SUMMARY
Different cancer cell compartments often communicate through soluble factors to facilitate tumor growth. Glioma stem cells (GSCs) are a subset of tumor cells that resist standard therapy to contribute to disease progression. How GSCs employ a distinct secretory program to communicate with and nurture each other over the non-stem tumor cell (NSTC) population is not well defined. Here, we show that GSCs preferentially secrete Sema3C and coordinately express PlexinA2/D1 receptors to activate Rac1/NF-κB signaling in an autocrine/paracrine loop to promote their own survival. Importantly, Sema3C is not expressed in neural progenitor cells (NPCs) or NSTCs. Disruption of Sema3C induced apoptosis of GSCs, but not NPCs or NSTCs, and suppressed tumor growth in orthotopic models of glioblastoma. Introduction of activated Rac1 rescued the Sema3C knockdown phenotype in vivo. Our study supports targeting Sema3C to break this GSC-specific autocrine/paracrine loop to improve glioblastoma treatment, potentially with a high therapeutic index.
INTRODUCTION
Glioblastoma (GBM) is a highly infiltrative and incurable primary brain tumor. Despite aggressive therapy, patients with GBM have a dismal prognosis with median survival of about 1 year (Stupp et al., 2009). Tumor control is short lived with the vast majority of patients progressing within 6 months of diagnosis (Stupp et al., 2009). Glioma stem cells (GSCs) contribute to this resistance because they can efficiently repair DNA damage and activate pro-survival pathways after cytotoxic therapy (Bao et al., 2006; Bleau et al., 2009; Chen et al., 2012; Eramo et al., 2006). GSCs and neural progenitor cells (NPCs) share many common properties including the ability to self-renew and establish a cellular hierarchy; however, the molecular mechanisms underlying these processes may differ. Strategies that exploit the differences between GSC and NPC biology would improve the therapeutic index and minimize potential side effects.
GSCs reside in stem-cell niches where they integrate extracellular signals including niche related factors such as VEGF, cell adhesion molecules and extracellular matrix components to support their growth and promote angiogenesis (Rosen and Jordan, 2009; Soeda et al., 2009; Vescovi et al., 2006; Zhou et al., 2009). While crosstalk between GSCs and endothelial cells has been demonstrated (Calabrese et al., 2007; Lu et al., 2012; Zhu et al., 2011), the signaling mechanisms GSCs employ to communicate with each other and promote their own survival within the greater NSTC population is not well understood. Recent studies reveal that cancer stem-like cells (CSCs) may produce and utilize autocrine or paracrine factors to protect themselves from differentiation and apoptosis (Scheel et al., 2011). In GBM, autocrine TGFp, VEGF and HGF/cMET signaling play important roles in the maintenance of GSC identity and tumorigenicity (Hamerlik et al., 2012; Ikushima et al., 2009; Joo et al., 2012). However, these pathways also play critical roles in normal physiology. Identification of molecular mechanisms that discriminate between normal and pathologic stem cell survival are essential.
To determine potential therapeutic targets that are differentially expressed by GSCs, we assessed the expression of secreted proteins that have been implicated in cancer. Class 3 semaphorins were initially identified as evolutionarily conserved axon guidance cues that instruct the assembly of the neural circuitry (Tran et al., 2009). Since their discovery, various class 3 semaphorins have been found to influence cancer growth, either positively or negatively depending on tumor type (Neufeld and Kessler, 2008; Tamagnone, 2012; Zhou et al., 2008). Sema3C stands out because it has consistently been shown to promote tumor progression and correlate with poor prognosis across multiple tumor types (Blanc et al., 2011; Esselens et al., 2010; Galani et al., 2002; Herman and Meadows, 2007; Miyato et al., 2012). Sema3C is overexpressed in malignant glioma cell lines (Rieger et al., 2003) and is amplified in GBM (Brennan et al., 2013). However, the expression and function of Sema3C and its receptors in CSCs and GBM remain unknown.
Neuropilins and plexins form a receptor complex for semaphorins. Neuropilins serve as the primary receptor for ligand binding, whereas plexins co-receptors transduce semaphorin signaling via their intracellular domain (Capparuccia and Tamagnone, 2009; Hota and Buck, 2012). The plexin intracellular domain interacts with the Rac1 GTPase to promote cell migration (Hota and Buck, 2012). The role of Rac1 in cancer has been underscored by its high frequency of activating mutations in melanoma (Hodis et al., 2012; Krauthammer et al., 2012) and dysregulation in colon (Esufali et al., 2007) and lung cancers (Zhou et al., 2013). While Rac1 is best known for its role in cytoskeletal organization, cell motility and growth, Rac1 also plays a role in cancer cell survival (Feng et al., 2011; Heasman and Ridley, 2008; Senger et al., 2002). In addition, Rac1 has been implicated in regulating cancer stem cell proliferation (Akunuru et al., 2011; Myant et al., 2013). However, the functional role of semaphorin signaling in regulating Rac1 activity in GBM and, in particular, in GSCs is unclear. In this study, we sought to investigate the role of Sema3C and its potential regulation of Rac1 in mediating GSC self-renewal and GBM growth.
RESULTS
Sema3C and Its Receptors PlexinA2/D1 Are Differentially Expressed in GSCs
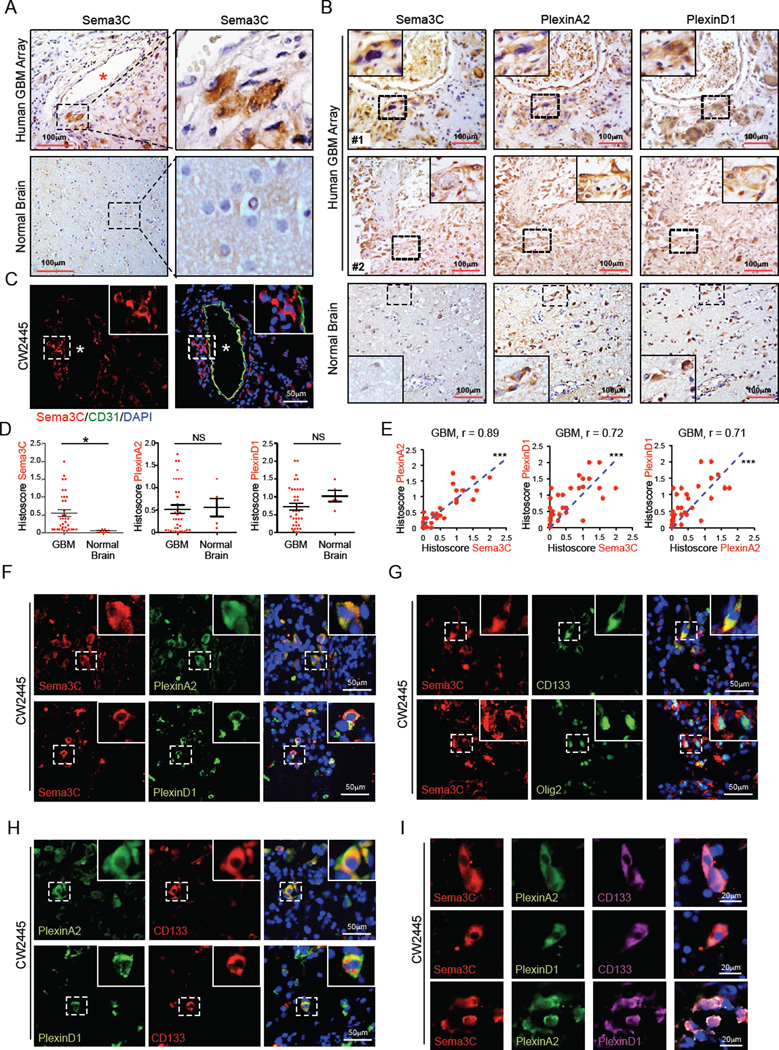
Sema3C is overexpressed in malignant glioma cell lines and amplified in GBM (Brennan et al., 2013; Rieger et al., 2003). However, its role in the pathogenesis of GBM is unclear. We first examined Sema3C expression in a panel of human GBM. Sema3C staining was strong in a subpopulation of GBM cells from seven different patient specimens compared to normal human brain tissue (Figure S1A). Using a GBM tissue microarray, Sema3C was overexpressed in a subpopulation of tumor cells in 30 of 35 (85.7%) GBM but was barely detectable in normal brain (Figure 1A). Sema3C binds to the receptors PlexinA2 and PlexinD1 (Gitler et al., 2004; Kodo et al., 2009; Law, 2012). In serial sections, Sema3C and PlexinA2/D1 expression was highly concordant (Figure 1B), with PlexinA2 expressed in 90% (32/35) GBM and PlexinD1 in all 35 GBM. Within normal brain, Sema3C staining was weak but its receptors stained strongly (Figure 1B, S1A). Notably, histoscore analysis showed that Sema3C levels were higher in GBM compared to normal brain and correlated with expression of PlexinA2 (p<0.0001) and PlexinD1 (p<0.0001) (Figure 1D, 1E, S1B).
Figure 1. Sema3C and Its Receptors Are Co-expressed In Stem Cell Marker+ GBM Cells.
(A and B) Immunohistochemical (IHC) staining of Sema3C, PlexinA2 and PlexinD1 in serial sections of human GBM tissue array. Sections were counterstained with hematoxylin. Asterisk denotes vessel lumen.
(C) Immunofluorescent (IF) staining of Sema3C (red) in relation to blood vessels marked by CD31 staining for endothelial cells (green) in human primary GBM tissues. * denotes vessel lumen.
(D and E) Histoscores (D) and correlation analysis (E) of human GBM tissue array stained for Sema3C, PlexinA2 and PlexinD1. *p< 0.05, ***p < 0.001.
(F) IF staining of Sema3C and PlexinA2/D1 on frozen sections of human primary GBM. Nuclei were counterstained with DAPI (blue).
(G–I) IF staining of Sema3C, PlexinA2/D1 and GSC markers CD133 and Olig2 on frozen sections of human primary GBM. Nuclei were counterstained with DAPI (blue).
See also Figure S1.
To investigate further the expression of Sema3C and its receptors, we performed co-immunofluorescence staining on human GBM specimens. Cells that expressed both Sema3C and PlexinA2/D1 clustered together, suggesting that they may participate in autocrine or paracrine signaling (Figure 1F, 1I). Sema3C and PlexinA2/D1 were co-expressed with GSC markers including CD133, Olig2 and Sox2 (Singh et al., 2004; Suva et al., 2014) (Figure 1G-1I, S1C–S1D). A subset of Sema3C–positive cells localized to perivascular niches (Figure 1A, 1C, S1E), where GSCs are commonly found (Calabrese et al., 2007). These findings suggest that Sema3C is co-expressed with its receptors in GSCs.
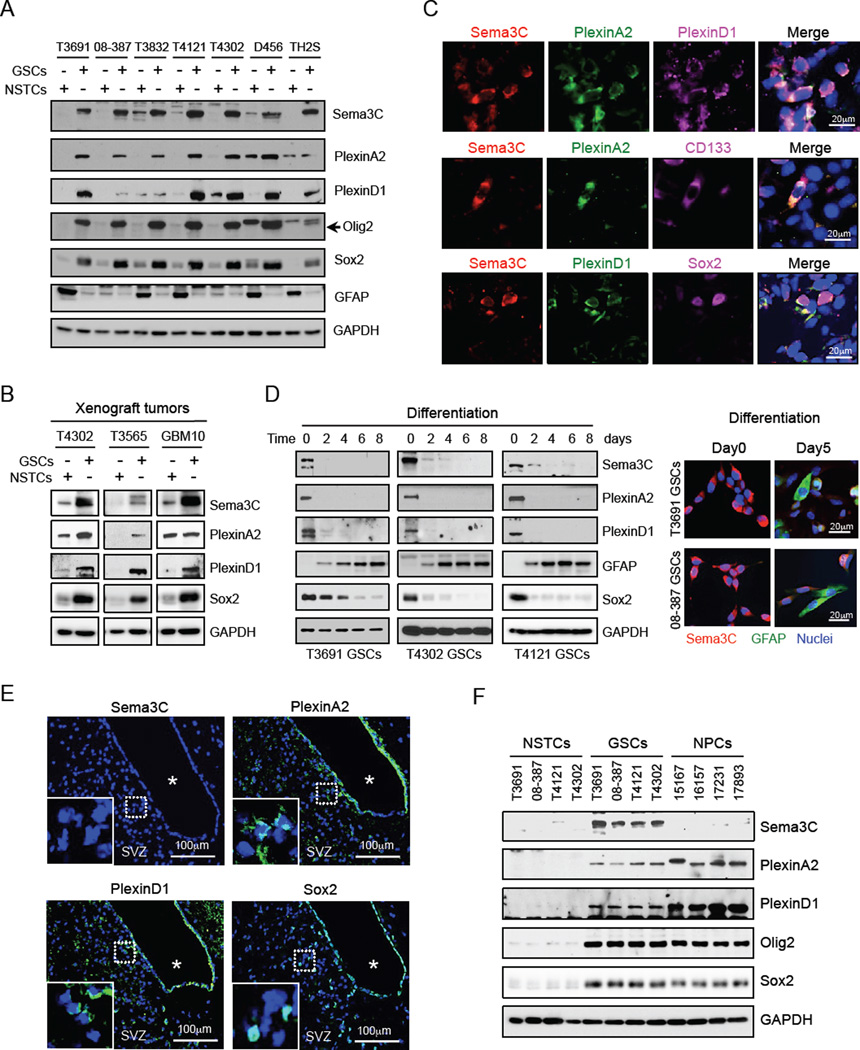
We next assessed the expression of Sema3C and PlexinA2/D1 in matched GSCs and NSTCs isolated from fresh GBM patient specimens that were propagated as xenografts and functionally validated (Cheng et al., 2013). Sema3C and PlexinA2/D1 were elevated in all seven GSC populations enriched for the stem cell markers CD133, Olig2 and Sox2 relative to matched NSTCs (Figure 2A, S2A). The semaphorin ligand-binding receptor (Neufeld and Kessler, 2008; Tamagnone, 2012; Zhou et al., 2008) Neuropilin1 (NRP1), but not NRP2, was ubiquitously expressed in both GSCs and NSTCs (Figure S2B). Of note, expression of other class 3 semaphorins, including Sema3A and Sema3B, was similar between GSCs and NSTCs (Figure S2C). To rule out the possibility that these patterns of expression were driven by cell culture conditions, we confirmed our analysis using freshly sorted GBM xenografts (Figure 2B). We further validated these results in situ by co-immunofluorescence staining of GSC tumorspheres (Figure S2D), GSC-derived GBM xenografts (Figure 2C, S2E–S2I) and fresh GBM patient specimens (Figure 1F-1I). Furthermore, induction of differentiation in GSCs by serum stimulation led to rapid loss of expression of Sema3C and its receptors (Figure 2D), suggesting a role for Sema3C signaling in GSCs.
Figure 2. GSCs Preferentially Co-express Sema3C and Its Receptors.
(A) Immunoblot (IB) analysis of Sema3C, PlexinA2 and PlexinD1 in GSCs and matched NSTCs derived from human GBM.
(B) IB analysis of Sema3C, PlexinA2 and PlexinD1 proteins in GSCs compared to NSTCs isolated via CD133-based sorting from T4302, T3565 and GBM10 xenograft tumors without intervening in vitro culture.
(C) IF staining of Sema3C, PlexinA2, PlexinD1 and GSC markers CD133 and Sox2 on frozen sections of T4302 GSC-derived GBM xenografts. Nuclei were counterstained with DAPI (blue).
(D) GSC differentiation was induced by serum (5% FBS). IB analysis of Sema3C, PlexinA2, PlexinD1, Sox2 and GFAP (astrocyte marker) proteins during GSC differentiation (left). IF staining of Sema3C (red) and GFAP (green) from day 0 to day 5 during GSC differentiation (right). Nuclei were counterstained with DAPI (blue).
(E) IF staining of Sema3C, PlexinA2, PlexinD1 or Sox2 in the subventricular zone (SVZ) in adult mouse brain. Sections were counterstained with DAPI. * denotes ventricle.
(F) IB analysis of Sema3C, PlexinA2 and PlexinD1 proteins in four GSCs, matched NSTCs and four human NPC lines.
See also Figure S2.
Because GSCs and NPCs share several properties, we examined the expression of Sema3C and its receptors in normal brain. Oncomine database analysis revealed reduced mRNA levels of Sema3C in human NPCs compared to GBM (Figure S2J). NPCs reside in the subventricular zone (SVZ) (Fuentealba et al., 2012; Shen et al., 2008). PlexinA2 and PlexinD1 were both expressed in a subpopulation of cells within the SVZ of mouse brain, an area enriched with Sox2-positive cells. Sema3C, in contrast, was undetectable within the SVZ (Figure 2E, S2K). Next, we compared expression of Sema3C and its receptors in four human NPCs, and four pairs of matched GSCs and NSTCs. Consistent with our in situ studies (Figure 1), high levels of Sema3C and its receptors were found in all four GSCs but not in NSTCs. Sema3C was undetectable in NPCs, but its receptors were expressed at high levels (Figure 2F, S2L). These results collectively reveal that GSCs preferentially co-express Sema3C and its receptors PlexinA2/D1 compared to normal brain progenitor cells and non-stem GBM cells.
Secreted Sema3C Promotes GSC Survival and Self-renewal Through PlexinA2/D1
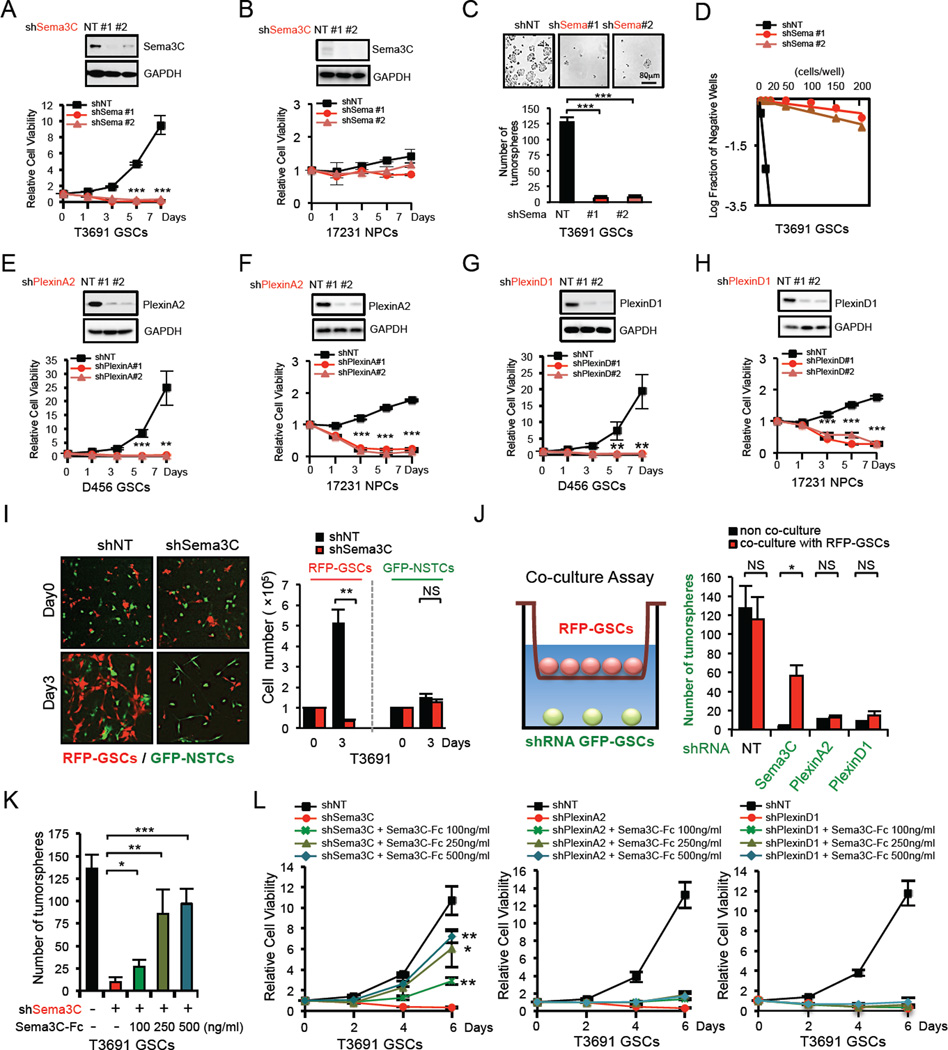
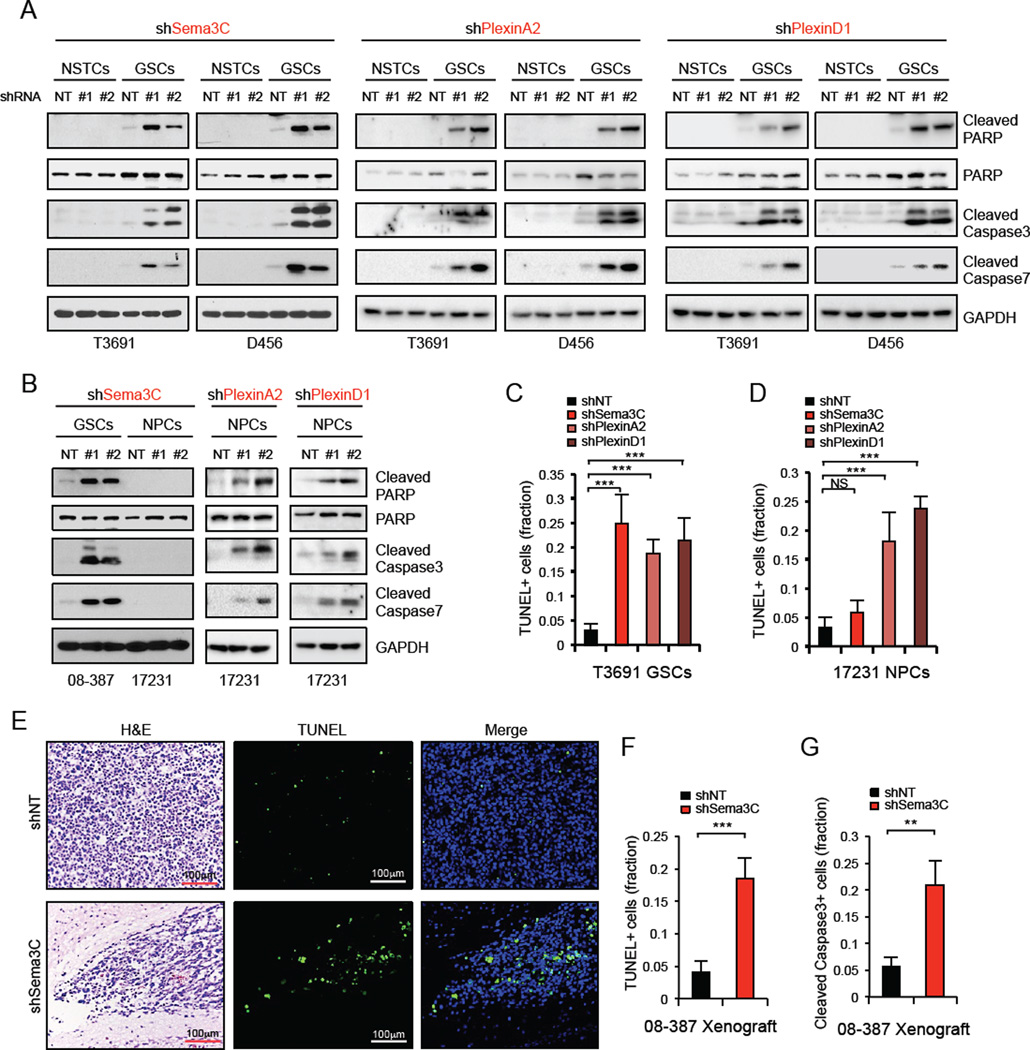
To explore the functional roles of Sema3C signaling in GSCs, we first investigated the effect of Sema3C knockdown on GSC proliferation and tumorsphere formation using two different, non-overlapping Sema3C small hairpin RNA (shRNA) sequences or control non-targeting (NT) shRNA. Reduction of Sema3C significantly decreased cell viability in all three GSC populations examined (Figure 3A, S3A, S3B) but had little effect on matched NSTCs (Figure S3C, S3D, S3L). In addition, Sema3C knockdown impaired GSC self-renewal as assessed by tumorsphere formation and in vitro limiting dilution assay (Figure 3C, 3D, S3E–H), the standard in vitro assay to assess self-renewal capacity (Pastrana et al., 2011). Moreover, knockdown of Sema3C did not impact expression of stem cell transcription factors Sox2 or Olig2 in GSCs (Figure 6A), suggesting that it does not alter stem cell identity but rather GSC survival (Figure 5).
Figure 3. GSC Viability and Self-Renewal Depend on Sema3C Secretion.
(A–D) Effects of Sema3C knockdown with two different shRNA sequences on cell viability in GSCs and NPCs, and tumorsphere formation of GSCs. Knockdown of Sema3C resulted in a decrease in cell viability in GSCs (A), but not in NPCs (B). shSema3C–GSCs showed reduced tumorsphere numbers (C). For the limiting dilution assay, GSCs expressing shNT or shSema3C were plated into 96-well plates with various seeding densities (1–200 cells per well, 12 wells per each condition). Seven days later, each well was evaluated for the presence or absence of tumorspheres (D).
(E–H) Effects of PlexinA2 or PlexinD1 knockdown with two different shRNA sequences on cell viability of GSCs (E, G) and NPCs (F, H).
(I) GSCs and matched NSTCs stably expressing RFP or GFP were mixed and plated on stem cell Matrigel-coated plates at 1:1 ratio and infected by lentivirus containing shNT or shSema3C. RFP-GSC or GFP-NSTC were counted at indicated times after infection (right). Representative images of mixed cells on day 0 and day 3 are shown (left).
(J) GFP-GSCs transduced with shNT, shSema3C, shPlexinA2 or shPlexinD1 were plated at low cell density (500 cells) at the base of transwells and co-cultured with RFP-GSCs (1×105 cells) that were seeded in the upper chambers of transwells. GFP-GSC tumorsphere number was counted on day 6 of co-culture (right). Representative diagram of the co-culture assay is shown (left).
(K and L) GSCs transduced with shNT, shSema3C, shPlexinA2 or shPlexinD1 were cultured with different doses of recombinant human Sema3C protein (Sema3C–Fc). GSC tumorsphere quantification is shown (K) and GSC viability was assessed by cell titer assay (L).
Data are means ± standard deviation (SD) (n = 3). *p< 0.05, **p < 0.01, ***p < 0.001. See also Figure S3.
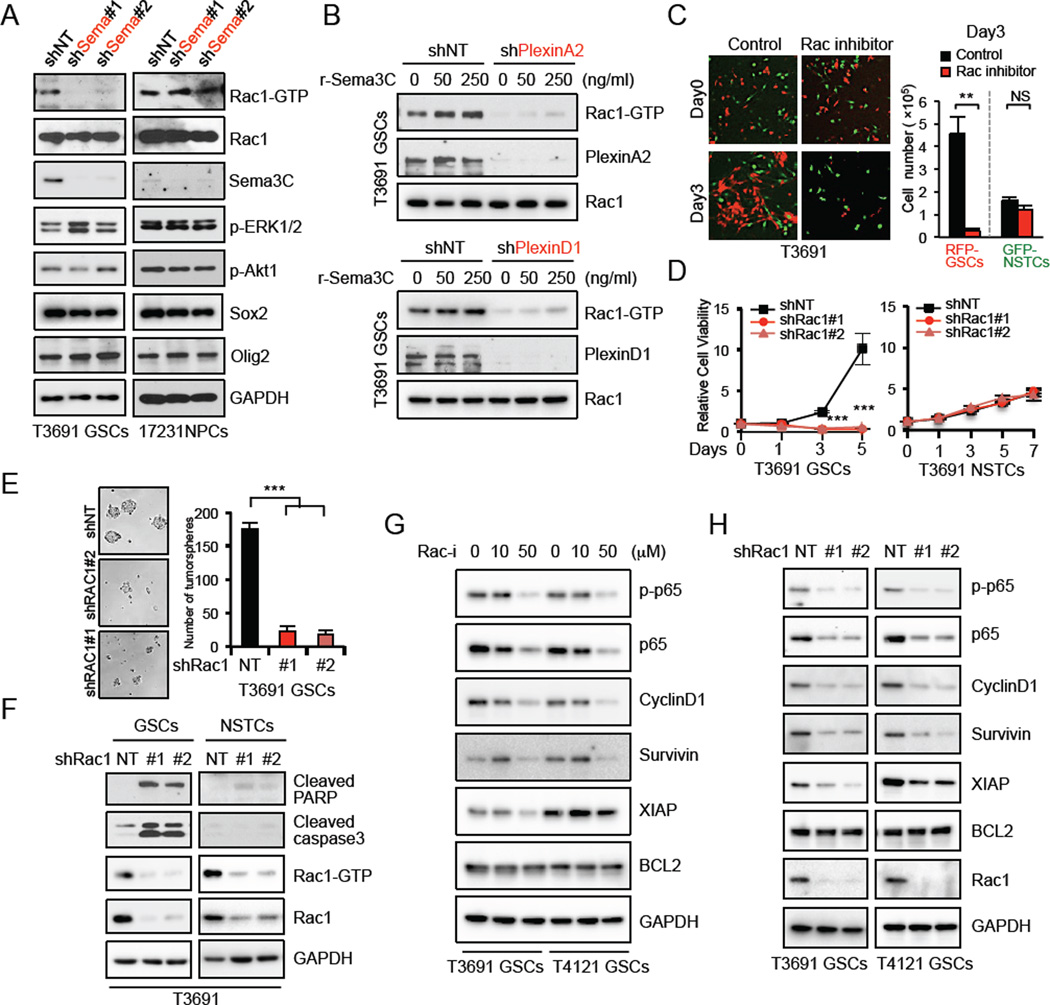
Figure 6. Sema3C Activates Rac1 To Promote Survival of GSCs.
(A) Detection of the GTP-bound form of active Rac1 in a pull-down assay from the lysates of GSCs and NPCs expressing shNT or shSema3C. Cell lysates from the indicated cells were analyzed for protein levels of total Rac1, Sema3C, p-ERK1/2, p-Akt1, Sox2 and Olig2.
(B) GSCs transduced with shNT, shPlexinA2 or shPlexinD1 were cultured with different doses of recombinant human Sema3C protein (r-Sema3C). IB analysis of active GTP-Rac1 by pull-down assay from the lysates of indicated GSCs.
(C) RFP-GSCs and matched GFP-NSTCs were mixed at a 1:1 ratio, plated on stem cell Matrigel-coated plates and treated with Rac1 inhibitor NSC23766 (50|JM). Representative images of mixed cells on day 0 and day 3 (left). Quantification of RFP-GSC and GFP-NSTC cell number 3 days after treatment (right). Data are means ± standard deviation (SD) (n = 3).
(D) Knockdown of Rac1 with two separate shRNA resulted in significantly decreased cell viability in GSCs but not in NSTCs. Data are means ± standard deviation (SD) (n = 3).
(E) Knockdown of Rac1 via two separate shRNAs resulted in decreased tumorsphere formation. Data are means ± standard deviation (SD) (n = 3).
(F) IB analysis of cleaved-PARP and -caspase3 in GSCs and matched NSTCs transduced with shNT or two separate shRac1. Active GTP-Rac1 by pull-down assay is shown.
(G and H) IB analysis of p-p65, p65, CyclinD1, Survivin, XIAP and BCL2 in two GSCs treated with Rac inhibitor (G) or transduced with shNT or two separate shRac1 (H). *p< 0.05, **p < 0.01, ***p < 0.001. See also Figure S6
Figure 5. Sema3C Depletion Induces Apoptosis of GSCs But Not NPCs.
(A and B) IB analysis of cleaved-caspase3, -caspase7 and -PARP proteins in GSCs and matched NSTCs (A), or GSCs and NPCs (B) in which Sema3C, PlexinA2 or PlexinD1 were knocked down by two separate shRNAs.
(C and D) Apoptotic cells in GSCs (C) or NPCs (D) expressing shNT, shSema3C, shPlexinA2 or shPlexinD1 were detected by TUNEL assay. The apoptotic index was assessed by the ratio of TUNEL-positive cells/total number of cells from eight randomly selected fields.
(E–G) Apoptotic cells in GBM xenografts derived from GSCs expressing shNT or shSema3C were detected in situ using the TUN EL assay (E and F) or cleaved-caspase3 staining (G). The apoptotic index was assessed by the ratio of TUNEL-positive cells or cleaved-caspase3-positive cells/total number of cells from eight randomly selected fields. Data are means ± standard deviation (SD) (n = 3). **p< 0.01, ***p < 0.001. See also Figure S5.
Reduction of PlexinA2 or PlexinD1 similarly decreased GSC viability and self-renewal (Figure 3E, 3G, S3I–J) but had little effect on NSTCs (Figure S3L). shRNA-resistant mutants of Sema3C or PlexinA2/D1 rescued cell survival that was reduced by knockdown of these genes (Figure S3M), supporting the specificity of these target shRNAs. To rule out the effects of different culture conditions on the specificity of Sema3C for GSCs over NSTCs, we performed a mixing experiment with matched GSCs and NSTCs that were differentially labeled with RFP or GFP proteins, respectively. The GSCs and NSTCs were plated at a 1:1 ratio together and transduced with Sema3C shRNA or NT shRNA. Sema3C knockdown strongly inhibited growth of RFP-GSCs but not GFP-NSTCs (Figure 3I), revealing that Sema3C preferentially supports GSCs.
An important attribute for a potential therapeutic target is its differential effects on tumor growth and normal physiology. Importantly, functional studies revealed that Sema3C knockdown had no significant impact on NPC growth (Figure 3B). In contrast, knockdown of either PlexinA2 or PlexinD1 resulted in rapid NPC death (Figure 3F, 3H). These data indicate that for patients with GBM, anti-Sema3C therapy may spare NPCs and therefore have limited brain toxicity.
These functional studies complement our expression studies and suggest a role for autocrine or paracrine Sema3C signaling in promoting the survival of GSCs specifically. To test this possibility, we performed a mixing experiment in which GSCs transduced with shSema3C were cultured alone or co-cultured with healthy GSCs at a 1:1 ratio. These populations were differentially labeled with GFP (GFP-GSC-shSema3C) or RFP (RFP-GSC). When cultured in the absence of Sema3C–expressing cells (RFP-GSC), cells with reduced Sema3C (GFP-GSC-shSema3C) readily died. In contrast, when these cells were co-cultured with Sema3C–expressing cells (RFP-GSC) they survived and formed tumorspheres (Figure S3N), suggesting that secretion of Sema3C from the RFP-GSC population could support the survival of GSCs in which Sema3C was abrogated.
To determine the dependency of this effect on soluble factors, we performed another co-culture experiment in which RFP-GSCs were cultured in an upper chamber and GFP-GSCs transduced with Sema3C, PlexinA2 or PlexinD1 shRNA were plated in the lower chamber (Figure 3J). The upper and lower chambers were separated by a permeable membrane that permitted the diffusion of small proteins but not migration of cells. Co-culture of RFP-GSCs rescued the sphere-forming ability of GSCs in which Sema3C was knocked down but not GSCs in which PlexinA2 or PlexinD1 were knocked down (Figure 3J). These results suggest that secretion of a soluble protein by RFP-GSCs acts through PlexinA2/D1 to support tumorsphere formation.
We next tested the ability of exogenous recombinant human Sema3C to rescue the viability and self-renewal capacity of GSCs in which Sema3C, PlexinA2, PlexinD1 or NRP1 were reduced. Exogenous Sema3C treatment rescued cell viability and tumorsphere formation in a dose-dependent manner in GSCs with reduced Sema3C, but had no appreciable effect on GSCs with reduced PlexinA2, PlexinD1 or NRP1 expression (Figure 3K-3L, S3O–S3P). Additional exogenous Sema3C slightly increased GSC viability and was not utilized by NSTCs (Figure S3Q–S3R). These results revealed that Sema3C secretion by GSCs acts through both PlexinA2/D1 and NRP1 receptors to mediate GSC self-renewal and sphere-forming capacity.
Our studies suggest that NPCs are sensitive to PlexinA2/D1 knockdown but not Sema3C knockdown. We hypothesized that other semaphorin ligands might engage these receptors to mediate NPC survival. We interrogated a murine brain in situ hybridization database (Allen Institute Brain Atlas database; http://mouse.brain-map.org) and found that Sema4A and Sema6A, the other known ligands of PlexinD1 and PlexinA2, respectively (Neufeld and Kessler, 2008; Zhou et al., 2008), are highly expressed in normal brain during mouse development (Figure S3S). We further validated their expression by Western blot in NPCs, GSCs and NSTCs (Figure S3T). Functional studies revealed that knockdown of Sema4A or Sema6A significantly inhibited NPC viability, but had only limited effect on GSCs (Figure S3U). These results suggest that GSCs and NPCs might utilize different semaphorin ligands to mediate cell survival.
Sema3C Knockdown Reduces Tumor Growth and Improves Animal Survival
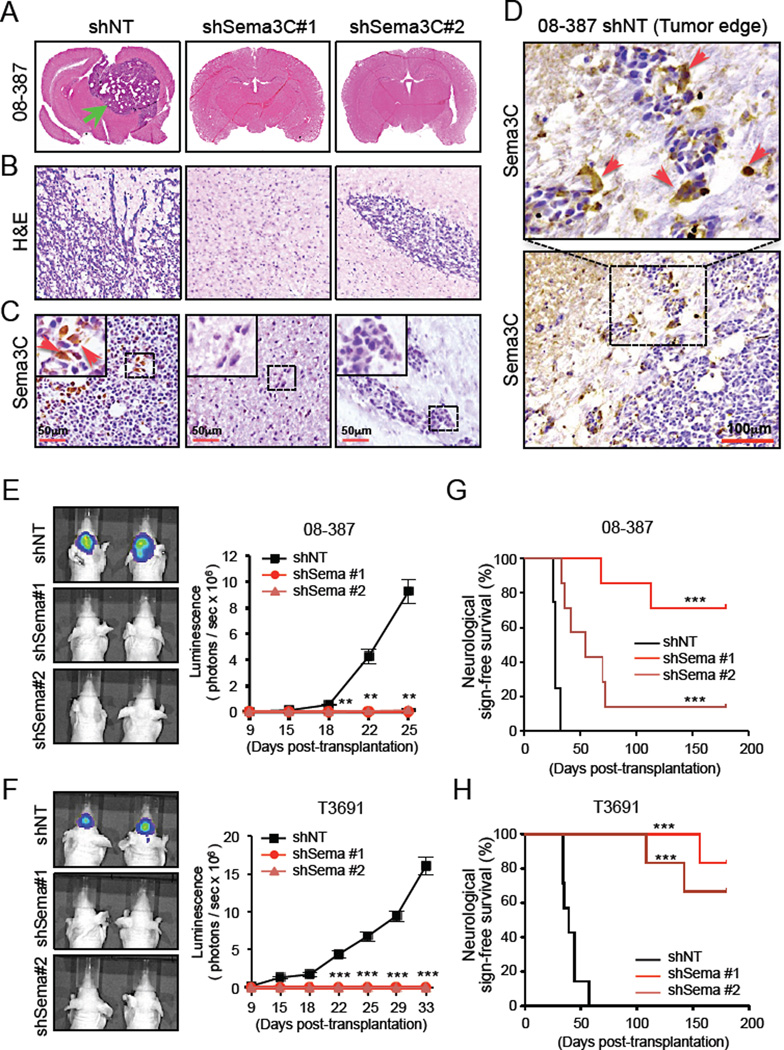
To determine the functional consequences of Sema3C knockdown in vivo, we established orthotopic xenografts utilizing shNT or shSema3C–expressing GSCs (shNT mice or shSema3C mice, respectively). Using two different GSC populations, shNT mice readily established intracranial tumors, whereas shSema3C mice demonstrated significantly impaired tumor formation, diminished tumor invasion and increased survival (Figure 4). When the first few shNT mice developed neurologic signs or clinically declined, a subset of mice in each group was sacrificed. shNT mice developed highly invasive tumors that extended to both hemispheres and exerted significant mass effect. In contrast, shSema3C mice had no clinical signs of tumor growth and only small nests of tumor cells were identified (Figure 4A, 4B, S4A). Sema3C was strongly expressed in a subpopulation of tumor cells in shNT mice but was undetectable in shSema3C mice (Figure 4C, S4A bottom). Of note, within shNT mice, a subset of cells expressing high levels of Sema3C localized to the invasive fingers of the tumor (Figure 4D), consistent with a role for Sema3C in facilitating cell invasion (Herman and Meadows, 2007; Miyato et al., 2012). These results are consistent with in vitro Boyden chamber migration assays (Figure S4B, S4C). Using 2 different orthotopic tumor models, Sema3C knockdown consistently impaired tumor growth, as shown by quantitative bioluminescent imaging, and significantly extended animal survival (Figure 4E-4H). In three of the four Sema3C knockdown tumor models, median survival was not reached by the conclusion of the observation period of 6 months post-transplantation (Figure 4G, 4H).
Figure 4. Targeting Sema3C Suppresses GSC-Mediated Tumor Growth and Improves Animal Survival.
GSCs transduced with shNT or shSema3C through lentiviral infection were intracranially transplanted into the brains of immunocompromised mice (2×104 cells per mouse). Mouse brains implanted with GSCs were harvested simultaneously to examine the impact of Sema3C disruption on GBM tumor growth (A–F). In the animal survival experiments (G and H), mice implanted with GSCs expressing shNT or shSema3C were maintained until the development of neurological signs or for 180 days, whichever came first.
(A) Representative images of cross-sections (hematoxylin and eosin stained) of mouse brains 25 days after transplantation. Arrow indicates a tumor formed from GSCs expressing shNT.
(B) Histological analysis of brain tumors derived from GSCs expressing shNT or shSema3C.
(C and D) IHC staining of Sema3C in GBM xenografts derived from GSCs expressing shNT or shSema3C. Staining at the center (C) or periphery (D) of shNT mouse tumors is shown. Sections were counterstained with hematoxylin. Arrows indicate Sema3C positive cells.
(E and F) GBM xenografts derived from luciferase-labeled GSCs expressing shNT or shSema3C were tracked by bioluminescence (right). Real-time images from animals on day 25 (E) and 33 (F) are shown (left). Error bars represent the mean ± SEM.
(G and H) Kaplan-Meier survival curves of mice implanted with 08–387 GSCs (G) and T3691 GSCs (H) expressing shNT or shSema3C.
**p< 0.01, ***p < 0.001. See also Figure S4.
To further evaluate whether the expression of Sema3C and its receptors in GBM correlated with patient survival, we queried the Oncomine database and found that high expression levels of Sema3C or its receptors PlexinA2 or PlexinD1 in human glioma inversely correlated with patient survival (Figure S4D–S4F). Together, these data suggest that Sema3C instructs the tumorigenic capacity and invasive phenotype of GSCs.
Sema3C Depletion Induces Apoptosis of GSCs But Not NPCs
A significant increase in cell death was observed after silencing of Sema3C and PlexinA2/D1 expression in GSCs in cell culture (Figure S5A). These findings raised the possibility that abrogation of Sema3C signaling may induce apoptosis of GSCs. Sema3C knockdown in GSCs increased apoptosis as assessed by Annexin-V/PI flow cytometry (Figure S5B) and TUNEL assay (Figure S5C–S5E). Similarly, knockdown of Sema3C and PlexinA2/D1 increased levels of cleaved caspase3/7, cleaved poly-ADP ribose polymerase (PARP) and the fraction of TUNEL-positive cells in GSCs but not in matched NSTCs (Figure 5A-5C, S5F–S5G). In NPCs, Sema3C knockdown showed no significant effect on these measures of apoptosis (Figure 5B, 5D, S5H). In contrast, PlexinA2/D1 knockdown in NPCs efficiently increased apoptosis (Figure 5B, 5D, S5G, S5H). Consistent with our in vitro findings, GBM tissue from shSema3C mice showed significantly elevated levels of apoptotic TUNEL-positive and cleaved caspase 3-positive tumor cells (Figure 5E-5G). These data support that Sema3C suppresses apoptosis of GSCs but not NSTCs or NPCs.
Sema3C Activates Rac1 to Mediate Survival of GSCs
To further understand a mechanism by which Sema3C protects GSCs from apoptosis, we investigated the interaction between Sema3C and its receptors PlexinA2/D1 and NRP1. Sema3C co-immunoprecipitated with PlexinA2, PlexinD1 or NRP1 (Figure S6A, S6B up). The receptors themselves interacted with each other (Figure S6A, S6B up), and knockdown of NRP1 abrogated the interaction with Sema3C and PlexinA2/D1 (Figure S6B down). These results suggest that Sema3C, PlexinA2/D1 and NRP1 might form a complex in GSCs, with NRP1 functioning as the ligand binding receptor. Plexins can engage many signaling pathways including Ras, Akt and Rac pathways (Neufeld and Kessler, 2008). Using a candidate approach, Sema3C knockdown significantly decreased GTP-bound, active Rac1 in GSCs but had little effect on ERK or Akt activation. In NPCs, however, activated Rac1 levels were unchanged after Sema3C knockdown (Figure 6A). In addition, phospho-kinase array analysis showed that Sema3C had no significant effects on p38 MAPK, JNK, p53 or STAT signaling (data not shown).
Rac1 is known to interact with the intracellular domain of plexins (Hota and Buck, 2012). To determine the role of plexins in transducing Sema3C signals, we knocked down PlexinA2 or PlexinD1 in GSCs and assessed activation of Rac1. Knockdown of either PlexinA2 or PlexinD1 inhibited Rac1 activity (Figure 6B). Furthermore, treatment of control GSCs with recombinant human Sema3C increased activated Rac1 levels in a dose-dependent manner but had minimal effect on GSCs with reduced PlexinA2/D1 (Figure 6B). PlexinA2/D1 mutants lacking the intracellular domain failed to rescue tumorsphere formation in GSCs in which PlexinA2/D1 were knocked down (data not shown). Together, these findings demonstrate that Sema3C engages the PlexinA2/D1 receptor complex to regulate Rac1 activation in GSCs but not NPCs.
GSCs Exhibit Increased Sensitivity to Rac1 Inhibition
Rac1 has been implicated in cancer cell survival (Senger et al., 2002; Velaithan et al., 2011) but its role in cancer stem cells is not well established. Inhibition of Rac1 by the pharmacologic inhibitor NSC23766 or knockdown approaches strongly inhibited GSC viability, reduced tumorsphere formation and induced apoptosis of GSCs (Figure 6C-6F, S6C–S6F), revealing that Rac1 signaling is necessary for GSC survival. However, Rac1 inhibition had minimal effects on NSTCs even though Rac1 activation was comparable between NSTCs and GSCs (Figure 6C-6F, S6C–S6F). Rac1 knockdown significantly increased apoptosis of GSCs but had minimal effect on NSTCs (Figure 6F, S6F). GSCs are therefore dependent on Rac1 signaling for their survival and are particularly sensitive to Rac1 inhibition.
To determine how Rac1 might regulate GSC survival, we assessed NF-κB signaling, a downstream pathway (Myant et al., 2013) that has been implicated in GBM cell survival. NF-κB activation promotes radiation resistance of GSCs and correlates with poor survival (Bhat et al., 2013; Bredel et al., 2011; Park et al., 2009). In two GSC populations, blocking Rac1 activation with a pharmacologic inhibitor or Rac1 shRNA reduced activation of p65/RelA and expression of NF-κB dependent target genes, cyclinD1, XIAP and Survivin (Figure 6G, 6H). Collectively, these data demonstrate that Rac1 regulation of NF-κB is vital for GSCs.
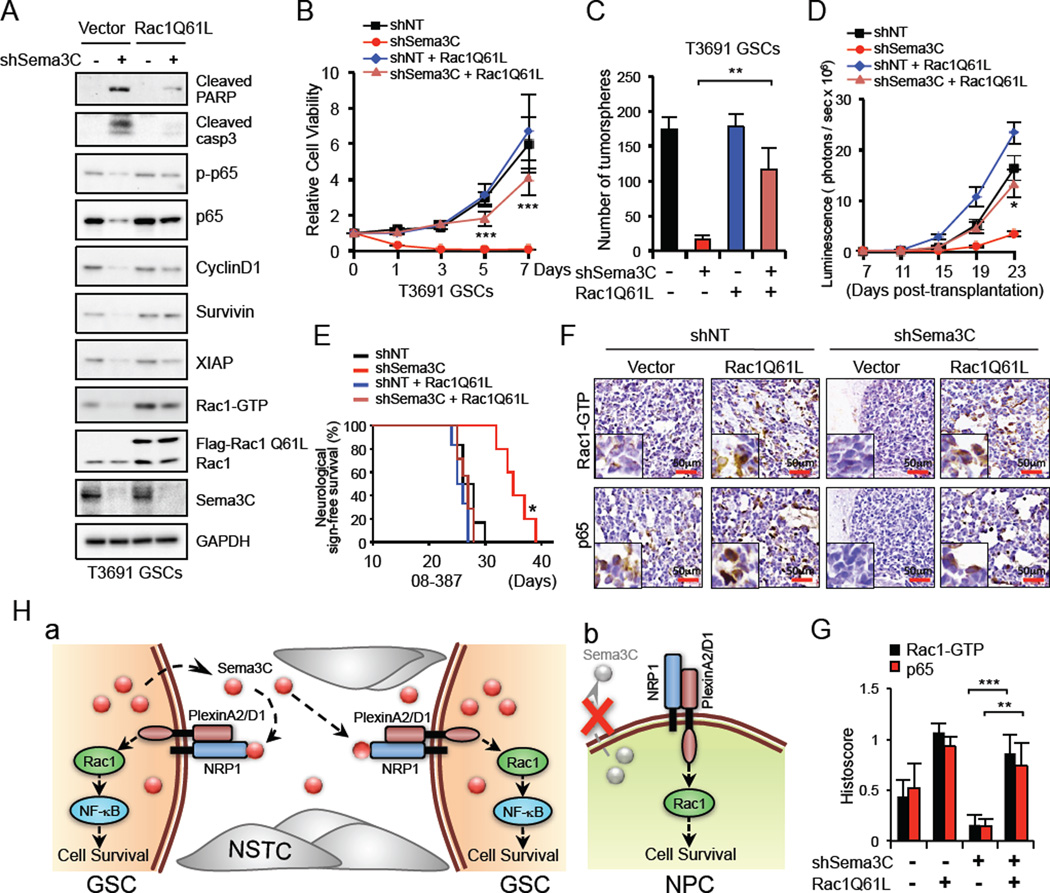
Rac1 Activation Rescues the Phenotype Caused by Sema3C Disruption in GSCs
To further define the role of Rac1 in Sema3C–mediated GSC survival, we introduced constitutively activated Rac1 (Flag-Rac1Q61L), which abolishes GTP hydrolysis and mimics the GTP-bound state (Wu et al., 2009), into GSCs in which Sema3C was knocked down. Ectopic expression of Flag-Rac1Q61L in shSema3C GSCs restored NF-κB activation, reduced apoptosis (Figure 7A) and rescued GSC proliferation and sphere-forming ability (Figure 7B, 7C, S7A). Similarly, in vivo, ectopic expression of activated Rac1 significantly accelerated tumor growth and reduced survival of shSema3C mice (Figure 7D, 7E, S7B). However, expression of activated Rac1 by itself did not significantly alter survival compared to shNT mice (Figure 7E). Ectopic expression of Rac1Q61L increased p65/RelA nuclear staining to rescue Sema3C knockdown in GBM xenografts (Figure 7F, 7G), suggesting that Sema3C regulates NF-κB activation through Rac1. Both in vitro and in vivo, activated Rac1 restored GSC migration and promoted tumor cell invasion despite Sema3C knockdown (Figure S7C, S7D). Collectively, these data suggest that Sema3C activates Rac1 in GSCs to drive tumor growth and invasion.
Figure 7. Ectopic Expression of a Constitutively Active Rac1 in GSCs Rescued the Phenotype Caused by Sema3C Disruption.
(A) IB analysis of cleaved-PARP, -caspase3, p-p65, p65 and NF-κB dependent gene expression in GSCs transduced with control vector or constitutively active Rac1 (Flag-Rac1Q61L) in combination with shNT or shSema3C.
(B and C) GSCs were treated as described in (A) and cell viability was assessed by cell titer assay (B). Quantification of GSC tumorsphere number is shown (C). Data are means ± standard deviation (SD) (n = 3).
(D and E) GSCs (08–387, labeled with luciferase) were treated as described in (A). 48 hr after infection, GSCs were transplanted into the brains of immunocompromised mice. GBM xenograft growth was tracked by bioluminescence (D). Error bars represent the mean ± SEM. Kaplan-Meier survival curves of different groups of mice are shown (E). Mice were maintained until the development of neurological signs.
(F and G) IHC staining of Rac1-GTP and p65 in GBM xenografts derived from GSCs treated as indicated (F). Sections were counterstained with hematoxylin. Histoscore analysis for Rac1-GTP and p65 is shown (G). Data are means ± standard deviation (SD) (n = 3).
(H) Proposed model for Sema3C signaling on the regulation of GSC survival. a. Sema3C and PlexinA2/D1 are differentially co-expressed in GSCs but not NSTCs. GSCs secrete and utilize Sema3C in an autocrine/paracrine loop to promote their survival by facilitating Rac1 signaling. NSTCs do not express or utilize Sema3C. b. PlexinA2/D1 are expressed in NPCs and contribute to their survival. NPCs do not express or require Sema3C.
*p< 0.05, **p < 0.01, ***p < 0.001. See also Figure S7.
DISCUSSION
In this study, we identified an autocrine/paracrine Sema3C–PlexinA2/D1 loop employed by GSCs to promote their survival and invasion (Figure 7H, a). In GBM, activation of Sema3C–PlexinA2/D1 signaling appears to be confined to the stem cell compartment. GSCs but not NSTCs preferentially express Sema3C and PlexinA2/D1. GSCs were dependent on Sema3C–PlexinA2/D1-Rac1 signaling for their survival and were able to utilize exogenous Sema3C. However, NSTCs were unresponsive to recombinant Sema3C, probably because they lacked PlexinA2/D1 expression. Interestingly, knockdown of Sema3C did not alter the expression of stem cell markers or cause differentiation of GSCs. This suggests that Sema3C regulates the survival of GSCs and contributes to the maintenance of their subpopulation rather than instructing cell-fate decisions. In preclinical models, abrogation of Sema3C significantly impaired tumor growth and invasion and extended animal survival in a Rac1-dependent manner.
GSCs have been implicated in GBM treatment failure because of their self-renewal properties and resistance to standard cytotoxic therapy. GSCs have evolved to co-opt core developmental programs to ensure their survival. Targeting GSCs remains a daunting task due to the many vital signaling pathways shared between cancerous and normal progenitor cell populations (Gilbertson and Rich, 2007). We therefore sought to identify differences in the regulation of these pathways in GSCs and NPCs.
In contrast to PlexinA2/D1 receptors, Sema3C was undetectable in cultured NPCs and native NPCs in the subventricular zone. Perturbations of Sema3C in NPCs had minimal functional impact. Intriguingly, knockdown of either PlexinA2 or PlexinD1 receptors was sufficient to induce apoptosis of both GSCs and NPCs (Figure 7H, b). These results suggest that under different selective pressures, GSCs and NPCs have independently evolved distinct molecular pathways that converge on PlexinA2/D1 and Rac1 to promote cell survival. We speculate that GSCs have evolved to overexpress and utilize Sema3C, whereas NPCs may engage other semaphorins, such as Sema4A and Sema6A (Figure S3S–U), known ligands for PlexinA2/D1 (Neufeld and Kessler, 2008; Zhou et al., 2008). Thus, these studies suggest that anti-Sema3C strategies may target GSCs and have a favorable therapeutic index.
Secreted proteins are logical therapeutic targets because they are exposed to the microenvironment and obviate the need for a drug to move intracellularly. Ligand sequestration drugs or monoclonal antibodies that block receptors, such as aflibercept (VEGF-Trap) and bevacizumab (Avastin) have been successful in some solid tumors (Lambrechts et al., 2013; Stewart et al., 2012) but not GBM (Gilbert et al., 2013). Our study provides a strong biological rationale for the development of similar types of Sema3C–directed therapies.
Rac1 is best known as a regulator of cell motility (Heasman and Ridley, 2008; Kaibuchi et al., 1999) and has been implicated as a driver of invasion and metastasis in many cancers, including GBM (Chan et al., 2005; Feng et al., 2011). Previous studies have suggested that Sema3C could promote migration and invasion of cancer cells, and its overexpression is associated with poor survival (Herman and Meadows, 2007; Martin-Satue and Blanco, 1999; Miyato et al., 2012). Our data also support a role for Sema3C in stimulating the invasion of GSCs through Rac1 activation. Sema3C–positive cells localized to the invasive nests of GBM, and knockdown of Sema3C reduced migration both in vitro and in mouse models. Re-introduction of constitutively active Rac1 in Sema3C–deficient tumors restored the invasive phenotype. Together, these data demonstrate that Sema3C–mediated activation of Rac1 contributes to the highly invasive phenotype of GSCs.
Our studies also extend the function of Rac1 to the survival of GSCs. Rac1 has been implicated in regulating apoptosis in several types of cancers. In the intestine, Rac1 regulates ROS production and NF-κB to stimulate the hyperproliferation and transformation of LGR5-positive stem cells in the setting of constitutive Wnt signaling (Myant et al., 2013). In hematopoietic cells, Rac1 regulates PI3K–Akt and p38 MAPK signaling pathways to promote survival (Nishida et al., 1999) and maintains hematopoietic stem/progenitor cells (Gu et al., 2003). In GSCs, we found that Sema3C can activate Rac1 through PlexinA2/D1 receptors. Rac1 inhibition by both pharmacologic or shRNA approaches reduced NF-κB activation and decreased levels of Survivin and XIAP to facilitate apoptosis of GSCs. Furthermore, constitutively active Rac1 rescued the Sema3C knockdown phenotype in mice. Examination of these tumors revealed increased levels of nuclear p65 staining. We did not detect significant regulation of PI3K–Akt or MAPK/ERK signaling by Sema3C or Rac1. These data suggest that Sema3C–mediated activation of Rac1 and NF-κB promotes GSC survival and tumorigenicity. Moreover, the dependency of GSCs on Rac1 for their survival is also of therapeutic significance. Our results are timely because a new generation of Rac1 inhibitors are just beginning to enter clinical trials and could be extended to patients with GBM.
In summary, we have identified Sema3C signaling as a central regulator of GSC survival and GBM progression. Targeting Sema3C will disrupt the GSC-specific autocrine/paracrine signaling loop potentially with limited normal brain toxicity. Our data support Sema3C as a promising therapeutic target for GBM.
EXPERIMENTAL PROCEDURES
Isolation and Culture of GSCs and NPCs
GBM surgical specimens were collected for this study in accordance with a Cleveland Clinic Institutional Review Board-approved protocol. GSCs and NSTCs were isolated and characterized from GBM surgical specimens or xenografts as previously described (Cheng et al., 2013). For the detailed procedure, please see Supplemental Experimental Procedures.
Immunofluorescence Staining, Immunohistochemistry and Immunoblot Analysis
Immunofluorescent staining of cells and tissues sections was performed as described (Guryanova et al., 2011). Immunohistochemistry staining of tissue section was performed with an ABC kit using DAB (3,30-Diaminobenzine) detection (Vector Lab) as previously described (Guryanova et al., 2011). Immunoblot analysis was performed as previously described (Huang et al., 2011). For the detailed procedure, please see Supplemental Experimental Procedures.
For methods related to Differentiation Assay, DNA Constructs and Lentiviral Transfection, Orthotopic Mouse Xenografts, Necropsy, Cell Viability Assays, TUNEL Assay, Rac1 Activation Assay, Immunoprecipitation, see the Supplemental Experimental Procedures.
Statistical Analysis
All grouped data are presented as mean ± standard deviation (SD). Difference between groups was assessed by one-way analysis of variance (ANOVA) or one-way ANOVA on ranks tests. All in vitro experiments were repeated at least three times. For the in vivo experiments, log rank survival analysis was performed. Graphpad Prism was used for all statistical analyses.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Amy Post Brain Tumor Foundation, Scott Hamilton CARES Award, Cleveland Clinic Foundation, and Clinical and Translational Science Collaborative of Cleveland, KL2TR000440 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and the NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.M. and J.S. performed and analyzed the experiments. W.Z, X.F., Q.W., A.R. and S.B. provided reagents and technical advice. R.P., S.B. and J.N.R. helped provide human specimens, and R.P. helped analyze staining. J.M. and J.S.Y. designed the overall research and wrote the manuscript.
REFERENCES
- Akunuru S, Palumbo J, Zhai QJ, Zheng Y. Rac1 targeting suppresses human non-small cell lung adenocarcinoma cancer stem cell activity. PloS one. 2011;6:e16951. doi: 10.1371/journal.pone.0016951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bhat KP, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD, et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer cell. 2013;24:331–346. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc V, Nariculam J, Munson P, Freeman A, Klocker H, Masters J, Williamson M. A role for class 3 semaphorins in prostate cancer. Prostate. 2011;71:649–658. doi: 10.1002/pros.21281. [DOI] [PubMed] [Google Scholar]
- Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredel M, Scholtens DM, Yadav AK, Alvarez AA, Renfrow JJ, Chandler JP, Yu IL, Carro MS, Dai F, Tagge MJ, et al. NFKBIA deletion in glioblastomas. The New England journal of medicine. 2011;364:627–637. doi: 10.1056/NEJMoa1006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Capparuccia L, Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment--two sides of a coin. J Cell Sci. 2009;122:1723–1736. doi: 10.1242/jcs.030197. [DOI] [PubMed] [Google Scholar]
- Chan AY, Coniglio SJ, Chuang YY, Michaelson D, Knaus UG, Philips MR, Symons M. Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene. 2005;24:7821–7829. doi: 10.1038/sj.onc.1208909. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C, De Maria R. Chemotherapy resistance of glioblastoma stem cells. Cell death and differentiation. 2006;13:1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- Esselens C, Malapeira J, Colome N, Casal C, Rodriguez-Manzaneque JC, Canals F, Arribas J. The cleavage of semaphorin 3C induced by ADAMTS1 promotes cell migration. The Journal of biological chemistry. 2010;285:2463–2473. doi: 10.1074/jbc.M109.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esufali S, Charames GS, Pethe VV, Buongiorno P, Bapat B. Activation of tumor-specific splice variant Rac1b by dishevelled promotes canonical Wnt signaling and decreased adhesion of colorectal cancer cells. Cancer research. 2007;67:2469–2479. doi: 10.1158/0008-5472.CAN-06-2843. [DOI] [PubMed] [Google Scholar]
- Feng H, Hu B, Liu KW, Li Y, Lu X, Cheng T, Yiin JJ, Lu S, Keezer S, Fenton T, et al. Activation of Rac1 by Src-dependent phosphorylation of Dock180(Y1811) mediates PDGFRalpha-stimulated glioma tumorigenesis in mice and humans. The Journal of clinical investigation. 2011;121:4670–4684. doi: 10.1172/JCI58559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell stem cell. 2012;10:698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani E, Sgouros J, Petropoulou C, Janinis J, Aravantinos G, Dionysiou-Asteriou D, Skarlos D, Gonos E. Correlation of MDR-1, nm23-H1 and H Sema E gene expression with histopathological findings and clinical outcome in ovarian and breast cancer patients. Anticancer Res. 2002;22:2275–2280. [PubMed] [Google Scholar]
- Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Developmental cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- Guryanova OA, Wu Q, Cheng L, Lathia JD, Huang Z, Yang J, MacSwords J, Eyler CE, McLendon RE, Heddleston JM, et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer cell. 2011;19:498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerlik P, Lathia JD, Rasmussen R, Wu Q, Bartkova J, Lee M, Moudry P, Bartek J, Jr, Fischer W, Lukas J, et al. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med. 2012;209:507–520. doi: 10.1084/jem.20111424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nature reviews Molecular cell biology. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Herman JG, Meadows GG. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. Int J Oncol. 2007;30:1231–1238. [PubMed] [Google Scholar]
- Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hota PK, Buck M. Plexin structures are coming: opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions. Cell Mol Life Sci. 2012;69:3765–3805. doi: 10.1007/s00018-012-1019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Wu Q, Guryanova OA, Cheng L, Shou W, Rich JN, Bao S. Deubiquitylase HAUSP stabilizes REST and promotes maintenance of neural progenitor cells. Nat Cell Biol. 2011;13:142–152. doi: 10.1038/ncb2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikushima H, Todo T, Ino Y, Takahashi M, Miyazawa K, Miyazono K. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell stem cell. 2009;5:504–514. doi: 10.1016/j.stem.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Joo KM, Jin J, Kim E, Ho Kim K, Kim Y, Gu Kang B, Kang YJ, Lathia JD, Cheong KH, Song PH, et al. MET signaling regulates glioblastoma stem cells. Cancer research. 2012;72:3828–3838. doi: 10.1158/0008-5472.CAN-11-3760. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Kodo K, Nishizawa T, Furutani M, Arai S, Yamamura E, Joo K, Takahashi T, Matsuoka R, Yamagishi H. GATA6 mutations cause human cardiac outflow tract defects by disrupting semaphorin-plexin signaling. Proc Natl Acad Sci U S A. 2009;106:13933–13938. doi: 10.1073/pnas.0904744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, Cheng E, Davis MJ, Goh G, Choi M, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nature genetics. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:1219–1230. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- Law JWaL, AY The role of semaphorins and their receptors in gliomas. J Signal Transduct. 2012;2012:14. doi: 10.1155/2012/902854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD, Phillips JJ, Cheresh DA, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Satue M, Blanco J. Identification of semaphorin E gene expression in metastatic human lung adenocarcinoma cells by mRNA differential display. J Surg Oncol. 1999;72:18–23. doi: 10.1002/(sici)1096-9098(199909)72:1<18::aid-jso5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Miyato H, Tsuno NH, Kitayama J. Semaphorin 3C is involved in the progression of gastric cancer. Cancer Sci. 2012;103:1961–1966. doi: 10.1111/cas.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myant KB, Cammareri P, McGhee EJ, Ridgway RA, Huels DJ, Cordero JB, Schwitalla S, Kalna G, Ogg EL, Athineos D, et al. ROS production and NF-kappaB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell. 2013;12:761–773. doi: 10.1016/j.stem.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- Nishida K, Kaziro Y, Satoh T. Anti-apoptotic function of Rac in hematopoietic cells. Oncogene. 1999;18:407–415. doi: 10.1038/sj.onc.1202301. [DOI] [PubMed] [Google Scholar]
- Park S, Hatanpaa KJ, Xie Y, Mickey BE, Madden CJ, Raisanen JM, Ramnarain DB, Xiao G, Saha D, Boothman DA, et al. The receptor interacting protein 1 inhibits p53 induction through NF-kappaB activation and confers a worse prognosis in glioblastoma. Cancer research. 2009;69:2809–2816. doi: 10.1158/0008-5472.CAN-08-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell stem cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger J, Wick W, Weller M. Human malignant glioma cells express semaphorins and their receptors, neuropilins and plexins. Glia. 2003;42:379–389. doi: 10.1002/glia.10210. [DOI] [PubMed] [Google Scholar]
- Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DL, Tudan C, Guiot MC, Mazzoni IE, Molenkamp G, LeBlanc R, Antel J, Olivier A, Snipes GJ, Kaplan DR. Suppression of Rac activity induces apoptosis of human glioma cells but not normal human astrocytes. Cancer research. 2002;62:2131–2140. [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell stem cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T, Kassam AB, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28:3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- Stewart MW, Grippon S, Kirkpatrick P. Aflibercept. Nature reviews Drug discovery. 2012;11:269–270. doi: 10.1038/nrd3700. [DOI] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- Suva ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD, Riggi N, Chi AS, Cahill DP, Nahed BV, et al. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell. 2014;157:580–594. doi: 10.1016/j.cell.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L. Emerging role of semaphorins as major regulatory signals and potential therapeutic targets in cancer. Cancer cell. 2012;22:145–152. doi: 10.1016/j.ccr.2012.06.031. [DOI] [PubMed] [Google Scholar]
- Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, Huganir RL, Ginty DD, Kolodkin AL. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature. 2009;462:1065–1069. doi: 10.1038/nature08628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velaithan R, Kang J, Hirpara JL, Loh T, Goh BC, Le Bras M, Brenner C, Clement MV, Pervaiz S. The small GTPase Rac1 is a novel binding partner of Bcl-2 and stabilizes its antiapoptotic activity. Blood. 2011;117:6214–6226. doi: 10.1182/blood-2010-08-301283. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nature reviews Drug discovery. 2009;8:806–823. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- Zhou C, Licciulli S, Avila JL, Cho M, Troutman S, Jiang P, Kossenkov AV, Showe LC, Liu Q, Vachani A, et al. The Rac1 splice form Rac1b promotes K-ras-induced lung tumorigenesis. Oncogene. 2013;32:903–909. doi: 10.1038/onc.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33:161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Zhu TS, Costello MA, Talsma CE, Flack CG, Crowley JG, Hamm LL, He X, Hervey-Jumper SL, Heth JA, Muraszko KM, et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer research. 2011;71:6061–6072. doi: 10.1158/0008-5472.CAN-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.