Domingues MR, Menezes AM. Life-course evidence of birth weight effects on bone mass: systematic review and meta-analysis. Osteoporos Int. 2013;24:7–18. doi: 10.1007/s00198-012-2114-7. [DOI] [PubMed] [Google Scholar]
- 59.Masoura S, Kalogiannidis I, Makedou K, Theodoridis T, Koiou K, Gerou S, Athanasiadis A, Agorastos T. Biomarkers of endothelial dysfunction in preeclampsia and neonatal morbidity: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2014;175:119–123. doi: 10.1016/j.ejogrb.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Metha G, Roach HI, Langley-Evans S, Taylor P, Reading I, Oreffo RO, Aihie-Sayer A, Clarke NM, Cooper C. Intrauterine exposure to a maternal low protein diet reduces adult bone mass and alters growth plate morphology in rats. Calcif Tissue Int. 2002;71:493–498. doi: 10.1007/s00223-001-2104-9. [DOI] [PubMed] [Google Scholar]
- 61.Mogren I, Lindahl B, Högberg U. Impaired fasting glucose and impaired glucose tolerance are related to both heredity and low birth weight. Scand J Public Health. 2003;31:382–388. doi: 10.1080/14034940210165136. [DOI] [PubMed] [Google Scholar]
- 62.Moritz KM, Mazzuca MQ, Siebel AL, Mibus A, Arena D, Tare M, Owens JA, Wlodek ME. Uteroplacental insufficiency causes a nephron deficit, modest renal insufficiency but no hypertension with ageing in female rats. J Physiol. 2009;587:2635–2646. doi: 10.1113/jphysiol.2009.170407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.National Center for Health Statistics, final natality data. [Retrieved February 4th, 2004]; from www.marchofdimes.com/peristats. [Google Scholar]
- 64.Newsome CA, Shiell AW, Fall CH, Phillips DI, Shier R, Law CM. Is birth weight related to later glucose and insulin metabolism?--A systematic review. Diabet Med. 2003;20:339–348. doi: 10.1046/j.1464-5491.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- 65.Norris SA, Osmond C, Gigante D, Kuzawa CW, Ramakrishnan L, Lee NR, Ramirez-Zea M, Richter LM, Stein AD, Tandon N, Fall CH COHORTS Group. Size at birth, weight gain in infancy and childhood, and adult diabetes risk in five low- or middle-income country birth cohorts. Diabetes Care. 2012;35:72–79. doi: 10.2337/dc11-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Sullivan L, Combes AN, Moritz KM. Epigenetics and developmental programming of adult onset diseases. Pediatr Nephrol. 2012;27:2175–2182. doi: 10.1007/s00467-012-2108-x. [DOI] [PubMed] [Google Scholar]
- 67.Ogonowski J, Miazgowski T, Engel K, Celewicz Z. Birth weight predicts the risk of gestational diabetes mellitus and pregravid obesity. Nutrition. 2014;30:39–43. doi: 10.1016/j.nut.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 68.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–685. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2007;292:R758–R763. doi: 10.1152/ajpregu.00311.2006. [DOI] [PubMed] [Google Scholar]
- 70.Ojeda NB, Hennington BS, Williamson DT, Hill ML, Betson NE, Sartori-Valinotti JC, Reckelhoff JF, Royals TP, Alexander BT. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension. 2012;60:114–122. doi: 10.1161/HYPERTENSIONAHA.112.192955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003;41:328–334. doi: 10.1161/01.hyp.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN. Diabetes in old male offspring of rat dams fed a reduced protein diet. Int J Exp Diabetes Res. 2001;2:139–143. doi: 10.1155/EDR.2001.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reinisch JM, Simon NG, Karwo WG, Gandelman R. Prenatal exposure to prednisone in humans and animals retards intra-uterine growth. Science. 1978;202:436–438. doi: 10.1126/science.705336. [DOI] [PubMed] [Google Scholar]
- 74.Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Gillman MW, Hennekens CH, Speizer FE, Manson JE. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med. 1999;130:278–284. doi: 10.7326/0003-4819-130-4_part_1-199902160-00005. [DOI] [PubMed] [Google Scholar]
- 75.Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women's future cardiovascular health: an underused opportunity to improve women's health? Epidemiol Rev. 2014;36:57–70. doi: 10.1093/epirev/mxt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ryckman KK, Rillamas-Sun E, Spracklen CN, Wallace RB, Garcia L, Tylavsky FA, Howard BV, Liu S, Song Y, Leblanc ES, White MV, Parikh NI, Robinson JG. Ethnic differences in the relationship between birth weight and type 2 diabetes mellitus in postmenopausal women. Diabetes Metab. 2014 doi: 10.1016/j.diabet.2014.03.003. pii: S1262-3636(14)00064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Romano T, Wark JD, Owens JA, Wlodek ME. Prenatal growth restriction and postnatal growth restriction followed by accelerated growth independently program reduced bone growth and strength. Bone. 2009;45:132–141. doi: 10.1016/j.bone.2009.03.661. [DOI] [PubMed] [Google Scholar]
- 78.Romano T, Wark JD, Wlodek ME. Calcium supplementation does not rescue the programmed adult bone deficits associated with perinatal growth restriction. Bone. 2010;47:1054–1063. doi: 10.1016/j.bone.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 79.Salonen M, Tenhola S, Laitinen T, Lyyra-Laitinen T, Romppanen J, Jääskeläinen J, Voutilainen R. Tracking serum lipid levels and the association of cholesterol concentrations, blood pressure and cigarette smoking with carotid artery intima-media thickness in young adults born small for gestational age. Circ J. 2010;74:2419–2425. doi: 10.1253/circj.cj-10-0398. [DOI] [PubMed] [Google Scholar]
- 80.Schlüssel MM, Vaz JS, Kac G. Birth weight and adult bone mass: a systematic literature review. Osteoporos Int. 2010;21:1981–1991. doi: 10.1007/s00198-010-1236-z. [DOI] [PubMed] [Google Scholar]
- 81.Schoof E, Girstl M, Frobenius W, Kirschbaum M, Dörr HG, Rascher W, Dötsch J. Decreased gene expression of 11beta-hydroxysteroid dehydrogenase type 2 and 15-hydroxyprostaglandin dehydrogenase in human placenta of patients with preeclampsia. J Clin Endocrinol Metab. 2001;86:1313–1317. doi: 10.1210/jcem.86.3.7311. [DOI] [PubMed] [Google Scholar]
- 82.Siebel AL, Mibus A, De Blasio MJ, Westcott KT, Morris MJ, Prior L, Owens JA, Wlodek ME. mproved lactational nutrition and postnatal growth ameliorates impairment of glucose tolerance by uteroplacental insufficiency in male rat offspring. Endocrinology. 2008;149:3067–3076. doi: 10.1210/en.2008-0128. [DOI] [PubMed] [Google Scholar]
- 83.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- 84.Spence D, Stewart MC, Alderdice FA, Patterson CC, Halliday HL. Intra-uterine growth restriction and increased risk of hypertension in adult life: a follow-up study of 50-year-olds. Public Health. 2012;126:561–565. doi: 10.1016/j.puhe.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 85.Stein AD, Zybert PA, van der Pal-de Bruin K, Lumey LH. Exposure to famine during gestation, size at birth, and blood pressure at age 59 y: evidence from the Dutch Famine. Eur J Epidemiol. 2006;21:759–765. doi: 10.1007/s10654-006-9065-2. [DOI] [PubMed] [Google Scholar]
- 86.Steiner AZ, D'Aloisio AA, DeRoo LA, Sandler DP, Baird DD. Association of intrauterine and early-life exposures with age at menopause in the Sister Study. Am J Epidemiol. 2010;172:140–148. doi: 10.1093/aje/kwq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int. 2005;68:2180–2188. doi: 10.1111/j.1523-1755.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 88.Styrud J, Eriksson UJ, Grill V, Swenne I. Experimental intrauterine growth retardation in the rat causes a reduction of pancreatic B-cell mass, which persists into adulthood. Biol Neonate. 2005;88:122–128. doi: 10.1159/000086136. [DOI] [PubMed] [Google Scholar]
- 89.Sugden MC, Holness MJ. Gender-specific programming of insulin secretion and action. J Endocrinol. 2002;75:757–767. doi: 10.1677/joe.0.1750757. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki K, Tanaka T, Kondo N, Minai J, Sato M, Yamagata Z. Is maternal smoking during early pregnancy a risk factor for all low birth weight infants? J Epidemiol. 2008;18:89–96. doi: 10.2188/jea.JE2007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tao H, Rui C, Zheng J, Tang J, Wu L, Shi A, Chen N, He R, Wu C, Li J, Yin X, Zhang P, Zhu Z, Tao J, Xiao J, Mao C, Xu Z. Angiotensin II-mediated vascular changes in aged offspring rats exposed to perinatal nicotine. Peptides. 2013;44:111–119. doi: 10.1016/j.peptides.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 92.Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142C:155–170. doi: 10.1016/j.jsbmb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thamotharan M, Garg M, Oak S, Rogers LM, Pan G, Sangiorgi F, Lee PW, Devaskar SU. Transgenerational inheritance of the insulin-resistant phenotype in embryo-transferred intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab. 2007;292:E1270–E1279. doi: 10.1152/ajpendo.00462.2006. [DOI] [PubMed] [Google Scholar]
- 94.Theys N, Bouckenooghe T, Ahn MT, Remacle C, Reusens B. Maternal low-protein diet alters pancreatic islet mitochondrial function in a sex-specific manner in the adult rat. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1516–R1525. doi: 10.1152/ajpregu.00280.2009. [DOI] [PubMed] [Google Scholar]
- 95.Tom SE, Cooper R, Kuh D, Guralnik JM, Hardy R, Power C. Fetal environment and early age at natural menopause in a British birth cohort study. Hum Reprod. 2010;25:791–798. doi: 10.1093/humrep/dep451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vos LE, Oren A, Bots ML, Gorissen WH, Grobbee DE, Uiterwaal CS. Birth size and coronary heart disease risk score in young adulthood. The Atherosclerosis Risk in Young Adults (ARYA) study. Eur J Epidemiol. 2006;21:33–38. doi: 10.1007/s10654-005-4658-8. [DOI] [PubMed] [Google Scholar]
- 97.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause. 2012;19:1081–1087. doi: 10.1097/gme.0b013e3182517bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia. 2011;54:504–507. doi: 10.1007/s00125-010-2008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, Barrett-Connor E, Bhargava SK, Birgisdottir BE, Carlsson S, de Rooij SR, Dyck RF, Eriksson JG, Falkner B, Fall C, Forsén T, Grill V, Gudnason V, Hulman S, Hyppönen E, Jeffreys M, Lawlor DA, Leon DA, Minami J, Mishra G, Osmond C, Power C, Rich-Edwards JW, Roseboom TJ, Sachdev HS, Syddall H, Thorsdottir I, Vanhala M, Wadsworth M, Yarbrough DE. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 100.Wilcox CS. Reactive oxygen species: roles in blood pressure and kidney function. Curr Hypertens Rep. 2002;4:160–166. doi: 10.1007/s11906-002-0041-2. [DOI] [PubMed] [Google Scholar]
- 101.Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA, Moritz KM. Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J Am Soc Nephrol. 2007;18:1688–1696. doi: 10.1681/ASN.2007010015. [DOI] [PubMed] [Google Scholar]
- 102.Wood CL, Wood AM, Harker C, Embleton ND. Bone mineral density and osteoporosis after preterm birth: the role of early life factors and nutrition. Int J Endocrinol. 2013;2013:902513. doi: 10.1155/2013/902513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 104.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1131–R1136. doi: 10.1152/ajpregu.00037.2003. [DOI] [PubMed] [Google Scholar]
- 105.Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension. 2008;51:1239–1247. doi: 10.1161/HYPERTENSIONAHA.107.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang Z, Huffman SL. Nutrition in pregnancy and early childhood and associations with obesity in developing countries. Matern Child Nutr. 2013;9(Suppl 1):105–119. doi: 10.1111/mcn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yarbrough DE, Barrett-Connor E, Morton DJ. Birth weight as a predictor of adult bone mass in postmenopausal women: the Rancho Bernardo Study. Osteoporos Int. 2000;11:626–630. doi: 10.1007/s001980070085. [DOI] [PubMed] [Google Scholar]
- 108.Yarde F, Broekmans FJ, van der Pal-de Bruin KM, Schönbeck Y, te Velde ER, Stein AD, Lumey LH. Prenatal famine, birthweight, reproductive performance and age at menopause: the Dutch hunger winter families study. Hum Reprod. 2013;28:3328–3336. doi: 10.1093/humrep/det331. [DOI] [PMC free article] [PubMed] [Google Scholar]