Abstract
Viral RNAs accumulate to high levels during infection and interact with a variety of cellular factors including miRNAs and RNA-binding proteins. Although many of these interactions exist to directly modulate replication, translation and decay of viral transcripts, evidence is emerging that abundant viral RNAs may in certain cases serve as a sponge to sequester host non coding RNAs and proteins. By effectively reducing the ability of cellular RNA binding proteins to regulate host cell gene expression, viral RNAs can alter the response to infection and favor viral replication. This review focuses on the potential contribution that sequestration of cellular proteins by viral RNAs makes to viral replication and cytopathology.
Introduction
Virus-host interactions play key roles in promoting viral replication and gene expression. In addition, these interactions can influence how the virus interfaces with cellular innate immune mechanisms, initiates cytopathology, and determines overall pathogenic outcomes of infections. Over the years, we have learned a great deal regarding how numerous viral proteins interact with host factors and promote viral infections. We also have an in-depth understanding of how viral mRNAs commandeer the host machinery to regulate their own stability and translation. However, with the discovery of a multitude of non-coding RNAs (such as miRNAs and long noncoding RNAs (lncRNAs)) within eukaryotic genomes, it is now abundantly clear that RNAs can also play vital roles as regulators of gene expression in their own right. Of course, miRNAs are the best-studied example of how a non-coding RNA can regulate gene expression through RNA-RNA interactions. However, there are a growing number of studies that indicate that RNAs, including mRNAs, can also interact with proteins and down-regulate their functions. The goal of this review is to summarize recent data demonstrating that viral RNAs can act as a decoy and interact directly with cellular proteins to sequester them away from their normal tasks. Given the large number of viral RNAs that can accumulate in an infected cell, we speculate that such virus encoded ‘sponges’ may be important but generally overlooked contributors to the successful replication.
Viral and cellular RNA sponges can target and down-regulate other RNAs
Cells contain a number of characterized ‘competing endogenous RNAs’ or ceRNAs that appear to serve as sponges to sequester and down-regulate the activity of miRNAs [1, 2]. Examples include the H19 lncRNA which targets the let7 miRNA [3] and the Lnc-Md-1 RNA which acts as a decoy for miR133 [4]. Abundant small circular RNAs generated by ‘backsplicing’ have also been implicated as miRNA sponges [5]. Interestingly, recent global analyses have suggested the presence of ceRNA-driven regulatory networks where combinations of ceRNAs, miRNAs and mRNAs determine important aspects of cellular processes and may play a role in diseases such as breast and gastric cancers [6, 7]. These RNA-driven networks have also been implicated in influencing the expression of key developmental regulators [8]. However, whether naturally occurring ceRNAs are abundant enough to significantly influence miRNA availability has recently been called into question through a quantitative analysis of miRNA122 and its endogenous target sites [9]. Thus, the ability of an RNA to act as an effective sponge and thereby modulate cellular functions is determined both by the affinity of the interaction and the abundance of the sponge itself.
The introduction of designer RNA sponges has been demonstrated to be an effective means of down-regulating the functions of major miRNAs such as miR23b [10], miR-101 [11], miR-151 [12] and others [13]. These synthetic RNA sponges have been delivered using a variety of vectors – including lentiviruses. This strongly implies that natural RNA sponges encoded by viruses may be expressed at high enough levels to wreak havoc on post-transcriptional regulatory networks in infected cells by targeting miRNAs. Indeed, there are already several examples of virally-encoded RNA sponges that do just that. These include the non-coding U-rich HSUR1 transcripts made by Herpesvirus saimiri [14] which targets cellular miR-27 for degradation, the m169 transcript of murine cytomegalovirus which also inhibits miR-27 [15], and the sponging of cellular miRNAs encoded by the 15a/16 cluster by Hepatitis B virus mRNAs [16]. Importantly, since viral transcripts are often present in very large quantities relative to natural cellular ceRNAs, these viral RNAs may very well be present in such high ratios relative to their targets that could indeed effectively sequester cellular miRNAs (thus overcoming the concerns raised by the Denzler et al study [9]). In summary, viruses have clearly borrowed/adapted the ‘ceRNA’ strategy of RNA sponging into their arsenal of host interactions to dysregulate cellular gene expression.
Viral RNAs may also serve as effective sponges of cellular RNA binding proteins
Countless interactions between cellular RNA binding proteins and viral RNAs have been reported over the last several decades. Many of these interactions have been documented to promote specific aspects of viral gene expression/replication. However, a largely overlooked consequence of abundant viral transcripts interacting with cellular proteins may be to reduce the effective cellular concentrations of free forms of the RNA binding proteins. If this occurs, one would expect to observe a significant diminution of the natural function on the sequestered cellular RNA binding protein. Let’s look at a few lines of evidence that suggest this could be the case.
First, there are two well-documented cases where the expression of a non-coding RNA segment results in a pathological condition due to sequestration of RNA binding proteins. In myotonic dystrophy, a CTG triplet repeat microsatellite in the 3’ UTR of the DMPK gene is expanded to hundreds of repeats [17]. The expansion of this repeat in the noncoding region of the transcript results in the accumulation of a ‘toxic’ RNA in nuclear foci that sequesters the important regulatory RNA binding protein muscleblind like 1 (MBNL1) [18]as well as dysregulates the expression of the CELF1 protein [19]. Both of these factors have been implicated in the regulation of cellular mRNA splicing and mRNA stability [20]. More recently, it has been recognized that a GGGGCC hexameric repeat expansion within the first intron of the C9ORF72 gene is the most common genetic cause of neurotoxicity associated with amyotrophic lateral sclerosis (ALS) and frontotemporal dementia [21]. The GGGGCC repeat-containing RNA accumulates in nuclear foci and sequester hnRNP H, hnRNP A1, ASF/SF2 and other RNA binding proteins in a fashion similar to the CUG repeat-containing mRNA in myotonic dystrophy [22–24]. Thus the sequestration of cellular RBPs by aberrantly expressed RNAs is a clearly documented cause of pathology. This sets the precedent for the possibility that abundantly expressed viral RNAs targeting cellular proteins can act in a similar fashion and cause pathology in host cells.
Two recent examples nicely document the ability of viral RNAs to sequester/sponge cellular proteins and lead to dysregulation of gene expression that may be associated with pathology. G3BP1, G3BP2 and CAPRIN1 are three cellular RNA binding proteins that are required for an efficient interferon-β response by promoting the translation of Interferon Stimulated Gene (ISG)-encoding mRNAs [25]. During infections with Dengue virus (DENV) and other insect-borne flaviviruses, large amounts of a small non-coding RNA encompassing the 3’ UTR of viral mRNAs accumulate in cells. The generation of this small flavivirus RNA or ‘sfRNA’ is not due to a subgenomic promoter, but instead is the result of stalling of the cellular exoribonuclease XRN1 at a novel knot-like RNA structure positioned just downstream of the large open reading frame of viral transcripts [26, 27]. Recently, Garcia-Blanco and coworkers have used RNA-protein immunoprecipitation (RIP) analysis and 3’UTR mimics to demonstrate that DENV sfRNA effectively binds to and down-regulates the activity of these three cellular proteins [25]. The consequence of sfRNA-mediated sponging of G3BP1, G3BP2 and CAPRIN1 proteins is to effectively antagonize the host interferon response against DENV in infected cells. Thus the ability of sfRNA to act as a protein sponge has obvious implications for viral pathogenesis.
The second example involves the sponging/sequestration of the cellular HuR protein. HuR is an important regulator of cellular RNA stability that plays a major role in regulating the expression of many short-lived AU-rich element containing mRNAs, including those encoding innate immune mediators and cell cycle proteins [28, 29]. Sindbis virus and several other members of the alphavirus family contain conserved high affinity HuR binding sites in the 3’ UTR [30]. Since the same 3’ UTR is present on both the genomic and abundant subgenomic mRNAs generated by these plus-sense members of the Togaviridae, viral RNAs containing this HuR binding site readily accumulate to high levels in infected cells. While viral transcripts usurp HuR to assist in their stabilization [31, 32], recent evidence using viral infections and transfections of noncoding viral 3’UTR RNA segments indicates that the viral 3’ UTR is also effectively serving as a sponge for HuR protein in infected cells [33]. HuR sponging by SINV results in the relocalization of HuR from the nucleus to the cytoplasm in infected cells, apparently through preventing HuR protein from shuttling back into the nucleus. HuR sponging by SINV also causes destabilization of numerous cellular mRNAs that normally rely on HuR binding to enhance their stability [33]. Finally, HuR-mediated polyadenylation and splicing events in the nucleus are also altered by viral UTR-mediated protein sponging in infected or transfected cells [33]. These major changes in cellular mRNA stability and nuclear mRNA processing that result in the dysregulation of cellular gene expression very likely play a role in viral-based cytopathology.
Interestingly, a recent report also documents a virally-encoded RNA-mediated protein sponge as a regulator of viral protein function. The abundant long noncoding PAN RNA made in Kaposi Sarcoma Herpesvirus (KSHV) infections can serve as a sponge for the viral LANA protein to sequester the viral factor away from histones. This activity assists in removing LANA from the viral DNA episome to facilitate the reactivation of the virus from latent infection [34]. Thus, viral RNA sponges can also contribute to virus-specific regulation of gene expression. Along these lines, it is important to note that RNA aptamers and nanoparticles have also been demonstrated to effectively serve as protein sponges, making these macromolecules candidates for antiviral therapeutics [35, 36]. Therefore the balance of viral RNA sponges to their targets is likely to be just as important to virally-encoded networks of gene expression as they are to cellular ceRNA-mediated networks noted above.
Are RNA-mediated protein sponges a major viral strategy to disrupt the function of host proteins during infections?
While the clear answer to this question awaits additional experimentation, one can put forward the argument that any cellular protein recruited by abundant viral RNAs to facilitate replication, stabilization or translation is simultaneously being prevented from carrying out its normal functions. Numerous cellular proteins including DDX6 [37], NF90 [38], p100 [39], polypyrimidine tract-binding protein (PTB) [40], poly(A) binding protein (PABP) [41], Y box-binding protein 1 [42], and La [43] have been demonstrated to interact with the 3’ UTR/sfRNA of Dengue virus. Could these cellular proteins be sequestered/sponged in a similar fashion to G3BP1, G3BP2 and CAPRIN1? Likewise, in Sindbis virus infections the cellular hnRNP K protein has been demonstrated to interact with the abundant subgenomic Sindbis virus mRNA [44] and packaged cellular proteins appear to play a role in enhanced SINV infectivity [45]. Therefore the potential exists for both DENV and SINV RNAs to sponge additional cellular proteins.
Methods are now fairly well-established for assessing the array of cellular proteins that can interact with viral RNAs [e.g. 46–48]. It should be very interesting to take a more comprehensive look at the interactions between cellular proteins and abundant viral RNAs and then specifically assess the functional consequences of these interactions in infected cells. One can experimentally separate the effects of sponging cellular RNA binding proteins from effects of RNA-protein interactions on viral replication/translation simply by expressing or transfecting the viral RNA regions on their own in the absence of any other viral gene products [25, 33]. Given the growing appreciation for RNA-mediated regulation in cell biology, there is a very good chance that protein sponging by viral RNAs plays a much greater role in viral pathogenesis than has been appreciated to date.
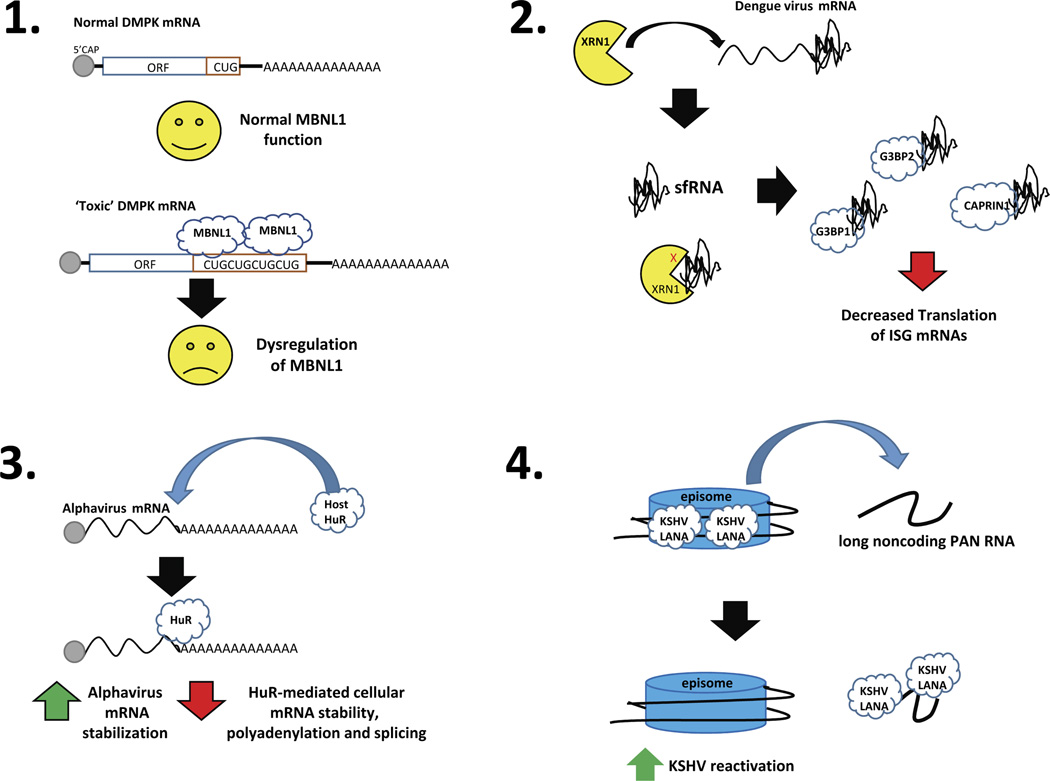
Figure 1.
The functional consequences of four examples of RNA-mediated protein sponging. Panel 1: Expanded CUG repeat sequences in the 3’ UTR of the DMPK mRNA serve as a sponge for the muscleblind (MBNL1) RNA binding protein, resulting in dysregulated RNA splicing that contributes to the disease myotonic dystrophy. Panel 2: Dengue virus mRNA contains a structure in its 3’ UTR that stalls and represses the cellular XRN1 exoribonuclease. The large amount of the stable decay intermediates – called sfRNA – is then able to sponge the indicated cellular RNA binding proteins and repress translation of mRNAs involved in innate immunity. Panel 3: Alphavirus mRNAs contain a high affinity binding site in their 3’ UTRs that binds the cellular HuR protein, resulting in increased viral RNA stability as well as sponging of the cellular factor that dysregulates its normal function. Panel 4: The KSHV PAN RNA serves as a sponge for the viral LANA protein, removing it from viral episomes and facilitating reactivation of latent virus.
Highlights.
Abundant viral RNAs can act as sponges for cellular factors
Dengue and Sindbis viral RNAs sequester select cellular RNA binding proteins
RNA-mediated sequestration of host proteins dysregulates gene expression
Protein sponging has significant implications for viral pathogenesis
Acknowledgements
We wish to thank members of the Wilusz laboratories for constructive feedback. Virus-related research in the laboratory is funded by grant award U54 AI-065357 from the NIAID / the Rocky Mountain Regional Center of Excellence.
Footnotes
Publisher's Disclaimer: This is a PDF .le of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. **one of the first two studies to describe competing endogenous RNAs
- 2. Tay Y, Kats L, Salmena L, Weiss D, Tan SM, Ala U, Karreth F, Poliseno L, Provero P, Di Cunto F, Lieberman J, Rigoutsos I, Pandolfi PP. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell. 2011;147:344–357. doi: 10.1016/j.cell.2011.09.029. **one of the first two studies to describe competing endogenous RNAs
- 3. Kallen AN, Zhou XB, Xu J, Qiao C, Ma J, Yan L, Lu L, Liu C, Yi JS, Zhang H, Min W, Bennett AM, Gregory RI, Ding Y, Huang Y. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell. 2013;52:101–112. doi: 10.1016/j.molcel.2013.08.027. *demonstrates a role for a lncRNA that’s been implicated in human disease in the downregulation of a major class of miRNAs in cells.
- 4.Legnini I, Morlando M, Mangiavacchi A, Fatica A, Bozzoni I. A feedforward regulatory loop between HuR and the long noncoding RNA linc-MD1 controls early phases of myogenesis. Mol Cell. 2014;53:506–514. doi: 10.1016/j.molcel.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. **provided major insight into the role of circular RNAs generated by backsplicing
- 6.Paci P, Colombo T, Farina L. Computational analysis identifies a sponge interaction network between long non-coding RNAs and messenger RNAs in human breast cancer. BMC Syst Biol. 2014;8:83. doi: 10.1186/1752-0509-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia T, Liao Q, Jiang X, Shao Y, Xiao B, Xi Y, Guo J. Long noncoding RNA associated-competing endogenous RNAs in gastric cancer. Sci Rep. 2014;4:6088. doi: 10.1038/srep06088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Xu Z, Jiang J, Xu C, Kang J, Xiao L, Wu M, Xiong J, Guo X, Liu H. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell. 2013;25:69–80. doi: 10.1016/j.devcel.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Denzler R, Agarwal V, Stefano J, Bartel DP, Stoffel M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol Cell. 2014;54:766–776. doi: 10.1016/j.molcel.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, Zhang K, Shi Z, Zhang A, Jia Z, Wang G, Pu P, Kang C, Han L. A lentivirus-mediated miR-23b sponge diminishes the malignant phenotype of glioma cells in vitro and in vivo. Oncol Rep. 2014;31:1573–1580. doi: 10.3892/or.2014.3012. [DOI] [PubMed] [Google Scholar]
- 11.Barbato C, Pezzola S, Caggiano C, Antonelli M, Frisone P, Ciotti MT, Ruberti F. A lentiviral sponge for miR-101 regulates RanBP9 expression and amyloid precursor protein metabolism in hippocampal neurons. Front Cell Neurosci. 2014;8:37. doi: 10.3389/fncel.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CL, Wu JC, Chen GY, Yuan PH, Tseng YW, Li KC, Hwang SM, Hu YC. Baculovirus-Mediated miRNA Regulation to Suppress Hepatocellular Carcinoma Tumorigenicity and Metastasis. Mol Ther. 2014 doi: 10.1038/mt.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bak RO, Mikkelsen JG. miRNA sponges: soaking up miRNAs for regulation of gene expression. Wiley Interdiscip Rev RNA. 2014;5:317–333. doi: 10.1002/wrna.1213. [DOI] [PubMed] [Google Scholar]
- 14. Guo YE, Riley KJ, Iwasaki A, Steitz JA. Alternative capture of noncoding RNAs or protein-coding genes by herpesviruses to alter host T cell function. Mol Cell. 2014;54:67–79. doi: 10.1016/j.molcel.2014.03.025. ** demonstrates multiple strategies, including miRNA sponges, used by gamma herpesviruses to downregulate select T cell genes.
- 15.Libri V, Helwak A, Miesen P, Santhakumar D, Borger JG, Kudla G, Grey F, Tollervey D, Buck AH. Murine cytomegalovirus encodes a miR-27 inhibitor disguised as a target. Proc Natl Acad Sci U S A. 2012;109:279–284. doi: 10.1073/pnas.1114204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu N, Zhang J, Jiao T, Li Z, Peng J, Cui Z, Ye X. Hepatitis B virus inhibits apoptosis of hepatoma cells by sponging the MicroRNA 15a/16 cluster. J Virol. 2013;87:13370–13378. doi: 10.1128/JVI.02130-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickson AM, Wilusz CJ. Repeat expansion diseases: when a good RNA turns bad. Wiley Interdiscip Rev RNA. 2010;1:173–192. doi: 10.1002/wrna.18. [DOI] [PubMed] [Google Scholar]
- 18.Lee KY, Li M, Manchanda M, Batra R, Charizanis K, Mohan A, Warren SA, Chamberlain CM, Finn D, Hong H, et al. Compound loss of muscleblind-like function in myotonic dystrophy. EMBO Mol Med. 2013;5:1887–1900. doi: 10.1002/emmm.201303275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasgupta T, Ladd AN. The importance of CELF control: molecular and biological roles of the CUG-BP, Elav-like family of RNA-binding proteins. Wiley Interdiscip Rev RNA. 2012;3:104–121. doi: 10.1002/wrna.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masuda A, Andersen HS, Doktor TK, Okamoto T, Ito M, Andresen BS, Ohno K. CUGBP1 and MBNL1 preferentially bind to 3' UTRs and facilitate mRNA decay. Sci Rep. 2012;2:209. doi: 10.1038/srep00209. *expands the function of these two mediators of myotonic dystrophy beyond splicing to include mRNA stability
- 21. Mizielinska S, Grönke S, Niccoli T, Ridler CE, Clayton EL, Devoy A, Moens T, Norona FE, Woollacott IO, Pietrzyk J, et al. C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science. 2014 Aug 7;:1256800. doi: 10.1126/science.1256800. pii. ** reveals molecular mechanisms that underlie the neurotoxicity of an expanded repeat.
- 22.Lee YB, Chen HJ, Peres JN, Gomez-Deza J, Attig J, Stalekar M, Troakes C, Nishimura AL, Scotter EL, Vance C, et al. Hexanucleotide repeats in ALS/FTD form length-dependent RNA foci, sequester RNA binding proteins, and are neurotoxic. Cell Rep. 2013;5:1178–1186. doi: 10.1016/j.celrep.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zamiri B, Reddy K, Macgregor RB, Jr, Pearson CE. TMPyP4 porphyrin distorts RNA G-quadruplex structures of the disease-associated RGGGGCC)n repeat of the C9orf72 gene and blocks interaction of RNA-binding proteins. J Biol Chem. 2014;289:4653–4659. doi: 10.1074/jbc.C113.502336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper-Knock J, Walsh MJ, Higginbottom A, Robin Highley J, Dickman MJ, Edbauer D, Ince PG, Wharton SB, Wilson SA, Kirby J, et al. Sequestration of multiple RNA recognition motif-containing proteins by C9orf72 repeat expansions. Brain. 2014;137:2040–2051. doi: 10.1093/brain/awu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bidet K, Dadlani D, Garcia-Blanco MA. G3BP1, G3BP2 and CAPRIN1 are required for translation of interferon stimulated mRNAs and are targeted by a dengue virus non-coding RNA. PLoS Pathog. 2014;10:e1004242. doi: 10.1371/journal.ppat.1004242. **Demonstrates RNA sponge activity of the abundant sfRNA made by Dengue virus.
- 26.Roby JA, Pijlman GP, Wilusz J, Khromykh AA. Noncoding subgenomic flavivirus RNA: multiple functions in West Nile virus pathogenesis and modulation of host responses. Viruses. 2014;6:404–427. doi: 10.3390/v6020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chapman EG, Costantino DA, Rabe JL, Moon SL, Wilusz J, Nix JC, Kieft JS. The structural basis of pathogenic subgenomic flavivirus RNA (sfRNA) production. Science. 2014;344:307–310. doi: 10.1126/science.1250897. ** determines a novel knot-like RNA structure that stalls and represses a major cellular exoribonuclease
- 28.Simone LE, Keene JD. Mechanisms coordinating ELAV/Hu mRNA regulons. Curr Opin Genet Dev. 2013;23:35–43. doi: 10.1016/j.gde.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srikantan S, Gorospe M. HuR function in disease. Front Biosci. 2012;17:189–205. doi: 10.2741/3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickson AM, Anderson JR, Barnhart MD, Sokoloski KJ, Oko L, Opyrchal M, Galanis E, Wilusz CJ, Morrison TE, Wilusz J. Dephosphorylation of HuR protein during alphavirus infection is associated with HuR relocalization to the cytoplasm. J Biol Chem. 2012;287:36229–36238. doi: 10.1074/jbc.M112.371203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garneau NL, Sokoloski KJ, Opyrchal M, Neff CP, Wilusz CJ, Wilusz J. The 3' untranslated region of sindbis virus represses deadenylation of viral transcripts in mosquito and mammalian cells. J Virol. 2008;82:880–892. doi: 10.1128/JVI.01205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sokoloski KJ, Dickson AM, Chaskey EL, Garneau NL, Wilusz CJ, Wilusz J. Sindbis virus usurps the cellular HuR protein to stabilize its transcripts and promote productive infections in mammalian and mosquito cells. Cell Host Microbe. 2010;8:196–207. doi: 10.1016/j.chom.2010.07.003. *First demonstration that Sindbis virus uses the cellular HuR protein to stabilize its RNA
- 33. Barnhart MD, Moon SL, Emch AW, Wilusz CJ, Wilusz J. Changes in cellular mRNA stability, splicing, and polyadenylation through HuR protein sequestration by a cytoplasmic RNA virus. Cell Rep. 2013;5:909–917. doi: 10.1016/j.celrep.2013.10.012. **Demonstrates RNA-mediated sponging of the cellular HuR protein by a non-coding region of an alphavirus transcript
- 34.Campbell M, Kim KY, Chang PC, Huerta S, Shevchenko B, Wang DH, Izumiya C, Kung HJ, Izumiya Y. A lytic viral long noncoding RNA modulates the function of a latent protein. J Virol. 2014;88:1843–1848. doi: 10.1128/JVI.03251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaworski E, Saifuddin M, Sampey G, Shafagati N, Van Duyne R, Iordanskiy S, Kehn-Hall K, Liotta L, Petricoin E, 3rd, Young M, et al. The use of Nanotrap particles technology in capturing HIV-1 virions and viral proteins from infected cells. PLoS One. 2014;9:e96778. doi: 10.1371/journal.pone.0096778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CH, Lee YJ, Kim JH, Lim JH, Kim JH, Han W, Lee SH, Noh GJ, Lee SW. Inhibition of hepatitis C virus (HCV) replication by specific RNA aptamers against HCV NS5B RNA replicase. J Virol. 2013;87:7064–7074. doi: 10.1128/JVI.00405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward AM, Bidet K, Yinglin A, Ler SG, Hogue K, Blackstock W, Gunaratne J, Garcia-Blanco MA. Quantitative mass spectrometry of DENV-2 RNA-interacting proteins reveals that the DEAD-box RNA helicase DDX6 binds the DB1 and DB2 3' UTR structures. RNA Biol. 2011;8:1173–1186. doi: 10.4161/rna.8.6.17836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomila RC, Martin GW, Gehrke L. NF90 binds the dengue virus RNA 3' terminus and is a positive regulator of dengue virus replication. PLoS One. 2011;6:e16687. doi: 10.1371/journal.pone.0016687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei Y, Huang Y, Zhang H, Yu L, Zhang M, Dayton A. Functional interaction between cellular p100 and the dengue virus 3' UTR. J Gen Virol. 2011;92:796–806. doi: 10.1099/vir.0.028597-0. [DOI] [PubMed] [Google Scholar]
- 40.Anwar A, Leong KM, Ng ML, Chu JJ, Garcia-Blanco MA. The polypyrimidine tract-binding protein is required for efficient dengue virus propagation and associates with the viral replication machinery. J Biol Chem. 2009;284:17021–17029. doi: 10.1074/jbc.M109.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polacek C, Friebe P, Harris E. Poly(A)-binding protein binds to the non-polyadenylated 3' untranslated region of dengue virus and modulates translation efficiency. J Gen Virol. 2009;90:687–692. doi: 10.1099/vir.0.007021-0. [DOI] [PubMed] [Google Scholar]
- 42.Paranjape SM, Harris E. Y box-binding protein-1 binds to the dengue virus 3'-untranslated region and mediates antiviral effects. J Biol Chem. 2007;282:30497–30508. doi: 10.1074/jbc.M705755200. [DOI] [PubMed] [Google Scholar]
- 43.Yocupicio-Monroy RM, Medina F, Reyes-del Valle J, del Angel RM. Cellular proteins from human monocytes bind to dengue 4 virus minus-strand 3' untranslated region RNA. J Virol. 2003;77:3067–3076. doi: 10.1128/JVI.77.5.3067-3076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnham AJ, Gong L, Hardy RW. Heterogeneous nuclear ribonuclear protein K interacts with Sindbis virus nonstructural proteins and viral subgenomic mRNA. Virology. 2007;367:212–221. doi: 10.1016/j.virol.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Sokoloski KJ, Snyder AJ, Liu NH, Hayes CA, Mukhopadhyay S, Hardy RW. Encapsidation of host-derived factors correlates with enhanced infectivity of Sindbis virus. J Virol. 2013;87:12216–12226. doi: 10.1128/JVI.02437-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward AM, Gunaratne J, Garcia-Blanco MA. Identification of dengue RNA binding proteins using RNA chromatography and quantitative mass spectrometry. Methods Mol Biol. 2014;1138:253–270. doi: 10.1007/978-1-4939-0348-1_16. [DOI] [PubMed] [Google Scholar]
- 47.Gebauer F, Preiss T, Hentze MW. From cis-regulatory elements to complex RNPs and back. Cold Spring Harb Perspect Biol. 2012;4:a012245. doi: 10.1101/cshperspect.a012245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riley KJ, Steitz JA. The "Observer Effect" in genome-wide surveys of protein-RNA interactions. Mol Cell. 2013;49:601–604. doi: 10.1016/j.molcel.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]