Abstract
Objectives
evaluate if CT features of intra-diverticular bladder cancer can predict clinical outcome.
Methods
retrospective study of 34 patients with intra-diverticular bladder cancer. Two radiologists independently evaluated all CT exams.
Results
CT tumor length and width were significantly associated with survival for both readers (HRs 1.31–1.62, p<0.001–0.043). No other tumor features were significantly associated with survival. The inter-reader agreement for the assessment of CT features was fair to substantial (k=0.34–0.78; CCC=0.56–0.66). There was no association between TUR pathology stage and survival (HR 2.10; p=0.21).
Conclusions
In patients with intra-diverticular bladder cancer, the tumor length and width measured on the pre-treatment CT predicted survival.
Keywords: bladder diverticulum, bladder cancer, imaging, CT
INTRODUCTION
Bladder diverticula are defined as outpouchings of the urothelial lining through the muscularis layer of the bladder wall. They may result from congenital weakness of the bladder wall at the level of the ureterovesical junction (e.g. Hutch’s diverticulum) or may be acquired as a result of increased intravesical pressure, typically in the context of lower urinary tract obstruction.
Bladder diverticula may sometimes harbor tumors, classically thought to be secondary to urinary stasis with chronic infection and inflammation leading to metaplasia and subsequent tumor formation. However, the transitional cell lining of a diverticulum is identical to that which covers the entire urinary drainage system, and therefore carcinogenic alteration related to exposure of urinary agents can similarly affect the lining of the diverticulum predisposing to cancer formation. Although diverticular tumors account for only about 1.5% of all bladder cancers (1), they pose distinct challenges for diagnosis and management and warrant special attention. First, although direct visualization of the bladder lumen through cystoscopy has been demonstrated to be an adequate first-line method for the diagnosis of bladder neoplasms, cystoscopic visualization of diverticular tumors may be difficult, particularly if the diverticula have narrow orifices or are otherwise difficult to access. Furthermore, diverticula are characterized by absence or thin muscularis propria layer, which increases the risk of perforation during biopsy or transurethral resection (TUR) and also theoretically facilitates extra-vesical tumor spread (2). Attempts to avoid perforation and tumor cell dissemination during biopsy often lead to understaging on TUR.
The role of imaging for the evaluation of intra-diverticular tumors is not well known since prior studies have been limited to case reports and small case series (3–11). Therefore, the purpose of this study was to describe the computed tomography (CT) features of intra-diverticular bladder cancer and evaluate if the imaging features can predict clinical outcome
MATERIALS AND METHODS
Patients
This retrospective study was compliant with the Health Insurance Portability and Accountability Act. The institutional review board issued a waiver of informed consent. The inclusion criteria for the study were: (i) cystoscopy-guided biopsy or resection of intra-diverticular bladder tumor performed between 2001 and 2010 at our institution; (ii) CT imaging performed before treatment; (iii) CT imaging study available in DICOM format through our institution’s picture archiving and communications system (PACS) and (iv) complete histopathology report available. Of 106 patients treated for intra-diverticular bladder tumors at our institution during the study period, 35 satisfied all the above inclusion criteria. One patient was excluded because of severe beam-hardening artifacts on CT caused by bilateral hip prosthesis. Thus, our final study population consisted of 34 patients. Clinical and demographic characteristics are presented in Table 1.
Table 1.
Patient Characteristics
| Age in years, mean (range) | 67 (42–87) |
| Gender, (%) n | |
| Male | 94% (32/34) |
| Female | 6% (2/34) |
| Time between CT and cystoscopy in days, mean (range) | 27 (0–169) |
| Number of intra-diverticular tumors per patient, (%)n | |
| 1 tumor | (91%) 31/34 |
| 2 tumors | (9%) 3/34 |
| Clinical stage*, (%) n | |
| Non-invasive (<T1) | 35% (12/34) |
| Invasive (≥ T1) | 65% (22/34) |
| Grade, (%) n | |
| Low | 9% (3/34) |
| High | 91% (31/34) |
| Tumor Histology, (%) n | |
| Transitional cell carcinoma | 76% (26/34) |
| Mixed transitional and squamous cell | 9% (3/34) |
| Neuroendocrine carcinoma | 6% (2/34) |
| Squamous cell carcinoma | 3% (1/34) |
| Adenocarcinoma | 3% (1/34) |
| Sarcomatoid carcinoma | 3% (1/34) |
| Presenting Complaint, (%) n | |
| Hematuria | 85% (29/34) |
| Treatment received, (%) n | |
| TUR alone | 29% (10/34) |
| TUR + intravesical BCG | 18%(6/34) |
| TUR + Cystectomy | 53%(18/34) |
Abbreviations: TUR: Transurethral resection. BCG: Bacillus Calmette-Guerin
Clinical stage based on physical exam, cystoscopy findings and pathological evaluation of transurethral resection specimen
CT acquisition
All CT imaging studies were performed on commercially available 16- or 64-detector-row CT scanners (GE Medical Systems, Toshiba, Philips) using the following scan parameters: tube voltage, 120KVp; tube current time product, 80–442 mAs; reconstruction slice thickness, 2.5–7.5 mm; pitch 0.75–1.75. Iodinated intravenous contrast material was administered in 32 of 34 (91%) of patients.
CT interpretation
Two radiologists retrospectively and independently reviewed all CT studies using a PACS workstation (Centricity; GE Medical System). Reader 1 (--; omitted for blinded review) had 6 years of experience in interpreting genitourinary CT studies, and reader 2 (--; omitted for blinded review) had 2 years of experience in interpreting CT studies. Both readers were aware that all patients had bladder cancer, but they were otherwise blinded to all clinical and histopathological findings as well as the original CT imaging reports. Both readers assessed the CT images for the following:
total number of bladder diverticula with tumors;
tumor involvement of the diverticular neck (connecting the diverticulum to the bladder lumen) (Fig 1);
tumor length (defined as the length of contact between the tumor and the diverticular wall) and the largest tumor diameter perpendicular to the tumor length (tumor width) (Fig 2);
tumor morphology (i.e., flat vs. polypoid) (Fig 3).
presence of extravesical tumor spread
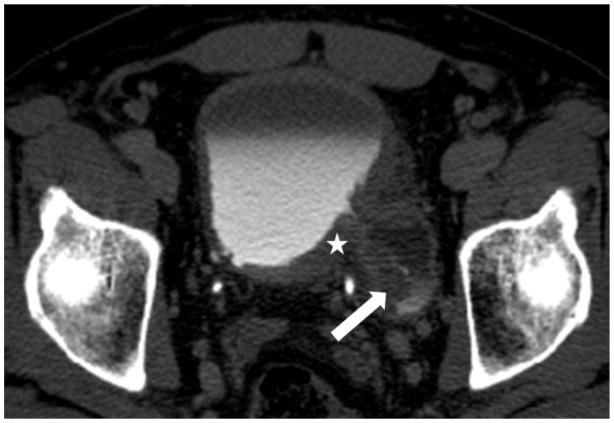
Figure 1.
Male age 53 years. CT urography scan shows a large soft tissue mass involving the diverticulum neck and right lateral wall of the bladder. The mass extends into the extravesical fat (arrows). The patient was found to have transitional cell carcinoma with sarcomatoid features, stage T3.
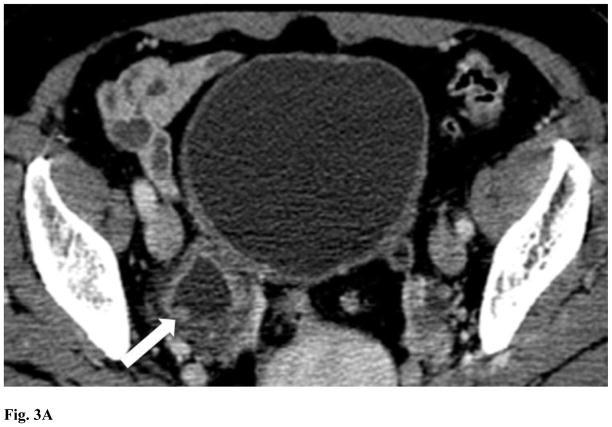
Figure 2.
Male age 66 years. Delayed CT scan showing an irregular polypoid soft tissue mass (arrow) within a diverticulum without transmural invasion. There is circumferential thickening of the left bladder wall involving the diverticulum neck (asterisk). Lines show the length of contact between the tumor and the diverticular wall (l) and the tumor depth perpendicular to diverticular wall (d). The patient was found to have transitional cell carcinoma, stage T1.
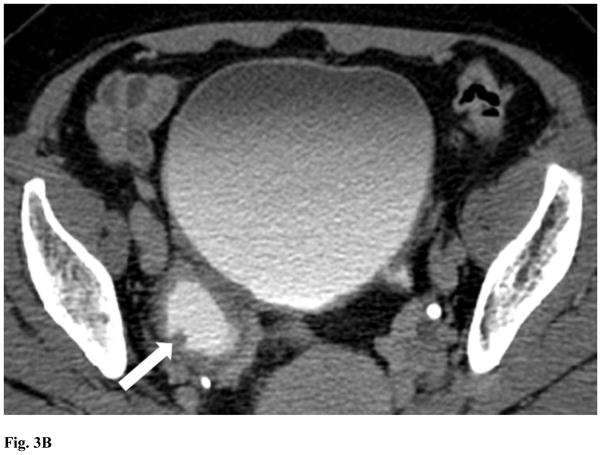
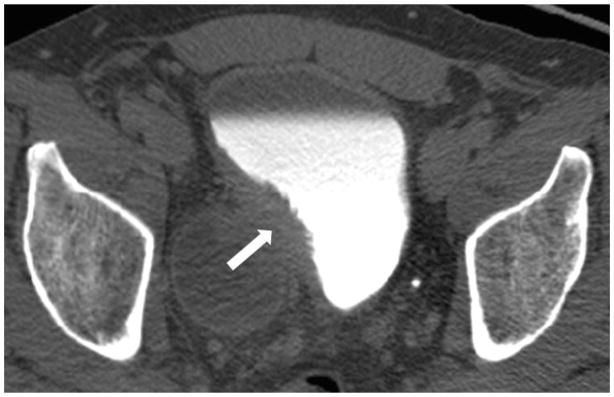
Figure 3.
Female age 56 years. (a) Post-contrast and (b) delayed CT scans show a small polypoid tumor (arrow) within a postero-lateral diverticulum. The tumor is confined to the wall of the diverticulum. The patient was found to have transitional cell carcinoma, stage T1.
Clinical and Histopathologic Data
TUR tumor histology was established according to the 2004 World Health Organization histological classification of tumors of the urinary tract (12). Cystectomy pathology tumor stages were recorded according to TNM stage (13). All patients were initially biopsied via TUR. Definitive treatment was at the discretion of the treating physician and consisted of TUR alone in 29% (10/34), TUR followed by intravesical Bacillus Calmette-Guerin (BCG) therapy in 18% (6/34), partial cystectomy in 15% (5/34) and radical cystectomy in 38% (13/34). Experienced genitourinary pathologists, who process all bladder cancer specimens at our institution, performed histopathologic analyses.
Statistical Analysis
Continuous variables were expressed as medians and ranges, categorical variables as percentages and frequencies. Two-sided p-values ≤ 0.05 were considered to indicate statistical significance. All statistical analyses were performed using commercially available software (SAS 9.2, SAS Institute, Cary, NC).
Inter-reader agreement levels for categorical variables were calculated using Cohen’s Kappa. For continuous variables, the concordance correlation coefficient (CCC) was used. Inter-reader agreement values were interpreted as follows: 0.01–0.20: slight agreement; 0.21–0.40: fair agreement; 0.41–0.60: moderate agreement; 0.61–0.80: substantial agreement; 0.81–1.0: almost perfect agreement.
Survival analysis was performed for TUR pathology stage and CT features separately for each reader. When more than 1 diverticular tumor was present in a single patient (which occurred in 3 of the 34 cases), the largest tumor was used for all calculations. Overall survival (OS) was calculated as the interval from the time of the CT to the time of death or last follow-up. Cox regression was used to examine the associations between CT features with OS, with a robust covariance matrix and an independent correlation structure to take into account multiple diverticula per patient. Hazard ratios (HR) and 95% confidence intervals were estimated for every feature.
RESULTS
Clinical and Pathological Evaluation
Clinical staging confirmed a total of 37 intra-diverticular bladder cancers in 34 patients. Tumor histologies were obtained by transurethral biopsy in all cases and consisted of transitional cell carcinoma in 26 patients, mixed transitional and squamous cell carcinoma in 3, neuroendocrine carcinoma in 2, squamous cell carcinoma in 1, adenocarcinoma in 1, and sarcomatoid carcinoma in 1 patient (Table 1). Eighteen (53%) patients underwent partial/radical cystectomy. In 8 of these 18 patients (44%), the tumor stage assigned on pathological review of the cystectomy specimen was higher than the clinical stage. In seven of these 18 patients (39%), extravesical extension was identified in the cystectomy specimen. The treatments received by all patients are summarized in Table 1. The mean follow-up for all patients was 1060 days (range, 11–2540 days).
CT features and inter-reader agreement
Reader 1 (R1) identified 28 tumors in 49 bladder diverticula and reader 2 (R2) identified 39 tumors in 51 bladder diverticula. Tumor involvement of the diverticular neck was identified in 15 patients (53.5%) by R1 and in 25 patients (64.1%) by R2. Tumor morphology was polypoid in 60.7% (17/28) and flat in 39.2% (11/28) of tumors according to R1, and polypoid in 46.1% (18/39) and flat in 53.8% (21/39) according to R2. Median tumor length was 23.5 mm (range, 3–78 mm) for R1 and 18.5 mm (range, 3–72 mm) for R2. Median tumor width was 14 mm (range, 2–71 mm) for R1 and 9 mm (range, 2–72 mm) for R2 (Table 2). Inter-reader agreement levels, also summarized in Table 2, ranged from fair to substantial (k=0.34–0.78; CCC=0.56–0.66). Of the 7 patients in whom extravesical extension was present on pathological analysis of the cystectomy specimen, extravesical extension on CT was identified in 6/7 (86%) patients by R1 and 5/7 patients (71%) by R2.
Table 2.
CT features and interreader agreement
| Reader 1 | Reader 2 | Inter-Reader Agreement | |||
|---|---|---|---|---|---|
| Patient | Intra-Diverticular Tumor | No | 18% (6/34) | 6%(2/34) | κ = 0.452 (0.030, 0.874) |
| Yes | 82%(28/34) | 94%(32/34) | |||
| Number of Intra-Diverticular Tumors per patient | No Tumor | 18%(6/34) | 6%(2/34) | κ = 0.344 (0.129, 0.559) | |
| 1 Tumor | 82%(28/34) | 74%(25/34) | |||
| 2 Tumors | none | 21%(7/34) | |||
| Tumor | Diverticular Neck Involvement* | No | 46%(13/28) | 36%(14/39) | κ = 0.635 (0.422, 0.871) |
| Yes | 54%(15/28) | 64%(25/39) | |||
| Tumor morphology* | Polypoid | 61%(17/28) | 46%(18/39) | κ = 0.779 (0.607, 1.000) | |
| Flat | 39%(11/28) | 54%(21/39) | |||
| Tumor Width (mm)* | Median (Range) | 14 (2–71) | 9 (2–72) | CCC = 0.666 (0.124, 0.964) | |
| Tumor Length (mm)* | 23.5 (3–78) | 18.5 (3–72) | CCC = 0.562 (0.147, 0.864) |
CT features (34 patients; 49 diverticula for reader 1, 51 diverticula for reader 2)
For diverticular tumors observed on CT scans.
κ: Kappa (95% CI); concordance correlation coefficient: CCC (95%CI)
Associations between TUR pathology, CT features and survival
During the follow-up period, 22/34 (65%) patients remained alive and 12/34 (35%) patients died. There was no association between TUR pathology stage and survival (HR 2.10; p=0.21). On CT, the length of contact between the tumor and the diverticular wall correlated significantly with overall survival for both readers (HR 1.45, p=0.007 for R1 and HR=1.55; p<0.001 for R2). In addition, tumor depth correlated significantly with overall survival for both readers (HR=1.62; p<0.001 for R1 and HR=1.31; p=0.043 for R2). Associations between CT features and survival are summarized in Table 3.
Table 3.
Associations between CT features and survival (OS = overall survival, PFS = progression-free survival)
| CT Feature | Reader 1 | Reader 2 | |||
|---|---|---|---|---|---|
| OS | OS | ||||
| HR (95%CI) | p-value | HR (95%CI) | p-value | ||
| Tumor presence | No | 1 | No event for patients with no tumor on CT, HR not estimable (NE). | ||
| Yes | 3.95 (0.51, 30.5) | 0.188 | |||
| Number of diverticula with tumor | 0 | 1 | NE | ||
| 1 | 3.95 (0.51, 30.5) | 0.188 | 1 | ||
| 2 | NE | 1.09 (0.30, 3.98) | 0.897 | ||
| Morphology | Polypoid | 1 | 1 | ||
| Flat | 0.36 (0.12, 1.12) | 0.077 | 0.35 (0.13, 0.93) | 0.036 | |
| Diverticular neck involvement | No | 1 | 1 | ||
| Yes | 1.64 (0.74, 3.61) | 0.221 | 1.20 (0.51, 2.84) | 0.671 | |
| Tumor length | 1.45 (1.11, 1.90) | 0.007 | 1.55 (1.22, 1.97) | <.001 | |
| Tumor width | 1.62 (1.29, 2.03) | <.001 | 1.31 (1.01, 1.71) | 0.043 | |
Note: OS= overall survival. HR= hazards ratio. P-values in bold font indicate statistically significant associations.
DISCUSSION
In this study we identified two CT features--namely, tumor width and the length of contact between the tumor and the diverticular wall--that were associated with survival and could serve as prognostic indicators in patients with intra-diverticular bladder tumors. This information is clinically relevant, as the pathology stage from the transurethral resection specimen, which is typically considered to guide treatment decisions in these patients, provided limited prognostic information in our study population (i.e. there was no association between TUR pathology stage and survival).
Literature regarding the use of imaging in intra-diverticular bladder tumors is limited and consists predominantly of case reports or small case series (3–11, 14–17). Our study population (n=34) is larger than the sum of patients included in all of the prior studies (3–11, 14–17), none of which correlated imaging findings with clinical outcomes. Some of the earlier reports evaluated the role of intravenous pyelography (IVP) (3)(6), a technique that was found to be of limited value and is no longer in routine clinical use. Other case series have shown that cross-sectional imaging with CT and magnetic resonance imaging (MRI) offer incremental value compared to IVP and cystography for the evaluation of intra-diverticular tumors. Dondalski et al. evaluated 6 patients with diverticular bladder tumors who underwent IVP/cystography and cross-sectional imaging (5 with CT and 1 with MRI) (4). Only 3 of the 6 tumors were seen as intraluminal filling defects on IVP/cystography. In one patient, CT showed a concentric tumor at the diverticular neck, with corresponding IVP/cystography showing only smooth narrowing in the same area. In a large diverticulum, CT and MRI showed a tumor that was not visualized on cystography due to obstruction at the diverticular orifice. Another study found that CT was able to document the absence of extravesical extension in all 5 patients in whom CT was performed for preoperative staging of intra-diverticular bladder tumors (5). Haecker et al. reported one case of intra-diverticular bladder tumor that was detected on both CT and endorectal ultrasound but was not detected on transabdominal ultrasound (7). Another case report described how a large pelvic mass detected on CT was characterized as a diverticular bladder tumor using multiplanar T2-weighted MRI (8).
The majority of patients (32 of 34) in our study were men, and most patients in our study (29 of 34) had either pure or mixed transitional cell carcinomas. In addition, the tumors of most patients demonstrated invasive (22 of 34) and/or high-grade (31 of 34) features on pathology. These clinical and demographic characteristics of our patient population are similar to those reported in the largest clinical series published to date, which included 39 patients (2), suggesting that our findings are relevant to all patients with intra-diverticular bladder tumors rather than only a specific subset of patients undergoing imaging. Although as mentioned earlier, it is difficult to estimate the prevalence of bladder diverticula in the general population, bladder diverticula should be considered potential sites of carcinoma and as such should be carefully evaluated, particularly in older patients with hematuria.
Our study was limited by its retrospective design and small sample size. Despite a prior report suggesting significant associations between pathology stage of intra-diverticular bladder tumors and 5-year survival (2), we did not attempt to correlate the final pathology stage obtained from cystectomy with clinical outcomes in our patient population. Although all patients in our study had histologically confirmed tumors, only 18 patients underwent cystectomy. Upstaging of 8 and the presence of extravesical tumor extension in 7 of the 18 patients who underwent cystectomy confirmed that, as expected, TUR tends to underestimate tumor extent in patients with intra-diverticular bladder tumors.
In addition, the study period was long (2001–2010) and thus the imaging protocols, including the CT scanners and acquisition parameters used, were heterogeneous. Modern imaging techniques using magnetic resonance imaging may play an expanded role in the assessment of these tumors; however at present there is no published data to support this. Given the rarity of intra-diverticular tumors it was felt that maximizing the sample size was desirable and that it was necessary to include all patients who had undergone pretreatment CT regardless of the technical parameters used. Another important limitation is the fact that readers were aware that all patients in the study had intra-diverticular bladder tumors. This may have influenced the readers’ confidence in establishing the presence of intra-diverticular tumors, so that findings that would otherwise have been considered indeterminate or even normal may have been deemed suspicious.
In summary, in patients with intra-diverticular bladder tumors, the length of contact between the tumor and diverticular wall and the tumor width are significantly associated with overall survival.
Footnotes
Conflict of interest statement
All authors state that none conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tamas EF, Stephenson AJ, Campbell SC, Montague DK, Trusty DC, Hansel DE. Histopathologic features and clinical outcomes in 71 cases of bladder diverticula. Archives of pathology & laboratory medicine. 2009;133(5):791–6. doi: 10.5858/133.5.791. [DOI] [PubMed] [Google Scholar]
- 2.Golijanin D, Yossepowitch O, Beck SD, Sogani P, Dalbagni G. Carcinoma in a bladder diverticulum: presentation and treatment outcome. J Urol. 2003;170(5):1761–4. doi: 10.1097/01.ju.0000091800.15071.52. [DOI] [PubMed] [Google Scholar]
- 3.Baniel J, Vishna T. Primary transitional cell carcinoma in vesical diverticula. Urology. 1997;50(5):697–9. doi: 10.1016/S0090-4295(97)00319-1. [DOI] [PubMed] [Google Scholar]
- 4.Dondalski M, White EM, Ghahremani GG, Patel SK. Carcinoma arising in urinary bladder diverticula: imaging findings in six patients. AJR American journal of roentgenology. 1993;161(4):817–20. doi: 10.2214/ajr.161.4.8372767. [DOI] [PubMed] [Google Scholar]
- 5.Lowe FC, Goldman SM, Oesterling JE. Computerized tomography in evaluation of transitional cell carcinoma in bladder diverticula. Urology. 1989;34(6):390–5. doi: 10.1016/0090-4295(89)90451-2. [DOI] [PubMed] [Google Scholar]
- 6.Montague DK, Boltuch RL. Primary neoplasms in vesical diverticula: report of 10 cases. J Urol. 1976;116(1):41–2. doi: 10.1016/s0022-5347(17)58664-4. [DOI] [PubMed] [Google Scholar]
- 7.Haecker A, Riedasch G, Langbein S, Alken P, Michel MS. Diverticular carcinoma of the urinary bladder: diagnosis and treatment problems. A case report. Medical principles and practice : international journal of the Kuwait University, Health Science Centre. 2005;14(2):121–4. doi: 10.1159/000083925. [DOI] [PubMed] [Google Scholar]
- 8.Durfee SM, Schwartz LH, Panicek DM, Russo P. MR imaging of carcinoma within urinary bladder diverticulum. Clin Imaging. 1997;21(4):290–2. doi: 10.1016/s0899-7071(96)00049-6. [DOI] [PubMed] [Google Scholar]
- 9.Matta EJ, Kenney AJ, Barre GM, Vanlangendonck RM., Jr Best cases from the AFIP: intradiverticular bladder carcinoma. Radiographics. 2005;25(5):1397–403. doi: 10.1148/rg.255045205. [DOI] [PubMed] [Google Scholar]
- 10.Siegel WH. Neoplasms in bladder diverticula. Urology. 1974;4(4):411–3. doi: 10.1016/0090-4295(74)90009-0. [DOI] [PubMed] [Google Scholar]
- 11.Moratalla MB, Salvador RL. An unusual cause of hematuria: Intradiverticular bladder carcinoma. European Journal of Radiology Extra. 2008;68(3):e133–e5. [Google Scholar]
- 12.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary system and male genital organ. Lyon: IARC Press; 2004. [Google Scholar]
- 13.AJCC cancer staging manual. 7. New York: Springer; 2011. [Google Scholar]
- 14.Omeroglu A, Paner GP, Wojcik EM, Siziopikou K. A carcinosarcoma/sarcomatoid carcinoma arising in a urinary bladder diverticulum. Archives of pathology & laboratory medicine. 2002;126(7):853–5. doi: 10.5858/2002-126-0853-ACSCAI. [DOI] [PubMed] [Google Scholar]
- 15.Melekos MD, Asbach HW, Barbalias GA. Vesical diverticula: etiology, diagnosis, tumorigenesis, and treatment. Analysis of 74 cases. Urology. 1987;30(5):453–7. doi: 10.1016/0090-4295(87)90378-5. [DOI] [PubMed] [Google Scholar]
- 16.Das S, Amar AD. Vesical diverticulum associated with bladder carcinoma: therapeutic implications. J Urol. 1986;136(5):1013–4. doi: 10.1016/s0022-5347(17)45191-3. [DOI] [PubMed] [Google Scholar]
- 17.Faysal MH, Freiha FS. Primary neoplasm in vesical diverticula. A report of 12 cases. Br J Urol. 1981;53(2):141–3. doi: 10.1111/j.1464-410x.1981.tb03153.x. [DOI] [PubMed] [Google Scholar]