Abstract
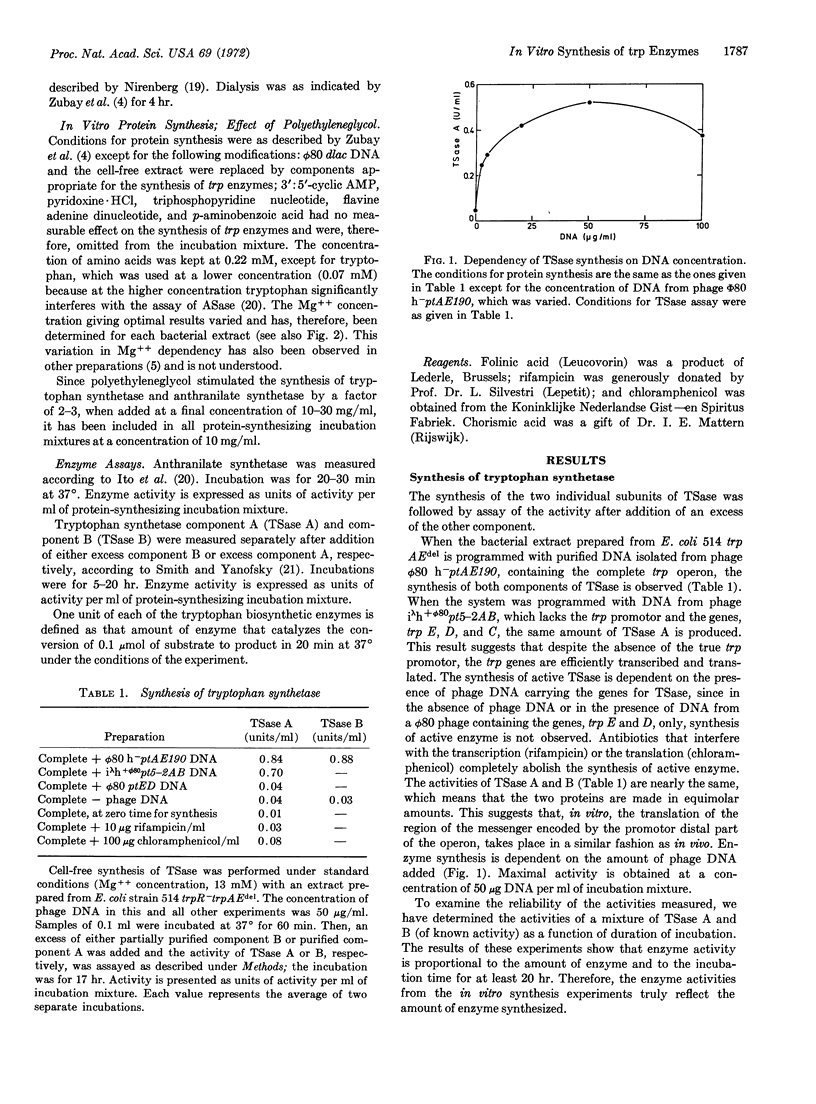
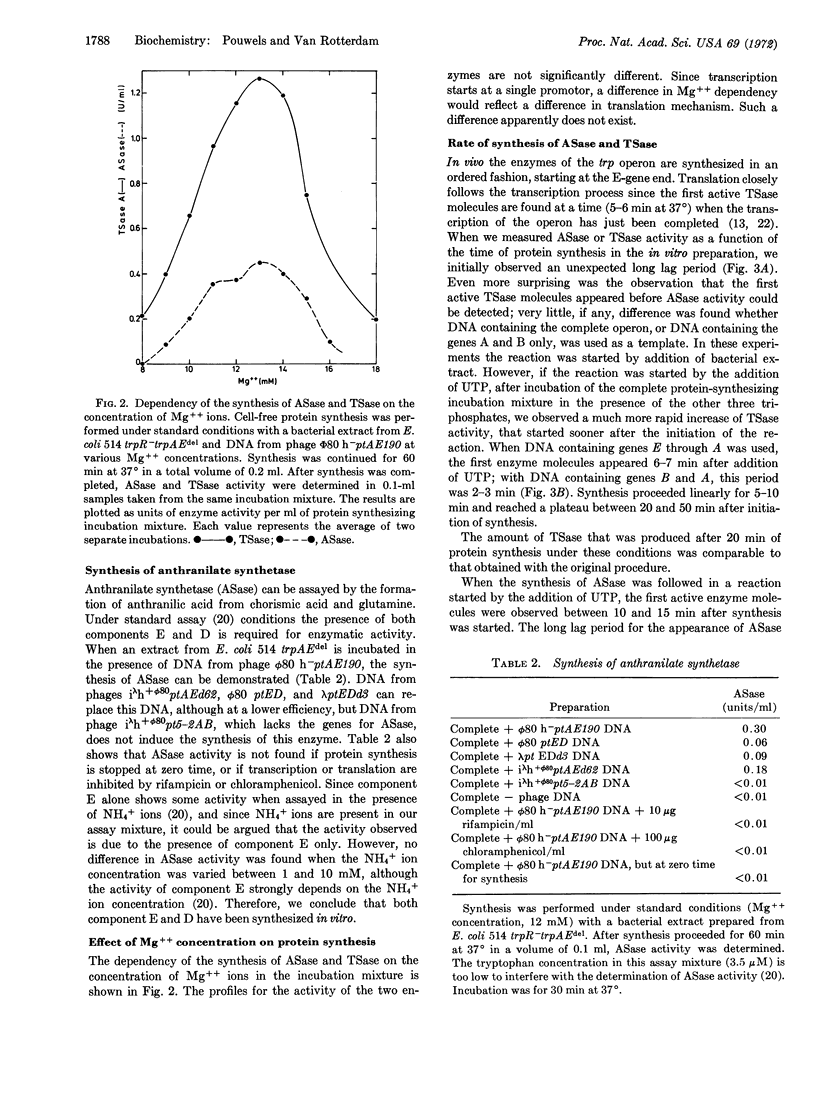
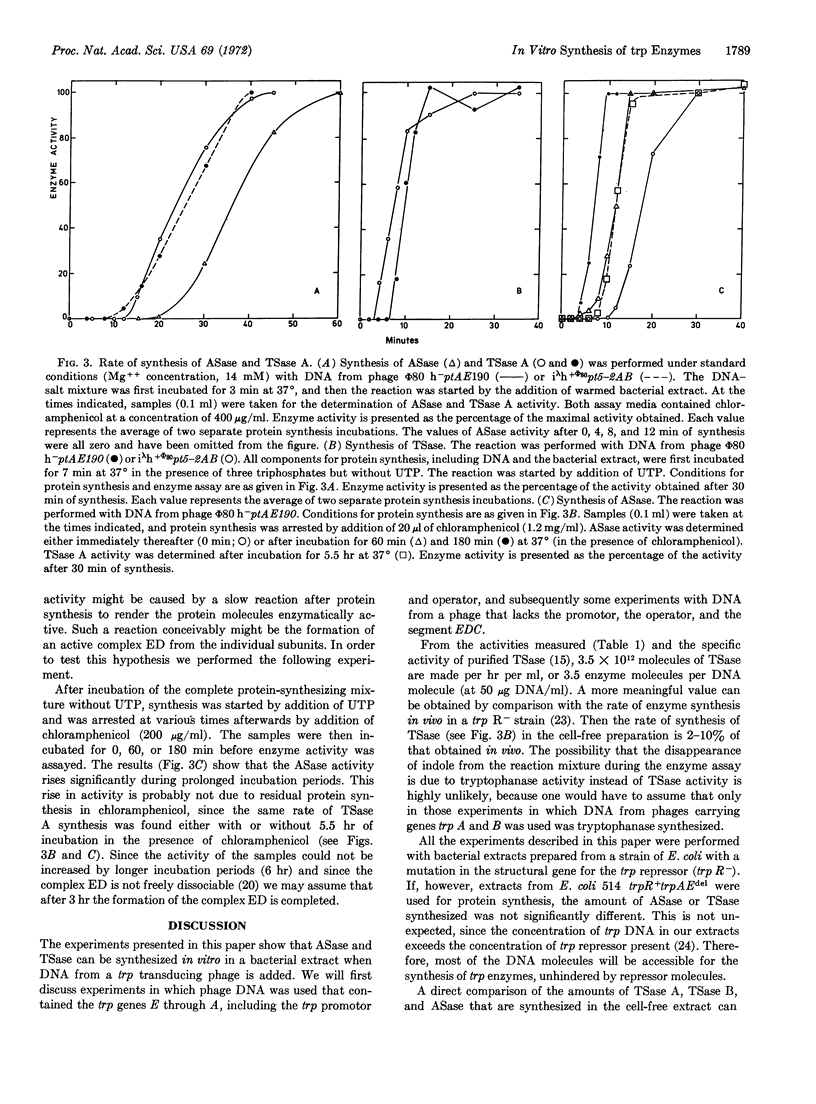
Two active enzymes of the tryptophan operon of E. coli, anthranilate synthetase (product of trp E and trp D) and tryptophan synthetase (EC 4.2.1.20) (product of trp B and trp A), have been synthesized in a DNA-dependent preparation for protein synthesis. Anthranilate synthetase and the two components of tryptophan synthetase (tryptophan synthetase A and B) are made in vitro in equimolar amounts, suggesting that regulation of the translation is similar in vivo and in vitro. Kinetics of the synthesis of the two enzymes suggest that transcription and translation proceed in vitro in a correct temporal sequence.
Keywords: anthranilate synthetase, tryptophan synthetase, DNA-dependent protein synthesis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R. F., Yanofsky C. The periodicity of RNA polymerase initiations: a new regulatory feature of transcription. Proc Natl Acad Sci U S A. 1968 May;60(1):313–320. doi: 10.1073/pnas.60.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb S. S., Okamoto K., Hall B. D. Isolation and characterization of non-defective transducing elements of bacteriophage phi-80. Virology. 1967 Feb;31(2):289–295. doi: 10.1016/0042-6822(67)90173-0. [DOI] [PubMed] [Google Scholar]
- Gratia J. P. Délétion et substitution de sites de restriction dans un phage hybride lambda 80. Ann Inst Pasteur (Paris) 1971 Jul;121(1):13–22. [PubMed] [Google Scholar]
- Greenblatt J., Schleif R. Arabinose C protein: regulation of the arabinose operon in vitro. Nat New Biol. 1971 Oct 6;233(40):166–170. doi: 10.1038/newbio233166a0. [DOI] [PubMed] [Google Scholar]
- HENNING U., HELINSKI D. R., CHAO F. C., YANOFSKY C. The A protein of the tryptophan synthetase of Escherichia coli. Purification, crystallization, and composition studies. J Biol Chem. 1962 May;237:1523–1530. [PubMed] [Google Scholar]
- Imamoto F., Morikawa N., Sato K. On the transcription of the tryptophan operon in Escherichia coli. 3. Multicistronic messenger RNA and polarity for transcription. J Mol Biol. 1965 Aug;13(1):169–182. doi: 10.1016/s0022-2836(65)80087-0. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Yanofsky C. Transcription of the tryptophan operon in polarity mutants of Escherichia coli. I. Characterization of the tryptophan messenger RNA of polar mutants. J Mol Biol. 1967 Aug 28;28(1):1–23. doi: 10.1016/s0022-2836(67)80073-1. [DOI] [PubMed] [Google Scholar]
- Ito J., Cox E. C., Yanofsky C. Anthranilate synthetase, an enzyme specified by the tryptophan operon of Escherichia coli: purification and characterization of component I. J Bacteriol. 1969 Feb;97(2):725–733. doi: 10.1128/jb.97.2.725-733.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Imamoto F. Sequential derepression and repression of the tryptophan operon in E. coli. Nature. 1968 Nov 2;220(5166):441–444. doi: 10.1038/220441a0. [DOI] [PubMed] [Google Scholar]
- Ito J., Yanofsky C. The nature of the anthranilic acid synthetase complex of Escherichia coli. J Biol Chem. 1966 Sep 10;241(17):4112–4114. [PubMed] [Google Scholar]
- MARUSHIGE K., YURA T., IMAI M. THE ROLE OF RIBOSOMES IN THE CELL-FREE FORMATION OF TRYPTOPHAN SYNTHETASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 May 18;87:90–100. doi: 10.1016/0926-6550(64)90050-7. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Baker R. F., Yanofsky C. Translation of the tryptophan messenger RNA of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1428–1435. doi: 10.1073/pnas.60.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Amber mutants of the trpR regulatory gene. J Mol Biol. 1969 Aug 28;44(1):185–193. doi: 10.1016/0022-2836(69)90413-6. [DOI] [PubMed] [Google Scholar]
- Salser W., Gesteland R. F., Bolle A. In vitro synthesis of bacteriophage lysozyme. Nature. 1967 Aug 5;215(5101):588–591. doi: 10.1038/215588a0. [DOI] [PubMed] [Google Scholar]
- Schweiger M., Gold L. M. DNA-dependent in vitro synthesis of bacteriophage enzymes. Cold Spring Harb Symp Quant Biol. 1969;34:763–766. doi: 10.1101/sqb.1969.034.01.086. [DOI] [PubMed] [Google Scholar]
- Wetekam W., Staack K., Ehring R. DNA-dependent in vitro synthesis of enzymes of the galactose operon of Escherichia coli. Mol Gen Genet. 1971;112(1):14–27. doi: 10.1007/BF00266928. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]
- YURA T., MARUSHIGE K., IMAIM, WATANABE I. The cell-free formation of tryptophan synthetase enzyme in Escherichia coli. Biochem Biophys Res Commun. 1962 Dec 19;9:545–550. doi: 10.1016/0006-291x(62)90123-7. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]
- Young E. T., 2nd Cell-free synthesis of bacteriophage T4 glucosyl transferase. J Mol Biol. 1970 Aug;51(3):591–604. doi: 10.1016/0022-2836(70)90010-0. [DOI] [PubMed] [Google Scholar]
- Zubay G., Gielow L., Englesberg E. Cell-free studies on the regulation of the arabinose operon. Nat New Biol. 1971 Oct 6;233(40):164–165. doi: 10.1038/newbio233164a0. [DOI] [PubMed] [Google Scholar]



