Abstract
Accumulation of misfolded α-synuclein (α-syn) protein in Lewy bodies and neurites is the cardinal pathologic feature of Parkinson disease (PD), but abnormal deposition of other proteins may also play a role. Cerebrospinal fluid (CSF) levels of proteins known to accumulate in PD may provide insight into disease-associated changes in protein metabolism and their relationship to disease progression. We measured CSF α-syn, Aβ1-42 and tau from seventy-seven non-demented PD and thirty control participants. CSF α-syn and Aβ1-42 were significantly lower in PD compared to controls. In contrast to increased CSF tau in Alzheimer disease, CSF tau did not significantly differ between PD and controls. CSF protein levels did not significantly correlate with ratings of motor function or performance on neuropsychological testing. As expected, CSF Aβ1-42 inversely correlated with [11C]-Pittsburgh Compound B (PiB) mean cortical binding potential, with PiB+ PD participants having lower CSF Aβ1-42 compared to PiB− PD participants. Furthermore, CSF α-syn positively correlated with Aβ1-42 in PD participants but not in controls, suggesting a pathophysiologic connection between the metabolisms of these proteins in PD.
1. Introduction
Parkinson disease (PD) is a debilitating, progressive, neurodegenerative disorder. Approximately 500,000 people in the United States alone had a diagnosis of PD in 2010, and the projected estimate is likely to double by 2040 (Kowal, et al., 2013). The pathologic hallmark of PD is the accumulation of fibrillar α-synuclein (α-syn) protein in Lewy bodies and Lewy neurites. Dominant mutations in the α-syn gene (SNCA) cause rare familial forms of PD, indicating a role for α-syn in the pathogenesis of PD (Kruger, et al., 1998,Narhi, et al., 1999,Polymeropoulos, et al., 1997). Approximately 80% of individuals with PD develop dementia within 20 years after the onset of motor symptoms, decreasing quality of life and reducing survival (Aarsland and Kurz, 2010,Hely, et al., 2008). The incidence of dementia correlates with increasing age, longer duration and severity of motor symptoms (Hobson and Meara, 2004,Riedel, et al., 2008).
Pathologic α-syn deposition accompanies degeneration of substantia nigra dopaminergic neurons underlying the motor features of PD. The development of dementia in PD is always accompanied by pathologic accumulation of α-syn in neocortex. However, growing evidence indicates that Aβ deposition also frequently occurs in dementia associated with PD, as shown in our previous autopsy study of PD patients with dementia, where 38% of cases had predominant synucleinopathy (Braak Lewy body stages 5-6); 59% had predominant synucleinopathy plus Aβ deposition (Braak amyloid stages B-C) with minimal or no cortical tauopathy; and only 3% had synucleinopathy plus Aβ deposition with at least moderate tauopathy (Braak tau stages 5-6) (Kotzbauer, et al., 2012). This suggests that neocortical synucleinopathy and Aβ deposition co-occur regardless of abnormal tau deposition in PD, unlike the widespread co-existence of Aβ accumulation with neocortical neurofibrillary tangles (NFTs) that defines Alzheimer disease (AD) according to National Institute of Aging-Reagan Institute (NIA-Reagan) criteria (Newell, et al., 1999). Accordingly, positron emission tomography (PET) with [11C]-Pittsburgh Compound B (PiB) has identified PD patients with elevated cortical PiB binding, corresponding to Aβ plaque deposition at autopsy (Burack, et al., 2010,Edison, et al., 2008,Foster, et al., 2010,Gomperts, et al., 2012,Maetzler, et al., 2009,Maetzler, et al., 2008).
The aim of the current study was to investigate CSF levels of these three proteins, α-syn, Aβ1-42 and tau, in PD patients without dementia, as these could provide important insights into the pathophysiology underlying PD. While some reports investigating the levels of α-syn in CSF showed no differences between control and PD (Aerts, et al., 2012,Borghi, et al., 2000,Foulds, et al., 2012,Park, et al., 2011), other studies report low levels of CSF α-syn in PD compared to controls (Hong, et al., 2010,Kang, et al., 2013,Mollenhauer, et al., 2011,Parnetti, et al., 2011,Shi, et al., 2011). These inconsistencies may be attributed to differences in the time of collection, CSF processing, assay platforms, antibody characteristics or ELISA protocols (Mollenhauer, et al., 2010). The design of the present study aimed to investigate the relationship between CSF α-syn, Aβ1-42, total tau and phosphorylated tau 181 (p tau181), to clarify the differences in the metabolism of these proteins in PD compared to healthy controls, and to determine how these CSF proteins relate to APOE genotype, PiB binding, disease severity and cognitive function.
2. Methods
2.1. Standard protocol approvals, registrations and patient consents
The Human Research Protection Office and the Radioactive Drug Research Committee at Washington University in Saint Louis approved this study. Written informed consent was obtained from all participants prior to collection of clinical data, imaging studies and CSF.
2.2. Study participants
The cross-sectional cohort included 77 PD participants and 30 healthy age-matched controls. The inclusion criteria for enrollment in the study are i) non-demented PD participants with a clinically definitive diagnosis of PD, based on the United Kingdom Parkinson Disease Society Brain Bank diagnostic criteria (Hughes, et al., 1992) and Clinical Dementia Rating (CDR global score) < 1 ii) healthy age-matched controls who had normal neurological exam, no family history of PD, and normal cognition (CDR = 0). The exclusion criteria for all participants were i) neurological diagnosis other than PD; ii) psychiatric disorder other than depression or anxiety; iii) a history of head trauma with loss of consciousness > 5 min; iv) inability to complete PET or magnetic resonance imaging (MRI); v) unwillingness to consent to brain donation for post-mortem analysis.
2.3. Clinical Assessments
Clinical assessments were performed by movement disorders specialists and trained research staff who were blinded to CSF data.
2.4. Motor examination
Severity of motor signs was evaluated by movement disorders specialists using the Unified Parkinson Disease Rating Scale motor sub-scale III (UPDRS-III) (Goetz, et al., 2008).
2.5. Evaluation of cognitive function
Participants completed neuropsychological testing to determine possible associations between cognitive performance and CSF protein levels. PD participants were tested while OFF medication overnight to minimize the potential confound of drugs on cognitive performance (Cools, 2006). Neuropsychological assessment included tests of memory (California Verbal Learning Test – short form (CVLT-sf) (Delis, et al., 2000); Logical Memory (LM) (Wechsler, 1997b)), attention (Digit Symbol (Wechsler, 1997a); Digit Span (Wechsler, 1997a)), language (Boston Naming Test (BNT) (Kaplan, et al., 2001)), visual-spatial function (Judgment of Line Orientation (JLO) (Lezak, et al., 2004); Spatial Relations Test (SRT) (Woodcock, et al., 2001)) and executive function (Trail Making Test (Lezak, 1995); Verbal Fluency (Delis, et al., 2001); Stroop Color-Word test (Delis, et al., 2001)). The age-adjusted scaled scores for each measure, based on the test manuals and published normative data (Ivnik, et al., 1996), were converted to Z-scores and averaged within cognitive domains and then across cognitive domains to obtain a single global cognitive Z-score for each participant. Specifically, the memory composite Z-score was the average of: LM-I, LM-II, CVLT trials 1-4 total, CVLT short delay, CVLT long delay free recall. Attention composite Z-score was the average of: Digit Span total and Digit Symbol. Visual-Spatial function composite Z-score was the average of: JLO and SRT. The language Z-score was the Z-score for the BNT. Executive function composite Z-score was computed as the average of: Trails B, Stroop Inhibition, Verbal Fluency category switching total correct responses and Verbal Fluency category switching total switching accuracy. Overall cognition was simply the average of all composite domain Z-scores.
Dementia was determined by the CDR scale, administered by raters trained and certified by the Washington University in Saint Louis, Knight Alzheimer's Disease Research Center. The CDR is a semi-structured interview with the participant and a collateral source that assesses cognitive and functional performance across six domains: memory, orientation, judgment and problem solving, community affairs, home and hobbies and personal care (Burke, et al., 1988,Morris, 1997). Participants with a global score of 1 or greater were considered to have dementia (n = 5), in accordance with the established criteria for dementia in PD (Emre, et al., 2007), and were excluded from further analyses; thus 77 non-demented PD participants were included in this study.
2.6. PiB PET imaging
In vivo brain β-amyloid load was assessed by PiB PET imaging, as described (Campbell, et al., 2013,Klunk, et al., 2004). PiB was synthesized on-site according to established procedures (Mathis, et al., 2003). PET data were acquired using a Siemens HR or HR+ ECAT PET scanner (CTI, Knoxville, TN). The mean cortical binding potential (MCBP) for PiB was calculated by averaging values from prefrontal cortex, precuneus, lateral temporal cortex, and gyrus rectus. MCBP cut-off ≥ 0.18 discriminated PiB positive (PiB+) from PiB negative (PiB−) participants (Mintun, et al., 2006).
2.7. CSF collection and processing
CSF was collected between January 2011 and January 2014. Most participants had lumbar punctures (LP) for CSF collection done preceding the PiB PET scans at the initial study visit; 19 participants (11 PD; 8 controls) completed LPs at their follow-up visit 1- 5 years after the PiB scan. Analyses comparing PiB scans and CSF levels were limited to only those participants with LP and PiB scans completed on the same day (66/77 (85.7 %), PD; 22/30 (73.3%) controls). CSF was consistently collected between 1300 and 1330 hrs. Approximately, 25 ml of CSF was obtained from the L3/L4 or L4/L5 lumbar space of participants, using a 22-guage-atraumatic Sprotte needle (Fagan, et al., 2006). CSF was inspected visually for blood contamination, and the first 1-2 ml was discarded if blood was initially present. Blood-free CSF was collected by gravity flow into a 50 ml polypropylene tube placed on wet ice. After collection was completed, the tube containing CSF was gently pulse - vortexed to prevent gradient effects before centrifuging at 2000 g for 15 minutes at 4°C. The s upernatant except for the last 500 μl was slowly removed using a sterile 25 ml pipette and then transferred to a new 50 ml tube on wet ice. From this tube, 0.5 ml and 1 ml CSF aliquots were prepared and frozen at − 80°C. The total time taken from collection to freezing was typically about 30 minutes.
2.8. Quantification of CSF proteins
Quantitative measurements of CSF α-syn, Aβ1-42, total tau and p tau181 were evaluated using sandwich enzyme–linked immunosorbent assays (ELISAs). CSF from 107 participants (77 PD; 30 controls) was distributed in duplicate on three ELISA plates with the same range of concentrations of standard recombinant protein duplicates on each plate. For each protein, the third sets of ELISAs were performed approximately twelve months after the completion of the first two sets of ELISAs. An assay for a given protein across plates, was conducted under the same conditions to eliminate procedural confounds. For p tau181 ELISA plates, standards and CSF from 75 participants (55 PD; 20 controls) in duplicate were distributed on two ELISA plates, both of which were processed on the same day.
Measures for α-syn were performed using the Covance α-syn ELISA kit (Covance Inc., Indianapolis, USA) in accordance with the guidelines provided in the kit manual with some modifications. To minimize matrix effects and improve the signal, a number of trial runs of CSF dilutions in assay diluent were empirically tested. A CSF dilution of 1:4 provided optimal signal for CSF with all values falling within the range of the standard curve.
CSF measurements for Aβ1-42, total tau and p tau181 were conducted using the Innotest ELISA Kits (Innogenetics Inc., Ghent, Belgium) according to the manufacturer's instructions. The p tau181/total tau ratio was chosen as the dependent variable for p tau181 analysis to reflect the percent phosphorylation of tau in each sample.
To address the possible contribution of α-syn from red blood cells due to blood contamination during LP, hemoglobin (Hb) levels in CSF from all 107 participants were quantified using the Human Hemoglobin ELISA Quantitation Set (Bethyl Laboratories Inc., Texas, USA) according to the kit's instructions.
2.9. Apolipoprotein (Apo) Eε4 genotyping
To determine possible differences in CSF protein levels based on genetic status, we obtained ApoE genotypes from 94 participants (69/77 PD; 25/30 controls). Genomic DNA was extracted from blood and the presence of an ApoE ε4 or ε2 allele was determined as previously described (Head, et al., 2012,Huang, 2010).
2.10. Measurement of α-syn and Hb from whole blood
Whole blood samples were collected in K2EDTA tubes and stored at − 80°C in 0.5 ml aliquots until use. For ELISA analysis, 0.1 ml whole blood samples were diluted in 10 volumes of lysis buffer (50 mM Tris, pH 7.6, 150 mM NaCl, 2 mM EDTA, 1% Triton-X-100) at 4°C and then centrifuged at 15,000 × g for 10 minutes at 4°C (Ar gyriou, et al., 2012). The supernatant was diluted 1:400,000 (α-syn) or 1:1,000,000 (Hb) prior to ELISA analysis using the methods described above for CSF.
2.11. Statistical analysis
Data were analyzed using GraphPad Prism software, version 4 (Graph Pad Inc.) and PASW version 22 (IBM, Chicago, IL). The concentrations of all proteins were determined using standard curves generated by curve fitting with nonlinear regression using a 4-parameter logistic regression model. Due to unequal group sizes, non-normal distribution of some variables, and rank-order ratings on the UPDRS-III, non-parametric tests (Chi-square, Mann-Whitney and Spearman correlation) were used for group comparisons and correlation analyses. To control for any possible confounds (e.g., age, education, gender), analysis of covariance was also used for group comparisons, including as covariates any demographic variables that were significantly different between groups. For each comparison or analysis, the result of the non-parametric statistical test is listed, followed by the P value determined by the statistical test. All tests were 2-tailed and P < 0.05 was considered statistically significant.
3. Results
3.1. Demographic and clinical information of participants
Table 1 summarizes the demographic and clinical information for all participants. There were no significant differences in age or education level between PD and control participants. However, there were a larger proportion of male PD participants than male control participants (57.1% PD; 33% controls); therefore, gender was included as a covariate in the between-group comparisons of CSF levels.
Table 1.
Demographics and clinical information of the study participantsa
| Controls | PD | P valueb | |
|---|---|---|---|
| Demographics | |||
| Participants, No. | 30 | 77 | NA |
| Age at LP, y | 64.8 (10.5) | 66.9 (8.5) | P = 0.28 |
| Education, y | 14.9 (2.8) | 15.9 (2.7) | P = 0.10 |
| Male/Female, No. | 10/20 | 44/33 | P = 0.03 |
| Clinical characteristics | |||
| Age at PD diagnosis, y | NA | 60.6 (8.4) | NA |
| Duration of motor impairment, y | NA | 4.9 (4.0) | NA |
| UPDRS-III score (OFF medications) | NA | 23.2 (9.1) | NA |
| LEDD, mg | NA | 810.9 (542.9) | NA |
| Median CSF measures (pg/ml) | |||
| α-syn | 2298.6 (650.3) | 1852.8 (743.1) | P < 0.001 |
| Aβ1-42 | 945.8 (141.2) | 780.8 (222.4) | P < 0.001 |
| Total tau | 243.3 (99.5) | 218.2 (110.1) | P = 0.07 |
| p tau181/total tau | 0.183 (0.022) | 0.178 (0.017) | P = 0.13 |
Abbreviations: PD, Parkinson Disease, NA, Not Applicable; LP, Lumbar Puncture; UPDRS, Unified Parkinson Disease Rating Scale; LEDD, Levodopa Equivalent Daily Dose.
Values are medians (SD) unless otherwise indicated.
Age at LP and education were tested using ANOVA. Gender was compared using chi-square test. All CSF measures were tested using Mann-Whitney U test.
3.2. Assay performance characteristics
The intra and inter-plate coefficients of variation (CV), which are measures of quality control between duplicate wells and between assay plates respectively, were within the range of the manufacturers’ recommendations (Supplementary Table 1). The relative luminescence units (RLUs; α-syn) or optical densities (ODs; Aβ1-42, total tau and p tau181) for the blank and highest recombinant protein concentration standard wells were within the range of the manufacturer validated RLUs or ODs.
3.3. CSF protein measurements
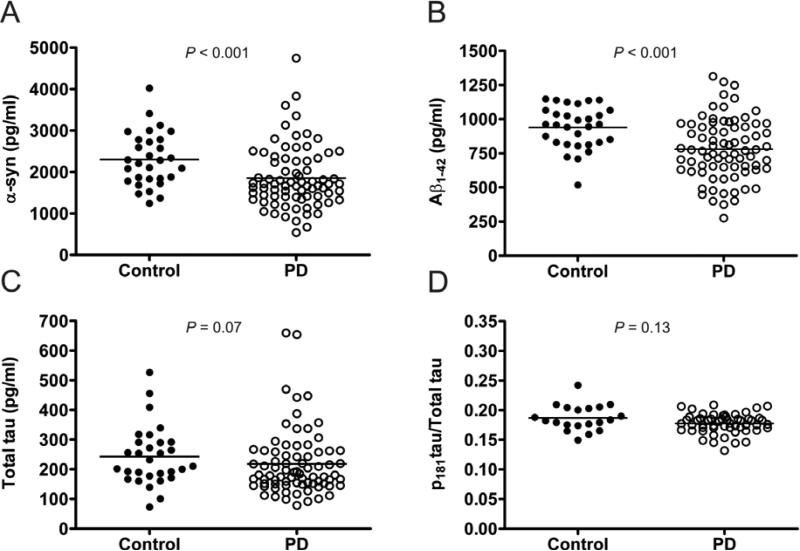
CSF levels of α-syn and Aβ1-42 were significantly lower (α-syn: Mann-Whitney U = 672, P < 0.001; Aβ1-42: Mann-Whitney U = 608, P < 0.001) in PD participants compared to controls (Table 1; Figures 1.A and 1.B). Furthermore, CSF Aβ1-42 levels remained significantly lower in PD participants compared to controls after excluding PiB+ individuals (Mann-Whitney U = 564, P = 0.003). Differences in CSF levels of total tau or p tau181/total tau between PD and control participants did not reach significance (total tau: Mann-Whitney U = 890, P = 0.07, p tau181/total tau: Mann-Whitney U = 422, P = 0.13) (Figures 1.C and 1.D). After controlling for gender, the group differences in α-syn and Aβ1-42 remained significant (ANCOVA; α-syn: F(1,104) = 7.62, P = 0.007; Aβ1-42: F(1,103) = 14.68, P < 0.001), and total tau and p tau181/total tau remained non-significant (total tau: F(1,103) = 1.03, P = 0.31, p tau181/total tau: F(1,74) = 3.58, P = 0.06). Overall, the percent median differences between PD and control participants were 23.8 and 19.8 for α–syn and Aβ1-42, respectively.
Figure 1. Comparison of CSF protein levels between control and PD participants.
Comparison of the levels of α-syn (A), Aβ1-42 (B), tau (C) and p tau181/total tau (D) in the CSF of control (n=30; solid circles) and PD (n=77; open circles) participants by Mann-Whitney U test. The medians for each group are represented by a horizontal line. Differences between control and PD were statistically significant for α-syn: P < 0.001 and Aβ1-42: P < 0.001. Group differences were not significant for tau: P = 0.07 and p tau181/total tau: 0.13.
3.4. Correlations between CSF Aβ1-42 and PiB binding in PD participants
Of the participants who completed LP and PiB scan on the same day, the mean cortical PiB binding was elevated in 1/22 (4.5 %) control participant and 11/66 (16.7%) PD participants. The proportion of PiB+ participants did not significantly differ between control and PD participants (χ2= 2.06, df = 1, P = 0.15). Within the PD group, PiB+ participants had significantly lower CSF Aβ1-42 levels than PiB− participants (Mann-Whitney U = 7.0, P < 0.001), with minimal overlap between the distributions of Aβ1-42 levels between the two groups (Figure 2). CSF Aβ1-42 inversely correlated with MCBP for PD (rho = − 0.46, n = 66, P < 0.001) and control (rho = −0.43, n = 22, P = 0.05) participants.
Figure 2. Correlation between CSF Aβ1-42 and MCBP levels in PD participants.
Scatterplots represent correlation (Spearman) between CSF Aβ1-42 levels and MCBP in PD (n=66; solid circles) participants. Each solid black circle is representative of a PD participant. The vertical dotted line indicates the MCBP cut-off score of 0.18 that discriminates PiB− (left) from PiB+ (right) participants. There was a significant inverse correlation between CSF Aβ1-42 and MCBP (rho = − 0.46; P < 0.001).
3.5. APOE genotype
The proportion of participants with at least one APOE ε4 allele was not significantly different between the control (5/25; 20%) and PD participants (15/69; 21.7%) (P = 0.86), nor did the proportion of participants with at least one APOE ε2 allele significantly differ between the control (2/25; 8%) and PD participants (14/69; 20.3%) (P = 0.16). PD participants who were APOE ε4+ had lower CSF Aβ1-42 levels (Mann-Whitney U = 170.00, P = 0.001) than APOE ε4− PD participants; however, CSF Aβ1-42 levels did not differ based on the APOE ε2 allele (P = 0.33).
3.6. Correlations among CSF α-syn, Aβ1-42 and tau
We analyzed correlations among CSF α-syn, Aβ1-42, total tau and p tau181/total tau within the PD and control groups. CSF α-syn significantly positively correlated with Aβ1-42 for PD participants (rho = 0.39, n = 77, P < 0.001), but the correlation between CSF α-syn and Aβ1-42 for control participants was not significant (rho = − 0.09, n = 30, P = 0.63) (Figure 3.A). In contrast, CSF α-syn and total tau levels displayed strong positive correlations in both PD and control groups (PD: rho = 0.78, n = 77, P < 0.001; controls: rho = 0.86, n = 30, P < 0.001) (Figure 3.B). However, CSF α-syn and p tau181/total tau were significantly correlated for controls but not PD participants (controls: rho = − 0.76, n = 20, P < 0.001).
Figure 3. Correlations among CSF α-syn, Aβ1-42 and tau in control and PD participants.
Correlations (Spearman) between the CSF levels of α-syn and Aβ1-42 (A) or tau (B) in control (n=30; blue solid circles) and PD (n=77; red solid circles) participants. A significant positive correlation between α-syn and Aβ1-42 is present in the PD group (rho = 0.39, P < 0.001) but not in the control group (rho = − 0.09, P =0.63), where there was an insignificant inverse correlation. Similar positive correlations between α-syn and tau were present in both the control and PD groups (control: rho = 0.86, P < 0.001; PD: rho = 0.78, P < 0.001). Lines (control; blue line, PD; red line) indicate trends within each group as determined by linear regression.
For PD participants, CSF Aβ1-42 positively correlated with total tau (rho = 0.24, n = 77, P = 0.04) and p tau181/total tau (rho = 0.47, n = 55, P < 0.001), but CSF Aβ1-42 did not correlate with total tau or p tau181/total tau for controls (total tau: rho = − 0.02, n = 30, P = 0.93; p tau181/total tau: rho = 0.21, n = 20, P = 0.38).
3.7. Effect of CSF Hb on CSF α-syn measurements
The CSF levels of Hb did not significantly differ between PD and controls (Mann-Whitney U =1086, P = 0.635) and the median (SD) CSF Hb concentrations were similar (PD: 520.8 (1492.8), controls: 533.2 (1322.7). Furthermore, there was no correlation between CSF levels of Hb and α-syn (rho = 0.007, n = 107, P = 0.94, Supplementary Figure 1). There were also no significant correlations between CSF Hb and CSF Aβ1-42, total tau or p tau181/total tau (data not shown). Furthermore, when samples with greater than 1000 ng/ml or 500 ng/ml of CSF Hb were excluded from the analyses, group differences or correlations where α-syn was included as a dependent variable continued to be significant (Supplementary Tables 2-4).
3.8. Correlations between CSF protein levels and clinical features
Comparisons of CSF levels with clinical features revealed that total tau and p tau181/total tau significantly correlated with age at LP (total tau: rho = 0.29, n = 77, P = 0.01; p tau181/total tau: rho = − 0.27, n = 55, P = 0.05) and age of PD onset (total tau: rho = 0.26, n = 77, P = 0.03; p tau181/total tau: rho = − 0.28, n = 55, P = 0.04). CSF Aβ1-42 positively correlated with Mini Mental State Examination (MMSE) scores (rho = 0.25, n = 74, P = 0.03) and Levodopa Equivalent Daily Dose (LEDD) (rho = 0.24, n = 72, P = 0.04) but did not significantly correlate with age at LP (rho = − 0.19, n = 77, P = 0.09) or age of PD onset (rho = − 0.21, n = 77, P = 0.07). We did not find any other significant correlations between CSF measures and clinical features, including, duration of PD, CDR sum of boxes, or UPDRS-III total scores (all P > 0.10). Furthermore, the relationship between CSF α-syn and Aβ1-42 remained significant with partial correlations (all P ≤ 0.001) after controlling for MMSE, LEDD, age at LP or PD onset.
3.9. Cognitive performance and CSF protein levels
PD participants performed worse than controls on visual-spatial function (F(1,104) = 3.17, P = 0.08) and executive function (F(1,105) = 6.52, P = 0.01) as well as overall cognition (F(1,106) = 6.34, P = 0.01). There were no significant differences in attention (P = 0.12), memory (P = 0.14) or language (P = 0.31) performances. Within the PD group, CSF protein levels did not significantly relate to cognitive performances (all P > 0.18).
3.10. Receiver operating characteristic (ROC) analysis
We performed ROC analysis to evaluate the ability of individual CSF protein measures or combinations of measures to discriminate between PD and control subjects. Consistent with the significant overlap between groups observed in Figure 1, ROC analysis indicated that CSF protein measures do not discriminate PD from control (Supplementary Figure 2).
Discussion
Analysis of our cross-sectional cohort of non-demented PD participants and age-matched controls revealed PD-associated differences in the CSF levels of α-syn and Aβ1-42. CSF levels of α-syn and Aβ1-42 were lower in PD participants compared to controls. Additional analysis revealed a significant positive correlation between CSF α-syn and Aβ1-42 in PD but not in control participants. CSF levels of total tau and p tau181/total tau did not significantly differ between PD and controls, in contrast to the elevated CSF total tau and p tau181/total tau profiles observed in AD (Mollenhauer, et al., 2011,Shi, et al., 2011,Zhang, et al., 2008). Despite the significant PD-related differences in CSF measures of α-syn and Aβ1-42, these measures were not sufficiently separated between groups to discriminate PD from control subjects for diagnostic purposes.
These CSF findings provide insight into PD-related differences in the metabolism of proteins observed to aggregate and accumulate in PD as well as in other neurodegenerative disorders. Neuronal accumulation of misfolded α-syn is a defining histopathologic feature of PD, and the development of dementia correlates with more widespread accumulation of misfolded α-syn (Armstrong, et al., 2013,Ballard, et al., 2006,Braak, et al., 2004,Compta, et al., 2011,Hurtig, et al., 2000). The decreased CSF levels of α-syn in PD relative to healthy age-matched controls in our study supports other studies comparing PD to healthy control subjects (Kang, et al., 2013,Shi, et al., 2011), DLB to healthy control subjects (Tateno, et al., 2012) and PD to other neurological disorders (Mollenhauer, et al., 2011,Parnetti, et al., 2011,Parnetti, et al., 2014b). Glial accumulation of aggregated α-syn in MSA also corresponds to low CSF α-syn levels (Hall, et al., 2012,Mollenhauer, et al., 2011). The low levels of CSF α-syn are in contrast to the high levels of CSF α-syn observed in AD (Korff, et al., 2013,Slaets, et al., 2014,Toledo, et al., 2013). Altered levels of CSF α-syn likely correspond to altered levels of soluble α-syn in the brain that may be caused by sequestration of α-syn protein into fibrillar aggregates. The pathophysiology of PD may also include changes in the synthesis rate or turnover of α-syn protein that could alter the extracellular release of α-syn or transport from the interstitial space to CSF.
The lack of correlation between CSF Hb and α-syn in our analysis contrasts with other studies that did observe a correlation between these CSF measurements (Hong, et al., 2010,Kang, et al., 2013,Shi, et al., 2011). CSF sample processing in our study, specifically centrifugation of CSF to remove red blood cells (RBCs), may have been more effective in reducing RBC-derived α-syn than it was in reducing RBC-derived Hb, possibly because Hb is more readily released from RBCs. Since α-syn associates with membranes, it may be less readily released from RBCs during centrifugation. For example, measurement of the RBC Hb to α-syn ratio in whole blood by the assays used in this CSF study predicted that 5000 ng/ml Hb should be accompanied by 15 ng/ml α-syn (data not shown), yet we did not detect any effect on α-syn levels in samples with Hb concentrations in this range. Moreover, we observed no change in the results of group comparisons and correlation analyses after excluding samples based on previously used Hb cut-off values.
Widespread accumulation of aggregated Aβ occurs in many but not all PD cases at autopsy. We previously showed widespread cortical Aβ deposition in 60% of PD patients who developed dementia (Kotzbauer, et al., 2012). In addition, 20-30% of living PD patients have elevated cortical PiB binding (Campbell, et al., 2013,Edison, et al., 2008,Foster, et al., 2010,Gomperts, et al., 2008,Maetzler, et al., 2009,Maetzler, et al., 2008). Positive PiB scans are associated with low CSF Aβ1-42 levels in AD (Fagan, et al., 2006), and we found the same relationship in our PD cohort. Other studies reported decreased CSF Aβ1-42 levels in PD relative to healthy controls (Kang, et al., 2013,Shi, et al., 2011). The low mean CSF Aβ1-42 levels in our primarily non-demented PD cohort indicates that changes in Aβ metabolism occur early in PD prior to the onset of dementia. Such changes in CSF Aβ levels may reflect changes in release, clearance or transport of the Aβ peptide (Mawuenyega, et al., 2010). The positive correlation between CSF α-syn and Aβ1-42 levels that occurs in PD but not in control participants does suggest that PD-associated changes in protein homeostasis within the brain or transport of proteins to CSF could have a common effect on α-syn and Aβ metabolism. Interestingly, a recent report showed a weak negative correlation between CSF α-syn and Aβ1-42 levels in AD (Toledo, et al., 2013), indicating that the positive correlation we observe may be unique to PD or at least distinct from the pathophysiology of AD. Another recent study did not find a correlation between α-syn and Aβ1-42 in a cohort of drug-naïve, early stage PD patients with shorter disease duration relative to our population (Kang, et al., 2013) suggesting that including more severely affected individuals may have permitted us to identify this correlation. Their drug-naïve cohort also had smaller percent differences in mean α-syn and Aβ1-42 levels relative to healthy controls probably reflecting the milder disease status of their participants or effects of medication in our cohort.
In contrast to AD in which CSF Aβ1-42 decreases and CSF total tau increases, we found no significant changes in CSF total tau between PD and controls, whereas others found low CSF total tau (Kang, et al., 2013,Mollenhauer, et al., 2011,Parnetti, et al., 2011,Shi, et al., 2011,Zhang, et al., 2008). Furthermore, we observed no changes in the levels of CSF p tau181/total tau for PD whereas AD leads to an increase in this ratio. CSF α-syn and total tau are strongly correlated in control samples, suggesting a shared mechanism by which these proteins move from their normal intracellular location to CSF. This correlation is similarly present in PD samples. We also observed a positive correlation between CSF Aβ1-42 and total tau or p tau181/total tau in PD samples, but this correlation was observed in the absence of group differences for tau measures. Elevated CSF total tau and p tau181/total tau levels correspond with the development of cognitive impairment in AD, which pathologically includes a combination of widespread accumulation of Aβ in plaques and tau in neurofibrillary tangles. In PD patients with dementia, accumulation of Aβ plaques is much less likely accompanied by widespread abnormal neocortical tau deposition (Kotzbauer, et al., 2012). Thus, the histopathologic differences in tau deposition and the contrasting changes in CSF total tau levels indicate clear differences in the role of tau in AD and PD disease, or alternatively indicate that AD and PD disease processes differentially alter tau metabolism. Studies of CSF total tau levels in PD participants with dementia could help to determine whether CSF total tau is increased in the minority of patients that have substantial neocortical tau pathology.
Accumulation of Aβ may be associated with decreased survival in PD patients with dementia (Kotzbauer, et al., 2012). In addition, low CSF Aβ1-42 levels are associated with an increased rate of cognitive decline and dementia-risk in PD (Compta, et al., 2013,Siderowf, et al., 2010), and greater cortical Aβ load related to a faster rate to dementia from disease onset (Compta, et al., 2011). Similar to previous cross-sectional studies of CSF protein levels (Kang, et al., 2013,Vemuri, et al., 2009), we did not find any associations between CSF protein levels and cognitive function in this non-demented cross-sectional cohort, other than a weak, yet significant correlation between CSF Aβ1-42 and MMSE. However, other studies found associations between CSF Aβ1-42 levels and either phonetic fluency or memory (Alves, et al., 2010,Compta, et al., 2009). Longitudinal analysis of motor and cognitive ratings may be more appropriate and could reveal whether CSF levels of Aβ1-42 as well as α-syn and tau predict disease progression. Alternatively, the correlation between CSF Aβ1-42 and α-syn suggests that the poorer prognosis found at autopsy in those PD patients who had abnormal Aβ compared to those that did not (Kotzbauer, et al., 2012) could reflect a higher α-syn burden in the brain rather than an additive effect of Aβ as suggested by previously observed correlations between Lewy Body density and Aβ plaque density (Lashley, et al., 2008,Pletnikova, et al., 2005). Further studies quantifying abnormal accumulation of both of these proteins could be revealing. A cause and effect relationship between accumulation of either protein and the rate of disease progression remains to be demonstrated, but transgenic mouse studies indicate that combined over-production of Aβ peptide and α-syn protein may have synergistic effects on pathologic protein accumulation and neurologic impairment (Clinton, et al., 2010,Masliah, et al., 2001). Longitudinal analysis of CSF protein levels could in turn reveal whether disease progression is associated with further changes in protein metabolism.
Emerging data on the detection of elevated levels of oligomeric and phosphorylated forms of CSF α-syn, along with changes in the levels of CSF lysosomal enzymes in PD indicates that these proteins could be useful biomarkers of pathology (Park, et al., 2011,Parnetti, et al., 2014a,Parnetti, et al., 2014b,Tokuda, et al., 2010,van Dijk, et al., 2013,Wang, et al., 2012). Measurement of other CSF proteins may yield additional biomarkers for PD, including proteins present in both the CSF and the cortical proteomes identified by proteomic analysis (Pan, et al., 2007). Combining CSF studies with longitudinal clinical data, volumetric brain MRI, post-mortem tissue studies, and molecular imaging approaches to measure pathologic protein accumulation or loss of synaptic function may provide further insights into the time course of pathologic processes. The ultimate goal would be to identify the earliest changes that predict cognitive impairment and provide a biomarker to assess efficacy of a therapeutic intervention to either halt or slow down the development of dementia in PD.
Supplementary Material
Highlights.
CSF levels of α-syn and Aβ1-42 were lower in PD participants compared to controls.
CSF total tau and p tau181/total tau did not differ between PD and controls.
CSF α-syn positively correlated with Aβ1-42 in PD participants but not in controls.
Acknowledgments
Funding/Support
Support for this work was provided by: grants NS075321, NS41509, NS058714, and NS48924 from the National Institute of Neurological Disorders and Stroke; grant UL1 TR000448 from the NIH National Center for Advancing Translational Sciences; the APDA Advanced Research Center for Parkinson Disease at Washington University in St Louis; the Greater St Louis Chapter of the APDA; and the Barnes Jewish Hospital Foundation (Elliot Stein Family Fund and Parkinson Disease Research Fund). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: The authors declare that no competing interests exist.
Author Contributions
Study concept and design: Drs. Campbell, Perlmutter and Kotzbauer; Acquisition of data: Drs. Campbell and Buddhala; Analysis and interpretation of data: All authors; Drafting of the manuscript: Drs. Buddhala and Kotzbauer; Critical revision of the manuscript for important intellectual content: All authors; Statistical analysis: Drs. Campbell and Buddhala; Obtained funding: Drs. Campbell, Perlmutter and Kotzbauer.
Drs. Kotzbauer and Campbell had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Additional contributions
We thank Drs. Johanna Hartlein, Mwiza Ushe, Scott Norris and Robert Bucelli for collecting CSF from study participants.
Disclosure Statement
The authors declare no conflicts of interest.
The Human Research Protection Office and the Radioactive Drug Research Committee at Washington University in Saint Louis approved this study. Written informed consent was obtained from all participants prior to collection of clinical data, imaging studies and CSF.
References
- Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. Journal of the neurological sciences. 2010;289(1-2):18–22. doi: 10.1016/j.jns.2009.08.034. doi:10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- Aerts MB, Esselink RA, Abdo WF, Bloem BR, Verbeek MM. CSF alpha-synuclein does not differentiate between parkinsonian disorders. Neurobiology of aging. 2012;33(2):430, e1–3. doi: 10.1016/j.neurobiolaging.2010.12.001. doi:10.1016/j.neurobiolaging.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Alves G, Bronnick K, Aarsland D, Blennow K, Zetterberg H, Ballard C, Kurz MW, Andreasson U, Tysnes OB, Larsen JP, Mulugeta E. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson's disease: the Norwegian ParkWest study. Journal of neurology, neurosurgery, and psychiatry. 2010;81(10):1080–6. doi: 10.1136/jnnp.2009.199950. doi:10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- Argyriou A, Dermentzaki G, Papasilekas T, Moraitou M, Stamboulis E, Vekrellis K, Michelakakis H, Stefanis L. Increased dimerization of alpha-synuclein in erythrocytes in Gaucher disease and aging. Neuroscience letters. 2012;528(2):205–9. doi: 10.1016/j.neulet.2012.08.069. doi:10.1016/j.neulet.2012.08.069. [DOI] [PubMed] [Google Scholar]
- Armstrong R, Kotzbauer P, Perlmutter J, Campbell M, Hurth K, Schmidt R, Cairns N. A quantitative study of α-synuclein pathology in fifteen cases of dementia associated with Parkinson disease. J Neural Transm. 2013:1–11. doi: 10.1007/s00702-013-1084-z. doi:10.1007/s00702-013-1084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Ziabreva I, Perry R, Larsen JP, O'Brien J, McKeith I, Perry E, Aarsland D. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931–4. doi: 10.1212/01.wnl.0000249130.63615.cc. doi:10.1212/01.wnl.0000249130.63615.cc. [DOI] [PubMed] [Google Scholar]
- Borghi R, Marchese R, Negro A, Marinelli L, Forloni G, Zaccheo D, Abbruzzese G, Tabaton M. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neuroscience letters. 2000;287(1):65–7. doi: 10.1016/s0304-3940(00)01153-8. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell and tissue research. 2004;318(1):121–34. doi: 10.1007/s00441-004-0956-9. doi:10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Burack MA, Hartlein J, Flores HP, Taylor-Reinwald L, Perlmutter JS, Cairns NJ. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology. 2010;74(1):77–84. doi: 10.1212/WNL.0b013e3181c7da8e. doi:10.1212/WNL.0b013e3181c7da8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke WJ, Miller JP, Rubin EH, Morris JC, Coben LA, Duchek J, Wittels IG, Berg L. Reliability of the Washington University Clinical Dementia Rating. Archives of neurology. 1988;45(1):31–2. doi: 10.1001/archneur.1988.00520250037015. [DOI] [PubMed] [Google Scholar]
- Campbell MC, Markham J, Flores H, Hartlein JM, Goate AM, Cairns NJ, Videen TO, Perlmutter JS. Principal component analysis of PiB distribution in Parkinson and Alzheimer diseases. Neurology. 2013 doi: 10.1212/WNL.0b013e31829e6f94. doi:10.1212/WNL.0b013e31829e6f94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(21):7281–9. doi: 10.1523/JNEUROSCI.0490-10.2010. doi:10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compta Y, Marti MJ, Ibarretxe-Bilbao N, Junque C, Valldeoriola F, Munoz E, Ezquerra M, Rios J, Tolosa E. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2009;24(15):2203–10. doi: 10.1002/mds.22594. doi:10.1002/mds.22594. [DOI] [PubMed] [Google Scholar]
- Compta Y, Parkkinen L, O'Sullivan SS, Vandrovcova J, Holton JL, Collins C, Lashley T, Kallis C, Williams DR, de Silva R, Lees AJ, Revesz T. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain : a journal of neurology. 2011;134(Pt 5):1493–505. doi: 10.1093/brain/awr031. doi:10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compta Y, Pereira JB, Rios J, Ibarretxe-Bilbao N, Junque C, Bargallo N, Camara A, Buongiorno M, Fernandez M, Pont-Sunyer C, Marti MJ. Combined dementia-risk biomarkers in Parkinson's disease: a prospective longitudinal study. Parkinsonism & related disorders. 2013;19(8):717–24. doi: 10.1016/j.parkreldis.2013.03.009. doi:10.1016/j.parkreldis.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neuroscience and biobehavioral reviews. 2006;30(1):1–23. doi: 10.1016/j.neubiorev.2005.03.024. doi:10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J, Ober B. California Verbal Learning Test-II. The Psychological Corporation; San Antonio, TX.: 2000. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis Kaplan Executive Function System. The Psychological Corporation; 2001. [Google Scholar]
- Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, Villemagne VL, O'Keefe G, Nagren K, Chaudhury KR, Masters CL, Brooks DJ. Amyloid load in Parkinson's disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. Journal of neurology, neurosurgery, and psychiatry. 2008;79(12):1331–8. doi: 10.1136/jnnp.2007.127878. doi:10.1136/jnnp.2007.127878. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2007;22(12):1689–707. doi: 10.1002/mds.21507. quiz 837. doi:10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Annals of neurology. 2006;59(3):512–9. doi: 10.1002/ana.20730. doi:10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Foster ER, Campbell MC, Burack MA, Hartlein J, Flores HP, Cairns NJ, Hershey T, Perlmutter JS. Amyloid imaging of Lewy body-associated disorders. Movement disorders : official journal of the Movement Disorder Society. 2010;25(15):2516–23. doi: 10.1002/mds.23393. doi:10.1002/mds.23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds PG, Yokota O, Thurston A, Davidson Y, Ahmed Z, Holton J, Thompson JC, Akiyama H, Arai T, Hasegawa M, Gerhard A, Allsop D, Mann DM. Post mortem cerebrospinal fluid alpha-synuclein levels are raised in multiple system atrophy and distinguish this from the other alpha-synucleinopathies, Parkinson's disease and Dementia with Lewy bodies. Neurobiology of disease. 2012;45(1):188–95. doi: 10.1016/j.nbd.2011.08.003. doi:10.1016/j.nbd.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, Movement Disorder Society URTF. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders : official journal of the Movement Disorder Society. 2008;23(15):2129–70. doi: 10.1002/mds.22340. doi:10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Locascio JJ, Marquie M, Santarlasci AL, Rentz DM, Maye J, Johnson KA, Growdon JH. Brain amyloid and cognition in Lewy body diseases. Movement disorders : official journal of the Movement Disorder Society. 2012;27(8):965–73. doi: 10.1002/mds.25048. doi:10.1002/mds.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, Mathis CA, Elmaleh DR, Shoup T, Fischman AJ, Hyman BT, Growdon JH, Johnson KA. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71(12):903–10. doi: 10.1212/01.wnl.0000326146.60732.d6. doi:10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, Ohrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, Nilsson C, Hakan W, Decraemer H, Nagga K, Minthon L, Londos E, Vanmechelen E, Holmberg B, Zetterberg H, Blennow K, Hansson O. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Archives of neurology. 2012;69(11):1445–52. doi: 10.1001/archneurol.2012.1654. doi:10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, Holtzman DM, Morris JC. Exercise Engagement as a Moderator of the Effects of APOE Genotype on Amyloid Deposition. Archives of neurology. 2012;69(5):636–43. doi: 10.1001/archneurol.2011.845. doi:10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Movement disorders : official journal of the Movement Disorder Society. 2008;23(6):837–44. doi: 10.1002/mds.21956. doi:10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson's disease in the United Kingdom. Movement disorders : official journal of the Movement Disorder Society. 2004;19(9):1043–9. doi: 10.1002/mds.20216. doi:10.1002/mds.20216. [DOI] [PubMed] [Google Scholar]
- Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Leverenz JB, Baird G, Montine TJ, Hancock AM, Hwang H, Pan C, Bradner J, Kang UJ, Jensen PH, Zhang J. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain : a journal of neurology. 2010;133(Pt 3):713–26. doi: 10.1093/brain/awq008. doi:10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Mechanisms linking apolipoprotein E isoforms with cardiovascular and neurological diseases. Current opinion in lipidology. 2010;21(4):337–45. doi: 10.1097/MOL.0b013e32833af368. doi:10.1097/MOL.0b013e32833af368. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. Journal of neurology, neurosurgery, and psychiatry. 1992;55(3):181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, Clark CM, Glosser G, Stern MB, Gollomp SM, Arnold SE. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology. 2000;54(10):1916–21. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological tests' norms above age 55: COWAT, BNT, MAE token, WRAT-R reading, AMNART, STROOP, TMT, and JLO. The Clinical Neuropsychologist. 1996;10(3):262–78. doi:10.1080/13854049608406689. [Google Scholar]
- Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, Coffey CS, Waligorska T, Taylor P, Pan S, Frasier M, Marek K, Kieburtz K, Jennings D, Simuni T, Tanner CM, Singleton A, Toga AW, Chowdhury S, Mollenhauer B, Trojanowski JQ, Shaw LM, the Parkinson's Progression Markers, I. Association of Cerebrospinal Fluid beta-Amyloid 1-42, T-tau, P-tau181, and alpha-Synuclein Levels With Clinical Features of Drug-Naive Patients With Early Parkinson Disease. JAMA neurology. 2013 doi: 10.1001/jamaneurol.2013.3861. doi:10.1001/jamaneurol.2013.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test 2nd Edition. Pro-ED; Austin, TX.: 2001. [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of neurology. 2004;55(3):306–19. doi: 10.1002/ana.20009. doi:10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Korff A, Liu C, Ginghina C, Shi M, Zhang J, Alzheimer's Disease Neuroimaging I. alpha-Synuclein in cerebrospinal fluid of Alzheimer's disease and mild cognitive impairment. Journal of Alzheimer's disease : JAD. 2013;36(4):679–88. doi: 10.3233/JAD-130458. doi:10.3233/JAD-130458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzbauer PT, Cairns NJ, Campbell MC, Willis AW, Racette BA, Tabbal SD, Perlmutter JS. Pathologic accumulation of alpha-synuclein and Abeta in Parkinson disease patients with dementia. Archives of neurology. 2012;69(10):1326–31. doi: 10.1001/archneurol.2012.1608. doi:10.1001/archneurol.2012.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson's disease in the United States. Movement disorders : official journal of the Movement Disorder Society. 2013;28(3):311–8. doi: 10.1002/mds.25292. doi:10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nature genetics. 1998;18(2):106–8. doi: 10.1038/ng0298-106. doi:10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lashley T, Holton JL, Gray E, Kirkham K, O'Sullivan SS, Hilbig A, Wood NW, Lees AJ, Revesz T. Cortical alpha-synuclein load is associated with amyloid-beta plaque burden in a subset of Parkinson's disease patients. Acta neuropathologica. 2008;115(4):417–25. doi: 10.1007/s00401-007-0336-0. doi:10.1007/s00401-007-0336-0. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. Oxford University Press; Oxford: 1995. [Google Scholar]
- Lezak MD, Howieson DB, Loring D. A Compendium of Tests and Assessment Techniques. Oxford University Press; 2004. [Google Scholar]
- Maetzler W, Liepelt I, Reimold M, Reischl G, Solbach C, Becker C, Schulte C, Leyhe T, Keller S, Melms A, Gasser T, Berg D. Cortical PIB binding in Lewy body disease is associated with Alzheimer-like characteristics. Neurobiology of disease. 2009;34(1):107–12. doi: 10.1016/j.nbd.2008.12.008. doi:10.1016/j.nbd.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Maetzler W, Reimold M, Liepelt I, Solbach C, Leyhe T, Schweitzer K, Eschweiler GW, Mittelbronn M, Gaenslen A, Uebele M, Reischl G, Gasser T, Machulla HJ, Bares R, Berg D. [11C]PIB binding in Parkinson's disease dementia. NeuroImage. 2008;39(3):1027–33. doi: 10.1016/j.neuroimage.2007.09.072. doi:10.1016/j.neuroimage.2007.09.072. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(21):12245–50. doi: 10.1073/pnas.211412398. doi:10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. Journal of medicinal chemistry. 2003;46(13):2740–54. doi: 10.1021/jm030026b. doi:10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330(6012):1774. doi: 10.1126/science.1197623. doi:10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–52. doi: 10.1212/01.wnl.0000228230.26044.a4. doi:10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, El-Agnaf OM, Marcus K, Trenkwalder C, Schlossmacher MG. Quantification of alpha-synuclein in cerebrospinal fluid as a biomarker candidate: review of the literature and considerations for future studies. Biomarkers in medicine. 2010;4(5):683–99. doi: 10.2217/bmm.10.90. doi:10.2217/bmm.10.90. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Doring F, Trenkwalder C, Schlossmacher MG. alpha-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet neurology. 2011;10(3):230–40. doi: 10.1016/S1474-4422(11)70014-X. doi:10.1016/S1474-4422(11)70014-X. [DOI] [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. International psychogeriatrics / IPA 9 Suppl. 1997;1:173–6. doi: 10.1017/s1041610297004870. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M. Both familial Parkinson's disease mutations accelerate alpha-synuclein aggregation. The Journal of biological chemistry. 1999;274(14):9843–6. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET. Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. Journal of neuropathology and experimental neurology. 1999;58(11):1147–55. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Pan S, Shi M, Jin J, Albin RL, Lieberman A, Gearing M, Lin B, Pan C, Yan X, Kashima DT, Zhang J. Proteomics identification of proteins in human cortex using multidimensional separations and MALDI tandem mass spectrometer. Molecular & cellular proteomics : MCP. 2007;6(10):1818–23. doi: 10.1074/mcp.M700158-MCP200. doi:10.1074/mcp.M700158-MCP200. [DOI] [PubMed] [Google Scholar]
- Park MJ, Cheon SM, Bae HR, Kim SH, Kim JW. Elevated levels of alpha-synuclein oligomer in the cerebrospinal fluid of drug-naive patients with Parkinson's disease. Journal of clinical neurology. 2011;7(4):215–22. doi: 10.3988/jcn.2011.7.4.215. doi:10.3988/jcn.2011.7.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnetti L, Chiasserini D, Bellomo G, Giannandrea D, De Carlo C, Qureshi MM, Ardah MT, Varghese S, Bonanni L, Borroni B, Tambasco N, Eusebi P, Rossi A, Onofrj M, Padovani A, Calabresi P, El-Agnaf O. Cerebrospinal fluid Tau/alpha-synuclein ratio in Parkinson's disease and degenerative dementias. Movement disorders : official journal of the Movement Disorder Society. 2011;26(8):1428–35. doi: 10.1002/mds.23670. doi:10.1002/mds.23670. [DOI] [PubMed] [Google Scholar]
- Parnetti L, Chiasserini D, Persichetti E, Eusebi P, Varghese S, Qureshi MM, Dardis A, Deganuto M, De Carlo C, Castrioto A, Balducci C, Paciotti S, Tambasco N, Bembi B, Bonanni L, Onofrj M, Rossi A, Beccari T, El-Agnaf O, Calabresi P. Cerebrospinal fluid lysosomal enzymes and alpha-synuclein in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2014a doi: 10.1002/mds.25772. doi:10.1002/mds.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnetti L, Farotti L, Eusebi P, Chiasserini D, De Carlo C, Giannandrea D, Salvadori N, Lisetti V, Tambasco N, Rossi A, Majbour NK, El-Agnaf O, Calabresi P. Differential role of CSF alpha-synuclein species, tau, and Abeta42 in Parkinson's Disease. Frontiers in aging neuroscience. 2014b;6:53. doi: 10.3389/fnagi.2014.00053. doi:10.3389/fnagi.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnikova O, West N, Lee MK, Rudow GL, Skolasky RL, Dawson TM, Marsh L, Troncoso JC. Abeta deposition is associated with enhanced cortical alpha-synuclein lesions in Lewy body diseases. Neurobiology of aging. 2005;26(8):1183–92. doi: 10.1016/j.neurobiolaging.2004.10.006. doi:10.1016/j.neurobiolaging.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Riedel O, Klotsche J, Spottke A, Deuschl G, Forstl H, Henn F, Heuser I, Oertel W, Reichmann H, Riederer P, Trenkwalder C, Dodel R, Wittchen HU. Cognitive impairment in 873 patients with idiopathic Parkinson's disease. Results from the German Study on Epidemiology of Parkinson's Disease with Dementia (GEPAD). Journal of neurology. 2008;255(2):255–64. doi: 10.1007/s00415-008-0720-2. doi:10.1007/s00415-008-0720-2. [DOI] [PubMed] [Google Scholar]
- Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Kim HM, Leverenz JB, Montine TJ, Ginghina C, Kang UJ, Cain KC, Wang Y, Aasly J, Goldstein D, Zhang J. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Annals of neurology. 2011;69(3):570–80. doi: 10.1002/ana.22311. doi:10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderowf A, Xie SX, Hurtig H, Weintraub D, Duda J, Chen-Plotkin A, Shaw LM, Van Deerlin V, Trojanowski JQ, Clark C. CSF amyloid {beta} 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75(12):1055–61. doi: 10.1212/WNL.0b013e3181f39a78. doi:10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaets S, Vanmechelen E, Le Bastard N, Decraemer H, Vandijck M, Martin JJ, De Deyn PP, Engelborghs S. Increased CSF alpha-synuclein levels in Alzheimer's disease: Correlation with tau levels. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2014 doi: 10.1016/j.jalz.2013.10.004. doi:10.1016/j.jalz.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Tateno F, Sakakibara R, Kawai T, Kishi M, Murano T. Alpha-synuclein in the cerebrospinal fluid differentiates synucleinopathies (Parkinson Disease, dementia with Lewy bodies, multiple system atrophy) from Alzheimer disease. Alzheimer disease and associated disorders. 2012;26(3):213–6. doi: 10.1097/WAD.0b013e31823899cc. doi:10.1097/WAD.0b013e31823899cc. [DOI] [PubMed] [Google Scholar]
- Tokuda T, Qureshi MM, Ardah MT, Varghese S, Shehab SA, Kasai T, Ishigami N, Tamaoka A, Nakagawa M, El-Agnaf OM. Detection of elevated levels of alpha-synuclein oligomers in CSF from patients with Parkinson disease. Neurology. 2010;75(20):1766–72. doi: 10.1212/WNL.0b013e3181fd613b. doi:10.1212/WNL.0b013e3181fd613b. [DOI] [PubMed] [Google Scholar]
- Toledo JB, Korff A, Shaw LM, Trojanowski JQ, Zhang J. CSF alpha-synuclein improves diagnostic and prognostic performance of CSF tau and Abeta in Alzheimer's disease. Acta neuropathologica. 2013 doi: 10.1007/s00401-013-1148-z. doi:10.1007/s00401-013-1148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk KD, Persichetti E, Chiasserini D, Eusebi P, Beccari T, Calabresi P, Berendse HW, Parnetti L, van de Berg WD. Changes in endolysosomal enzyme activities in cerebrospinal fluid of patients with Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2013;28(6):747–54. doi: 10.1002/mds.25495. doi:10.1002/mds.25495. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Knopman DS, Petersen RC, Jack CR, Jr., Alzheimer's Disease Neuroimaging I. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology. 2009;73(4):287–93. doi: 10.1212/WNL.0b013e3181af79e5. doi:10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shi M, Chung KA, Zabetian CP, Leverenz JB, Berg D, Srulijes K, Trojanowski JQ, Lee VM, Siderowf AD, Hurtig H, Litvan I, Schiess MC, Peskind ER, Masuda M, Hasegawa M, Lin X, Pan C, Galasko D, Goldstein DS, Jensen PH, Yang H, Cain KC, Zhang J. Phosphorylated alpha-synuclein in Parkinson's disease. Science translational medicine. 2012;4(121):121ra20. doi: 10.1126/scitranslmed.3002566. doi:10.1126/scitranslmed.3002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - III. The Psychological Corporation; San Antonio, TX.: 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale III. The Psychological Corporation; San Antonio, TX.: 1997b. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson Tests of Achievement. Riverside Publishing Company; Itasca, IL.: 2001. [Google Scholar]
- Zhang J, Sokal I, Peskind ER, Quinn JF, Jankovic J, Kenney C, Chung KA, Millard SP, Nutt JG, Montine TJ. CSF multianalyte profile distinguishes Alzheimer and Parkinson diseases. American journal of clinical pathology. 2008;129(4):526–9. doi: 10.1309/W01Y0B808EMEH12L. doi:10.1309/W01Y0B808EMEH12L. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.