Abstract
Holoprosencephaly (HPE) is the most common developmental defect of the forebrain characterized by inadequate or absent midline division of the forebrain into cerebral hemispheres, with concomitant midline facial defects in the majority of cases. Understanding the pathogenesis of HPE requires knowledge of the relationship between the developing brain and the facial structures during embryogenesis. A number of signalling pathways control and coordinate the development of the brain and face, including Sonic hedgehog (SHH), Bone Morphogenetic Protein (BMP), Fibroblast Growth Factor (FGF), and Nodal signalling. Mutations in these pathways have been identified in animal models of HPE and human patients. Due to incomplete penetrance and variable expressivity of HPE, patients carrying defined mutations may not manifest the disease at all, or have a spectrum of defects. It is currently unknown what drives manifestation of HPE in genetically at risk individuals, but it has been speculated that other gene mutations and environmental factors may combine as cumulative insults. HPE can be diagnosed in utero by a high-resolution prenatal ultrasound or a fetal magnetic resonance imaging, sometimes in combination with molecular testing from chorionic villi or amniotic fluid sampling. Currently, there are no effective preventive methods for HPE. Better understanding of the mechanisms of gene-environment interactions in HPE would provide avenues for such interventions.
Introduction
Holoprosencephaly (HPE; OMIM 236100) is characterized by inadequate or absent midline division of the developing forebrain into cerebral hemispheres (Fig. 1), with concomitant midline facial defects in about 80% of the cases.1 In 1–10% of cases, HPE may be associated with jaw defects.2–4 HPE is the most common developmental defect of the forebrain with an incidence of 1 in 250 conceptuses and about 1 in every 10,000 at term.5, 6 The etiology includes both genetic and environmental causes (e.g. maternal diabetes, prenatal exposure to ethyl alcohol, retinoic acid).7–9 An important feature of HPE is incomplete penetrance and variable expressivity. Patients carrying defined mutations may not manifest the disease at all, or have a spectrum of defects ranging from mild defects referred to as microforms (hypotelorism, midfacial hypoplasia, a single maxillary central incisor) that are generally non-lethal, to severe (cyclopia, proboscis), which are usually lethal.6, 10 It is currently unknown what drives manifestation of HPE in genetically at risk individuals, but it has been speculated that other gene mutations and environmental factors may combine as cumulative insults.11 Currently, there are no effective preventive methods for HPE, and children who survive require long-term multidisciplinary care at a significant financial and emotional cost.
Fig. 1. Holoprosencephaly in humans and mice.
(A) Normal human brain MRI. The two cerebral hemispheres are completely separated. The septum (arrow) and the corpus callosum (arrowhead) are present (reprinted with permission from Geng et al. 20091, American Society for Clinical Investigation). (B) MRI of a neonate with alobar HPE showing a monoventricle (MV) and a large dorsal cyst (DC) posteriorly (reprinted with permission from Hahn et al. 201024, John Wiley and Sons). (C) Transverse section of wild type mouse brain at birth. (D) Nonseparation of the brain and a large monoventricle in Twsg1−/− mouse at birth (C and D reprinted with permission from Petryk et al. 200429, Elsevier).
Much of the understanding about the embryological and genetic basis of HPE has been gained by studying mouse and other animal models (chicken, zebrafish) of HPE.12 Mouse models offer an experimental advantage because of a similar embryonic development of the forebrain and face between mice and humans, the ability to engineer disease-causing mutations, and because variables like environment and genetic background can be controlled.1, 13
Morphogenetic events during brain and face development are controlled and coordinated by what appears to be only a handful of extracellular signalling networks, such as Sonic hedgehog (SHH), Bone Morphogenetic Protein (BMP), Fibroblast Growth Factor (FGF), Nodal, and retinoid signalling. All these signalling pathways are used in a reiterated fashion not only during brain and face development, but also during embryonic development as a whole. These factors are often produced together in signalling centers from where they specify and pattern surrounding cells and tissues. Numerous regulatory loops and crosstalk between the different signalling pathways buffer against perturbations, making the system somewhat resilient to genetically or environmentally caused fluctuations. Place, time, and duration of the signalling activity are all important. Signal strength is often fine-tuned by accessory molecules (co-receptors, agonist, antagonists). Once signalling falls outside the limits of tolerance, a phenotypic change may become manifest.
Signalling from the ventral midline is critical for normal midface development, a process in which SHH plays a key role.8, 14, 15 Mutations in SHH are the most common genetic cause of HPE in humans6 and many of the HPE genes encode proteins that either directly or indirectly regulate SHH expression or signalling. Some of the environmental factors, such as retinoic acid16, 17 or ethyl alcohol18 also converge on SHH signalling. Nevertheless, SHH mutations account for only a minority of overall HPE cases and carrying a mutation alone does not mean that an individual will manifest HPE. Only about 37% of human carriers of SHH mutations develop HPE, while others display mild signs or no signs at all19, highlighting the complexity of HPE pathogenesis and complicating the process of genetic counselling.
PHENOTYPIC VARIABILITY OF HPE
Both brain and facial manifestations of HPE are highly variable. A generally accepted classification of cerebral defects in humans with HPE incudes four main subtypes from the most severe to the least severe based on the degree of separation of the cerebral hemispheres: alobar, semilobar, lobar HPE and middle interhemispheric variant MIHV) HPE.20, 21 In alobar HPE, there is lack of midline separation of the cerebral hemispheres with a single large ventricle (monoventricle, Fig. 1). In semilobar HPE, interhemispheric fissure is incomplete with a partial separation of the hemispheres posteriorly. In lobar HPE, interhemispheric fissure is mostly present except for the most rostral portion of the frontal lobes (frontal neocortex). Finally, in MIHV HPE, the interhemispheric fissure is formed anteriorly and posteriorly, with fusion of the cerebral hemispheres centrally.22, 23 In addition, the so called septopreoptic HPE, in which the nonseparation is restricted to the septal and/or preoptic regions is considered by some authors a very mild subtype within the HPE spectrum.24 Inclusion of other less severe defects, such as agenesis of the corpus callosum or arhinencephaly (absence of the olfactory tracts and bulbs), as mild forms of HPE remains controversial.21 It should be noted that a precise distinction between these subtypes is not always possible because HPE represents a continuum of hemispheric nonseparation.21, 24
Facial defects also show various degrees of severity and involve both upper and lower face (Fig. 2 A–E). Separation of the eyes may range from normal to closely spaced (hypotelorism) to the most severe cyclopia. Failure of proper separation of the frontonasal region results in proboscis (elongated nose-like structure, usually above the cyclopic eye in humans and either above or below the cyclopic eye in mice), reduced distance between the nares, or a single nostril. Other defects include midline cleft lip with or without cleft palate, midface hypoplasia, a single maxillary central incisor, micrognathia or agnathia.25, 26 Milder defects (hypotelorism, midfacial hypoplasia, a single maxillary central incisor) are also referred to as microforms.27 In the majority of cases (60–90%) there is a correlation between the severity of midline facial defects and the severity of brain defects.5, 28 HPE microforms are usually associated with normal hemispheric separation, although mild midline defects, such as agenesis of corpus callosum may be present.
Fig. 2. Spectrum of craniofacial phenotypes in humans (A–E) and Twsg1−/− mice (F–J).
(A) Single central incisor; (B) Microcephaly, midface hypoplasia with bilateral cleft lip and palate; (C) Cyclopia with proboscis above the fused eye; (D) Hypotelorism and a single nostril. Images A-D are reprinted with permission from Muenke and Cohen 200081, John Wiley and Sons. (E) Agnathia with downward displacement of the ears and microstomia (reprinted with permission from Schiffer et al, 2002149, John Wiley and Sons); (F) Wild type; (G) severe anterior truncation; (H) cyclopia with proboscis); (I) single nostril with agnathia, (J) agnathia. Images F–J are reprinted with permission from Petryk et al., 200429, Elsevier).
This phenotypic variability can also be reproduced experimentally in animal models. As described above, mice deficient in BMP binding proteins, including Twsg1−/− mice29 and Chordin−/−;Noggin+/− double mutants30 show a spectrum of craniofacial defects similar to human HPE. While these mutants are not considered traditional HPE mouse models, their phenotype more faithfully reflects a human HPE than other mouse models of classical human mutations (e.g. SHH mutations) because of incomplete penetrance and a range of defects. In contrast, HPE is fully penetrant in Shh null embryos with all of them exhibiting severe HPE.31 While homozygosity for Shh has profound effects on craniofacial development in mice, intermediate levels of SHH signaling obtained by pharmacologic intervention do result in phenotypic variability in avian embryos.32, 33
The mechanisms of the incomplete penetrance and variable expressivity remain poorly understood. The examples mentioned above offer some possible explanations. Most importantly, there appears to be a gene dosage effect with resultant variable functional thresholds of encoded proteins.11, 34 For example, in mice, haploinsufficiency for Shh does not lead to HPE, while homozygosity for Shh mutation results in severe HPE. This scenario illustrates a threshold for SHH signalling below which the phenotype is always severe. In humans, however, haploinsufficiency may lead to HPE, but in the majority of cases it is either not enough to evoke the HPE phenotype or leads to only mild defects19, presumably due to variable levels of SHH signalling. Additional genetic or environmental factors (e.g. in utero exposure to teratogens) can lower or raise the threshold below which the disease manifests itself. Several models for modulating HPE severity have been proposed. First, two or more HPE genes may interact in generating the phenotype, for example SHH and TGIF or SHH and ZIC235. Chordin−/−;Noggin+/− double mutants30 are an example of digenic inheritance in mice. Second, mutations may involve other genes that are not classical HPE genes, but are nonetheless important for normal forebrain development through other signaling pathways that act either concurrently or at different developmental times, for example by altering proliferation and/or apoptosis of critical cell types.11 The effect of concurrent partial defects in more than one pathway, or at multiple steps in one pathway on variable manifestation of disease has been shown in other diseases in humans.36 Third, there is a clear effect of a genetic background on the penetrance of HPE phenotype in mice carrying a predisposing mutation with a C57BL/6 background conferring an increased susceptibility29, suggesting a role for genetic modifiers.37 Finally, presence of phenotypic variability in the same genetic background and under the same environmental conditions in mice suggests stochastic and/or epigenetic contribution.38 All of these factors support a “multiple hit” hypothesis11 in the pathogenesis of HPE.
ETIOLOGY OF HPE
GENETIC CAUSES
Genetic causes include chromosomal aberrations and single gene mutations. Chromosomal abnormalities, such as trisomy 13 (most prevalent), trisomy 18, triploidy are the most common cause of HPE (24–45% of cases)4, 8, 39, 40 and frequently result in embryonic or perinatal mortality. HPE that is due to gene mutations can be classified into syndromic and nonsyndromic. Syndromic HPE is part of multiple malformation syndromes, for example Smith-Lemli-Opitz syndrome (OMIM 270400) caused by mutation in the gene encoding sterol delta-7-reductase, Pallister–Hall syndrome (OMIM 146510) due to mutation in the GLI3 gene, or Rubinstein–Taybi syndrome (OMIM 180849) caused by mutation in the gene encoding the transcriptional coactivator CREB-binding protein. At least 13 chromosomal loci have been associated with nonsyndromic HPE4, 9 of them including known HPE genes (Table 1). About 25% of these point mutations or microdeletions involve SHH (most common), zinc finger protein of the cerebellum 2 (ZIC2), SIX homeobox 3 (SIX3), and TGIF.41 Other genes include glioma-associated oncogene family zinc finger 2 (GLI2), patched homolog 1 (PTCH1), NODAL, forkhead box H1 (FOXH1), teratocarcinoma-derived growth factor 1 (TDGF1, also known as CRIPTO), dispatched homolog 1 (DISP1).4 Mutations in these genes account for about 25% of HPE cases, with point mutations being more common in live born children than in fetuses who are more likely to have microdeletions.1, 4, 9 New candidate HPE loci continue to be identified by high resolution cytogenetic techniques such as microarray-based comparative genomic hybridization (array CGH) and subtelomeric multiplex ligation-dependent probe amplification (MLPA).9 HPE has been reported to be about twice as common in females than in males.40, 42, 43
Table 1.
Genes (loci) contributing to HPE reprinted with permission from Roessler and Muenke 20104, John Wiley and Sons).
| Human gene | (Human locus) | Chromosome | Molecular function |
|---|---|---|---|
| — | HPE1 | 21q22.3 | (unknown) |
| SIX3 | HPE2 | 2p21 | Forebrain and eye development |
| SHH | HPE3 | 7q36 | Ventral CNS patterning |
| TGIF | HPE4 | 18p11.3 | Transcriptional repressor including retinoids |
| ZIC2 | HPE5 | 13q32 | Axis formation and dorsal brain development |
| — | HPE6 | 2q37.1-q37.3 | (unknown) |
| PTCH1 | HPE7 | 9q22.3 | Receptor for hedgehog ligands |
| — | HPE8 | 14q13 | (unknown) |
| GLI2 | HPE9 | 2q14 | Transcription factor mediating hedgehog signaling |
| — | HPE10 | -- | (unknown) |
| DISP1 | — | 1q42 | Release of hedgehog ligands |
| NODAL | — | 10q | TGFβ-like ligand involved in midline and laterality establishment |
| FOXH1 | — | 8q24.3 | Transcription factor for NODAL signaling |
ENVIRONMENTAL FACTORS
Animal studies have shown that exposure to teratogens can lead to HPE if it occurs during a specific developmental stage. In mice, E7.5 is the most sensitive window for teratogen-induced HPE16, 44 (3rd to 4th week post-fertilization in humans).27 Environmental risk factors have been reviewed in detail elsewhere7, 45 The most extensively studied risk factor for HPE (as well as other birth defects) is maternal diabetes, particularly with pregestational onset.46 The incidence of HPE among infants of diabetic mothers is about 1–2%47, which is very significant on a population scale given the high prevalence of diabetes. The association between in utero exposure to ethanol and HPE has been demonstrated in a number of animal studies44, 48, 49 and some human studies50, 51, although a frequent confounding variable in humans is cigarette smoking during pregnancy.43 Similar association has been shown for retinoic acid16, 52, which continues to be prescribed for the treatment of acne, sun-damaged skin, psoriasis, prevention of nonmelanoma skin cancer, and for cancer chemotherapy. Since cholesterol is important for SHH processing and signaling53, and marked reduction in cholesterol levels in mice can produce HPE54, it has been speculated that cholesterol-lowering drugs, such as statins, could put offspring at risk for HPE.55 However, human data are inconclusive. The link between HPE and other risk factors, including Infections during pregnancy (e.g. cytomegalovirus infection), medications (e.g. antiepileptics, salicylates, antibiotics), the use of assisted reproductive technologies56, among others, remains tenuous and is based mostly on either case reports or animal studies.
It has been hypothesized that even subteratogenic doses of some of these agents may cause HPE when acting in concert with other environmental or genetic variables.11 For example mice homozygous for Tgif mutation have a normal phenotype, but show greater sensitivity to RA-induced teratogenesis than wild type mice even in a heterozygous state.57 Likewise, haploinsufficiency for Shh or Gli2 in mice predisposes them to teratogenic effects of ethanol.49 It is conceivable that individuals carrying mutations that predispose to HPE would show a similar heightened sensitivity to environmental influences. The exact mechanism of teratogenic effects is unknown. Hyperglycemia in diabetes58, reduced SHH signaling after alcohol18, 49 or RA exposure17, increased apoptosis59, or oxidative stress, which is increased after each of these exposures60–62, have been proposed as possible mechanisms.
DEVELOPMENT OF FOREBRAIN AND MIDLINE FACIAL STRUCTURES
Understanding the pathogenesis of HPE requires knowledge of the relationship between the developing brain and the developing facial structures. During gastrulation, the three germ layers are established in the head. The neural tube occupies the midline of the embryo and is flanked by paraxial mesoderm, underlain by prechordal mesoderm and endoderm, and covered by surface ectoderm (Fig. 3). These tissues play important roles during development of the craniofacial complex, and aberrant structural or signalling interactions among some of them may contribute to the phenotypic presentation of patients with HPE. The neural ectoderm is initially continuous with the sheet of surface ectoderm. During gastrulation, the boundary of the neural ectoderm and surface ectoderm is established by the signalling interactions among the FGF, WNT, and BMP pathways (reviewed by Groves et al.63) and then through a series of morphogenetic movements the neural tube closes and detaches from the surface ectoderm.64 The progenitors of the facial skeleton and portions of the calvarium arise from the neural crest cells. This is a migratory population of cells that are derived from the cephalic ectoderm that is situated at the border between the presumptive neural and surface ectoderm.63, 65 Cells located at the border undergo an epithelial-to-mesenchymal transformation and migrate ventro-laterally to form the facial primordia.66 Eventually, these cells will give rise to the connective tissues of the face.67
Fig. 3. Spatial relationships during development of the head.
(A) A bright field image of a HH10145 chicken embryo in ovo. Dorsal view showing regionalization of the neural tube into the prosencephalon (p), mesencephalon (ms), and metencephalon (mt). The anterior neural pore (anp) is located at the anterior end of the neural tube, and the neural tube is flanked by paraxial mesoderm (pm). (B) A sagittal section of a HH10 embryo that has been stained with bis-benzimide and imaged using an epifluorescent microscope (Leica) showing the prosencephalon (p), mesencephalon (ms), the anterior neural pore (anp), the pharyngeal endoderm (pe), the facial ectoderm (fe), and the prechordal mesoderm (asterisk).
PRIMARY PATTERNING AND GROWTH OF THE BRAIN
The developing brain is divided into regions along the anterior-posterior axis, exhibits bilateral symmetry and has a dorsal-ventral polarity.68, 69 Regionalization of the brain into the prosencephalon, mesencephalon, and rhombencephalon represents the earliest partitioning of the brain. Signalling from the mesendoderm establishes the bilateral symmetry of the prosencephalon.70 The prosencephalon is further divided into telencephalon at its most anterior end and diencephalon caudally.71 While the mesencephalon and rhombencephalon are also further divided, these regions are less relevant for our understanding of the pathogenesis of HPE. For the correct specification of the telencephalon, the balance between SHH, FGF, and BMP signalling is critical.72, 73 Shh is expressed in the ventral signalling center, BMP/WNT in the dorsal signalling center, and FGF8 in the anlage of the septum (Fig. 4). Signalling by SHH separates the single eye field into a left and right eye field that will form the optic cups.74
Fig. 4. Signalling centers instructing telencephalon.
Frontolateral view of the telencephalon indicating its distinct signalling centers important for regional differentiation in relation to other developing head structures: the ventral center secreting SHH (red), the rostral center secreting FGF8 (blue), and the dorsal center secreting BMPs and Wnt (green). Cross-regulation between the rostral, dorsal, and ventral signalling centres plays an essential role in patterning the early telencephalon. Cx, cortex; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; S, septum (reprinted with permission from Hoch et al, 2009146, Elsevier).
Expansion of the brain has a direct effect on the morphogenesis of the face. As the brain grows, the position of the facial prominences is affected.75 Furthermore, the rate of brain growth during early stages of skull development impacts facial morphogenesis. Mice with slower growing brains have faces that appear more advanced and are longer, presumably because growth of the facial anlagen on a smaller platform produces a larger and apparently more advanced face.76 While these early effects of growth on facial development may have an effect on the facial features of patients with HPE, the specific contributions are unknown. The molecular interactions between the brain and face may be more relevant for understanding the facial phenotype in these individuals.
SIGNALING INTERACTIONS BETWEEN THE BRAIN AND THE FACE
In addition to the physical effect that the growing brain has on facial development, the brain participates in a reciprocal series of signalling interactions along with the surface ectoderm and adjacent neural crest cells.77 Our research has revealed a signalling relay system that operates in the brain to control Shh expression in the telencephalon and adjacent surface cephalic ectoderm.14, 78 Blocking SHH signalling in the brain alters the dorso-ventral polarity of the forebrain, prevents the onset of Shh expression in the basal telencephalon, and inhibits the subsequent induction of Shh expression in the Frontonasal Ectodermal Zone (FEZ).78 In contrast, activating SHH signalling in the brain ventralizes the forebrain, expands Shh expression in the basal telencephalon, and alters the pattern of Shh expression in the FEZ.14 Both of these treatments lead to significant changes in facial morphology. Further, by varying the level of SHH signal activation in the brain, phenotypic variation is produced that is continuous with normal variation.79 Blocking SHH to varying degrees in the brain produces phenotypes that are consistent with the range of phenotypes that appear in patients with loss-of-function mutations in Shh80–82, and activating SHH in the brain to varying degrees produces phenotypes that span the range observed in patients with gain-of-function mutations in the Shh pathway.83 Moreover, all of these phenotypes are associated with changes in the level and pattern of expression of Shh in the FEZ. Thus, SHH signalling in the brain directly affects facial development by controlling the induction and spatial organization of the FEZ, a signalling center that regulates facial development.15 However, most importantly, these experiments revealed a fundamental non-linear relationship between SHH signalling and phenotypic outcome that may help explain the extreme phenotypic variability that is observed among patients with mutations that cause HPE (Fig. 5). For example, by using a doubling dilution of the SHH inhibitor we observed a very large change in facial shape over small changes in the concentration of available ligand suggesting that for very small changes in pathway activation large variation can be produced.77, 79
Fig. 5. Non-linearities in signaling may produce phenotypic variation.
A model illustrating how non-linear properties of a signalling pathway can produce phenotypic variation. By reducing SHH signalling a large amount of phenotypic variance could be produced due to the non-linear nature of the SHH pathway (adapted with permission from Hallgrimsson et al, 2009147, Springer).
GROWTH OF THE FACE
The stereotypical changes in the facial phenotype of patients with mild forms of HPE (hypotelorism, midfacial hypoplasia, cleft lip with or without cleft palate) may be a direct result of the signalling interactions among the brain, surface ectoderm, and adjacent neural crest cells. The FEZ is an important signalling center that regulates patterning and growth of the upper jaw in amniotes.15, 84, 85 The spatial organization of the gene expression patterns in the FEZ is highly associated with facial shape79, 85, and changes to the shape of the expression domains may directly result in the facial phenotypes observed in patients with HPE. For example, reduced SHH signalling in the brain of patients may lead to midfacial hypoplasia and clefting as a result of alterations to the initial growth patterns of the facial primordia prior to fusion of the primary palate anlagen. The surface ectoderm that covers the midline of the developing upper jaw expresses a series of signalling molecules including: Shh, Fgf8, Wnt9b, Bmp2, Bmp4, and Bmp7 among others.86–88 Shh is expressed in the ectoderm that will form the roof of the mouth and forms a boundary with cells expressing Fgf8 and Wnt9b in more dorsal ectoderm.15, 84 This ectoderm has the ability to promote growth and patterning of the upper jaw15, in part by regulating expression of Bmp2, -4, and -7 in the adjacent mesenchyme that then controls growth of the upper jaw anlagen.84, 89 Thus, the changes that occur in the brains of patients with HPE may have a direct impact on the developing face through the regulation of the signalling interactions that occur among these adjacent tissues during the earliest periods of their development.
KEY SIGNALING PATHWAYS IMPLICATED IN HPE - EXPERIENCE FROM ANIMAL STUDIES
NODAL SIGNALLING
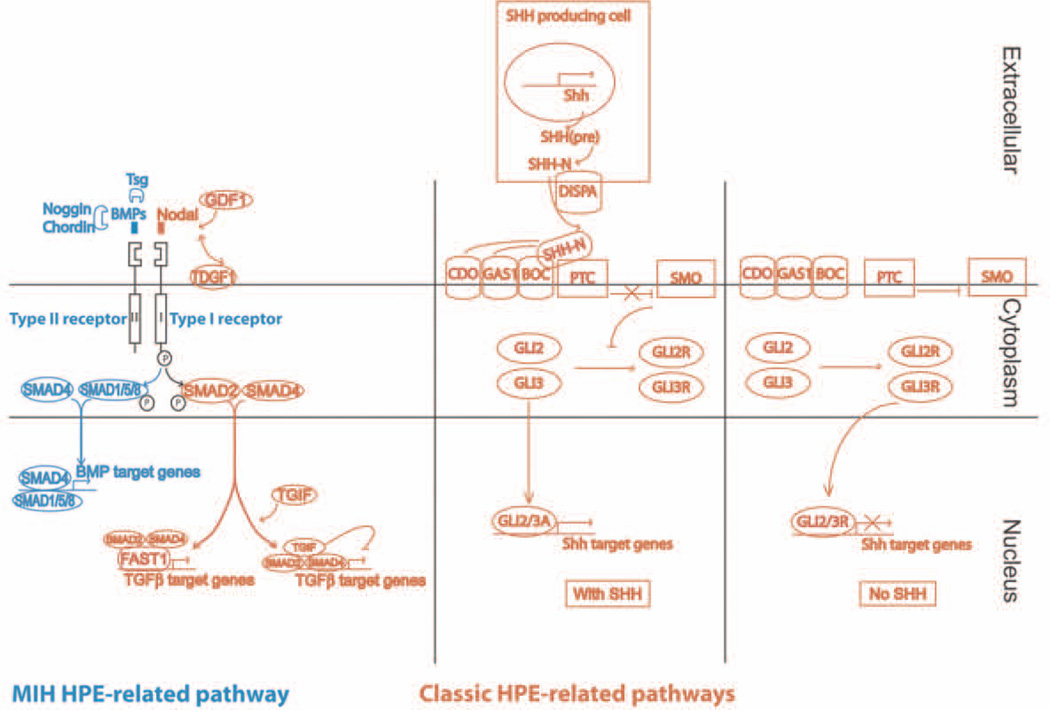
Studies in zebrafish and mouse have shown that signalling by the Transforming Growth Factor-β (TGF-β) superfamily member Nodal is critical for the initial specification of the prechordal plate.90, 91 Nodal signals through a receptor complex consisting of type I serine-threonine kinase receptors (Activin receptor-like kinase (Alk)4/ActrIb/Acvr1b or Alk7) and type II receptors (ActRIIA and ActRIIB) (Fig. 6). Their activation leads to the phosphorylation of Smad2 and Smad3, which following binding to Smad4 allows their translocation to the nucleus, where the complex interacts with transcriptional regulators such as the helix-loop-helix transcription factor FoxH1/FAST192 to activate transcription of prechordal-plate specific genes such has Goosecoid. Nodal signalling is regulated by the membrane-bound extracellular co-factors Tdgf1 (Cripto) and Cfc1 (Cryptic), which are members of the epidermal growth factor (EGF)-cysteine rich (CFC) family. Nodal is critical for mesoderm formation at the beginning of gastrulation and mutant mice die prior to the onset of brain development. Mice deficient in ActRIIA, Smad2 or FoxH1 exhibit rather severe forms of HPE due to failure to properly form prechordal mesendoderm.93–96 Allelic series with Nodal91 or Cripto hypmorph alleles97 support the concept of signalling thresholds in HPE and genetic combinations of haploinsufficient mice (Nodal, Alk4, Gdf1) illustrate how several heterozygote mutations within one pathway suffice for the development of HPE.98 A common feature in all these mutants is that expression of Shh is reduced or missing. Mutations in several components of the Nodal pathway or downstream targets (TDGF1, TGIF, FAST1) have been identified in human cases of HPE.99
Fig. 6. Schematic representation of core pathways involved in forebrain development and HPE.
Classic HPE-related pathways that signal predominantly at the ventral midline are shown in orange. The BMP pathway shown in blue signals predominantly at the dorsal midline and is involved in MIH (reprinted with permission from Fernandes and Hébert, 2008148, John Wiley and Sons).
HEDGEHOG SIGNALLING
Hedgehog (HH) signalling is essential for the development of the ventral forebrain.31 Proteins of the HH family undergo extensive posttranslational modification before they are secreted as active signalling molecules. The insoluble and inactive precursor molecule is autocatalytically cleaved and is subject to two covalent lipid modifications. First, a cholesterol moiety is added at its N-terminus100 followed by a palmitate moiety at its C-terminus that allows it to associate with lipid rafts and to be transported to the cell surface.101 Perturbations in either lipid modification have been associated with HPE in humans (mutations in DHCR7102) or mice (loss of function in the Hedgehog acyltranferase (Hhat) required for palmitoylation103). The now active multimeric HH protein remains lipid anchored at the cell membrane and requires the transmembrane protein Dispatched1 (DISP1) for its release. The HH receptor Patched (PTCH) functions as inhibitor of Smoothened (SMO). Binding of HH to PTCH releases SMO and the signal can be transduced intracellularly. Loss of Disp1 in mice leads to HPE including cyclopia.103–105 A similar phenotype is seen in Smo-deficient mice106, whereas Ptch-deficient mice show a constitutive HH pathway activation and dorsal expansion of the Shh expression domain.107 Canonical HH signalling proceeds through the glioblastoma gene products (Gli) family of transcription factors (Gli1, Gli2, Gli3), which are orthologues of Drosophila cubitus interruptus (Ci) and can either act as transcriptional activators or repressors. Gli independent signalling pathways (non-canonical signalling) have also been described.108 Loss of Gli2, which appears to be responsible for the majority of the HH-dependent transcriptional response, is neonatal lethal in the mouse with defects in early brain and spinal cord development109, 110 and several mutations in GLI2 have been reported in HPE cases.111 Deficiency of Gli3, which in the absence of HH is proteolytically processed into a transcriptional repressor, thus acting as HH pathway antagonist, leads to a ventralized forebrain and exencephaly.112 An increase in Gli3 is observed in mice deficient for both Tgif1 and its homologue Tgif2 resulting in HPE with features similar to loss of Shh.113 There are several HH-binding proteins, such as Cdo, Boc, Gas1, which may function as coreceptors with PTCH1 and enhance HH signaling.114 Loss of these proteins leads to a reduction of HH signalling that dependent on the genetic background leads to HPE of variable severity.115–117 Crosses to Shh-haploinsufficent mice (Gas1−/−;Shh+/−, Cdo−/−;Shh+/−) result in more severe phenotypes, establishing a genetic interaction between these molecules, and exemplify that amount and location of the SHH ligand is important for ventral forebrain patterning.115, 118 A lack of the cholesterol moiety in SHH leads to ectopic SHH signalling, which impairs correct forebrain development as well.119
RETINOID SIGNALLING
TG-interacting factor (TGIF) was originally discovered through its ability to bind a retinoic acid response element120 indicating a link to retinoid signalling. Retinoic acid (RA) is the active derivative of vitamin A, which is exclusively provided through the diet. RA plays a critical role in anterior-posterior patterning of the central nervous system. RA is produced by retinal dehydrogenases that convert vitamin A to the main biologically active metabolite, all-trans RA. All-trans RA mobilizes to the nucleus and binds to nuclear retinoic acid receptors, which act as transcription factors. Levels of RA are under tight regulation. Though Vitamin A is hydrolyzed by ubiquitous alcohol deyhdrogenases, the following irreversible conversion to all-trans RA catalyzed by retinaldehyde dehydrogenases is rate limiting. At the same time, RA-degrading enzymes such as the cytochrome P450 family member CYP26A1 tightly control RA levels. TGIF regulates the expression of genes controlling both RA synthesis and degradation.121
FIBROBLAST GROWTH FACTOR SIGNALLING
FGF8 is a member of the FGF family of secreted signaling proteins. FGF receptors are receptor tyrosine kinases that can activate several signalling cascades, most notably the MAP kinase pathway through Ras/Raf/Erk, which can lead to the phosphorylation and activation of key transcription factors.122 Reduction in Fgf8 in the mouse results in hypoplasia of the developing forebrain due to reduced cell proliferation and increased cell death, and midline anomalies.72 FGF8 can induce Zic2 expression in the dorsal signalling center.123 Both mutations in ZIC2124 and FGF8125 can cause HPE in humans.
BONE MORPHOGENETIC PROTEIN SIGNALLING
Members of the BMP subgroup of the TGF-β family of secreted signalling proteins are involved in the organization of the medial-lateral axis of the developing telencephalon and are required for formation of a morphologically defined dorsal midline. BMPs signal similar to Nodal through a receptor complex consisting of type I serine-threonine kinase receptors (Alk1, Alk2, Alk3 (also known as BmprIa) or Alk6 (BmprIb)) and type II receptors (BmprII or ActRII)126. Transduction of the signal is either via the BMP-specific Smad1, 5 and 8 effector proteins or via non-BMP specific signal transduction pathways such as MAPK/PI3K/Akt.126 BMP signaling activity in vivo is highly regulated at several levels of the pathway; in the intracellular space, where SMAD1 serves as a signaling hub integrating BMP, WNT, and FGF signals127, at the receptor level, and in the extracellular space, where secreted BMP-binding proteins like Noggin, Chordin, Twisted gastrulation (TWSG1) or Gremlin control BMP activity.128, 129 Dosage and timing of BMP signalling are important. Both a reduction in BMP signalling, as seen in Bmpr1a:Bmpr1b double mutant mice130, or increase, as seen in Chordin/Noggin mutant mice131 or Noggin mutant mice132, can affect forebrain and craniofacial development. Chordin and Noggin appear to antagonize the inhibitory effects of BMP signalling on the Fgf8 and Shh expression domains. Mutant mice display phenotypes remarkably analogous to the wide range of malformations seen in human HPE131, 132. In contrast, reduction of BMP signalling in Bmpr1a:Bmpr1b double mutant mice affects the development of the dorsal midline including the choroid plexus and cortical hem.130 This phenotype resembles the midline interhemispheric (MIH) subclass of HPE, which is rarer and generally milder than other types of HPE. TWSG1 can act as a BMP agonist133 or antagonist.129 Mice deficient in TWSG1 display a range of forebrain defects resembling human HPE29 reminiscent of a gain of BMP activity in this mutant. In contrast to this a study, Zakin and De Robertis 2004134 showed that removal of one copy of Bmp4 in a TWSG1-deficient mouse without overt phenotype on its own resulted in HPE with anophtalmia, reminiscent of a loss of BMP activity, supporting TWSG1’s role as a context dependent modulator of BMP signaling. Interestingly, human TWSG1 maps to the HPE4 locus on Chromosome 18p11.3 within 5 Mbp of TG-interacting factor (TGIF)135. Whereas mutations in TGIF have been described, minimal evidence for alterations in TWSG1 was found in humans, suggesting that coding sequence alterations of TWSG1 are not a common direct cause of human HPE.136
DIAGNOSIS AND CLINICAL MANAGEMENT
HPE can be diagnosed in utero by a high-resolution prenatal ultrasound137 or a fetal magnetic resonance imaging24, sometimes in combination with molecular testing from chorionic villi or amniotic fluid sampling if a mutation has been previously identified in a probant.138 Postnatally, presence of midline facial defects usually prompts neuroimaging for diagnostic confirmation. Recommendations for the molecular evaluation of newly diagnosed HPE patients (and their parents) have been summarized by Pineda-Alvarez et al.139 Molecular testing includes a high-resolution karyotype as first tier approach since cytogenetic abnormalities are the most common cause of HPE, DNA sequencing of most common HPE genes (SHH, ZIC2, SIX3) as second tier, followed by other complementary studies, including array CGH, MLPA, and next generation sequencing. In patients who show features of Smith-Lemli-Opitz syndrome140 (microcephaly, micrognathia, mental retardation, syndactyly of the second and third toes, incomplete development of the male genitalia), biochemical testing includes measurement of 7-dehydro-cholesterol level, which is elevated in this syndrome.
Patients with HPE have a number of medical problems, including seizures, developmental delay, hormonal deficiencies due to hypothalamic and pituitary dysfunction (diabetes insipidus being most common), abnormalities of tone and movement, temperature instability, abnormal sleep pattern, feeding difficulties, chronic lung disease, hydrocephalus and others that require a long-term follow-up and interdisciplinary care.20, 141 In addition to reconstructive surgeries, patients require other therapeutic interventions that address specific medical problems mentioned above (e.g. antiepileptics, hormonal replacement, physical therapy). Survival depends on the severity of brain and facial malformations, presence of chromosomal abnormalities, other congenital anomalies, and organ involvement (e.g. respiratory problems). Patients with mild forms of HPE may have a normal life expectancy.
Genetic counseling should be offered with a caveat that the presence of a mutation in one of the HPE-associated genes does not predict the severity of the disease due to remarkable variability in expressivity and overall poor genotype-phenotype correlation, which reflects a multifactorial etiology of the disease.138, 142 Gene mutations or chromosomal aberrations usually occur de novo, although familial HPE has been reported, usually with an autosomal dominant mode of inheritance (in which case the risk may be as high as 50%). For sporadic cases, the recurrence risk after an isolated case is 13–14%.26
Conclusion
In conclusion, HPE is a developmental disorder of the forebrain and midline facial structures that epitomizes the complexity of interactions between different developmental pathways and environmental influences in defining the pathology and its severity. Remarkable progress has already been made in identifying genetic basis of a significant proportion of cases and, with advances in molecular techniques, new candidate genes as well as mutations outside of the coding regions are likely to be found. Large epidemiological studies and animal models have facilitated detection of a number of environmental factors. However, our understanding of the mechanisms of gene-environment interactions remains rudimentary. Although the role of the modifier genes is implied, their identification has not been successful so far. Finally, future efforts should focus on designing preventive strategies. While supplementation of women of childbearing age with folic acid has been effective in preventing neural tube defects143, this has not been the case in HPE. Recent findings from the National Birth Defects Prevention Study suggested that folic acid supplementation during the periconceptional period was somewhat protective, but the association was of borderline statistical significance.56 Likewise, maternal diet supplementation with methyl donors did not reduce the incidence of midline facial defects in Twsg1−/− mice.144 Unlike genetic aberrations, environmental factors or a biological response they evoke are modifiable as long as the underlying mechanisms can be identified. While avoidance of teratogens is the most effective primary prevention method, it is not always feasible. Further, individual susceptibility may vary due to other intrinsic factors (e.g. silent gene mutations) that act in combination to cause HPE in at risk individuals. Therefore, further research into the mechanisms of gene-environment interactions and development of suitable animal models (chicken, zebrafish, mouse) will be critical for gaining insight into such interactions and providing avenues for future preventive interventions.
Acknowledgments
Grant support: This work was partially supported by the National Institutes of Health grants R56DE023530 to A.P, and R01DE019638, R01DE021708, R01DE018234 to R.M. DG was supported by the University of Zurich, the University of Alberta, and grants from the Swiss National Science Foundation.
Footnotes
Anna Petryk, No conflict of interest.
Daniel Graf, No conflict of interest
Ralph Marcucio, No conflict of interest
Contributor Information
Anna Petryk, Department of Pediatrics, University of Minnesota, 2450 Riverside Ave., Minneapolis, MN 55454, petry005@umn.edu.
Daniel Graf, School of Dentistry, University of Alberta, Edmonton, Canada, dgraf@ualberta.ca.
Ralph Marcucio, Department of Orthopaedic Surgery, Orthopaedic Trauma Institute, University of California, San Francisco, 2550 23rd. St, San Francisco, CA 94110, Ralph.marcucio@ucsf.edu.
References
- 1.Geng X, Oliver G. Pathogenesis of holoprosencephaly. J Clin Invest. 2009;119:1403–1413. doi: 10.1172/JCI38937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pauli RM, Pettersen JC, Arya S, Gilbert EF. Familial agnathia-holoprosencephaly. Am J Med Genet. 1983;14:677–698. doi: 10.1002/ajmg.1320140411. [DOI] [PubMed] [Google Scholar]
- 3.Lipinski RJ, Godin EA, O'Leary-Moore SK, Parnell SE, Sulik KK. Genesis of teratogen-induced holoprosencephaly in mice. Am J Med Genet C Semin Med Genet. 2010;154C:29–42. doi: 10.1002/ajmg.c.30239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roessler E, Muenke M. The molecular genetics of holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:52–61. doi: 10.1002/ajmg.c.30236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orioli IM, Castilla EE. Epidemiology of holoprosencephaly: Prevalence and risk factors. Am J Med Genet C Semin Med Genet. 2010;154C:13–21. doi: 10.1002/ajmg.c.30233. [DOI] [PubMed] [Google Scholar]
- 6.Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet. 1996;14:357–360. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MM, Jr, Shiota K. Teratogenesis of holoprosencephaly. Am J Med Genet. 2002;109:1–15. doi: 10.1002/ajmg.10258. [DOI] [PubMed] [Google Scholar]
- 8.Muenke M, Beachy PA. Genetics of ventral forebrain development and holoprosencephaly. Curr Opin Genet Dev. 2000;10:262–269. doi: 10.1016/s0959-437x(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 9.Bendavid C, Rochard L, Dubourg C, Seguin J, Gicquel I, Pasquier L, Vigneron J, Laquerriere A, Marcorelles P, Jeanne-Pasquier C, et al. Array-CGH analysis indicates a high prevalence of genomic rearrangements in holoprosencephaly: an updated map of candidate loci. Hum Mutat. 2009;30:1175–1182. doi: 10.1002/humu.21016. [DOI] [PubMed] [Google Scholar]
- 10.Shiota K, Yamada S. Early pathogenesis of holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:22–28. doi: 10.1002/ajmg.c.30248. [DOI] [PubMed] [Google Scholar]
- 11.Ming JE, Muenke M. Multiple hits during early embryonic development: digenic diseases and holoprosencephaly. Am J Hum Genet. 2002;71:1017–1032. doi: 10.1086/344412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gongal PA, French CR, Waskiewicz AJ. Aberrant forebrain signaling during early development underlies the generation of holoprosencephaly and coloboma. Biochim Biophys Acta. 2011;1812:390–401. doi: 10.1016/j.bbadis.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Schachter KA, Krauss RS. Murine models of holoprosencephaly. Curr Top Dev Biol. 2008;84:139–170. doi: 10.1016/S0070-2153(08)00603-0. [DOI] [PubMed] [Google Scholar]
- 14.Hu D, Marcucio RS. A SHH-responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm. Development. 2009;136:107–116. doi: 10.1242/dev.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu D, Marcucio R, Helms JA. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development. 2003;130:1749–1758. doi: 10.1242/dev.00397. [DOI] [PubMed] [Google Scholar]
- 16.Sulik KK, Dehart DB, Rogers JM, Chernoff N. Teratogenicity of low doses of all-trans retinoic acid in presomite mouse embryos. Teratology. 1995;51:398–403. doi: 10.1002/tera.1420510605. [DOI] [PubMed] [Google Scholar]
- 17.Helms JA, Kim CH, Hu D, Minkoff R, Thaller C, Eichele G. Sonic hedgehog participates in craniofacial morphogenesis and is down-regulated by teratogenic doses of retinoic acid. Dev Biol. 1997;187:25–35. doi: 10.1006/dbio.1997.8589. [DOI] [PubMed] [Google Scholar]
- 18.Ahlgren SC, Thakur V, Bronner-Fraser M. Sonic hedgehog rescues cranial neural crest from cell death induced by ethanol exposure. Proc Natl Acad Sci U S A. 2002;99:10476–10481. doi: 10.1073/pnas.162356199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen MM., Jr Perspectives on holoprosencephaly: Part I. Epidemiology, genetics, and syndromology. Teratology. 1989;40:211–235. doi: 10.1002/tera.1420400304. [DOI] [PubMed] [Google Scholar]
- 20.Hahn JS, Barnes PD, Clegg NJ, Stashinko EE. Septopreoptic holoprosencephaly: a mild subtype associated with midline craniofacial anomalies. AJNR Am J Neuroradiol. 2010;31:1596–1601. doi: 10.3174/ajnr.A2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcorelles P, Laquerriere A. Neuropathology of holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:109–119. doi: 10.1002/ajmg.c.30249. [DOI] [PubMed] [Google Scholar]
- 22.Fertuzinhos S, Krsnik Z, Kawasawa YI, Rasin MR, Kwan KY, Chen JG, Judas M, Hayashi M, Sestan N. Selective depletion of molecularly defined cortical interneurons in human holoprosencephaly with severe striatal hypoplasia. Cereb Cortex. 2009;19:2196–2207. doi: 10.1093/cercor/bhp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver DD, Solomon BD, Akin-Samson K, Kelley RI, Muenke M. Cyclopia (synophthalmia) in Smith-Lemli-Opitz syndrome: First reported case and consideration of mechanism. Am J Med Genet C Semin Med Genet. 2010;154C:142–145. doi: 10.1002/ajmg.c.30241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn JS, Barnes PD. Neuroimaging advances in holoprosencephaly: Refining the spectrum of the midline malformation. Am J Med Genet C Semin Med Genet. 2010;154C:120–132. doi: 10.1002/ajmg.c.30238. [DOI] [PubMed] [Google Scholar]
- 25.Cohen MM, Jr, Sulik KK. Perspectives on holoprosencephaly: Part II. Central nervous system, craniofacial anatomy, syndrome commentary, diagnostic approach, and experimental studies. J Craniofac Genet Dev Biol. 1992;12:196–244. [PubMed] [Google Scholar]
- 26.Kauvar EF, Solomon BD, Curry CJ, van Essen AJ, Janssen N, Dutra A, Roessler E, Muenke M. Holoprosencephaly and agnathia spectrum: Presentation of two new patients and review of the literature. Am J Med Genet C Semin Med Genet. 2010;154C:158–169. doi: 10.1002/ajmg.c.30235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kauvar EF, Muenke M. Holoprosencephaly: recommendations for diagnosis and management. Curr Opin Pediatr. 2010;22:687–695. doi: 10.1097/MOP.0b013e32833f56d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demyer W, Zeman W, Palmer CG. The Face Predicts the Brain: Diagnostic Significance of Median Facial Anomalies for Holoprosencephaly (Arhinencephaly) Pediatrics. 1964;34:256–263. [PubMed] [Google Scholar]
- 29.Petryk A, Anderson RM, Jarcho MP, Leaf I, Carlson CS, Klingensmith J, Shawlot W, O'Connor MB. The mammalian twisted gastrulation gene functions in foregut and craniofacial development. Developmental biology. 2004;267:374–386. doi: 10.1016/j.ydbio.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. doi: 10.1242/dev.129.21.4975. [DOI] [PubMed] [Google Scholar]
- 31.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 32.Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA. Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. J Clin Invest. 2004;114:485–494. doi: 10.1172/JCI19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dipple KM, McCabe ER. Phenotypes of patients with "simple" Mendelian disorders are complex traits: thresholds, modifiers, and systems dynamics. Am J Hum Genet. 2000;66:1729–1735. doi: 10.1086/302938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nanni L, Ming JE, Bocian M, Steinhaus K, Bianchi DW, Die-Smulders C, Giannotti A, Imaizumi K, Jones KL, Campo MD, et al. The mutational spectrum of the sonic hedgehog gene in holoprosencephaly: SHH mutations cause a significant proportion of autosomal dominant holoprosencephaly. Hum Mol Genet. 1999;8:2479–2488. doi: 10.1093/hmg/8.13.2479. [DOI] [PubMed] [Google Scholar]
- 36.Vockley J, Rinaldo P, Bennett MJ, Matern D, Vladutiu GD. Synergistic heterozygosity: disease resulting from multiple partial defects in one or more metabolic pathways. Mol Genet Metab. 2000;71:10–18. doi: 10.1006/mgme.2000.3066. [DOI] [PubMed] [Google Scholar]
- 37.Nadeau JH. Modifier genes in mice and humans. Nat Rev Genet. 2001;2:165–174. doi: 10.1038/35056009. [DOI] [PubMed] [Google Scholar]
- 38.Feinberg AP, Irizarry RA. Evolution in health and medicine Sackler colloquium: Stochastic epigenetic variation as a driving force of development, evolutionary adaptation, and disease. Proc Natl Acad Sci U S A. 2010;107(Suppl 1):1757–1764. doi: 10.1073/pnas.0906183107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solomon BD, Rosenbaum KN, Meck JM, Muenke M. Holoprosencephaly due to numeric chromosome abnormalities. Am J Med Genet C Semin Med Genet. 2010;154C:146–148. doi: 10.1002/ajmg.c.30232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Croen LA, Shaw GM, Lammer EJ. Holoprosencephaly: epidemiologic and clinical characteristics of a California population. Am J Med Genet. 1996;64:465–472. doi: 10.1002/(SICI)1096-8628(19960823)64:3<465::AID-AJMG4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 41.Dubourg C, Lazaro L, Pasquier L, Bendavid C, Blayau M, Le Duff F, Durou MR, Odent S, David V. Molecular screening of SHH, ZIC2, SIX3, and TGIF genes in patients with features of holoprosencephaly spectrum: Mutation review and genotype-phenotype correlations. Hum Mutat. 2004;24:43–51. doi: 10.1002/humu.20056. [DOI] [PubMed] [Google Scholar]
- 42.Olsen CL, Hughes JP, Youngblood LG, Sharpe-Stimac M. Epidemiology of holoprosencephaly and phenotypic characteristics of affected children: New York State, 1984–1989. Am J Med Genet. 1997;73:217–226. doi: 10.1002/(sici)1096-8628(19971212)73:2<217::aid-ajmg20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 43.Croen LA, Shaw GM, Lammer EJ. Risk factors for cytogenetically normal holoprosencephaly in California: a population-based case-control study. Am J Med Genet. 2000;90:320–325. [PubMed] [Google Scholar]
- 44.Aoto K, Shikata Y, Higashiyama D, Shiota K, Motoyama J. Fetal ethanol exposure activates protein kinase A and impairs Shh expression in prechordal mesendoderm cells in the pathogenesis of holoprosencephaly. Birth Defects Res A Clin Mol Teratol. 2008;82:224–231. doi: 10.1002/bdra.20447. [DOI] [PubMed] [Google Scholar]
- 45.Johnson CY, Rasmussen SA. Non-genetic risk factors for holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:73–85. doi: 10.1002/ajmg.c.30242. [DOI] [PubMed] [Google Scholar]
- 46.Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, Cleves MA, Riehle-Colarusso TJ, Waller DK, Reece EA. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199(237):e231–e239. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barr M, Jr, Hanson JW, Currey K, Sharp S, Toriello H, Schmickel RD, Wilson GN. Holoprosencephaly in infants of diabetic mothers. J Pediatr. 1983;102:565–568. doi: 10.1016/s0022-3476(83)80185-1. [DOI] [PubMed] [Google Scholar]
- 48.Higashiyama D, Saitsu H, Komada M, Takigawa T, Ishibashi M, Shiota K. Sequential developmental changes in holoprosencephalic mouse embryos exposed to ethanol during the gastrulation period. Birth Defects Res A Clin Mol Teratol. 2007;79:513–523. doi: 10.1002/bdra.20367. [DOI] [PubMed] [Google Scholar]
- 49.Kietzman HW, Everson JL, Sulik KK, Lipinski RJ. The teratogenic effects of prenatal ethanol exposure are exacerbated by Sonic Hedgehog or GLI2 haploinsufficiency in the mouse. PLoS One. 2014;9:e89448. doi: 10.1371/journal.pone.0089448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ronen GM, Andrews WL. Holoprosencephaly as a possible embryonic alcohol effect. Am J Med Genet. 1991;40:151–154. doi: 10.1002/ajmg.1320400206. [DOI] [PubMed] [Google Scholar]
- 51.Bonnemann C, Meinecke P. Holoprosencephaly as a possible embryonic alcohol effect: another observation. Am J Med Genet. 1990;37:431–432. doi: 10.1002/ajmg.1320370328. [DOI] [PubMed] [Google Scholar]
- 52.Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, Curry CJ, Fernhoff PM, Grix AW, Jr, Lott IT, et al. Retinoic acid embryopathy. N Engl J Med. 1985;313:837–841. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- 53.Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 54.Lanoue L, Dehart DB, Hinsdale ME, Maeda N, Tint GS, Sulik KK. Limb, genital, CNS, facial malformations result from gene/environment-induced cholesterol deficiency: further evidence for a link to sonic hedgehog. Am J Med Genet. 1997;73:24–31. [PubMed] [Google Scholar]
- 55.Edison RJ, Muenke M. Mechanistic and epidemiologic considerations in the evaluation of adverse birth outcomes following gestational exposure to statins. Am J Med Genet A. 2004;131:287–298. doi: 10.1002/ajmg.a.30386. [DOI] [PubMed] [Google Scholar]
- 56.Miller EA, Rasmussen SA, Siega-Riz AM, Frias JL, Honein MA. Risk factors for non-syndromic holoprosencephaly in the National Birth Defects Prevention Study. Am J Med Genet C Semin Med Genet. 2010;154C:62–72. doi: 10.1002/ajmg.c.30244. [DOI] [PubMed] [Google Scholar]
- 57.Bartholin L, Powers SE, Melhuish TA, Lasse S, Weinstein M, Wotton D. TGIF inhibits retinoid signaling. Mol Cell Biol. 2006;26:990–1001. doi: 10.1128/MCB.26.3.990-1001.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kousseff BG. Gestational diabetes mellitus (class A): a human teratogen? Am J Med Genet. 1999;83:402–408. [PubMed] [Google Scholar]
- 59.Sulik KK, Cook CS, Webster WS. Teratogens and craniofacial malformations: relationships to cell death. Development. 1988;103(Suppl):213–231. doi: 10.1242/dev.103.Supplement.213. [DOI] [PubMed] [Google Scholar]
- 60.Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod Toxicol. 2007;24:31–41. doi: 10.1016/j.reprotox.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Davis WL, Crawford LA, Cooper OJ, Farmer GR, Thomas D, Freeman BL. Generation of radical oxygen species by neural crest cells treated in vitro with isotretinoin and 4-oxo-isotretinoin. J Craniofac Genet Dev Biol. 1990;10:295–310. [PubMed] [Google Scholar]
- 62.Kay HH, Grindle KM, Magness RR. Ethanol exposure induces oxidative stress and impairs nitric oxide availability in the human placental villi: a possible mechanism of toxicity. Am J Obstet Gynecol. 2000;182:682–688. doi: 10.1067/mob.2000.104201. [DOI] [PubMed] [Google Scholar]
- 63.Groves AK, LaBonne C. Setting appropriate boundaries: fate, patterning and competence at the neural plate border. Developmental Biology. 2014;389:2–12. doi: 10.1016/j.ydbio.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Developmental dynamics : an official publication of the American Association of Anatomists. 2001;221:117–145. doi: 10.1002/dvdy.1144. [DOI] [PubMed] [Google Scholar]
- 65.Evans DJ, Noden DM. Spatial relations between avian craniofacial neural crest and paraxial mesoderm cells. Dev Dyn. 2006 doi: 10.1002/dvdy.20663. [DOI] [PubMed] [Google Scholar]
- 66.Noden DM, Marcucio R, Borycki AG, Emerson CP., Jr Differentiation of avian craniofacial muscles: I. Patterns of early regulatory gene expression and myosin heavy chain synthesis. Developmental Dynamics. 1999;216:96–112. doi: 10.1002/(SICI)1097-0177(199910)216:2<96::AID-DVDY2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 67.Noden DM, Trainor PA. Relations and interactions between cranial mesoderm and neural crest populations. J Anat. 2005;207:575–601. doi: 10.1111/j.1469-7580.2005.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murdoch JN, Copp AJ. The relationship between sonic Hedgehog signaling, cilia, and neural tube defects. Birth defects research. Part A, Clinical and molecular teratology. 2010;88:633–652. doi: 10.1002/bdra.20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Le Dreau G, Marti E. Dorsal-ventral patterning of the neural tube: a tale of three signals. Developmental neurobiology. 2012;72:1471–1481. doi: 10.1002/dneu.22015. [DOI] [PubMed] [Google Scholar]
- 70.Muller F, Albert S, Blader P, Fischer N, Hallonet M, Strahle U. Direct action of the nodal-related signal cyclops in induction of sonic hedgehog in the ventral midline of the CNS. Development. 2000;127:3889–3897. doi: 10.1242/dev.127.18.3889. [DOI] [PubMed] [Google Scholar]
- 71.Rubenstein JL, Shimamura K, Martinez S, Puelles L. Regionalization of the prosencephalic neural plate. Annual Review of Neuroscience. 1998;21:445–477. doi: 10.1146/annurev.neuro.21.1.445. [DOI] [PubMed] [Google Scholar]
- 72.Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- 73.Ohkubo Y, Chiang C, Rubenstein JL. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- 74.Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995;121:3267–3278. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- 75.Diewert VM, Lozanoff S. A morphometric analysis of human embryonic craniofacial growth in the median plane during primary palate formation. Journal of Craniofacial Genetics and Developmental Biology. 1993;13:147–161. [PubMed] [Google Scholar]
- 76.Boughner JC, Wat S, Diewert VM, Young NM, Browder LW, Hallgrimsson B. Short-faced mice and developmental interactions between the brain and the face. J Anat. 2008;213:646–662. doi: 10.1111/j.1469-7580.2008.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marcucio RS, Young NM, Hu D, Hallgrimsson B. Mechanisms that underlie co-variation of the brain and face. Genesis. 2011;49:177–189. doi: 10.1002/dvg.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Dev Biol. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 79.Young NM, Chong HJ, Hu D, Hallgrimsson B, Marcucio RS. Quantitative analyses link modulation of sonic hedgehog signaling to continuous variation in facial growth and shape. Development. 2010;137:3405–3409. doi: 10.1242/dev.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeMyer W. The face predicts the brain: diagnostic significance of median facial anomialies for holoprosencephaly (arhinencephay) Pediatrics. 1964 Aug;:256–263. [PubMed] [Google Scholar]
- 81.Muenke M, Cohen MM., Jr Genetic approaches to understanding brain development: holoprosencephaly as a model. Ment Retard Dev Disabil Res Rev. 2000;6:15–21. doi: 10.1002/(SICI)1098-2779(2000)6:1<15::AID-MRDD3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 82.Muenke MBP. Holoprosencephaly. In: Scriver CRBA, Sly WS, Valle D, editors. The metabolic & molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 6203–6230. [Google Scholar]
- 83.Balk K, Biesecker LG. The clinical atlas of Greig cephalopolysyndactyly syndrome. Am J Med Genet A. 2008;146A:548–557. doi: 10.1002/ajmg.a.32167. [DOI] [PubMed] [Google Scholar]
- 84.Hu D, Marcucio RS. Unique organization of the frontonasal ectodermal zone in birds and mammals. Dev Biol. 2009;325:200–210. doi: 10.1016/j.ydbio.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Young NM, Hu D, Lainoff AJ, Smith FJ, Diaz R, Tucker AS, Trainor PA, Schneider RA, Hallgrimsson B, Marcucio RS. Embryonic bauplans and the developmental origins of facial diversity and constraint. Development. 2014;141:1059–1063. doi: 10.1242/dev.099994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ashique AM, Fu K, Richman JM. Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development. 2002;129:4647–4660. doi: 10.1242/dev.129.19.4647. [DOI] [PubMed] [Google Scholar]
- 87.Foppiano S, Hu D, Marcucio RS. Signaling by bone morphogenetic proteins directs formation of an ectodermal signaling center that regulates craniofacial development. Dev Biol. 2007;312:103–114. doi: 10.1016/j.ydbio.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geetha-Loganathan P, Nimmagadda S, Antoni L, Fu K, Whiting CJ, Francis-West P, Richman JM. Expression of WNT signalling pathway genes during chicken craniofacial development. Dev Dyn. 2009;238:1150–1165. doi: 10.1002/dvdy.21934. [DOI] [PubMed] [Google Scholar]
- 89.Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin's finches. Science. 2004;305:1462–1465. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- 90.Sampath K, Rubinstein AL, Cheng AM, Liang JO, Fekany K, Solnica-Krezel L, Korzh V, Halpern ME, Wright CV. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature. 1998;395:185–189. doi: 10.1038/26020. [DOI] [PubMed] [Google Scholar]
- 91.Lowe LA, Yamada S, Kuehn MR. Genetic dissection of nodal function in patterning the mouse embryo. Development. 2001;128:1831–1843. doi: 10.1242/dev.128.10.1831. [DOI] [PubMed] [Google Scholar]
- 92.Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- 93.Heyer J, Escalante-Alcalde D, Lia M, Boettinger E, Edelmann W, Stewart CL, Kucherlapati R. Postgastrulation Smad2-deficient embryos show defects in embryo turning and anterior morphogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12595–12600. doi: 10.1073/pnas.96.22.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoodless PA, Pye M, Chazaud C, Labbe E, Attisano L, Rossant J, Wrana JL. FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse. Genes & development. 2001;15:1257–1271. doi: 10.1101/gad.881501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- 96.Song J, Oh SP, Schrewe H, Nomura M, Lei H, Okano M, Gridley T, Li E. The type II activin receptors are essential for egg cylinder growth, gastrulation, and rostral head development in mice. Developmental biology. 1999;213:157–169. doi: 10.1006/dbio.1999.9370. [DOI] [PubMed] [Google Scholar]
- 97.Chu J, Ding J, Jeays-Ward K, Price SM, Placzek M, Shen MM. Non-cell-autonomous role for Cripto in axial midline formation during vertebrate embryogenesis. Development. 2005;132:5539–5551. doi: 10.1242/dev.02157. [DOI] [PubMed] [Google Scholar]
- 98.Andersson O, Reissmann E, Jornvall H, Ibanez CF. Synergistic interaction between Gdf1 and Nodal during anterior axis development. Developmental biology. 2006;293:370–381. doi: 10.1016/j.ydbio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 99.Cohen MM., Jr Holoprosencephaly: clinical, anatomic, and molecular dimensions. Birth defects research. Part A, Clinical and molecular teratology. 2006;76:658–673. doi: 10.1002/bdra.20295. [DOI] [PubMed] [Google Scholar]
- 100.Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nature genetics. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 101.Buglino JA, Resh MD. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. The Journal of biological chemistry. 2008;283:22076–22088. doi: 10.1074/jbc.M803901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wassif CA, Maslen C, Kachilele-Linjewile S, Lin D, Linck LM, Connor WE, Steiner RD, Porter FD. Mutations in the human sterol delta7-reductase gene at 11q12-13 cause Smith-Lemli-Opitz syndrome. American journal of human genetics. 1998;63:55–62. doi: 10.1086/301936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dennis JF, Kurosaka H, Iulianella A, Pace J, Thomas N, Beckham S, Williams T, Trainor PA. Mutations in Hedgehog acyltransferase (Hhat) perturb Hedgehog signaling, resulting in severe acrania-holoprosencephaly-agnathia craniofacial defects. PLoS genetics. 2012;8:e1002927. doi: 10.1371/journal.pgen.1002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kawakami T, Kawcak T, Li YJ, Zhang W, Hu Y, Chuang PT. Mouse dispatched mutants fail to distribute hedgehog proteins and are defective in hedgehog signaling. Development. 2002;129:5753–5765. doi: 10.1242/dev.00178. [DOI] [PubMed] [Google Scholar]
- 105.Ma Y, Erkner A, Gong R, Yao S, Taipale J, Basler K, Beachy PA. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell. 2002;111:63–75. doi: 10.1016/s0092-8674(02)00977-7. [DOI] [PubMed] [Google Scholar]
- 106.Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell. 2001;106:781–792. [PubMed] [Google Scholar]
- 107.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 108.Jenkins D. Hedgehog signalling: emerging evidence for non-canonical pathways. Cellular signalling. 2009;21:1023–1034. doi: 10.1016/j.cellsig.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 109.Ding Q, Motoyama J, Gasca S, Mo R, Sasaki H, Rossant J, Hui CC. Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development. 1998;125:2533–2543. doi: 10.1242/dev.125.14.2533. [DOI] [PubMed] [Google Scholar]
- 110.Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–2770. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- 111.Roessler E, Du YZ, Mullor JL, Casas E, Allen WP, Gillessen-Kaesbach G, Roeder ER, Ming JE, Ruiz i Altaba A, Muenke M. Loss-of-function mutations in the human GLI2 gene are associated with pituitary anomalies and holoprosencephaly-like features. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13424–13429. doi: 10.1073/pnas.2235734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- 113.Taniguchi K, Anderson AE, Sutherland AE, Wotton D. Loss of Tgif function causes holoprosencephaly by disrupting the SHH signaling pathway. PLoS genetics. 2012;8:e1002524. doi: 10.1371/journal.pgen.1002524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kang JS, Zhang W, Krauss RS. Hedgehog signaling: cooking with Gas1. Science's STKE : signal transduction knowledge environment. 2007;2007:pe50. doi: 10.1126/stke.4032007pe50. [DOI] [PubMed] [Google Scholar]
- 115.Allen BL, Tenzen T, McMahon AP. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes & development. 2007;21:1244–1257. doi: 10.1101/gad.1543607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cole F, Krauss RS. Microform holoprosencephaly in mice that lack the Ig superfamily member Cdon. Current biology : CB. 2003;13:411–415. doi: 10.1016/s0960-9822(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 117.Zhang W, Hong M, Bae GU, Kang JS, Krauss RS. Boc modifies the holoprosencephaly spectrum of Cdo mutant mice. Disease models & mechanisms. 2011;4:368–380. doi: 10.1242/dmm.005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Seppala M, Depew MJ, Martinelli DC, Fan CM, Sharpe PT, Cobourne MT. Gas1 is a modifier for holoprosencephaly and genetically interacts with sonic hedgehog. The Journal of clinical investigation. 2007;117:1575–1584. doi: 10.1172/JCI32032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang X, Litingtung Y, Chiang C. Ectopic sonic hedgehog signaling impairs telencephalic dorsal midline development: implication for human holoprosencephaly. Human molecular genetics. 2007;16:1454–1468. doi: 10.1093/hmg/ddm096. [DOI] [PubMed] [Google Scholar]
- 120.Bertolino E, Reimund B, Wildt-Perinic D, Clerc RG. A novel homeobox protein which recognizes a TGT core and functionally interferes with a retinoid-responsive motif. The Journal of biological chemistry. 1995;270:31178–31188. doi: 10.1074/jbc.270.52.31178. [DOI] [PubMed] [Google Scholar]
- 121.Gongal PA, Waskiewicz AJ. Zebrafish model of holoprosencephaly demonstrates a key role for TGIF in regulating retinoic acid metabolism. Human molecular genetics. 2008;17:525–538. doi: 10.1093/hmg/ddm328. [DOI] [PubMed] [Google Scholar]
- 122.Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Developmental biology. 2005;287:390–402. doi: 10.1016/j.ydbio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 123.Hayhurst M, Gore BB, Tessier-Lavigne M, McConnell SK. Ongoing sonic hedgehog signaling is required for dorsal midline formation in the developing forebrain. Developmental neurobiology. 2008;68:83–100. doi: 10.1002/dneu.20576. [DOI] [PubMed] [Google Scholar]
- 124.Roessler E, Lacbawan F, Dubourg C, Paulussen A, Herbergs J, Hehr U, Bendavid C, Zhou N, Ouspenskaia M, Bale S, et al. The full spectrum of holoprosencephaly-associated mutations within the ZIC2 gene in humans predicts loss-of-function as the predominant disease mechanism. Human mutation. 2009;30:E541–E554. doi: 10.1002/humu.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arauz RF, Solomon BD, Pineda-Alvarez DE, Gropman AL, Parsons JA, Roessler E, Muenke M. A Hypomorphic Allele in the FGF8 Gene Contributes to Holoprosencephaly and Is Allelic to Gonadotropin-Releasing Hormone Deficiency in Humans. Molecular syndromology. 2010;1:59–66. doi: 10.1159/000302285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cellular signalling. 2004;16:291–299. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 127.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gazzerro E, Canalis E. Bone morphogenetic proteins and their antagonists. Reviews in endocrine & metabolic disorders. 2006;7:51–65. doi: 10.1007/s11154-006-9000-6. [DOI] [PubMed] [Google Scholar]
- 129.Ross JJ, Shimmi O, Vilmos P, Petryk A, Kim H, Gaudenz K, Hermanson S, Ekker SC, O'Connor MB, Marsh JL. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature. 2001;410:479–483. doi: 10.1038/35068578. [DOI] [PubMed] [Google Scholar]
- 130.Fernandes M, Gutin G, Alcorn H, McConnell SK, Hebert JM. Mutations in the BMP pathway in mice support the existence of two molecular classes of holoprosencephaly. Development. 2007;134:3789–3794. doi: 10.1242/dev.004325. [DOI] [PubMed] [Google Scholar]
- 131.Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- 132.Lana-Elola E, Tylzanowski P, Takatalo M, Alakurtti K, Veistinen L, Mitsiadis TA, Graf D, Rice R, Luyten FP, Rice DP. Noggin null allele mice exhibit a microform of holoprosencephaly. Human molecular genetics. 2011;20:4005–4015. doi: 10.1093/hmg/ddr329. [DOI] [PubMed] [Google Scholar]
- 133.Oelgeschlager M, Larrain J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zakin L, De Robertis EM. Inactivation of mouse Twisted gastrulation reveals its role in promoting Bmp4 activity during forebrain development. Development. 2004;131:413–424. doi: 10.1242/dev.00946. [DOI] [PubMed] [Google Scholar]
- 135.Graf D, Timmons PM, Hitchins M, Episkopou V, Moore G, Ito T, Fujiyama A, Fisher AG, Merkenschlager M. Evolutionary conservation, developmental expression, and genomic mapping of mammalian Twisted gastrulation. Mammalian genome : official journal of the International Mammalian Genome Society. 2001;12:554–560. doi: 10.1007/s0033501-0005-x. [DOI] [PubMed] [Google Scholar]
- 136.Kauvar EF, Hu P, Pineda-Alvarez DE, Solomon BD, Dutra A, Pak E, Blessing B, Proud V, Shanske AL, Stevens CA, et al. Minimal evidence for a direct involvement of twisted gastrulation homolog 1 (TWSG1) gene in human holoprosencephaly. Molecular genetics and metabolism. 2011;102:470–480. doi: 10.1016/j.ymgme.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wenghoefer M, Ettema AM, Sina F, Geipel A, Kuijpers-Jagtman AM, Hansmann H, Borstlap WA, Berge S. Prenatal ultrasound diagnosis in 51 cases of holoprosencephaly: craniofacial anatomy, associated malformations, and genetics. Cleft Palate Craniofac J. 2010;47:15–21. doi: 10.1597/08-036.1. [DOI] [PubMed] [Google Scholar]
- 138.Mercier S, Dubourg C, Belleguic M, Pasquier L, Loget P, Lucas J, Bendavid C, Odent S. Genetic counseling and "molecular" prenatal diagnosis of holoprosencephaly (HPE) Am J Med Genet C Semin Med Genet. 2010;154C:191–196. doi: 10.1002/ajmg.c.30246. [DOI] [PubMed] [Google Scholar]
- 139.Pineda-Alvarez DE, Dubourg C, David V, Roessler E, Muenke M. Current recommendations for the molecular evaluation of newly diagnosed holoprosencephaly patients. Am J Med Genet C Semin Med Genet. 2010;154C:93–101. doi: 10.1002/ajmg.c.30253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Curry CJ, Carey JC, Holland JS, Chopra D, Fineman R, Golabi M, Sherman S, Pagon RA, Allanson J, Shulman S, et al. Smith-Lemli-Opitz syndrome-type II: multiple congenital anomalies with male pseudohermaphroditism and frequent early lethality. Am J Med Genet. 1987;26:45–57. doi: 10.1002/ajmg.1320260110. [DOI] [PubMed] [Google Scholar]
- 141.Levey EB, Stashinko E, Clegg NJ, Delgado MR. Management of children with holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:183–190. doi: 10.1002/ajmg.c.30254. [DOI] [PubMed] [Google Scholar]
- 142.Solomon BD, Mercier S, Velez JI, Pineda-Alvarez DE, Wyllie A, Zhou N, Dubourg C, David V, Odent S, Roessler E, et al. Analysis of genotype-phenotype correlations in human holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:133–141. doi: 10.1002/ajmg.c.30240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 144.Billington CJ, Jr, Schmidt B, Zhang L, Hodges JS, Georgieff MK, Schotta G, Gopalakrishnan R, Petryk A. Maternal diet supplementation with methyl donors and increased parity affect the incidence of craniofacial defects in the offspring of twisted gastrulation mutant mice. J Nutr. 2013;143:332–339. doi: 10.3945/jn.112.168906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 146.Hoch RV, Rubenstein JL, Pleasure S. Genes and signaling events that establish regional patterning of the mammalian forebrain. Semin Cell Dev Biol. 2009;20:378–386. doi: 10.1016/j.semcdb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 147.Hallgrimsson B, Jamniczky H, Young NM, Rolian C, Parsons TE, Boughner JC, Marcucio RS. Deciphering the Palimpsest: Studying the Relationship Between Morphological Integration and Phenotypic Covariation. Evol Biol. 2009;36:355–376. doi: 10.1007/s11692-009-9076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fernandes M, Hebert JM. The ups and downs of holoprosencephaly: dorsal versus ventral patterning forces. Clin Genet. 2008;73:413–423. doi: 10.1111/j.1399-0004.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- 149.Schiffer C, Tariverdian G, Schiesser M, Thomas MC, Sergi C. Agnathia-otocephaly complex: report of three cases with involvement of two different Carnegie stages. Am J Med Genet. 2002;112:203–208. doi: 10.1002/ajmg.10672. [DOI] [PubMed] [Google Scholar]