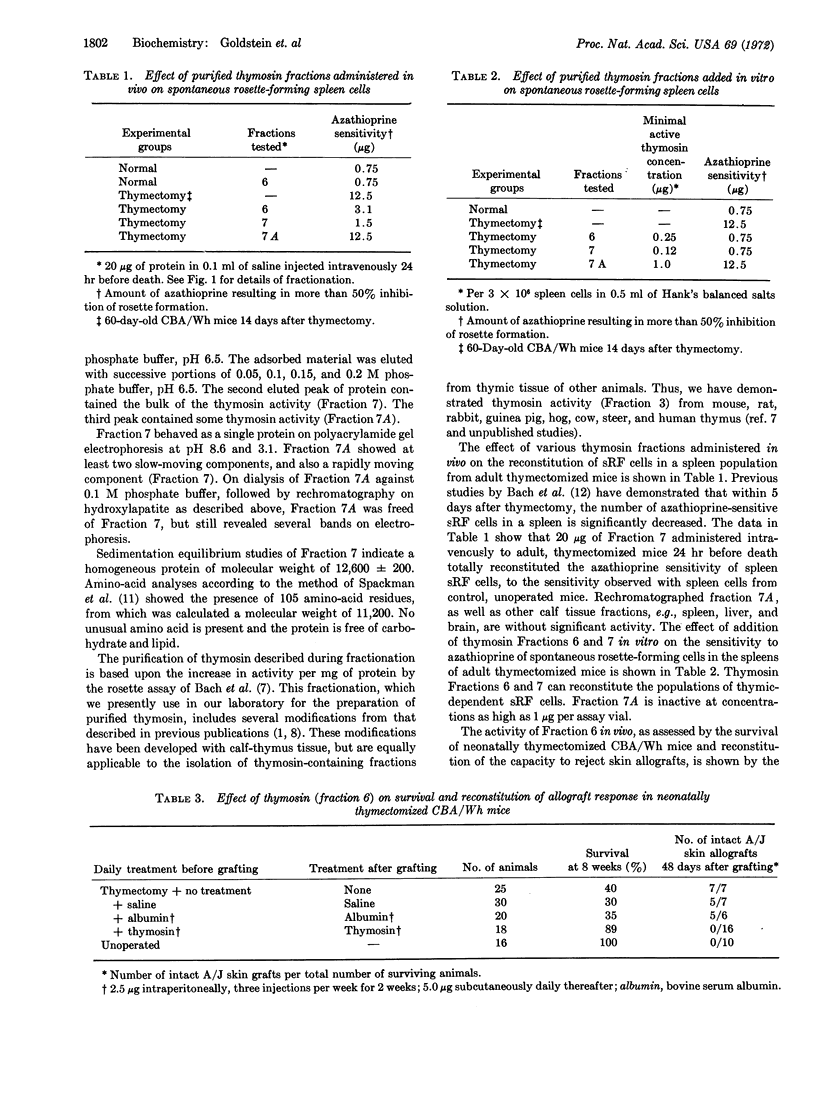
Abstract
The purification and chemical properties of thymosin, obtained from bovine thymus tissue, are described. The biological activity of the thymic hormone has been assessed by a newly developed rosette assay, which permits measurement of thymus-dependent lymphoid cells. Thymosin activity is associated with a physico-chemically homogeneous protein of molecular weight 12,600. The hormonal activity is evident in in vitro incubation assay, after injection into adult thymectomized mice, and in prolonging survival of neonatally thymectomized mice and the reconstitution of their response to a skin allograft.
Keywords: thymus, rosette-forming cells, thymectomy, lymphoid cells, mouse
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asanuma Y., Goldstein A. L., White A. Reduction in the incidence of wasting disease in neonatally thymectomized CBA-W mice by the injection of thymosin. Endocrinology. 1970 Mar;86(3):600–610. doi: 10.1210/endo-86-3-600. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Dardenne M. Antigen recognition by T lymphocytes. II. Similar effects of azathioprine, antilymphocyte serum, and anti-theta serum on rosette-forming lymphocytes in normal and neonatally thymectomized mice. Cell Immunol. 1972 Jan;3(1):11–21. doi: 10.1016/0008-8749(72)90221-3. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Dardenne M., Davies A. J. Early affect of adult thymectomy. Nat New Biol. 1971 May 26;231(21):110–111. doi: 10.1038/newbio231110a0. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Dardenne M., Goldstein A. L., Guha A., White A. Appearance of T-cell markers in bone marrow rosette-forming cells after incubation with thymosin, a thymic hormone. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2734–2738. doi: 10.1073/pnas.68.11.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Goldstein A. L., Asanuma Y., Battisto J. R., Hardy M. A., Quint J., White A. Influence of thymosin on cell-mediated and humoral immune responses in normal and in immunologically deficient mice. J Immunol. 1970 Feb;104(2):359–366. [PubMed] [Google Scholar]
- Goldstein A. L., Asanuma Y., White A. The thymus as an endocrine gland: properties of thymosin, a new thymus hormone. Recent Prog Horm Res. 1970;26:505–538. doi: 10.1016/b978-0-12-571126-5.50016-9. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., Guha A., Howe M. L., White A. Ontogenesis of cell-mediated immunity in murine thymocytes and spleen cells and its acceleration by thymosin, a thymic hormone. J Immunol. 1971 Mar;106(3):773–780. [PubMed] [Google Scholar]
- Goldstein A. L., Slater F. D., White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proc Natl Acad Sci U S A. 1966 Sep;56(3):1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Law L. W., Goldstein A. L., White A. Influence of thymosin on immunological competence of lymphoid cells from thymectomized mice. Nature. 1968 Sep 28;219(5161):1391–1392. doi: 10.1038/2191391a0. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Osoba D. Current concepts of the immunological function of the thymus. Physiol Rev. 1967 Jul;47(3):437–520. doi: 10.1152/physrev.1967.47.3.437. [DOI] [PubMed] [Google Scholar]
- Zisblatt M., Goldstein A. L., Lilly F., White A. Acceleration by thymosin of the development of resistance to murine sarcoma virus-induced tumor in mice. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1170–1174. doi: 10.1073/pnas.66.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]


