Abstract
Varicelloviruses in primates comprise the prototypic human varicella-zoster virus (VZV) and its non-human primate homologue simian varicella virus (SVV). Both viruses cause varicella as a primary infection, establish latency in ganglionic neurons and reactivate later in life to cause herpes zoster in their respective hosts. VZV is endemic worldwide and although varicella is usually a benign disease in childhood, VZV reactivation is a significant cause of neurological disease in the elderly and in immunocompromised individuals. The pathogenesis of VZV infection remains ill-defined, mostly due to the species restriction of VZV that impedes studies in experimental animal models. SVV infection of non-human primates parallels virological, clinical, pathological and immunological features of human VZV infection, thereby providing an excellent model to study the pathogenesis of varicella and herpes zoster in its natural host. In this review, we discuss recent studies that provided novel insight in both the virus and host factors involved in the three elementary stages of Varicellovirus infection in primates: primary infection, latency and reactivation.
Keywords: Varicella-zoster virus, Simian varicella virus, Primary infection, Latency, Reactivation Humans, Non-human primates, Dissemination, Immunity
Introduction
Varicella (chickenpox) and herpes zoster (HZ; shingles) are common human diseases. However, it was not until early 20th century that Von Bokay described that varicella was linked with exposure to HZ [1] and not until the mid-20th century that Garland and Hope-Simpson hypothesized that HZ reflected reactivation of latent virus acquired during varicella [2, 3]. In 1958, Weller was the first to isolate and culture the causative virus of varicella and HZ and demonstrated that both diseases were caused by the same virus: varicella-zoster virus (VZV) [4]. While considerable insight into VZV biology has been gained in the last decades, two important properties of the virus have seriously hampered studies on VZV pathogenesis in humans: (1) despite high titers of cell-free VZV in vesicular skin fluid, the virus is notoriously cell-associated in cell culture precluding synchronized infections; and (2) the virus does not cause overt disease in experimental animal models because it is highly species-specific [reviewed in 5, 6].
In 1967 Clarkson and colleagues reported on an outbreak of varicella-like disease in vervet monkeys introduced into an established breeding colony [7]. Subsequent epidemic outbreaks of varicella-like illness were described in various non-human primate species at primate centers [8–12]. The causative agent was isolated and identified as a herpesvirus, termed simian varicella virus (SVV), based on both the cytopathic effect observed in affected tissues and cell culture, and virion morphology by electron microscopy [7]. SVV causes a natural disease in non-human primates with clinical, pathological and immunological features resembling human varicella infection [13–18].
VZV and SVV are members of the Varicellovirus genus, which belongs to the subfamily of Alphaherpesviridae and family of Herpesviridae [19]. Phylogenetic analysis of SVV and VZV genomes suggests that both viruses originated from an ancestral virus that most likely emerged in the earliest primates in Africa [19, 20]. About 20–30 million years ago Hominoidae diverged from Old World monkeys, each carrying their own primordial Varicellovirus, eventually giving rise to two closely related viruses that co-evolved with their respective hosts [19–21]. Herein, we present an updated overview of the virus and host factors involved in the pathogenesis of VZV and SVV in their natural host.
Molecular biology
VZV and SVV virions are pleomorphic to spherical in shape, 170 – 200 nm in diameter and, like all herpesviruses, composed of four elements: a DNA core located within a capsid that is surrounded by a layer of tegument and this complex is packaged within a lipid envelope (Figure 1A and 1B) [5, 22]. The VZV genome is composed of about 125 kilobase pairs of linear double-stranded DNA and encodes for at least 70 unique open reading frames (ORFs) [24]. The SVV genome is similar in size and structure and encodes 71 unique ORFs of which 68 (96%) share extensive homology with corresponding VZV genes [25, 26]. SVV and VZV genomes deviate most in the leftward terminus of the genome: SVV does not encode a homologue of VZV ORF2, but contains 2 additional ORFs: ORFA and ORFLE [25, 26]. Tegument proteins surround the capsid and allow the virus to release a collection of already synthesized proteins into newly infected cells and immediately alter the host environment to inhibit antiviral defenses and support viral replication. VZV tegument proteins include transcriptional activators encoded by ORFs 4, 10, 62 and 63, protein kinases ORFs 47 and 66 and the protein products encoded by ORFs 9, 10, 11 and possibly ORFs 12, 22, 38, 53, 57 and 64/69, as well as host proteins [5, 27–31]. Of these, ORFs 47, 62 and 63 inhibit NFκB and IFNα signaling pathways and ORF66 impairs MHC class I surface expression [32–35]. VZV and SVV encode 9 different glycoproteins: glycoprotein B (gB), gC, gE, gH, gI, gL, gK, gM and gN [36]. Notably, unlike other Alphaherpesviruses VZV and SVV do not encode for a gD orthologue [24, 25]. Viral glycoproteins are incorporated in the envelope and are involved in viral attachment and entry into the host cell, envelopment of the virus, viral egress and cell-to-cell spread [36].
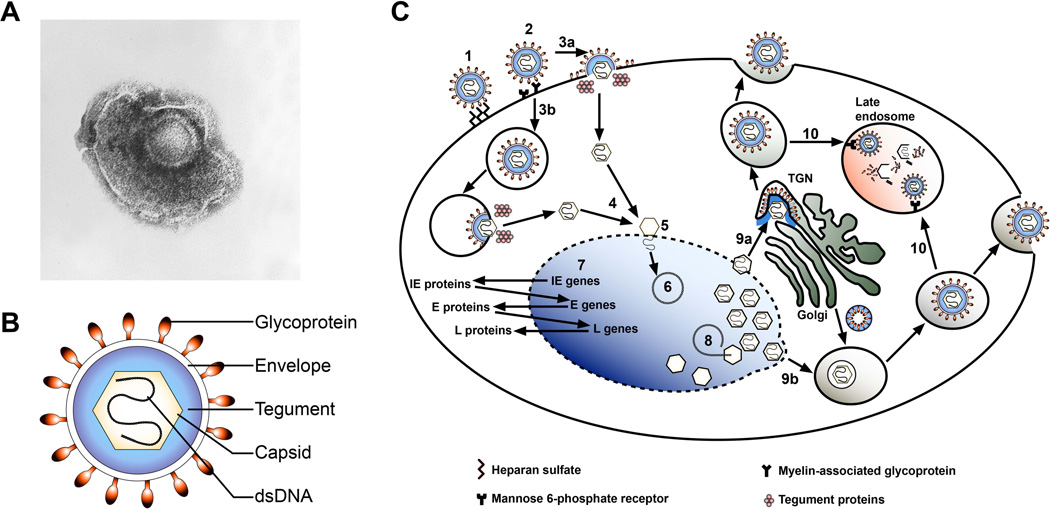
Figure 1. Structure and lytic replication cycle of VZV and SVV.
(A) Electron microscopy image of VZV (obtained from the CDC/Dr. Erskine Palmer; B.G. Partin [23]). (B) Schematic representation of the VZV/SVV particle. Virions are composed of a double-stranded (ds) DNA core located within the capsid, which is surrounded by a layer of tegument proteins and a lipid envelope carrying the viral glycoproteins. (C) Schematic representation of lytic VZV infection of the host cell. Initial attachment of VZV particles to the cell occurs via surface heparan sulfates (1), after which the virus engages with mannose 6-phosphate receptor (MPRci) and/or myelin-associated glycoprotein (2). Like other herpesviruses, VZV is assumed to enter the cells by direct fusion with the plasma membrane (3a) or via endocytosis (3b) followed by fusion and release of tegument proteins and capsids into the cytoplasm. Capsids are transported to the nuclear membrane (4), where viral DNA is released into the nucleus (5). The viral genome circularizes (6) and gene expression most likely occurs in a coordinated temporal fashion, analogous to that of HSV [43]: first immediate early (IE) are expressed, then early (E) genes and subsequently late (L) viral genes (7). Following DNA replication nascent viral DNA is packaged within newly assembled capsids in the nucleus (8). Assembly and egress may occur by one of two possible routes [47–49]: Naked nucleocapsids may be released in the cytoplasm and acquire their tegument proteins, envelope and glycoproteins in the trans-Golgi network (TGN) (9a). Alternatively, capsids gain their envelope from the nucleus and acquire their tegument proteins and glycoproteins by fusion with Golgi-derived vesicles (9b). Virus particles egress by exocytosis, although most particles are degraded in late endosomes through their association with MPRci (10) [38].
By analogy to other herpesviruses, VZV and SVV are assumed to enter the host cell by fusion of the viral envelope with the cell membrane or by endocytosis (Figure 1C) [5]. VZV particles initially attach non-specifically to cell membrane-expressed heparan sulfate [37], followed by specific interactions with one or more of the currently known VZV entry receptors: cation-independent mannose 6-phosphate receptor (MPRci) and myelin-associated glycoprotein [38, 39]. Although insulin degrading enzyme (IDE) was initially reported to serve as an additional entry receptor for VZV [40], more recent studies suggest that IDE binds to an immature form of gE in the cytoplasm and does not function as an entry receptor [41]. Genetic similarities in glycoprotein sequences [25], and analogous tissue tropism in vivo and in vitro [4, 5, 13–18, 42], suggest that SVV may utilize the same entry receptors as VZV. Fusion of the viral envelope with the plasma membrane or endosomal membrane releases viral tegument proteins and the nucleocapsid into the cytoplasm, after which the nucleocapsid is transported to the nuclear membrane to release the viral genome into the nucleus [5]. After circularization of viral DNA, VZV and SVV genes are most likely transcribed in a temporal cascaded fashion, analogous to the closely related herpes simplex virus (HSV) [43], to support the generation of virus progeny [5, 44, 45]. VZV particles are assembled in the cytoplasm and released by exocytosis, possibly involving egress at filopodia located in viral highways [46–49]. Most particles, however, are degraded in MPRci-expressing late endosomes, which may explain why VZV and SVV are notoriously cell-associated in vitro [38, 46–49].
Clinical features
Primary infection
VZV is spread from varicella or herpes zoster patients to susceptible individuals by aerosols or direct contact with vesicular fluid [50, 51]. The onset of clinical illness (after an incubation period of about 2 weeks) is characterized by fever, malaise, headache and loss of appetite concurrent with the appearance of a generalized characteristic cutaneous rash referred to as varicella [5, 52]. Varicella patients have a transient lymphopenia, granulocytopenia and frequently develop a mild hepatitis [5, 52]. Whereas varicella is a typically benign disease in children, adults and immunocompromised individuals are at high risk of developing severe disease upon primary VZV infection [5, 52]. Common complications of varicella include secondary bacterial infections in children and pneumonia in adults [5, 52]. Occasionally, varicella may cause VZV infection of the central nervous system (CNS) resulting in complications ranging in severity from relatively mild cerebellar ataxia to life-threatening meningoencephalitis, meningitis or large- and small-vessel vasculitis [52–54]. Varicella during pregnancy is associated with higher morbidity or even mortality in mother and infant [5, 52]. Note that not all individuals develop skin rash following primary VZV infection, as evidenced by the detection of virus-specific humoral immunity in individuals without a history of varicella [55, 56].
SVV is transmitted via aerosols or direct contact and both natural and experimental infection result in a generalized vesicular cutaneous rash that appears 7–14 days after inoculation [7–18]. Additional clinical manifestations include fever, loss of appetite, lethargy and development of a mild hepatitis [7–12, 15, 16, 18]. The susceptibility to and disease severity of SVV varies among different monkey species. African green monkeys, patas monkeys and cynomolgus macaques are highly susceptible to SVV infection and may develop severe, potentially life-threatening disease including pneumonia and hepatitis [16, 17]. Contrastingly, rhesus macaques are less susceptible to SVV infection and disease severity is limited compared to other non-human primate species [11, 57, 58]. The geographic origin of rhesus macaques may also affect their susceptibility to SVV. Infection of Indian rhesus macaques resulted in skin rash and the establishment of ganglionic viral latency [16], whereas Chinese rhesus macaques developed latency in the absence of clinical signs of acute SVV infection [17].
Reactivation
Herpes zoster results from reactivation of endogenous latent VZV and occurs most frequently in the elderly and immunocompromised individuals. The disease is characterized by a localized vesicular skin rash in the dermatomal distribution of one or more adjacent sensory nerves [5]. Dermatomes innervated by thoracic, cervical or the ophthalmic branch of the trigeminal ganglia (TG) are most frequently involved, the latter route of reactivation leads to herpes zoster ophthalmicus (HZO) [3]. Skin rash is preceded or accompanied by acute neuritis with severe local pain and hyperesthesia (reviewed in [5, 59, 60]). Post-herpetic neuralgia (PHN), a pain that persists beyond 3 months following HZ, is the most common complication (40% of HZ patients >60 years of age will develop PHN) and is highly resistant to treatment [60]. Patients may develop PHN in the absence of skin rash, which is referred to as zoster sine herpete [5, 59, 60]. HZO causes ocular complications, like keratitis and uveitis in 50–72% of patients (reviewed in [61, 62]). VZV reactivation may result in a variety of CNS diseases - including meningitis, meningoencephalitis, meningoradiculitis, cerebellitis and myelopathy [36] - and a broad spectrum of vasculopathies (recently reviewed in [63]). HZ increases the risk of developing stroke, transient ischemic attacks and myocardial infarctions, particularly in patients <40 years old [64, 65]. Moreover, recent studies suggest that VZV is a major cause of giant cell arteritis [63, 66, 67], which is the most common form of vasculitis in individuals >50 years old and is associated with headache and visual loss [68]. Additionally, reactivation of latent VZV from the enteric nervous system may cause gastrointestinal disease [69]. HZ is more severe in immunocompromised individuals, posing the risk of visceral dissemination and development of pneumonia, hepatitis or CNS disease [5, 59, 60]. Importantly, all complications of VZV reactivation may occur in the absence of skin rash [5, 59, 60].
SVV reactivation, either due to stress or immunosuppression, results in recrudescent cutaneous rash restricted to a single dermatome [70]. Severe immunosuppression may result in disseminated SVV reactivation, comparable to the generalized zoster that is occasionally observed in immunocompromised humans [57, 71]. Because low levels of SVV DNA and transcripts, but not infectious virus, may persists in peripheral blood and organs for prolonged periods of time after resolution of varicella in African green monkeys and cynomolgus macaques following intratracheal inoculation, a model of “natural” SVV infection was developed [72–74]. SVV naïve monkeys exposed to an experimentally infected monkey established latency in the absence of detectable viral nucleic acids in non-ganglionic tissues after resolution of varicella [74]. SVV reactivation can be experimentally induced in “naturally” infected African green monkeys, cynomolgus macaques and Indian rhesus macaques using various combinations of immunosuppression and stress [70, 71]. Disadvantages of this model, however, include the inability to control the amount of virus received by the animals and lack of a detectable viremia in most monkeys. Although ocular complications following reactivation have not been reported, intraocular SVV infection of African green monkeys resembled the immunopathology observed in humans with ocular VZV infections [75]. No data is available regarding the incidence of PHN, CNS complications or vasculopathies in non-human primates following SVV reactivation, emphasizing the need to investigate these issues in future studies.
Pathogenesis of Varicelloviruses in primates
The pathogenesis of VZV relies on coordinated infection of multiple cell types including epithelial cells, lymphocytes and ganglionic neurons. The clinical outcome depends on counteracting host innate and virus-specific adaptive immune responses. Whereas humoral immunity is supportive, VZV-specific T-cell immunity is considered pivotal for recovery from varicella and prevention of virus reactivation and HZ (reviewed in [76, 77]). Major breakthroughs on both virus and host factors involved in VZV pathogenesis have been obtained by the introduction of the experimental humanized severe combined immune-deficient (SCID-hu) mouse model, in which transplanted human fetal tissues are infected with VZV via different routes and cell types. Studies on the SCID-hu mouse model have recently been reviewed comprehensively and will not be discussed here in detail [78]. Disadvantages of this mouse model, however, include the use of fetal tissues, lack of an adaptive immune system – both of which are instrumental in VZV pathogenesis – and the inability of the virus to reactivate and cause HZ. These shortcomings are largely overcome in the SVV nonhuman primate model, which allows investigation of the complex and dynamic virus-host interactions involved in primary, latent and recurrent Varicellovirus infection in its natural host.
Primary infection
Early target cells
During primary infection VZV may target epithelial cells of the upper respiratory tract or gain access to the host via the conjunctiva or lower respiratory tract [5]. Initial virus replication may occur in the pharyngeal lymphoid tissue comprising Waldeyer’s tonsillar ring, resulting in transmission of VZV to lymphocytes [5]. This view is supported by the detection of VZV in tonsils obtained from a child undergoing tonsillectomy three days prior to the onset of varicella [79]. Alternatively, VZV may be transferred to tracheobronchial lymph nodes via airway-resident dendritic cells (DC) or alveolar macrophages (AM) [80, 81], which is supported by productive VZV infection of macrophages and DC in vitro and the ability of VZV-infected DC to transmit virus to fibroblasts and T-cells [82, 83].
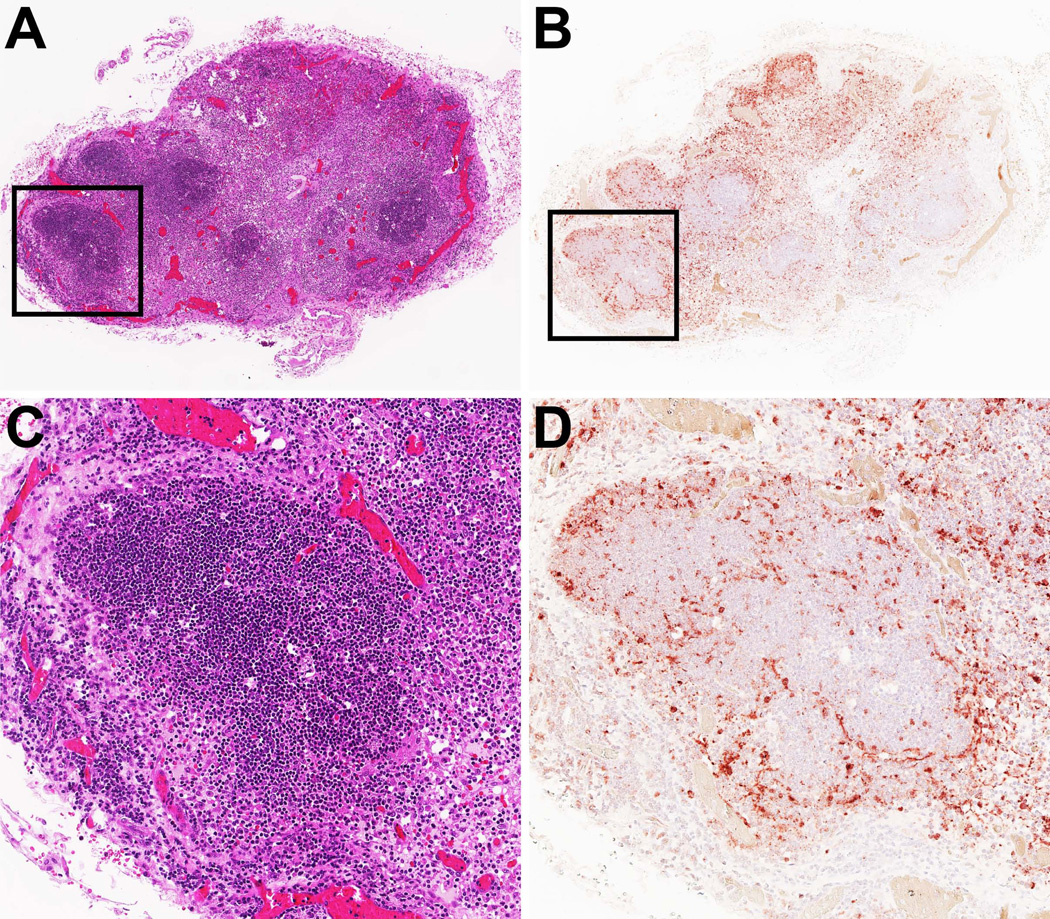
Primary SVV infection is most often studied following intratracheal or intrabronchial infection, thereby bypassing the putative “porte d'entrée” of SVV in the upper respiratory tract or tonsils during natural infection [13–18]. Nevertheless, intratracheal SVV infection results in virus replication in the respiratory tract and predominant infection of epithelial cells, AM and airway DC, which supports transport of SVV to lymph nodes via infected DC or AM [84]. Pronounced histopathology and high viral loads detected in lymph nodes and tonsils during primary SVV infection concur with virus replication and transfer to lymphocytes in lymphoid organs (Figure 2) [84, 85]. Future studies using alternative inoculation routes (e.g. nose or throat) are necessary to definitively identify the early target cells of SVV.
Figure 2. Detection of SVV in tonsils of non-human primates during primary infection.
Serial sections of a tonsil from an African green monkey obtained at 9 days after SVV infection were stained with hematoxylin and eosin (A and C) or examined immunohistochemically using rabbit anti-SVV nucleocapsid antibodies (B and D). (A and B): magnification 25×. (C and D): 100× magnification of the area indicated by the black squares in panels A and B.
Viremia
The detection of infectious virus and VZV DNA in peripheral blood mononuclear cells (PBMC) just prior to and immediately after the onset of cutaneous lesions implicate cell-associated viremic spread of VZV to the skin and other susceptible organs [86–89]. VZV was detected in various PBMC subsets during varicella, but the low frequencies of VZV-infected lymphocytes in blood (0.001–0.01%) precluded detailed analysis of their phenotype [88, 90]. Tonsil-derived activated memory T-cells could be productively infected with VZV in vitro and transferred virus to human melanoma (MeWo) cells [91, 92]. Initially, VZV was reported to preferentially infect human T-cells expressing the skin homing markers C-C type chemokine receptor type 4 (CCR4) and cutaneous lymphocyte antigen (CLA) [91]. More recently, however, it was shown that VZV does not preferentially infect specific T-cell subsets, but instead VZV infection results in extensive remodeling of infected T-cells thereby inducing an activated migratory skin-tropic (CCR4+ CLA+) phenotype [93]. In addition to T-cells, low-abundant VZV-infected B-cells and monocytes may also contribute to hematogenic spread of VZV [88, 90]. VZV-infected lymphocytes were rapidly cleared from the circulation [86–89], presumably due to a combination of virus-induced apoptosis [94] and the evoking of virus-specific adaptive immune responses [95].
SVV infection results in the detection of infectious virus and viral DNA in PBMC already from 2 days post infection (dpi), peaking at 7–10 dpi and declining rapidly after the onset of skin rash [13–18, 84]. Monkeys infected with a recombinant SVV strain expressing enhanced green fluorescent protein [96], which facilitates detection of low frequencies of infected cells ex-vivo and in-situ, revealed that the majority of SVV-infected cells in blood are memory T-cells [84]. No preference was observed for SVV infection of memory T-cells expressing CCR4 nor activated antigen-experienced T-cells (CD137+ T-cells [97, 98]), in vitro and in vivo [84], consistent with a scenario of infection-induced alteration of T-cell phenotype. Additionally, we detected low numbers of SVV-infected DC, B-cells, NK cells and monocytes in blood during viremia [84], suggesting the role of additional lymphocyte subsets in hematogenic spread of SVV. Again, the swift clearance of SVV-infected PBMC from blood may result from induced virus-specific adaptive immune responses [14–17] and, by analogy to VZV [94], SVV-induced apoptosis.
Skin
VZV reaches the skin via hematogenous spread, thereby resulting in a generalized cutaneous rash. This is evidenced by the detection of viral antigens in dermal endothelial and perivascular cells in the earliest stages of skin infection [99] and the observed correlation between the magnitude of viremia with the severity of rash during primary VZV [100]. Furthermore, direct intravenous injection of VZV-infected T-cells into SCID-hu mice resulted in virus delivery to and infection of human fetal skin xenografts [101]. VZV replication in skin may be linked with keratinocyte differentiation, as viral gene and protein expression increased from basal to superficial keratinocyte layers [102]. This can be in part explained by the absence of MPRci in the superficial but not basal epidermis, resulting in diversion and degradation of viral particles in late endosomes of basal keratinocytes [38]. In addition, VZV needs to overcome local epidermal innate immune responses, including type I interferons, to produce the characteristic vesicular skin rash [101, 103, 104].
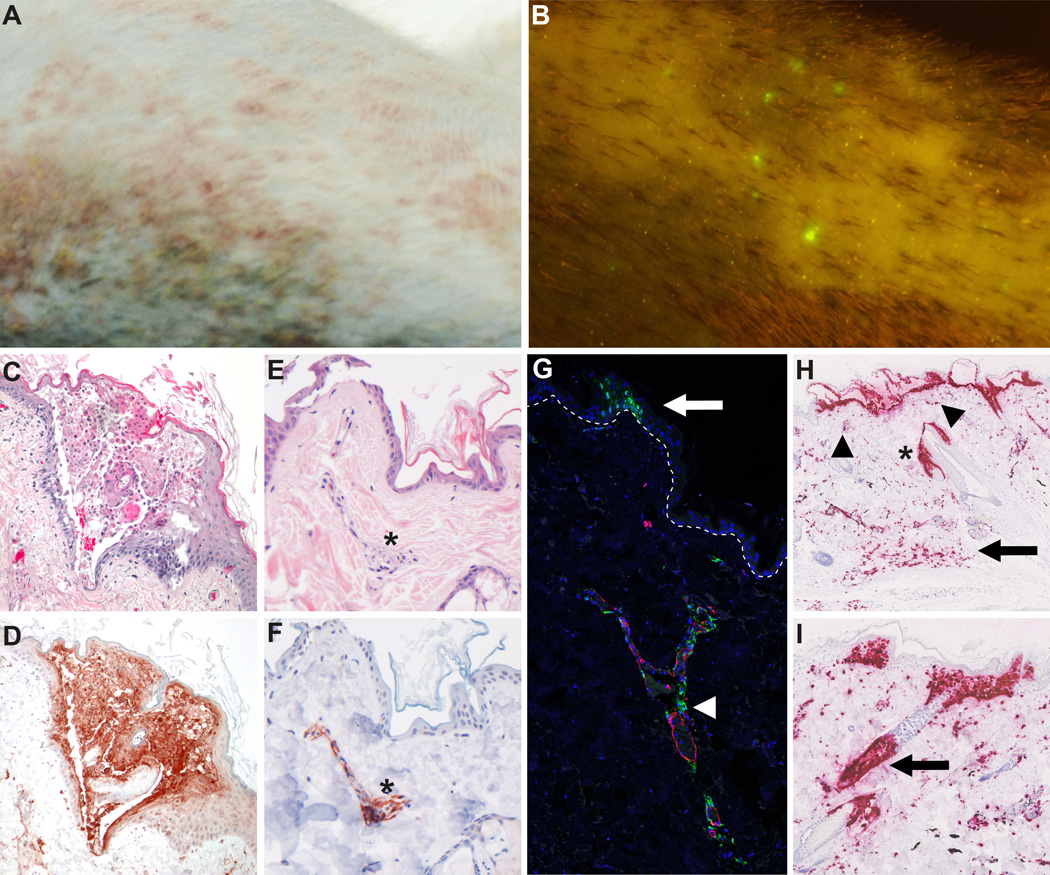
Likewise VZV, SVV reaches the skin via viremic spread resulting in widespread skin rash (Figure 3A–D). Viremic SVV DNA load correlates with the severity of rash [16, 84] and dermal perivascular cells are the first cells in the skin infected with SVV (Figure 3E-3G) [84]. The skin vasculature is composed of an upper horizontal superficial plexus just beneath the epidermal surface and a deep vascular plexus that supplies the hair bulbs and sweat glands [105]. Consequently, virus-infected memory T-cells may transfer SVV or VZV to endothelial cells and/or perivascular macrophages, DC or dendrocytes that in turn transfer virus to adjacent epidermal or hair follicle keratinocytes via cell-to-cell spread. Indeed, agglomerates of SVV-infected cells were observed in the deep dermis and proximal to the epidermis and hair follicles in advanced varicella lesions (Figure 3H). Interestingly, epidermal SVV infection was typically associated with concurrent infection of the underlying hair follicle (Figure 3I). Collectively, the data suggest that both VZV and SVV are transported to skin via a cell-associated viremia, most likely involving memory T-cells that transfer virus to perivascular cells or directly transmit virus to keratinocytes [84, 101].
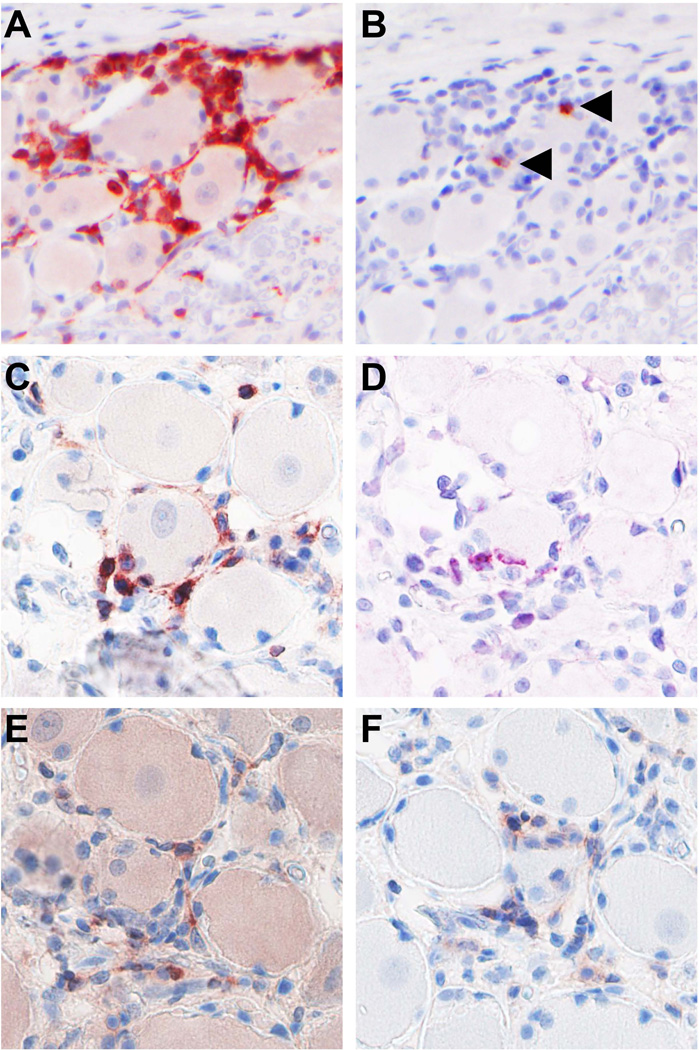
Figure 3. Detection of SVV in skin lesions of non-human primates during primary infection.
(A) Macroscopic image of vesicular skin rash in an SVV-infected African green monkey (AGM) at 8 days post-infection (dpi). (B) Infection of AGM with recombinant SVV expressing enhanced green fluorescent protein (SVV-EGFP) allows macroscopic detection of virus replication in the living animal [84]. Image shows the macroscopic detection of EGFP fluorescence on skin of an AGM infected with SVV-EGFP at 8 dpi. (C and D) Microscopic images of typical vesicular varicella skin rash. Consecutive sections of varicella blisters obtained from a SVV-infected AGM at 8 dpi were stained with hematoxylin and eosin (H&E; C) or analyzed by immunohistochemistry using rabbit anti-SVV nucleocapsid antibodies (D). Magnification: 100×. (E and F) Consecutive sections of early skin lesions obtained from an AGM at 9 dpi with SVV-EGFP were stained with H&E (E) or analyzed by immunohistochemistry using rabbit anti-SVV nucleocapsid antibodies (F). Early lesions were selected based on the detection of EGFP in otherwise macroscopically normal appearing skin. Note the co-localization of SVV antigens and dermal blood vessels (asterisks). Magnification: 200 ×. (G) Confocal image of a skin section obtained from an SVV-infected AGM at 9 dpi and stained for SVV (green), smooth muscle actin (red) and DAPI (blue). Image shows extensive co-localization of SVV-infected cells and blood vessel (arrowhead) and early stages of epidermal SVV infection (arrow). Dashed line indicates the dermal-epidermal border. Magnification: 50×. (H and I) Detection of SVV anti-sense ORF61 RNA (red) by in situ hybridization (Advanced Cell Diagnostics, Hayward, CA) in skin sections obtained from a SVV-infected AMG at 9 dpi. Magnification: 100×. (H) Advanced skin lesions contain large numbers of SVV-infected cells in the deep dermis (arrow) and superficial dermis (arrowheads) in addition to the epidermal blisters. Asterisk indicated SVV-infected sebaceous gland and adjacent hair follicle. (I) Image shows a starting epidermal SVV infection with concomitant infection of the underlying hair follicle (arrow).
Ganglia
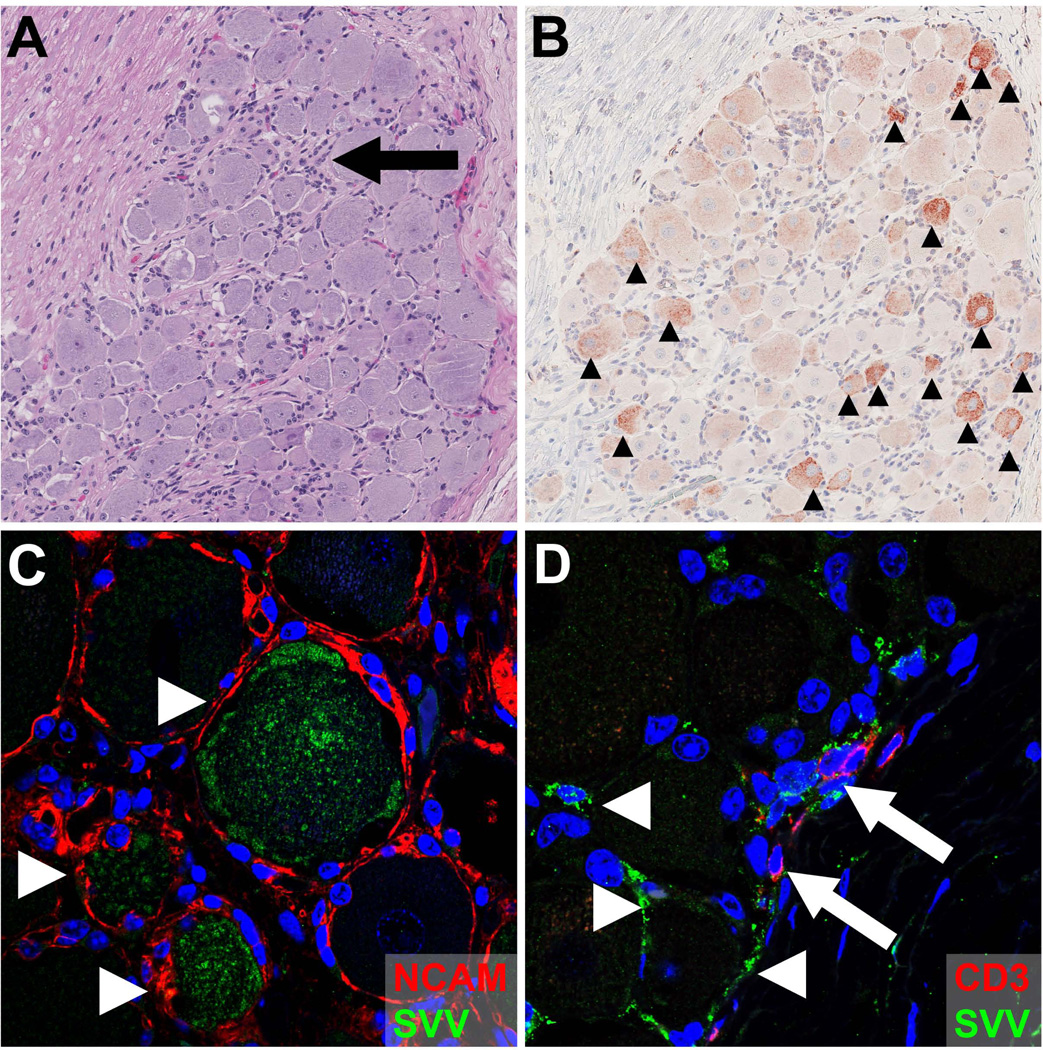
Two different routes have been proposed by which VZV and SVV infect ganglionic neurons: (1) retrograde axonal transport from infected skin and (2) hematogenous spread by infected lymphocytes. Transaxonal spread of VZV to dorsal root ganglia (DRG) is supported by the observation that HZ predominantly occurs at the anatomic site of varicella vaccine inoculation and sites most severely affected by varicella [3, 106]. Also, VZV can infect and migrate within axons to neuronal cell bodies in vitro [107, 108]. Retrograde axonal transport of SVV to sensory ganglia is supported by the detection of SVV proteins in neurons, but not neuron-surrounding satellite glial cells (SGC) during primary SVV infection at ≥ 9 days post-infection (Figure 4) [84]. Given that sensory nerve endings in skin are located in close proximity to the cutaneous vasculature at the dermal-epidermal junction and hair follicles [109], both SVV and VZV may concurrently infect keratinocytes and neurons via cell-to-cell spread.
Figure 4. Detection of SVV in sensory ganglia of non-human primates during primary infection.
Consecutive sections of thoracic dorsal root ganglia obtained from an African green monkey (AGM) at 9 days post-infection (dpi) with SVV were stained with hematoxylin and eosin (A) or examined immunohistochemically using rabbit anti-SVV nucleocapsid antibodies (B). (A) Acute SVV infection is associated with diffuse, and occasionally focal lymphocyte infiltrates (arrow). (B) Detection of SVV antigens is mostly restricted to neurons (arrowheads). Magnification: 100×. (C) Confocal image of sacral dorsal root ganglia (DRG) obtained from an SVV-infected AGM at 9 dpi were stained for SVV (green), neural cell adhesion molecule (NCAM) (red) and DAPI (blue). Image shows SVV-infected NCAM-positive neurons (arrowheads). Magnification: 400×. (D) Confocal image of thoracic DRG obtained from an AGM at 9 dpi were stained for SVV (green), CD3 (red) and DAPI (blue). Image shows occasional SVV-infected non-neuronal cells (arrowheads), including SVV-infected T-cells (arrows). Magnification: 400×.
Alternatively or additionally, virus-infected lymphocytes – most likely memory T-cells – may transport the virus to ganglia (Figure 4D). Hematogenous spread of VZV is supported by the establishment of latency in bilateral DRG and enteric ganglia of a child that received a localized subcutaneous injection of the VZV vaccine virus [110]. Furthermore, intravenous injection of VZV-infected PBMC caused infection of enteric ganglia in guinea pigs and VZV-infected T-cells spread the virus to human fetal ganglia in the SCID-hu mouse model [69, 111, 112]. In support of hematogenous spread of SVV, ganglionic DNA burden was dependent on the viremic SVV DNA load [16, 17], but not associated with the severity of varicella in the innervated dermatome during primary infection [84]. Also, SVV reached sensory ganglia prior to the onset of skin rash [17, 113], indicating that extensive virus replication in skin was not a prerequisite for neuronal infection. Thus, definitive identification of the route(s) by which VZV and SVV enter (sensory) ganglia needs to be addressed in future research (Figure 5).
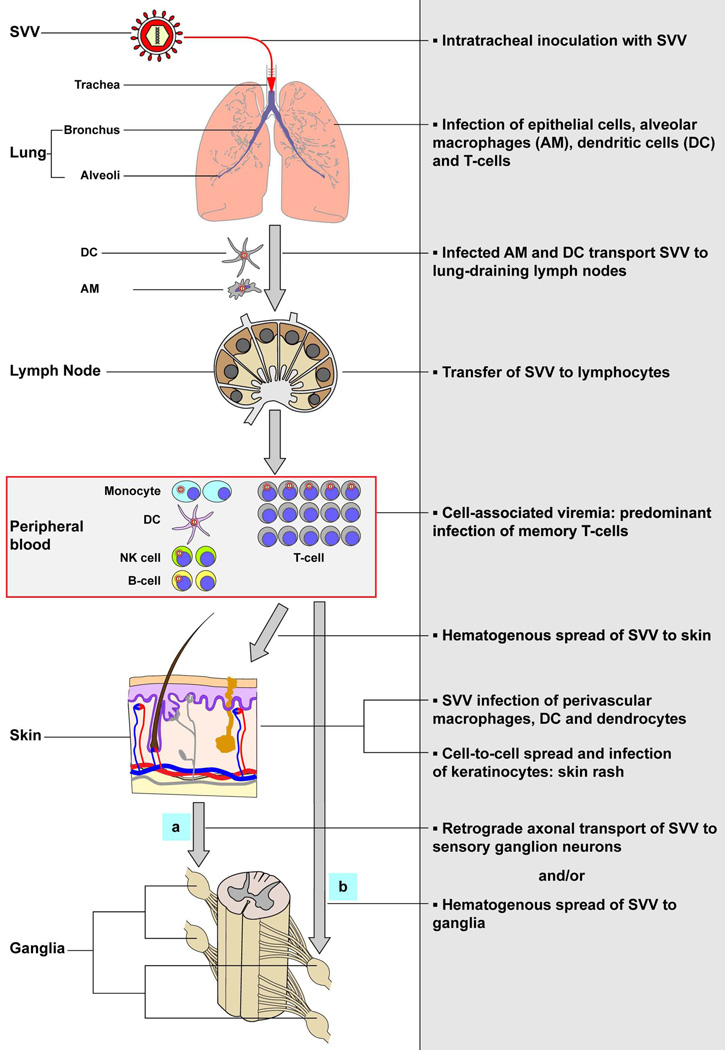
Figure 5. Model of the pathogenesis of primary SVV infection.
Upon intratracheal inoculation of non-human primates, SVV replicates in the lower respiratory tract and infects lung epithelial cells, alveolar macrophages (AM), dendritic cells (DC) and T-cells. SVV-infected AM and DC may transport the virus to draining lymph nodes and subsequently transfer SVV to local lymphocytes resulting in a cell-associated viremia. Memory T-cells are the predominant SVV-infected lymphocyte subset during viremia and may play a central role in dissemination of SVV to its target organs. SVV reaches the skin by the hematogenous route, presumable via virus-infected memory T-cells, which results in the infection of perivascular macrophages, DC and dendrocytes. Subsequently, SVV may infect epidermal and hair follicle keratinocytes via cell-to-cell spread and cause vesicular skin lesions. SVV may enter ganglia by (a) retrograde axonal transport and/or (b) by viremic spread via virus-infected lymphocytes. Figure adapted from reference [84].
Latency
VZV establishes latency in neurons of sensory ganglia (e.g. DRG, TG, geniculate and vestibular ganglia) and autonomic ganglia (e.g. nodose, enteric and thoracic sympathetic ganglia) [69, 114–117]. In the absence of in vitro models that mimic VZV latency [118–122], most studies are restricted to analyses of latently infected human DRG and TG obtained at autopsy. Latent VZV DNA is maintained in a circular configuration in 0.8%–6.9% of TG neurons, with approximately 5–7 genome copies/neuron [123–126]. For over 15 years, the central dogma of VZV latency was neuronal expression of a variable but restricted set of viral transcripts and corresponding proteins encoded by ORFs 4, 21, 29, 62, 63 and 66 [127]. However, Zerboni et al. and our group have recently demonstrated that ascites-derived mouse monoclonal and rabbit polyclonal anti-VZV antibodies non-specifically reacted with blood group A1-associated antigens expressed within neuronal cytoplasmatic Golgi-related vacuoles [128, 129]. This resulted in alleged neuronal VZV protein staining. VZV genome-wide transcriptional profiling of sensory ganglia detected 10 viral transcripts of all kinetic classes, expressed in variable patterns, which questions the true latent state of the virus [130]. A major confounding factor in most studies is the extensive post-mortem interval (PMI), frequently >24 hr, at which human ganglia were obtained [131–136]. Indeed, we have recently shown that the number and quantity of VZV transcripts in human TG increases when PMI progresses [137]. At <9 hr post-mortem, VZV transcription was highly restricted and possibly limited to ORF63, an immediate early gene that is abundantly expressed during lytic infection [137]. The mechanisms by which VZV latency is established and maintained are largely unknown, but most likely include epigenetic silencing of the viral genome [138] and local immune control. VZV establishes latency in the SCID-hu mouse model [112], suggesting that local innate immune responses are sufficient to control virus replication in ganglia and force VZV into latency. Neurons or the interacting SGC may produce antiviral factors like type I interferon, sequester viral proteins in promyelocytic leukemia protein nuclear bodies or degrade viral proteins/particles in autophagosomes [103, 139–141].
Latent SVV infections are established in neurons of sensory ganglia along the entire neuraxis [142–144]. Viremic spread of SVV suggests that the virus, like VZV, may also reach autonomic ganglia such as enteric ganglia. SVV latency is associated with the predominant expression of anti-sense ORF61 transcripts [16, 145], although additional transcripts may occasionally be expressed at low level [146, 147]. Deletion of both sense and anti-sense ORF61 transcripts did not impair establishment of SVV latency [148], although its effect on reactivation was not tested. The (epigenetic) state of the latent SVV genome is largely unknown, but by analogy to VZV and HSV, epigenetic silencing of SVV DNA is anticipated to contribute to the establishment of latency. Whereas depletion of B-cells and CD8 T-cells does not affect SVV latency, reduced CD4 T-cell immunity impairs the establishment of SVV latency [147].
Overall, the data suggest that transcription is highly restricted during latency of both VZV and SVV. Notably, the absence of a specific transcript that is predominantly expressed during latency distinguishes VZV from HSV, which expresses the non-coding latency-associated transcripts (LAT) and microRNAs [149, 150], and SVV that expresses the anti-sense ORF61 RNA [16, 145]. These observations warrant future studies to detect specific VZV transcripts in latently infected human ganglia with short post-mortem intervals using methodologies like next generation sequencing [137, 151]. Compared to HSV-1, VZV latency is not associated with retention of virus-specific T-cells in human ganglia [152–156]. This is, however, contradictive to the protective role of VZV-specific T-cells preventing HZ [157–159]. Future research on the SVV non-human primate model may provide more insight on the role of virus-specific T-cells, either systemic or local within ganglia, in preventing latent SVV reactivation.
Reactivation
Although the stimuli that drive VZV reactivation are unknown, the increased risk of developing HZ with advancing age is most likely attributable to waning of VZV-specific T-cell immunity [157–159]. Whereas most individuals develop only one HZ episode during life, the detection of VZV DNA in PBMC and saliva of healthy immune-competent VZV seropositive individuals implies that periodic asymptomatic VZV reactivation does occur, albeit at low frequency [160–162]. Unlike other Alphaherpesviruses, VZV reactivation is most likely associated with an initial phase of replication and spread within ganglia before anterograde transaxonal spread to the innervating dermal-epidermal junctions leading to HZ skin lesions. This is supported by the detection of mature VZV virions, nuclear inclusion bodies, and VZV proteins and nucleic acids in neurons, their surrounding SGC and fibroblasts in ganglia of HZ patients [163–165]. Pathological changes include profound hemorrhagic necrosis and inflammation, and degeneration of motor and sensory nerve roots, distal peripheral nerves and spinal cord [163, 164, 166]. Immune infiltrates in HZ ganglia were mainly composed of T-cells, which changed in time from mainly granzyme B (GrB)-expressing CD4 T-cells to predominantly non-cytolytic GrB-negative CD8 T-cells [167, 168]. VZV infection of human fetal ganglia induced local production and secretion of the T-cell attractant CXCL10 in vitro [169].
SVV reactivation is induced by stress or immunosuppression, resulting in anterograde axonal transport of virus to skin and HZ. Analogous to VZV, SVV reactivation induced a transient infiltration of predominantly non-cytolytic GrB-negative CD8 T-cells in DRG of cynomolgus macaques (Figure 6) [170]. Moreover, the number of ganglion-infiltrating CD8 T-cells correlated with local expression of CXCL10 transcripts (Figure 6C–F), but not SVV transcripts or antigens. The data suggest that the influx and retention of CD8 T-cells in ganglia at 4 days to 4 months post-HZ is mainly chemokine- rather than antigen-driven [170]. At present it remains unclear which cells produce CXCL10 and whether CXCL10 is the primary cause of T-cell infiltration or secondarily due to interferon-γ secreted by activated infiltrating lympocytes. Because ganglia from HZ patients are extremely rarely obtained, the SVV model provides the ideal platform to study the specificity, phenotype and function of ganglion-infiltrating T-cells at various times after reactivation [171].
Figure 6. T-cell infiltrates in ganglia of non-human primates following SVV reactivation.
Ganglia were obtained at 2 months after tacrolimus-induced immunosuppression of a latently SVV-infected cynomolgus macaque, which showed signs of subclinical reactivation at time of necropsy [70]. Consecutive sections of a sacral dorsal root ganglion (DRG) were immunohistochemically stained for CD3 (A) and granzyme B (B), demonstrating that SVV reactivation induces extensive infiltration of T-cells that generally do not express the cytolytic T-cell marker granzyme B. Arrowheads indicate rare granzyme B-positive cells. Consecutive sections of a cervical DRG were immunohistochemically stained for CD3 (A), CD8 (E) and CD4 (F), or analyzed by in situ hybridization for CXCL10 transcripts (Advanced Cell Diagnostics, Hayward, CA) (D). Magnification: 400×.
Future perspectives
The burden of HZ and PHN will increase in countries with an aging population. The live-attenuated VZV Oka vaccine is currently licensed in over 60 countries worldwide to prevent VZV disease in children and the elderly ([172, 173] and reviewed in [174]). The varicella vaccine, administered using a 2-dose regimen, prevents varicella in up to 98% of vaccinated children [172]. The 1-dose zoster vaccine regimen, containing a higher dose of infectious VZV Oka virus, is advocated for individuals over 60 years of age and prevents the development of HZ in 51% and PHN in 67% of vaccinees [175]. The limited protective effect and technical difficulties to produce the cell-free zoster vaccine, along with the risk of disease due to reactivation of the vaccine virus, argues for the development of a safer and more efficacious 2nd generation VZV vaccine. Rational design of novel VZV vaccines requires detailed insights into the pathogenesis of the virus. Since the latent phase is largely imperceptible to local adaptive immune control, novel intervention strategies should be aimed at limiting both the latent VZV load and prevention of VZV reactivation by inducing and boosting VZV immunity, respectively. Combined studies on the virus and host factors involved in VZV pathogenesis in humans and the SVV non-human primate model provide an ideal framework to identify and test the therapeutic efficacy of a novel polyvalent VZV vaccine, based on immune-dominant viral proteins targeted by protective T-cells in VZV-infected individuals. Furthermore, this strategy may provide novel intervention strategies that inhibit the detrimental effects of VZV infection (e.g. PHN) and those that induce local ganglionic innate immune responses that control VZV latency.
Acknowledgements
The authors would like to thank Albert D.M.E. Osterhaus for his continued support and insightful discussions, Sarah Getu for technical assistance, and Ravi Mahalingam for providing the tissue sections used in figure 6. This work was supported in part by Public Health Service grant AG032958 from the National Institutes of Health.
Footnotes
Conflicts of interest statement:
The authors declare to have no conflicts of interest.
References
- 1.Von Bokay J. Uber den atiologischen Zusammenhang der varizellen mit gewissen Fallen von Herpes Zoster. Wien Klini Wochensch. 1909;22:1323–1326. [Google Scholar]
- 2.Garland J. Varicella following exposure to herpes zoster. N Engl J Med. 1943:336–337. [Google Scholar]
- 3.Hope-Simpson RE. The Nature of Herpes Zoster: A Long-Term Study and a New Hypothesis. Proc R Soc Med. 1965;58:9–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Weller TH, Witton HM, Bell EJ. The etiologic agents of varicella and herpes zoster; isolation, propagation, and cultural characteristics in vitro. J Exp Med. 1958;108:843–868. doi: 10.1084/jem.108.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvin AM, Gilden D. Varicella-zoster virus. In: Knipe DM, Howley PM, editors. Fields Virology. 6th edn. Philadelphia: Lippincott Williams and Wilkins; 2013. pp. 2015–2057. [Google Scholar]
- 6.Weller TH. Varicella and herpes zoster: a perspective and overview. J Infect Dis. 1992;166(Suppl 1):S1–S6. [PubMed] [Google Scholar]
- 7.Clarkson MJ, Thorpe E, McCarthy K. A virus disease of captive vervet monkeys (Cercopithecus aethiops) caused by a new herpesvirus. Arch Gesamte Virusforsch. 1967;22:219–234. doi: 10.1007/BF01240517. [DOI] [PubMed] [Google Scholar]
- 8.Allen WP, Felsenfeld AD, Wolf RH, et al. Recent studies on the isolation and characterization of Delta herpesvirus. Lab Anim Sci. 1974;24:222–228. [PubMed] [Google Scholar]
- 9.Blakely GA, Lourie B, Morton WG, et al. A varicella-like disease in macaque monkeys. J Infect Dis. 1973;127:617–625. doi: 10.1093/infdis/127.6.617. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy K, Thorpe E, Laursen AC, et al. Exanthematous disease in patas monkeys caused by a herpes virus. Lancet. 1968;292:856–857. doi: 10.1016/s0140-6736(68)91006-4. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt NJ, Arvin AM, Martin DP, et al. Serological investigation of an outbreak of simian varicella in Erythrocebus patas monkeys. J Clin Microbiol. 1983;18:901–904. doi: 10.1128/jcm.18.4.901-904.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf RH, Smetana HF, Allen WP, et al. Pathology and clinical history of Delta herpesvirus infection in patas monkeys. Lab Anim Sci. 1974;24:218–221. [PubMed] [Google Scholar]
- 13.Dueland AN, Martin JR, Devlin ME, et al. Acute simian varicella infection. Clinical, laboratory, pathologic, and virological features. Lab Invest. 1992;66:762–773. [PubMed] [Google Scholar]
- 14.Gray WL, Gusick NJ, Fletcher TM, et al. Simian varicella virus antibody response in experimental infection of African green monkeys. J Med Primatol. 1995;24:246–251. doi: 10.1111/j.1600-0684.1995.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 15.Gray WL, Williams RJ, Chang R, et al. Experimental simian varicella virus infection of St. Kitts vervet monkeys. J Med Primatol. 1998;27:177–183. doi: 10.1111/j.1600-0684.1998.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 16.Messaoudi I, Barron A, Wellish M, et al. Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans. PLoS Pathog. 2009;5:e1000657. doi: 10.1371/journal.ppat.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouwendijk WJ, Mahalingam R, Traina-Dorge V, et al. Simian varicella virus infection of Chinese rhesus macaques produces ganglionic infection in the absence of rash. J Neurovirol. 2012;18:91–99. doi: 10.1007/s13365-012-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wenner HA, Abel D, Barrick S, et al. Clinical and pathogenetic studies of Medical Lake macaque virus infections in cynomolgus monkeys (simian varicella) J Infec Dis. 1997;135:611–622. doi: 10.1093/infdis/135.4.611. [DOI] [PubMed] [Google Scholar]
- 19.McGeoch DJ, Rixon FJ, Davison AJ. Topics in herpesvirus genomics and evolution. Virus Res. 2006;117:90–104. doi: 10.1016/j.virusres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Grose C. Pangaea and the out-of-Africa model for varicella-zoster virus evolution and phylogeography. J Virol. 2012;86:9558–9565. doi: 10.1128/JVI.00357-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens NJ, Seiffert ER, O’Connor PM, et al. Palaeontological evidence for an Oligocene divergence between Old World monkeys and apes. Nature. 2013;497:611–614. doi: 10.1038/nature12161. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher TM, Gray WL. Simian varicella virus: characterization of virion and infected cell polypeptides and the antigenic cross-reactivity with varicella-zoster virus. J Gen Virol. 1992;73:1209–1215. doi: 10.1099/0022-1317-73-5-1209. [DOI] [PubMed] [Google Scholar]
- 23.CDC/Dr. Erskine Palmer; B.G. Partin. Available from: http://phil.cdc.gov/phil/details.asp?pid=1878.
- 24.Davison AJ, Scott JE. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 25.Gray WL, Starnes B, White MW, et al. The DNA sequence of the simian varicella virus genome. Virology. 2001;284:123–130. doi: 10.1006/viro.2001.0912. [DOI] [PubMed] [Google Scholar]
- 26.Mahalingam R, Gray WL. The simian varicella virus genome contains an invertible 665 base pair terminal element that is absent in the varicella zoster virus genome. Virology. 2007;366:387–393. doi: 10.1016/j.virol.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Che X, Oliver SL, Reichelt M, et al. ORF11 protein interacts with the ORF9 essential tegument protein in varicella-zoster virus infection. J Virol. 2013;87:5106–5117. doi: 10.1128/JVI.00102-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinchington PR, Hougland JK, Arvin AM, et al. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J Virol. 1992;66:359–366. doi: 10.1128/jvi.66.1.359-366.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinchington PR, Bookey D, Turse SE. The transcriptional regulatory proteins encoded by varicella-zoster virus open reading frames (ORFs) 4 and 63, but not ORF 61, are associated with purified virus particles. J Virol. 1996;69:4274–4282. doi: 10.1128/jvi.69.7.4274-4282.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leisenfelder SA, Kinchington PR, Moffat JF. Cyclin-dependent kinase 1/cyclin B1 phosphorylates varicella-zoster virus IE62 and is incorporated into virions. J Virol. 2008;82:12116–12125. doi: 10.1128/JVI.00153-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson D, Colman KL, Davison AJ. Characterization of the putative protein kinases specified by varicella-zoster virus genes 47 and 66. J Gen Virol. 1994;75:317–326. doi: 10.1099/0022-1317-75-2-317. [DOI] [PubMed] [Google Scholar]
- 32.Ambagala AP, Cohen JI. Varicella-zoster virus IE63, a major viral latency protein, is required to inhibit the alpha interferon-induced antiviral response. J Virol. 2007;81:7844–7851. doi: 10.1128/JVI.00325-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisfeld AJ, Yee MB, Erazo A, et al. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J Virol. 2007;81:9034–9049. doi: 10.1128/JVI.00711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sen N, Sommer M, Che X, et al. Varicella-zoster virus immediate-early protein 62 blocks interferon regulatory factor 3 (IRF3) phosphorylation at key serine residues: a novel mechanism of IRF3 inhibition among herpesviruses. J Virol. 2010;84:9240–9253. doi: 10.1128/JVI.01147-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandevenne P, Lebrun M, El Mjiyad N, et al. The varicella-zoster virus ORF47 kinase interferes with host innate immune response by inhibiting the activation of IRF3. PLoS One. 2011;6:e16870. doi: 10.1371/journal.pone.0016870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storlie J, Maresova L, Jackson W, et al. Comparative analyses of the 9 glycoprotein genes found in wild-type and vaccine strains of varicella-zoster virus. J Infect Dis. 2008;197(Suppl 2):S49–S53. doi: 10.1086/522127. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Z, Gershon MD, Ambron R, et al. Infection of cells by varicella zoster virus: inhibition of viral entry by mannose 6-phosphate and heparin. Proc Natl Acad Sci USA. 1995;92:3546–3550. doi: 10.1073/pnas.92.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen JJ, Zhu Z, Gershon AA, et al. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell. 2004;119:915–926. doi: 10.1016/j.cell.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Suenaga T, Satoh T, Somboonthum P, et al. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc Natl Acad Sci USA. 2010;107:866–871. doi: 10.1073/pnas.0913351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, Ali MA, Cohen JI. Insulin degrading enzyme is a cellular receptor mediating varicella-zoster virus infection and cell-to-cell spread. Cell. 2006;127:305–316. doi: 10.1016/j.cell.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpenter JE, Jackson W, de Souza GA, et al. Insulin-degrading enzyme binds to the nonglycosylated precursor of varicella-zoster virus gE protein found in the endoplasmatic reticulum. J Virol. 2010;84:847–855. doi: 10.1128/JVI.01801-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheatham WJ, Dolan TF, Dower JC, et al. Varicella: report of two fatal cases with necropsy, virus isolation, and serologic studies. Am J Pathol. 1956;32:1015–1035. [PMC free article] [PubMed] [Google Scholar]
- 43.Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th edn. Philadelphia: Lippincott Williams and Wilkins; 2013. pp. 1823–1897. [Google Scholar]
- 44.Lenac Rovis T, Bailer SM, Pothineni VR, et al. Comprehensive analysis of varicella-zoster virus proteins using a new monoclonal antibody collection. J Virol. 2013;87:6943–6954. doi: 10.1128/JVI.00407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reichelt M, Brady J, Arvin AM. The replication cycle of varicella-zoster virus: analysis of the kinetics of viral protein expression, genome synthesis, and virion assembly at the single-cell level. J Virol. 2009;83:3904–3918. doi: 10.1128/JVI.02137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carpenter JE, Hutchinson JA, Jackson W, et al. Egress of light particles among filopodia on the surface of varicella-zoster virus-infected cells. J Virol. 2008;82:2821–2835. doi: 10.1128/JVI.01821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gershon AA, Sherman DL, Zhu Z, et al. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hambleton S, Gershon MD, Gershon AA. The role of the trans-Golgi network in varicella zoster virus biology. Cell Mol Life Sci. 2004;61:3047–3056. doi: 10.1007/s00018-004-4269-7. [DOI] [PubMed] [Google Scholar]
- 49.Harson R, Grose C. Egress of varicella-zoster virus from the melanoma cell: a tropism for the melanocyte. J Virol. 1995;69:4994–5010. doi: 10.1128/jvi.69.8.4994-5010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grose C. Variation on a theme by Fenner: the pathogenesis of chickenpox. Pediatrics. 1981;68:735–737. [PubMed] [Google Scholar]
- 51.LeClair JM, Zaia JA, Levin MJ, et al. Airborne transmission of chickenpox in a hospital. N Engl J Med. 1980;302:450–453. doi: 10.1056/NEJM198002213020807. [DOI] [PubMed] [Google Scholar]
- 52.Heininger U, Seward JF. Varicella. Lancet. 2006;368:1365–1376. doi: 10.1016/S0140-6736(06)69561-5. [DOI] [PubMed] [Google Scholar]
- 53.Johnson R, Milbourn PE. Central nervous system manifestations of chickenpox. Can Med Assoc J. 1970;102:831–834. [PMC free article] [PubMed] [Google Scholar]
- 54.Koskiniemi M, Piiparinen H, Rantalaiho T, et al. Acute central nervous system complications in varicella zoster virus infections. J Clin Virol. 2002;25:293–301. doi: 10.1016/s1386-6532(02)00020-3. [DOI] [PubMed] [Google Scholar]
- 55.Barak M, Weinberger R. Outcome of IgM- and IgG-seropositive cases of varicella zoster in pregnancy. J Reprod Med. 2004;49:38–40. [PubMed] [Google Scholar]
- 56.Christiansen D, Barret ED. Comparison of varicella history with presence of varicella antibody in refugees. Vaccine. 2004;22:4233–4237. doi: 10.1016/j.vaccine.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 57.Kolappaswamy K, Mahalingam R, Traina-Dorge V, et al. Disseminated simian varicella virus infection in an irradiated rhesus macaque (Macaca mulatta) J Virol. 2007;81:411–415. doi: 10.1128/JVI.01825-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ward TM, Traina-Dorge V, Davis KA, et al. Recombinant simian varicella expressing respiratory syncytial virus antigens are immunogenic. J Gen Virol. 2008;89:741–750. doi: 10.1099/vir.0.83453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl 1):S1–S26. doi: 10.1086/510206. [DOI] [PubMed] [Google Scholar]
- 60.Gilden D, Nagel MA, Cohrs RJ, et al. The variegate neurological manifestations of varicella zoster virus infection. Curr Neurol Neurosci Rep. 2013;13:374. doi: 10.1007/s11910-013-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liesegang TJ. Varicella-zoster virus eye disease. Cornea. 1999;18:511–531. [PubMed] [Google Scholar]
- 62.Ritterband DC, Friedberg DN. Virus infections of the eye. Rev Med Virol. 1998;8:187–201. doi: 10.1002/(sici)1099-1654(1998100)8:4<187::aid-rmv221>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 63.Nagel MA, Gilden D. Update on varicella zoster virus vasculopathy. Curr Infect Dis Rep. 2014;16:407. doi: 10.1007/s11908-014-0407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Breuer J, Pacou M, Gauthier A, et al. Herpes zoster as a risk factor for stroke and TIA: a retrospective cohort study in the UK. Neurology. 2014;82:206–212. doi: 10.1212/WNL.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sreenivasan N, Basit S, Wohlfahrt J, et al. The short- and long-term risk of stroke after herpes zoster - a nationwide population-based cohort study. PLoS One. 2013;8:e69156. doi: 10.1371/journal.pone.0069156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gilden D. Association of Varicella Zoster Virus with Giant Cell Arteritis. Monoclon Antib Immunodiagn Immunother. 2014;33:168–172. doi: 10.1089/mab.2014.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagel MA, Khmeleva N, Boyer PJ, et al. Varicella zoster virus in the temporal artery of a patient with giant cell arteritis. J Neurol Sci. 2013;335:228–230. doi: 10.1016/j.jns.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ness T, Bley TA, Schmidt WA, et al. The diagnosis and treatment of giant cell arteritis. Dtsch Arztebl Int. 2013;110:376–385. doi: 10.3238/arztebl.2013.0376. quiz 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen JJ, Gershon AA, Li Z, et al. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J Neurovirol. 2011;17:578–589. doi: 10.1007/s13365-011-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mahalingam R, Traina-Dorge V, Wellish M, et al. Latent simian varicella virus reactivates in monkeys treated with tacrolimus with or without exposure to irradiation. J Neurovirol. 2010;16:342–354. doi: 10.3109/13550284.2010.513031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahalingam R, Traina-Dorge V, Wellish M, et al. Simian varicella virus reactivation in cynomolgus monkeys. Virology. 2007;368:50–59. doi: 10.1016/j.virol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 72.White TM, Mahalingam R, Traina-Dorge V, et al. Simian varicella virus DNA is present and transcribed months after experimental infection of adult African green monkeys. J Neurovirol. 2002;8:191–203. doi: 10.1080/13550280290049705. [DOI] [PubMed] [Google Scholar]
- 73.White TM, Mahalingam R, Traina-Dorge V, et al. Persistence of simian varicella virus DNA in CD(+) and CD8(+) blood mononuclear cells for years after intratracheal inoculation of African green monkeys. Virology. 2002;10:192–198. doi: 10.1006/viro.2002.1664. [DOI] [PubMed] [Google Scholar]
- 74.Mahalingam R, Traina-Dorge V, Wellish M, et al. Naturally acquired simian varicella virus infection in African green monkeys. J Virol. 2002;76:8548–8550. doi: 10.1128/JVI.76.17.8548-8550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Metcalf JF, Christianson MD, Brady AG. Ocular inoculation of monkeys with simian varicella virus: clinical and histopathologic observations. Invest Ophthalmol Vis Sci. 1995;36:41–51. [PubMed] [Google Scholar]
- 76.Steain M, Slobedman B, Abendroth A. The host immune response to varicella zoster virus. Future virology. 2012;7:1205–1220. [Google Scholar]
- 77.Ouwendijk WJ, Laing KJ, Verjans GM, et al. T-cell immunity to human alphaherpesviruses. Curr Opin Virol. 2013;3:452–460. doi: 10.1016/j.coviro.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zerboni L, Sen N, Oliver SL, et al. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014;12:197–210. doi: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tomlinson TH. Giant cell formation in the tonsils in the prodromal stage of chickenpox: Report of a case. Am J Pathol. 1939;15:523–526. [PMC free article] [PubMed] [Google Scholar]
- 80.Kirby AC, Coles MC, Kaye PM. Alveolar macrophages transport pathogens to lung draining lymph nodes. J Immunol. 2009;183:1983–1989. doi: 10.4049/jimmunol.0901089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thornton EE, Looney MR, Bose O, et al. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J Exp Med. 2012;209:1183–1199. doi: 10.1084/jem.20112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abendroth A, Morrow G, Cunningham AL, et al. Varicella-zoster virus infection of human dendritic cells and transmission to T cells: implications for virus dissemination in the host. J Virol. 2001;75:6183–6192. doi: 10.1128/JVI.75.13.6183-6192.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arbeit RD, Zaia JA, Valerio MA, et al. Infection of human peripheral blood mononuclear cells by varicella-zoster virus. Intervirology. 1982;18:56–65. doi: 10.1159/000149304. [DOI] [PubMed] [Google Scholar]
- 84.Ouwendijk WJ, Mahalingam R, de Swart RL, et al. T-Cell tropism of simian varicella virus during primary infection. PLoS Pathog. 2013;9:e1003368. doi: 10.1371/journal.ppat.1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dueland AN, Martin JR, Devlin ME, et al. Acute simian varicella infection. Clinical, laboratory, pathologic, and virologic features. Lab Invest. 1992;66:762–773. [PubMed] [Google Scholar]
- 86.Asano Y, Itakura N, Hiroishi Y, et al. Viremia is present in incubation period in 7 nonimmunocompromised children with varicella. J Pediatr. 1985;106:69–71. doi: 10.1016/s0022-3476(85)80468-6. [DOI] [PubMed] [Google Scholar]
- 87.Asano Y, Itakura N, Hiroishi Y, et al. Viral replication and immunologic responses in children naturally infected with varicella-zoster virus and in varicella vaccine recipients. J Infect Dis. 1985;152:863–868. doi: 10.1093/infdis/152.5.863. [DOI] [PubMed] [Google Scholar]
- 88.Koropchak CM, Graham G, Palmer J, et al. Investigation of varicella-zoster virus infection by polymerase chain reaction in the immunocompetent host with acute varicella. J Infect Dis. 1991;163:1016–1022. doi: 10.1093/infdis/163.5.1016. [DOI] [PubMed] [Google Scholar]
- 89.Ozaki T, Ichikawa T, Matsui Y, et al. Viremic phase in nonimmunocompromised children with varicella. J Pediatr. 1984;104:85–87. doi: 10.1016/s0022-3476(84)80596-x. [DOI] [PubMed] [Google Scholar]
- 90.Mainka C, Fuss B, Geiger H, et al. Characterization of viremia at different stages of varicella-zoster virus infection. J Med Virol. 1998;56:91–98. doi: 10.1002/(sici)1096-9071(199809)56:1<91::aid-jmv15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 91.Ku CC, Padilla JA, Grose C, et al. Tropism of varicella-zoster virus for human tonsillar CD4(+) T lymphocytes that express activation, memory, and skin homing markers. J Virol. 2002;76:11425–11433. doi: 10.1128/JVI.76.22.11425-11433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soong W, Schultz JC, Patera AC, et al. Infection of human T lymphocytes with varicella-zoster virus: an analysis with viral mutants and clinical isolates. J Virol. 2000;74:1864–1870. doi: 10.1128/jvi.74.4.1864-1870.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sen N, Mukherjee G, Sen A, et al. Single-cell mass cytometry analysis of human tonsil T cell remodeling by varicella zoster virus. Cell Rep. 2014;24:633–645. doi: 10.1016/j.celrep.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Konig A, Homme C, Hauroder B, et al. The varicella-zoster virus induces apoptosis in vitro in subpopulations of primary human peripheral blood mononuclear cells. Microbes Infect. 2003;5:879–889. doi: 10.1016/s1286-4579(03)00177-1. [DOI] [PubMed] [Google Scholar]
- 95.Arvin AM, Koropchak CM, Williams BR, et al. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J Infect Dis. 1986;154:422–429. doi: 10.1093/infdis/154.3.422. [DOI] [PubMed] [Google Scholar]
- 96.Mahalingam R, Wellish M, White T, et al. Infectious simian varicella virus expressing the green fluorescent protein. J Neurovirol. 1998;4:438–444. doi: 10.3109/13550289809114543. [DOI] [PubMed] [Google Scholar]
- 97.Jing L, Schiffer JT, Chong TM, et al. CD4 T-cell memory responses to viral infections of humans show pronounced immunodominance independent of duration or viral persistence. J Virol. 2013;87:2617–2627. doi: 10.1128/JVI.03047-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolfl M, Kuball J, Ho WY, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tyzzer EE. The Histology of the Skin Lesions in Varicella. J Med Res. 1906;14:361–392. 7. [PMC free article] [PubMed] [Google Scholar]
- 100.Malavige GN, Jones L, Kamaladasa SD, et al. Viral load, clinical disease severity and cellular immune responses in primary varicella zoster virus infection in Sri Lanka. PLoS One. 2008;3:e3789. doi: 10.1371/journal.pone.0003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ku CC, Zerboni L, Ito H, et al. Varicella-zoster virus transfer to skin by T Cells and modulation of viral replication by epidermal cell interferon-alpha. J Exp Med. 2004;200:917–925. doi: 10.1084/jem.20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jones M, Dry IR, Frampton D, et al. RNA-seq analysis of host and viral gene expression highlights interaction between varicella zoster virus and keratinocyte differentiation. PLoS Pathog. 2014;10:e1003896. doi: 10.1371/journal.ppat.1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reichelt M, Wang L, Sommer M, et al. Entrapment of viral capsids in nuclear PML cages is an intrinsic antiviral host defense against varicella-zoster virus. PLoS Pathog. 2011;7:e1001266. doi: 10.1371/journal.ppat.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sen N, Che X, Rajamani J, et al. Signal transducer and activator of transcription 3 (STAT3) and survivin induction by varicella-zoster virus promote replication and skin pathogenesis. Proc Natl Acad Sci USA. 2012;109:600–605. doi: 10.1073/pnas.1114232109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Braverman IM. The cutaneous microcirculation. J Investig Dermatol Symp Proc. 2000;5:3–9. doi: 10.1046/j.1087-0024.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 106.Hardy I, Gershon AA, Steinberg SP, et al. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group. N Engl J Med. 1991;325:1545–1550. doi: 10.1056/NEJM199111283252204. [DOI] [PubMed] [Google Scholar]
- 107.Grigoryan S, Kinchington PR, Yang IH, et al. Retrograde axonal transport of VZV: kinetic studies in hESC-derived neurons. J Neurovirol. 2012;18:462–470. doi: 10.1007/s13365-012-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Markus A, Grigoryan S, Sloutskin A, et al. Varicella-zoster virus (VZV) infection of neurons derived from human embryonic stem cells: direct demonstration of axonal infection, transport of VZV, and productive neuronal infection. J Virol. 2011;85:6220–6233. doi: 10.1128/JVI.02396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Myers MI, Peltier AC, Li J. Evaluating dermal myelinated nerve fibers in skin biopsy. Muscle Nerve. 2013;47:1–11. doi: 10.1002/mus.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gershon AA, Chen J, Davis L, et al. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans Am Clin Climatol Assoc. 2012;123:17–33. discussion 33-15. [PMC free article] [PubMed] [Google Scholar]
- 111.Gan L, Wang M, Chen JJ, et al. Infected peripheral blood mononuclear cells transmit latent varicella zoster virus infection to the guinea pig enteric nervous system. J Neurovirol. 2014 doi: 10.1007/s13365-014-0259-1. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zerboni L, Ku CC, Jones CD, et al. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc Natl Acad Sci U S A. 2005;102:6490–6495. doi: 10.1073/pnas.0501045102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mahalingam R, Wellish M, Soike K, et al. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology. 2001;279:339–342. doi: 10.1006/viro.2000.0700. [DOI] [PubMed] [Google Scholar]
- 114.Furuta Y, Takasu T, Suzuki S, et al. Detection of latent varicella-zoster virus infection in human vestibular and spiral ganglia. J Med Virol. 1997;51:214–216. doi: 10.1002/(sici)1096-9071(199703)51:3<214::aid-jmv12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 115.Gilden DH, Gesser R, Smith J, et al. Presence of VZV and HSV-1 DNA in human nodose and celiac ganglia. Virus Genes. 2001;23:145–147. doi: 10.1023/a:1011883919058. [DOI] [PubMed] [Google Scholar]
- 116.Mahalingam R, Wellish M, Wolf W, et al. Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. N Engl J Med. 1990;323:627–631. doi: 10.1056/NEJM199009063231002. [DOI] [PubMed] [Google Scholar]
- 117.Nagel MA, Rempel A, Huntington J, et al. Frequency and abundance of alpha-Herpesvirus DNA in human yhoracic sympathetic ganglia. J Virol. 2014;88:8189–8192. doi: 10.1128/JVI.01070-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Christensen J, Steain M, Slobedman B, et al. Differentiated neuroblastoma cells provide a highly efficient model for studies of productive varicella-zoster virus infection of neuronal cells. J Virol. 2011;85:8436–8442. doi: 10.1128/JVI.00515-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dukhovny A, Sloutskin A, Markus A, et al. Varicella-zoster virus infects human embryonic stem cell-derived neurons and neurospheres but not pluripotent embryonic stem cells or early progenitors. J Virol. 2012;86:3211–3218. doi: 10.1128/JVI.06810-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goodwin TJ, McCarthy M, Osterrieder N, et al. Three-dimensional normal human neural progenitor tissue-like assemblies: a model of persistent varicella-zoster virus infection. PLoS Pathog. 2013;9:e1003512. doi: 10.1371/journal.ppat.1003512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee KS, Zhou W, Scott-McKean JJ, et al. Human sensory neurons derived from induced pluripotent stem cells support varicella-zoster virus infection. PLoS One. 2012;7:e53010. doi: 10.1371/journal.pone.0053010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu X, Seitz S, Pointon T, et al. Varicella zoster virus infection of highly pure terminally differentiated human neurons. J Neurovirol. 2013;19:75–81. doi: 10.1007/s13365-012-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clarke P, Beer T, Cohrs R, et al. Configuration of latent varicella-zoster virus DNA. J Virol. 1995;69:8151–8154. doi: 10.1128/jvi.69.12.8151-8154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kennedy PG, Grinfeld E, Gow JW. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc Natl Acad Sci U S A. 1998;95:4658–4662. doi: 10.1073/pnas.95.8.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Levin MJ, Cai GY, Manchak MD, et al. Varicella-zoster virus DNA in cells isolated from human trigeminal ganglia. J Virol. 2003;77:6979–6987. doi: 10.1128/JVI.77.12.6979-6987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang K, Lau TY, Morales M, et al. Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal Ganglia at the single-cell level. J Virol. 2005;79:14079–14087. doi: 10.1128/JVI.79.22.14079-14087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cohen JI, Straus SE, Arvin AM. Varicella-zoster virus replication, pathogenesis, and management. In: Knipe DM, Howley PM, editors. Fields Virology. 5th edn. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 2773–2818. [Google Scholar]
- 128.Ouwendijk WJ, Flowerdew SE, et al. Immunohistochemical detection of intra-neuronal VZV proteins in snap-frozen human ganglia is confounded by antibodies directed against blood group A1-associated antigens. J Neurovirol. 2012;18:172–180. doi: 10.1007/s13365-012-0095-0. [DOI] [PubMed] [Google Scholar]
- 129.Zerboni L, Sobel RA, Lai M, et al. Apparent expression of varicella-zoster virus proteins in latency resulting from reactivity of murine and rabbit antibodies with human blood group a determinants in sensory neurons. J Virol. 2012;86:578–583. doi: 10.1128/JVI.05950-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nagel MA, Choe A, Traktinskiy I, et al. Varicella-zoster virus transcriptome in latently infected human ganglia. J Virol. 2011;85:2276–2287. doi: 10.1128/JVI.01862-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cohrs RJ, Srock K, Barbour MB, et al. Varicella-zoster virus (VZV) transcription during latency in human ganglia: construction of a cDNA library from latently infected human trigeminal ganglia and detection of a VZV transcript. J Virol. 1994;68:7900–7908. doi: 10.1128/jvi.68.12.7900-7908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cohrs RJ, Barbour M, Gilden DH. Varicella-zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J Virol. 1996;70:2789–2796. doi: 10.1128/jvi.70.5.2789-2796.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cohrs RJ, Gilden DH. Varicella zoster virus transcription in latently-infected human ganglia. Anticancer Res. 2003;23:2063–2069. [PubMed] [Google Scholar]
- 134.Cohrs RJ, Gilden DH. Prevalence and abundance of latently transcribed varicella-zoster virus genes in human ganglia. J Virol. 2007;81:2950–2956. doi: 10.1128/JVI.02745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Croen KD, Ostrove JM, Dragovic LJ, et al. Patterns of gene expression and sites of latency in human nerve ganglia are different for varicella-zoster and herpes simplex viruses. Proc Natl Acad Sci USA. 1988;85:9773–9777. doi: 10.1073/pnas.85.24.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kennedy PG, Grinfeld E, Bell JE. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J Virol. 2000;74:11893–11898. doi: 10.1128/jvi.74.24.11893-11898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ouwendijk WJ, Choe A, Nagel MA, et al. Restricted varicella-zoster virus transcription in human trigeminal ganglia obtained soon after death. J Virol. 2012;86:10203–10206. doi: 10.1128/JVI.01331-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gary L, Gilden DH, Cohrs RJ. Epigenetic regulation of varicella-zoster virus open reading frames 62 and 63 in latently infected human trigeminal ganglia. J Virol. 2006;80:4921–4926. doi: 10.1128/JVI.80.10.4921-4926.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yordy B, Iijima N, Huttner A, et al. A neuron-specific role for autophagy in antiviral defense against herpes simplex virus. Cell Host Microbe. 2012;12:334–345. doi: 10.1016/j.chom.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 141.van Velzen M, Laman JD, Kleinjan A, et al. Neuron-interacting satellite glial cells in human trigeminal ganglia have an APC phenotype. J Immunol. 2009;183:2456–2461. doi: 10.4049/jimmunol.0900890. [DOI] [PubMed] [Google Scholar]
- 142.Kennedy PG, Grinfeld E, Traina-Dorge V, et al. Neuronal localization of simian varicella virus DNA in ganglia of naturally infected African green monkeys. Virus Genes. 2004;28:273–276. doi: 10.1023/b:viru.0000025774.19557.39. [DOI] [PubMed] [Google Scholar]
- 143.Mahalingam R, Smith D, Wellish M, et al. Simian varicella virus DNA in dorsal root ganglia. Proc Natl Acad Sci USA. 1991;88:2750–2752. doi: 10.1073/pnas.88.7.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mahalingam R, Clarke P, Wellish M, et al. Prevalence and distribution of latent simian varicella virus DNA in monkey ganglia. Virology. 1992;188:193–197. doi: 10.1016/0042-6822(92)90749-f. [DOI] [PubMed] [Google Scholar]
- 145.Ou Y, Davis KA, Traina-Dorge V, et al. Simian varicella virus expresses a latency-associated transcript that is antisense to open reading frame 61 (ICP0) mRNA in neural ganglia of latently infected monkeys. J Virol. 2007;81:8149–8156. doi: 10.1128/JVI.00407-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meyer C, Kerns A, Barron A, et al. Simian varicella virus gene expression during acute and latent infection of rhesus macaques. J Neurovirol. 2011;17:600–612. doi: 10.1007/s13365-011-0057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meyer C, Dewane J, Kerns A, et al. Age and immune status of rhesus macaques impact simian varicella virus gene expression in sensory ganglia. J Virol. 2013;87:8294–8306. doi: 10.1128/JVI.01112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meyer C, Kerns A, Haberthur K, et al. Attenuation of the adaptive immune response in rhesus macaques infected with simian varicella virus lacking open reading frame 61. J Virol. 2013;87:2151–2163. doi: 10.1128/JVI.02369-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stevens JG, Wagner EK, Devi-Rao GB, et al. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987;235:1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 150.Umbach JL, Kramer MF, Jurak I, et al. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature. 2008;454:780–783. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Depledge DP, Kundu S, Jensen NJ, et al. Deep sequencing of viral genomes provides insight into the evolution and pathogenesis of varicella zoster virus and its vaccine in humans. Mol Biol Evol. 2014;31:397–409. doi: 10.1093/molbev/mst210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gebhardt T, Wakim LM, Eidsmo L, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 153.St Leger AJ, Jeon S, Hendricks RL. Broadening the repertoire of functional herpes simplex virus type 1-specific CD8+ T cells reduces viral reactivation from latency in sensory ganglia. J Immunol. 2013;191:2258–2265. doi: 10.4049/jimmunol.1300585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Theil D, Derfuss T, Paripovic I, et al. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol. 2003;163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van Velzen M, Jing L, Osterhaus AD, et al. Local CD4 and CD8 T-cell reactivity to HSV-1 antigens documents broad viral protein expression and immune competence in latently infected human trigeminal ganglia. PLoS Pathog. 2013;9:e1003547. doi: 10.1371/journal.ppat.1003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Verjans GM, Hintzen RQ, van Dun JM, et al. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci USA. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Burke BL, Steele RW, Beard OW, et al. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142:291–293. [PubMed] [Google Scholar]
- 158.Levin MJ, Smith JG, Kaufhold RM, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003;188:1336–1344. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 159.Miller AE. Selective decline in cellular immune response to varicella-zoster in the elderly. Neurology. 1980;30:582–587. doi: 10.1212/wnl.30.6.582. [DOI] [PubMed] [Google Scholar]
- 160.Devlin ME, Gilden DH, Mahalingam R, et al. Peripheral blood mononuclear cells of the elderly contain varicella-zoster virus DNA. J Infect Dis. 1992;165:619–622. doi: 10.1093/infdis/165.4.619. [DOI] [PubMed] [Google Scholar]
- 161.Nagel MA, Choe A, Cohrs RJ, et al. Persistence of varicella zoster virus DNA in saliva after herpes zoster. J Infect Dis. 2011;204:820–824. doi: 10.1093/infdis/jir425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.van Velzen M, Ouwendijk WJ, Selke S, et al. Longitudinal study on oral shedding of herpes simplex virus 1 and varicella-zoster virus in individuals infected with HIV. J Med Virol. 2013;85:1669–1677. doi: 10.1002/jmv.23634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Esiri MM, Tomlinson AH, Herpes Zoster. Demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci. 1972;15:35–48. doi: 10.1016/0022-510x(72)90120-7. [DOI] [PubMed] [Google Scholar]
- 164.Nagashima K, Nakazawa M, Endo H. Pathology of the human spinal ganglia in varicella-zoster virus infection. Acta Neuropathol. 1975;33:105–117. doi: 10.1007/BF00687537. [DOI] [PubMed] [Google Scholar]
- 165.Shibuta H, Ishikawa T, Hondo R, et al. Varicella virus isolation from spinal ganglion. Arch Gesamte Virusforsch. 1974;45:382–385. doi: 10.1007/BF01242884. [DOI] [PubMed] [Google Scholar]
- 166.Head H, Campbell AW. The pathology of Herpes Zoster and its bearing on sensory localisation. Brain. 1900;23:353–523. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 167.Gowrishankar K, Steain M, Cunningham AL, et al. Characterization of the host immune response in human Ganglia after herpes zoster. J Virol. 2010;84:8861–8870. doi: 10.1128/JVI.01020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Steain M, Sutherland JP, Rodriguez M, et al. Analysis of T cell responses during active varicella-zoster virus reactivation in human ganglia. J Virol. 2014;88:2704–2716. doi: 10.1128/JVI.03445-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Steain M, Gowrishankar K, Rodriguez M, et al. Upregulation of CXCL10 in human dorsal root ganglia during experimental and natural varicella-zoster virus infection. J Virol. 2011;85:626–631. doi: 10.1128/JVI.01816-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ouwendijk WJ, Abendroth A, Traina-Dorge V, et al. T-cell infiltration correlates with CXCL10 expression in ganglia of cynomolgus macaques with reactivated simian varicella virus. J Virol. 2013;87:2979–2982. doi: 10.1128/JVI.03181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mahalingam R, Traina-Dorge V, Wellish M, et al. Simian varicella virus reactivation in cynomolgus monkeys. Virology. 2007;368:50–59. doi: 10.1016/j.virol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 172.Shapiro ED, Vazquez M, Esposito D, et al. Effectiveness of 2 doses of varicella vaccine in children. J Infect Dis. 2011;203:312–315. doi: 10.1093/infdis/jiq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Takahashi M, Otsuka T, Okuno Y, et al. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2:1288–1290. doi: 10.1016/s0140-6736(74)90144-5. [DOI] [PubMed] [Google Scholar]
- 174.Gershon AA, Gershon MD. Perspectives on vaccines against varicella-zoster virus infections. Curr Top Microbiol Immunol. 2010;342:359–372. doi: 10.1007/82_2010_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]