Abstract
The contribution of acetylcholine to psychiatric illnesses remains an area of active research. For example, increased understanding of mechanisms underlying cholinergic modulation of cortical function has provided insight into attentional dysfunction in schizophrenia. Acetylcholine normally enhances cortical sensitivity to external stimuli and decreases corticocortical communication, increasing focused attention. However, increases in ACh signaling can lead to symptoms related to anxiety and depression. For example, while stress-induced ACh release can result in adaptive responses to environmental stimuli, chronic elevations in cholinergic signaling may produce maladaptive behaviors. Here, we review several innovations in human imaging, molecular genetics and physiological control of circuits that have begun to identify mechanisms linking altered cholinergic neuromodulation to schizophrenia and depression.
Introduction
Acetylcholine (ACh) is a potent regulator of neuronal activity throughout the peripheral and central nervous system [1,2]; however, the specific contributions of cholinergic neuromodulation to circuit function in the healthy brain and in psychiatric illness have been difficult to dissect, due to its pleiotropic actions on neuronal excitability, synaptic transmission, and network dynamics. In the last few years, technological innovations in the areas of molecular genetics, physiology, and human imaging have provided new ways to understand how neuromodulation shapes circuits and behavior. In this review, we outline recent progress in understanding how cholinergic signaling contributes to circuits involved in two groups of psychiatric disorders, schizophrenia and major depressive disorder (MDD). Continued technical innovation will continue to bring us closer to the ideal of translating fundamental neuronal mechanisms to the understanding and treatment of psychiatric illness.
Cholinergic sources and receptors
The two sources of ACh in the CNS are (1) projection nuclei that diffusely innervate distal areas and (2) local interneurons that are interspersed among their cellular targets. Cholinergic projection nuclei include the pedunculopontine (PPT) and laterodorsal (LDT) tegmental areas and the basal forebrain complex, including the medial septum [3–5]. In contrast, cholinergic interneurons are typified by the tonically active cells of the striatum and nucleus accumbens [6]. There is also evidence for a small population of cholinergic interneurons in the neocortex [7,8] and hippocampus [9].
The actions of ACh are mediated by two major classes of receptors: metabotropic muscarinic receptors (mAChRs) and ionotropic nicotinic receptors (nAChRs) [reviewed in 10,11]. Briefly, mAChRs are G protein-coupled and categorized by signaling through either Gαq (M1, M3, M5 subtypes) or Gαi (M2, M4 subtypes). In contrast, nAChRs function as nonselective, excitatory cation channels and occur as either homomeric or heteromeric assemblies of a large family of alpha- (α2-α7) and beta- (β2-β4) subunits.
Considerable debate has focused on whether cholinergic signaling occurs via traditional synapses with closely apposed pre- and postsynaptic membranes or via volume transmission mediated by diffusion through the extracellular space [12,13]. While a detailed discussion of this topic is beyond the present scope, several studies have suggested that ACh acts primarily by volume transmission. There is an anatomical mismatch between the sites of ACh release and the location of cholinergic receptors [14–16], and extracellular levels of ACh fluctuate in a manner that appears to be inconsistent with localized clearance of a synaptic transmitter [17–19]. More recently, however, it has become clear that volume transmission may be insufficient for the rapid transfer of cholinergic signals measured using electrochemical recordings in behavioral tasks such as prefrontal cortex (PFC)-dependent cue-detection or sustained attention [20,21]. In addition, optogenetic stimulation of endogenous Ach release has revealed fast excitatory transients mediated by nAChRs in neocortical GABAergic interneurons [22–24]. These rapid cholinergic signals are a key element in a cortical network underlying auditory fear learning [25]. The development of tools allowing more precise stimulation of ACh neurons in vivo [22–24] has been an innovation that has already altered our view of cholinergic neuromodulation.
Cholinergic function and dysfunction in neuropsychiatric disease
The neuromodulatory effects of ACh signaling are critical for normal function of numerous brain systems. Accordingly, abnormalities in the cholinergic system are known to contribute to a number of psychiatric and neurological illnesses. In the periphery, autoantibodies to muscle nAChRs contribute to myasthenia gravis [26,27]. Moreover, loss of cholinergic neurons and receptors in the brain contribute to the cognitive decline in Alzheimer’s disease [28] and may contribute to the progression of Alzheimer’s and Parkinson’s disease [29]. More recently, advances in understanding the modulation of ACh signaling in cortical and subcortical circuits has revived interest in understanding how cholinergic dysfunction may contribute to psychiatric illnesses such as schizophrenia and depression. Here, we will review the evidence for cholinergic involvement in these two disorders.
ACh in schizophrenia and attention
Schizophrenia consists of positive symptoms, such as disordered thoughts, delusions, and hallucinations, and negative symptoms, such as blunted affect and social withdrawal. In addition, cognitive disturbances including reduced attention and working memory are frequently present [30]. Prevailing hypotheses of the pathophysiology underlying schizophrenia have largely focused on monoamines like dopamine and serotonin. However, there is increasing evidence from clinical and preclinical studies that aberrant ACh signaling may also contribute to the disease.
Epidemiological studies have long shown that schizophrenics exhibit higher rates of tobacco smoking than the general population, suggesting that patients may use nicotine (an agonist of nAChRs) for self-medication [31,32]. Moreover, genome-wide association studies link copy number variations of a locus containing the α7 nAChR with elevated risk for schizophrenia [33]. Indeed, reduced α7 nAChR expression has been observed in the hippocampus and cingulate cortex of post-mortem brains from schizophrenic patients [34,35]. In addition, individuals with schizophrenia show greatly decreased upregulation of high affinity nAChRs as a result of smoking compared to control subjects, suggesting that the high rates of smoking in schizophrenia may be influenced by the diminished effect of nicotine on this cholinergic receptor subclass [31,32]. However, these findings are partially confounded by observations that antipsychotic treatment may also reduce nAChR binding [36]. Nicotinic stimulation produces well known enhancements of attention and working memory [37,38]; however, nAChR agonists have yielded mixed results in clinical trials, and pharmacotherapies directed at nicotinic signaling remain an area of active research [39].
Aberrant signaling through mAChRs is also implicated in schizophrenia, as postmortem studies of patients have revealed a decrease in expression of these receptors throughout the brain, including the prefrontal cortex and hippocampus [40–42]. Perhaps more telling is the observation that muscarinic antagonists produce psychosis-like symptoms in healthy individuals and exacerbate existing symptoms in schizophrenia patients [43,44]. Notably, a recent study of the mixed M1/M4 agonist xanomeline found significant cognitive improvements in patients, though peripheral side effects continue to pose problems for muscarinic-based therapies [45].
Despite these clinical observations, mechanistic links between the cellular actions of ACh and the pathophysiology of schizophrenia have been difficult to establish. Instead, current research has focused largely on the contribution of cholinergic activity to normal behaviors that are known to be disrupted in schizophrenic patients, such as attention and working memory.
Cholinergic activity in the rodent neocortex has been linked to control of circuits underlying attention, cue detection, and short-term memory, cognitive abilities that are disrupted in schizophrenic patients [30]. Lesions of cholinergic inputs arising from the basal forebrain impair tests of sustained attention [46–48]. In addition, stimulation ofα4β2 nAChRs in the medial prefrontal cortex enhances performance in a visual attention task [49], while genetic deletion of these receptors in the mPFC impairs visual attention [50] and auditory discrimination [51]. Similarly, stimulation of α7 nAChRs in PFC modulates glutamatergic signaling through NMDA receptors, influencing circuits important for working memory performance [52]. Notably, transient rises in prefrontal ACh are significantly correlated with cue detection, suggesting that the temporal dynamics of cholinergic signaling are also critical for normal behavior [20]. In primates, locally applied ACh enhances the attentional modulation of neuronal activity in the primary visual cortex, while the muscarinic antagonist scopolamine reduces the effects of attention [53]. Similarly, optogenetic activation of cholinergic neurons in the basal forebrain enhanced visual responsiveness in mouse cortex and improved performance on a visual discrimination task [54]. Taken together, these findings suggest that cholinergic actions across both ionotropic and metabotropic receptors and diverse brain areas contribute to cognitive processing.
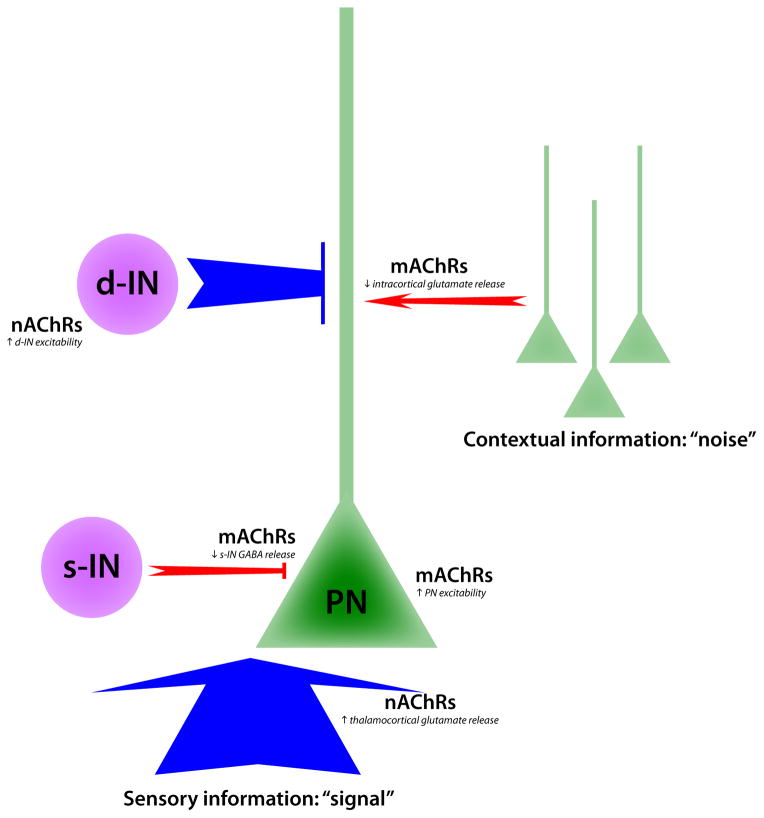
At the cellular level, ACh increases the excitability of many classes of neurons, including pyramidal cells and dendrite-targeting GABAergic interneurons via activation of both mAChRs and nAChRs [22,55–59]. ACh also modulates synaptic transmission. Activation of α4β2 nAChRs on thalamocortical terminals enhances glutamate release in both sensory and association cortex [60–62], whereas activation of mAChRs on terminals of soma-targeting parvalbumin-expressing interneurons decreases the probability of GABA release [63]. GABAergic inhibition normally reduces the response of cortical neurons to feed-forward excitation [64,65], and decreased GABA release therefore enhances the ability of thalamocortical inputs to stimulate pyramidal neuron firing [63]. In contrast, mAChRs located on pyramidal cell axon terminals suppress cortico-cortical transmission [60,62,66,67]. The simultaneous enhancement of feed-forward inputs from the thalamus and suppression of intra-cortical feed-back may increase the “signal-to-noise” ratio in cortical networks [68]. The most recent data therefore suggest that the role of cholinergic modulation in the cortex is to make neurons more sensitive to external stimuli, thereby increasing focused attention to sensory input (Fig. 1).
Figure 1.
Circuit mechanisms underlying cholinergic modulation of attention. In the schematic, ACh enhances sensory-evoked signals and limits the impact of noisy contextual inputs by strengthening pathways shown in blue and weakening pathways shown in red. Activation of nAChRs increases glutamate release from thalamocortical afferents, while mAChR activity increases the excitability of pyramidal neurons (PNs) and reduces GABA release from soma-targeting interneurons (s-INs). At the same time, mAChRs reduce intracortical glutamate release while nAChRs increase the excitability of dendrite-targeting interneurons (d-INs) that regulate synaptic integration. increases the release of glutamate from thalamocortical terminals.
Acetylcholine signaling in stress and depression
As in schizophrenia, the primary medications for MDD target the monoamine system, but the contribution of the cholinergic system to affective disorders is becoming more evident. Recently, human imaging studies have revived the idea, first proposed in the 1970’s [69], that increased cholinergic signaling can contribute to depression. Peripheral administration of the acetylcholinesterase (AChE) antagonist physostigmine induces symptoms of anxiety and depression in human subjects by decreasing the breakdown of ACh and increasing levels of the neurotransmitter in the brain [70]. Furthermore, in SPECT imaging studies, individuals with major depressive disorder or bipolar disorder who are actively depressed have lower availability for the radiotracer 5IA binding to nAChRs throughout the brain. This result is observed despite the absence of altered nAChR number in postmortem cortical tissue [71,72]. Increasing ACh levels in human brain by physostigmine challenge also displaces 5IA binding [73]. Thus, individuals who are actively depressed appear to have significantly higher levels of extracellular ACh than healthy subjects, suggesting that increased ACh signaling may contribute to the etiology of depression.
Rodent studies confirm that increasing ACh levels by treating with physostigmine acutely can induce anxiety- and depression-like behaviors, whereas chronic treatment with the serotonergic antidepressant fluoxetine increases levels and activity of AChE, particularly in the hippocampus [74]. Local administration of physostigmine or knockdown of AChE in the hippocampus is sufficient to increase anxiety- and depression-like behaviors that can be reversed by administration of fluoxetine, suggesting that they are consistent with symptoms of depression [74]. Taken together, these studies show that hyperactive ACh signaling in hippocampus can contribute to depressive symptoms.
The effects of increasing ACh release on the dynamics of hippocampal activity are complex, and the specific alterations linked to regulation of depression-like symptoms are unclear. Increasing ACh signaling in the CA1 region of the hippocampus using physostigmine in a hippocampal slice results in depolarization of postsynaptic interneurons, mediated through M2/M3 mAChRs [75]. Optogenetic stimulation of cholinergic terminals in CA1 also increases activity of α4/β2* nAChRs and depolarizes a subpopulation of GABAergic interneurons in the stratum lacunosum moleculare [23], suggesting that one mechanism underlying the effects of cholinergic signaling on anxiety and depression may be activation of inhibitory interneurons. In contrast, lower levels of cholinergic stimulation in CA1 hyperpolarizes a subset of interneurons via M4 mAChRs and entrains others into a rhythmic bursting pattern [75]. Elevated ACh may therefore modulate hippocampal activity by switching CA1 networks from a quiescent or stable bursting state, to a more depolarized state with a higher level of firing.
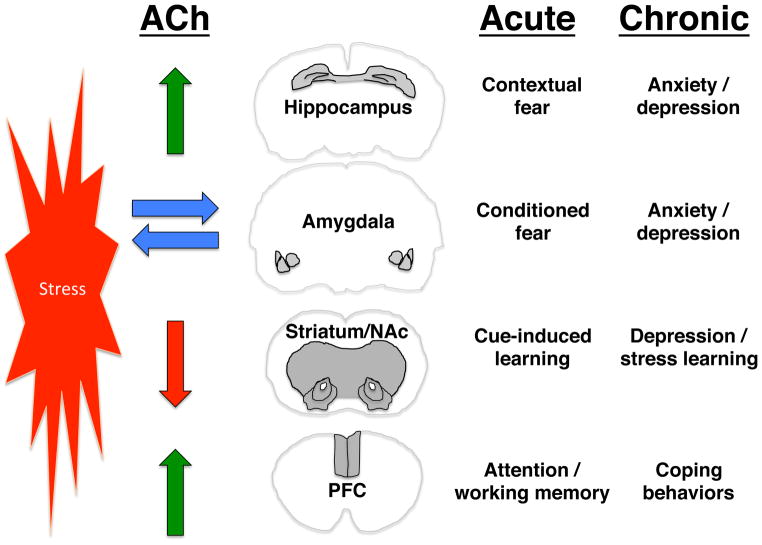
Cholinergic signaling in the hippocampus, amygdala, prefrontal cortex (PFC), and striatum modulates behavioral responses to stressors [76–80]. Despite the fact that global and hippocampal increases in ACh tone result in anxiety- and depression-like behaviors [74], the effects of cholinergic signaling on stress-related behaviors are complex and vary across brain areas. Stress induces release of ACh in the hippocampus and PFC [81] but not the amygdala [82], perhaps because the basal firing rate of medial septal neurons innervating the amygdala is high [83] and stress cannot further increase ACh levels. Similarly, basal ACh tone in the striatum is high due to tonic firing of intrinsic cholinergic interneurons, and behaviorally relevant stimuli result in a pause in their firing leading to cue-dependent learning [84]. These findings suggest that healthy behavior is dependent on appropriately balanced cholinergic signaling across brain regions (Fig. 2).
Figure 2.
Circuits involved in cholinergic modulation of mood and anxiety. The schematic demonstrates the differential effects of stress on ACh release. Stress induces increases in ACh release in the hippocampus and prefrontal cortex (PFC), has less of an effect on the already high levels of ACh in the amygdala, and decreases firing of the cholinergic interneurons in the striatum/nucleus accumbens (NAc). Relief of stress also increases ACh release in the PFC. The acute effects of stress-induced changes in ACh signaling area likely to be adaptive and to lead to behaviors that promote learning to change behavior and avoid stressors, whereas chronic stress may result in maladaptive plasticity downstream of ACh signaling that can lead to anxiety and mood disorders.
Supporting this view, stress impairs PFC-mediated working memory [85], but cholinergic signaling through α7-type nAChRs is important for tuning glutamatergic signaling in the dorsolateral PFC and improving working memory [52]. Accordingly, both stress [18] and relief from stress [82] can increase ACh levels in the PFC. Similarly, silencing striatal cholinergic interneurons leads to depression-like behaviors [80]. Thus, increased ACh signaling in the brain may have differential effects (Fig. 2) depending on whether there is already high cholinergic tone in a particular brain area at baseline (amygdala, striatum), and whether the brain area is involved in avoidance behaviors (amygdala, hippocampus) or adaptive coping behaviors (PFC, striatum).
The ability to withdraw from stressful stimuli and decrease exploration in response to an immediate threat is likely to be highly favored in evolution. Following chronic stress, this adaptive response can lead to maladaptive induction of a depression-like state [86,87]. Exposure to an acute stress may lead to adaptive behavioral responses mediated through ACh release in the hippocampus, whereas chronic increases in ACh signaling can lead to mood disorders, perhaps by evoking synaptic plasticity in different neuronal subtypes throughout the hippocampus [88] and contributing to encoding memories of stimuli associated with stressful events [89]. Similarly, plasticity in the amygdala strengthens associations between environmental cues and stressful events and therefore is also likely to contribute to maladaptive learning leading to mood and anxiety disorders [78,90].
CONCLUSIONS
Despite decades of work, a complete understanding of the role of ACh in brain function remains elusive. However, recent methodological advances for monitoring and manipulating cholinergic systems have broadened our knowledge of the cellular mechanisms underlying ACh signaling. Similarly, new human imaging studies have highlighted the role for distinct cholinergic systems in behavior. One principal conclusion to be drawn from the wealth of current data is that cholinergic modulation is best viewed as the synergistic alteration of neuronal function at the synaptic, cellular, and network levels. Thus, improved therapies for neuropsychiatric disorders such as schizophrenia and depression will require interventions directed at specific cholinergic receptor subtypes and cell classes. For example, GABAergic interneurons provide an intriguing focus for their role in linking nAChR activity, cortical circuit function, and behavior. Future research directions must continue to emphasize the interactions of clinical and preclinical studies, ultimately bridging bench and bedside.
Highlights.
ACh boosts attention by enhancing sensory stimuli and decreasing cortico-cortical communication.
Increased ACh signaling can lead to symptoms of depression in humans and animal models.
Novel techniques have helped elucidate the role of ACh in schizophrenia and depression.
Acknowledgments
This work was supported by a Smith Family Award, a Sloan Research Fellowship, and NIH grant MH099045 (MJH) and NIH grants DA014241, MH077681 and DA033945 (MRP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Changeux J-P. Allosteric receptors: from electric organ to cognition. Ann Rev Pharmacol Toxicol. 2010;50:1–38. doi: 10.1146/annurev.pharmtox.010909.105741. [DOI] [PubMed] [Google Scholar]
- 2.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesulam MM. Structure and Function of Cholinergic Pathways in the Cerebral Cortex, Limbic System, Basal Ganglia, and Thalamus of the Human Brain. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the Fourth Generation of Progress. New York, New York: Raven Press; 1995. [Google Scholar]
- 4.Zaborszky L. The modular organization of brain systems. Basal forebrain: the last frontier. Progress in brain research. 2002;136:359–372. doi: 10.1016/s0079-6123(02)36030-8. [DOI] [PubMed] [Google Scholar]
- 5.Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. NeuroImage. 2008;42:1127–1141. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apicella P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci. 2007;30:299–306. doi: 10.1016/j.tins.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Benagiano V, Virgintino D, Flace P, Girolamo F, Errede M, Roncali L, Ambrosi G. Choline acetyltransferase-containing neurons in the human parietal neocortex. European journal of histochemistry : EJH. 2003;47:253–256. doi: 10.4081/835. [DOI] [PubMed] [Google Scholar]
- 8.von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H. Functional characterization of intrinsic cholinergic interneurons in the cortex. J Neurosci. 2007;27:5633–5642. doi: 10.1523/JNEUROSCI.4647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frotscher M, Vida I, Bender R. Evidence for the existence of non-GABAergic, cholinergic interneurons in the rodent hippocampus. Neuroscience. 2000;96:27–31. doi: 10.1016/s0306-4522(99)00525-4. [DOI] [PubMed] [Google Scholar]
- 10.Picciotto MR, Caldarone BJ, King SL, Zachariou V. Nicotinic receptors in the brain: links between molecular biology and behavior. Neuropsychopharmacol. 2000;22:451–465. doi: 10.1016/S0893-133X(99)00146-3. [DOI] [PubMed] [Google Scholar]
- 11.Wess J. Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends in Pharmacological Sciences. 2003;24:414–420. doi: 10.1016/S0165-6147(03)00195-0. [DOI] [PubMed] [Google Scholar]
- 12.Zoli M, Jansson A, Sykova E, Agnati LF, Fuxe K. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Tr Pharmacol Sci. 1999;20:142–150. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]
- 13.Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nature reviews Neuroscience. 2009;10:383–390. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wainer BH, Bolam JP, Freund TF, Henderson Z, Totterdell S, Smith AD. Cholinergic synapses in the rat brain: a correlated light and electron microscopic immunohistochemical study employing a monoclonal antibody against choline acetyltransferase. Brain Res. 1984;308:69–76. doi: 10.1016/0006-8993(84)90918-1. [DOI] [PubMed] [Google Scholar]
- 15.Arroyo-Jimenez MM, Bourgeois JP, Marubio LM, Le Sourd AM, Ottersen OP, Rinvik E, Fairen A, Changeux JP. Ultrastructural localization of the alpha4-subunit of the neuronal acetylcholine nicotinic receptor in the rat substantia nigra. J Neurosci. 1999;19:6475–6487. doi: 10.1523/JNEUROSCI.19-15-06475.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill JA, Jr, Zoli M, Bourgeois J-P, Changeux J-P. Immunocytochemical localization of a neuronal nicotinic receptor: the β2 subunit. J Neurosci. 1993;13:1551–1568. doi: 10.1523/JNEUROSCI.13-04-01551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajnal A, Pothos EN, Lenard L, Hoebel BG. Effects of feeding and insulin on extracellular acetylcholine in the amygdala of freely moving rats. Brain Res. 1998;785:41–48. doi: 10.1016/s0006-8993(97)01291-2. [DOI] [PubMed] [Google Scholar]
- 18.Laplante F, Stevenson CW, Gratton A, Srivastava LK, Quirion R. Effects of neonatal ventral hippocampal lesion in rats on stress-induced acetylcholine release in the prefrontal cortex. Journal of Neurochemistry. 2004;91:1473–1482. doi: 10.1111/j.1471-4159.2004.02831.x. [DOI] [PubMed] [Google Scholar]
- 19.Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Sarter M, Bruno JP. Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci. 2004;20:1545–1554. doi: 10.1111/j.1460-9568.2004.03614.x. [DOI] [PubMed] [Google Scholar]
- 20**.Parikh V, Kozak R, Martinez V, Sarter M, Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. This elegant study used electrochemical recording of ACh transients in the prefrontal cortex of behaving rats. The authors showed that fast cholinergic signals were correlated with successful cue detection, suggesting that cortical ACh supports cognitive performance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parikh V, Ji J, Decker MW, Sarter M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:3518–3530. doi: 10.1523/JNEUROSCI.5712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Arroyo S, Bennett C, Aziz D, Brown SP, Hestrin S. Prolonged disynaptic inhibition in the cortex mediated by slow, non-alpha7 nicotinic excitation of a specific subset of cortical interneurons. J Neurosci. 2012;32:3859–3864. doi: 10.1523/JNEUROSCI.0115-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Bell KA, Shim H, Chen CK, McQuiston AR. Nicotinic excitatory postsynaptic potentials in hippocampal CA1 interneurons are predominantly mediated by nicotinic receptors that contain alpha4 and beta2 subunits. Neuropharmacology. 2011;61:1379–1388. doi: 10.1016/j.neuropharm.2011.08.024. This study, along with [22], used optogenetic stimulation of cholinergic fibers to evoke fast excitatory potentials in the cortex and hippocampus, mediated by activation of nicotinic receptors. In both cases, excitation was limited to a specific subset of GABAergic interneurons, indicating circuit-specific actions of ACh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett C, Arroyo S, Berns D, Hestrin S. Mechanisms generating dual- component nicotinic EPSCs in cortical interneurons. J Neurosci. 2012;32:17287–17296. doi: 10.1523/JNEUROSCI.3565-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. The authors show that foot shock-associated disinhibition in auditory cortex is mediated by cholinergic activation of layer 1 interneurons that subsequently inhibit layer 2/3 parvalbumin-expressing interneurons. Blockade of this pathway abolishes associative fear-learning. [DOI] [PubMed] [Google Scholar]
- 26.Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973;180:871–872. doi: 10.1126/science.180.4088.871. [DOI] [PubMed] [Google Scholar]
- 27.Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116:2843–2854. doi: 10.1172/JCI29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 29.Picciotto M, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- 30.Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- 31.Adams CE, Stevens KE. Evidence for a role of nicotinic acetylcholine receptors in schizophrenia. Front Biosci. 2007;12:4755–4772. doi: 10.2741/2424. [DOI] [PubMed] [Google Scholar]
- 32.D'Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62:1564–1573. doi: 10.1016/j.neuropharm.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–236. doi: 10.1038/nature07229. Along with earlier studies identifying an association between the CHRNA7 locus and phenotypes associated with schizophrenia, this study shows that copy number variations in this locus are associated with the illness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Ruiz CM, Haroutunian VH, Long P, Young AH, Davis KL, Perry EK, Court JA. Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2003;54:1222–1233. doi: 10.1016/s0006-3223(03)00348-2. [DOI] [PubMed] [Google Scholar]
- 35.Marutle A, Zhang X, Court J, Piggott M, Johnson M, Perry R, Perry E, Nordberg A. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J Chem Neuroanat. 2001;22:115–126. doi: 10.1016/s0891-0618(01)00117-x. [DOI] [PubMed] [Google Scholar]
- 36.Terry AV, Jr, Gearhart DA, Mahadik SP, Warsi S, Davis LW, Waller JL. Chronic exposure to typical or atypical antipsychotics in rodents: temporal effects on central alpha7 nicotinic acetylcholine receptors. Neuroscience. 2005;136:519–529. doi: 10.1016/j.neuroscience.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacology (Berl) 1998;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- 39.Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem Pharmacol. 2013;86:1122–1132. doi: 10.1016/j.bcp.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry. 2000;48:381–388. doi: 10.1016/s0006-3223(00)00918-5. [DOI] [PubMed] [Google Scholar]
- 41.Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E. Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2002;7:1083–1091. doi: 10.1038/sj.mp.4001199. [DOI] [PubMed] [Google Scholar]
- 42.Scarr E, Sundram S, Keriakous D, Dean B. Altered hippocampal muscarinic M4, but not M1, receptor expression from subjects with schizophrenia. Biol Psychiatry. 2007;61:1161–1170. doi: 10.1016/j.biopsych.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 43.Osterholm RK, Camoriano JK. Transdermal scopolamine psychosis. JAMA. 1982;247:3081. [PubMed] [Google Scholar]
- 44.Tandon R, Shipley JE, Greden JF, Mann NA, Eisner WH, Goodson JA. Muscarinic cholinergic hyperactivity in schizophrenia. Relationship to positive and negative symptoms. Schizophr Res. 1991;4:23–30. doi: 10.1016/0920-9964(91)90006-d. [DOI] [PubMed] [Google Scholar]
- 45.Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dube S, Mallinckrodt C, Bymaster FP, McKinzie DL, Felder CC. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- 46.McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J Neurosci. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- 48.Turchi J, Sarter M. Cortical acetylcholine and processing capacity: effects of cortical cholinergic deafferentation on crossmodal divided attention in rats. Brain Res Cogn Brain Res. 1997;6:147–158. doi: 10.1016/s0926-6410(97)00027-x. [DOI] [PubMed] [Google Scholar]
- 49.Howe WM, Ji J, Parikh V, Williams S, Mocaer E, Trocme-Thibierge C, Sarter M. Enhancement of attentional performance by selective stimulation of alpha4beta2(*) nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacology. 2010;35:1391–1401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, Spijker S, Mansvelder HD. Nicotinic acetylcholine receptor beta2 subunits in the medial prefrontal cortex control attention. Science. 2011;333:888–891. doi: 10.1126/science.1207079. In this remarkable study, the authors find that mice lacking the α4β2 nicotinic receptor show impairments in focused attention. This impairment is rescued by re-expressing the receptors in the prefrontal cortex, demonstrating a clear link between region-specific cholinergic signaling and behavior. [DOI] [PubMed] [Google Scholar]
- 51.Horst NK, Heath CJ, Neugebauer NM, Kimchi EY, Laubach M, Picciotto MR. Impaired auditory discrimination learning following perinatal nicotine exposure or beta2 nicotinic acetylcholine receptor subunit deletion. Behav Brain Res. 2012;231:170–180. doi: 10.1016/j.bbr.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Yang Y, Paspalas CD, Jin LE, Picciotto MR, Arnsten AF, Wang M. Nicotinic alpha7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc Natl Acad Sci U S A. 2013;110:12078–12083. doi: 10.1073/pnas.1307849110. This study shows that alpha 7 nAChRs in the primate PFC regulate NMDA signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci. 2013;16:1857–1863. doi: 10.1038/nn.3552. The authors show that optogenetic stimulation of ACh release in the mouse visual cortex improves perception on a trial-by-trial basis. Conversely, inactivation of the basal forebrain impairs performance, suggesting a causal role for cholinergic activity in the modulation of sensory processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Couey J, Meredith R, Spijker S, Poorthuis R, Smit A, Brussaard A, Mansvelder H. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- 57.Gulledge AT, Park SB, Kawaguchi Y, Stuart GJ. Heterogeneity of phasic cholinergic signaling in neocortical neurons. J Neurophysiol. 2007;97:2215–2229. doi: 10.1152/jn.00493.2006. [DOI] [PubMed] [Google Scholar]
- 58.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 59.McCormick DA, Prince DA. Two types of muscarinic response to acetylcholine in mammalian cortical neurons. Proc Natl Acad Sci U S A. 1985;82:6344–6348. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 61.Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- 62.Oldford E, Castro-Alamancos MA. Input-specific effects of acetylcholine on sensory and intracortical evoked responses in the “barrel cortex” in vivo. Neuroscience. 2003;117:769–778. doi: 10.1016/s0306-4522(02)00663-2. [DOI] [PubMed] [Google Scholar]
- 63.Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 65.Higley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J Neurosci. 2006;26:448–457. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsieh CY, Cruikshank SJ, Metherate R. Differential modulation of auditory thalamocortical and intracortical synaptic transmission by cholinergic agonist. Brain Research. 2000;880:51–64. doi: 10.1016/s0006-8993(00)02766-9. [DOI] [PubMed] [Google Scholar]
- 67.Kimura F, Baughman RW. Distinct muscarinic receptor subtypes suppress excitatory and inhibitory synaptic responses in cortical neurons. J Neurophysiol. 1997;77:709–716. doi: 10.1152/jn.1997.77.2.709. [DOI] [PubMed] [Google Scholar]
- 68.Hasselmo ME. The role of acetylcholine in learning and memory. Current opinion in neurobiology. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- 70.Risch SC, Cohen RM, Janowsky DS, Kalin NH, Murphy DL. Mood and behavioral effects of physostigmine on humans are accompanied by elevations in plasma beta-endorphin and cortisol. Science. 1980;209:1545–1546. doi: 10.1126/science.7433977. [DOI] [PubMed] [Google Scholar]
- 71**.Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A, Chen JI, Cosgrove KP, Kerestes R, Ghose S, et al. Persistent beta2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:851–859. doi: 10.1176/appi.ajp.2012.11101546. This is the first study to use a nicotinic tracer to measure ACh occupancy of nAChRs in depressed human subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hannestad JO, Cosgrove KP, DellaGioia NF, Perkins E, Bois F, Bhagwagar Z, Seibyl JP, McClure-Begley TD, Picciotto MR, Esterlis I. Changes in the cholinergic system between bipolar depression and euthymia as measured with [123I]5IA single photon emission computed tomography. Biol Psychiatry. 2013;74:768–776. doi: 10.1016/j.biopsych.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73*.Esterlis I, Hannestad JO, Bois F, Sewell RA, Tyndale RF, Seibyl JP, Picciotto MR, Laruelle M, Carson RE, Cosgrove KP. Imaging changes in synaptic acetylcholine availability in living human subjects. J Nucl Med. 2013;54:78–82. doi: 10.2967/jnumed.112.111922. This study demonstrates conclusively that a nicotinic tracer can be used to measure changes in ACh levels in awake, behaving human subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74*.Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A. 2013;110:3573–3578. doi: 10.1073/pnas.1219731110. Evidence that increased ACh signaling in the hippocampus results in anxiety- and depression-like behaviors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bell LA, Bell KA, McQuiston AR. Synaptic muscarinic response types in hippocampal CA1 interneurons depend on different levels of presynaptic activity and different muscarinic receptor subtypes. Neuropharmacology. 2013;73:160–173. doi: 10.1016/j.neuropharm.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Belujon P, Grace AA. Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Ann N Y Acad Sci. 2011;1216:114–121. doi: 10.1111/j.1749-6632.2010.05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 79.Gozzi A, Jain A, Giovannelli A, Bertollini C, Crestan V, Schwarz AJ, Tsetsenis T, Ragozzino D, Gross CT, Bifone A. A neural switch for active and passive fear. Neuron. 2010;67:656–666. doi: 10.1016/j.neuron.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 80*.Warner-Schmidt JL, Schmidt EF, Marshall JJ, Rubin AJ, Arango-Lievano M, Kaplitt MG, Ibanez-Tallon I, Heintz N, Greengard P. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci U S A. 2012;109:11360–11365. doi: 10.1073/pnas.1209293109. This paper provides elegant evidence that striatal cholinergic neurons are critical for depression-like behaviors and antidepressant response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laplante F, Sibley DR, Quirion R. Reduction in acetylcholine release in the hippocampus of dopamine D5 receptor-deficient mice. Neuropsychopharmacology. 2004;29:1620–1627. doi: 10.1038/sj.npp.1300467. [DOI] [PubMed] [Google Scholar]
- 82.Mark GP, Rada PV, Shors TJ. Inescapable stress enhances extracellular acetylcholine in the rat hippocampus and prefrontal cortex but not the nucleus accumbens or amygdala. Neuroscience. 1996;74:767–774. doi: 10.1016/0306-4522(96)00211-4. [DOI] [PubMed] [Google Scholar]
- 83.Whalen PJ, Kapp BS, Pascoe JP. Neuronal activity within the nucleus basalis and conditioned neocortical electroencephalographic activation. J Neurosci. 1994;14:1623–1633. doi: 10.1523/JNEUROSCI.14-03-01623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schulz JM, Reynolds JN. Pause and rebound: sensory control of cholinergic signaling in the striatum. Trends Neurosci. 2013;36:41–50. doi: 10.1016/j.tins.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seligman ME, Maier SF. Failure to escape traumatic shock. J Exp Psychol. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- 87.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 88.Drever BD, Riedel G, Platt B. The cholinergic system and hippocampal plasticity. Behav Brain Res. 2011;221:505–514. doi: 10.1016/j.bbr.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 89.Nijholt I, Farchi N, Kye M, Sklan EH, Shoham S, Verbeure B, Owen D, Hochner B, Spiess J, Soreq H, et al. Stress-induced alternative splicing of acetylcholinesterase results in enhanced fear memory and long-term potentiation. Mol Psychiatry. 2004;9:174–183. doi: 10.1038/sj.mp.4001446. [DOI] [PubMed] [Google Scholar]
- 90.Mansvelder HD, Mertz M, Role LW. Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits. Semin Cell Dev Biol. 2009;20:432–440. doi: 10.1016/j.semcdb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]