Abstract
Longitudinal cerebral metabolite changes in pig-tailed macaques inoculated with the simian immunodeficiency virus SIVsmmFGb were evaluated with in vivo proton MRS at 3T. Blood sample collection, and MRS were carried out before and 2, 4, 8, 12, 16, 20 and 24 weeks after SIV inoculation. Significant reduction of N-acetylaspartate (NAA)/creatine (Cr) and Choline (Cho)/Cr ratios in prefrontal grey matter (PGM) and glutamate/glutamine (Glx)/Cr ratios in striatum, and increase of myo-inositol (mI)/Cr in striatum were observed during acute SIV infection. The metabolite alterations during the SIVsmmFGb infection are largely in agreement with previous findings in other non-human primate models and HIV patients. Also, NAA/Cr in PGM and striatum and Glx/Cr in striatum are negatively correlated with the percentage of CD8+ T cells after the SIV infection, suggesting the interaction between brain metabolite and immune dysfunction. The present study complements previous studies by describing the time course of alterations of brain metabolites during SIVsmmFGb infection. The findings further demonstrate the efficacy of the SIVsmmFGb-infected macaque as a model to characterize central nervous system infection using novel neuroimaging approaches and also as a tool for exploration of novel and advanced neuroimaging techniques in HIV/AIDS studies.
Keywords: neuro-AIDS, SIV, in vivo proton MRS, macaque monkey, immune dysfunction
Introduction
Non-human primates (NHPs) infected with Simian Immunodeficiency Virus (SIV) exhibit neuropathological symptoms similar to those seen in HIV+ patients, and have been employed extensively to study the mechanisms of the disease or therapeutical treatment (Apetrei et al, 2012; Haigwood, 2004; Hu, 2005; Staprans and Feinberg, 2004; Van Rompay, 2012; Williams et al, 2008). SIVsmmFGb is a novel strain of SIV, which induces neuropathology (as measured by significant levels of SIV in the brain parenchyma, multi-nucleated giant cells (MNGCs), and SIV-encephalitis (SIVE)) in 90–100% of infected pig-tailed macaques while most SIV strains in 25–40% of infected macaques (Novembre et al, 1998; O’Neil et al, 2004; Reeve et al, 2009), suggesting SIVsmmFGb infected pig-tailed macaques are an excellent model system for studies on neuropathogenesis and therapeutic development.
Neurochemical alterations associated with the inflammatory process and neuronal loss have been commonly observed in previous magnetic resonance spectroscopy (MRS) studies of HIV patients (Chang, 1995; Chang et al, 1999; Ernst et al, 2003; Ernst et al, 2010; Lentz et al, 2011; Salvan et al, 1997; Tracey et al, 1996; Vion-Dury et al, 1998). Also, rhesus macaques inoculated with a SIV251 swarm (Kodama et al, 1993; Strickland et al, 2011), have been employed to investigate the metabolite alterations of brain injury during SIV infection (Fuller et al, 2004; Gonzalez et al, 2000; Greco et al, 2004; Lentz et al, 2005; Williams et al, 2005; Wu et al, 2013a; Wu et al, 2013b), demonstrating in vivo MRS is a promising approach to evaluate progressive neurochemical changes in the brain injury of SIV NeuroAIDS. Our previous studies have demonstrated that SIVsmmFGb is highly neuropathogenic in pig-tailed macaques, but the temporal evolution of brain infection remains not fully understood. In the present study, in vivo MRS was used to investigate temporal cerebral metabolite alterations in pig-tailed macaques infected with SIVsmmFGb.
Materials and Methods
Pig-tailed macaques (Macacanemestrina) (male, 4 years old, n=5), were infected by intravenous inoculation with 100 TCID50 of the SIVsmmFGb isolate (Novembre et al, 1998; O’Neil et al, 2004).
In vivo proton MRS examination
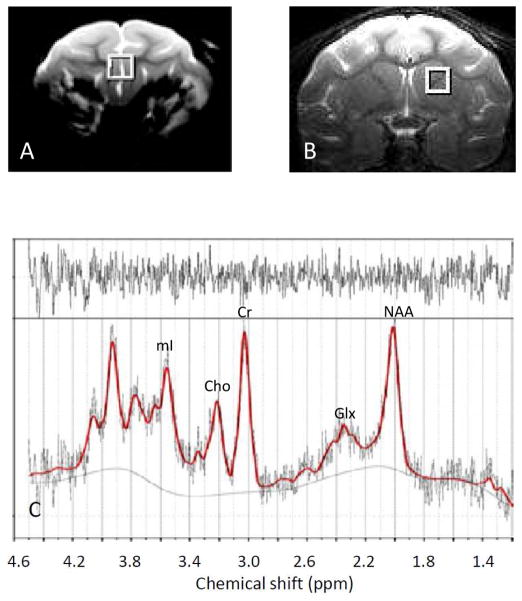
The single voxel spectra were acquired with the point-resolved spectroscopy (PRESS) sequence with Chemical Shift Selective (CHESS) water suppression and TR/TE =1500ms/30ms by using a circular surface coil (ID=7cm) on a Siemens 3T Trio scanner (Siemens Medical Solutions, PA, USA). A volume of 6mm × 6mm × 6 mm (or 0.22 ml) within prefrontal grey matter (PGM) or striatum was chosen as regions of interest (ROI) (Fig 1A and 1B) and scanned before SIV inoculation (baseline) and at 2, 4, 8, 12, 16, 20 and 24 wpi. For each scan, animals were anesthetized and maintained with 1–1.5% isoflurane mixed with 100% oxygen and the animal head was immobilized with a custom-built head holder. Vital physiological parameters including heart rate, breathing rate, rectal temperature, blood pressure, oxygen saturation, etc, were monitored continuously during scanning. Baseline MRS data were acquired in three separate scanning sessions for each animal, with at least one week between sessions. Animals were euthanized after their final scanning session.
Fig. 1.
Voxels of interest illustrated in A): prefrontal grey matter (PGM) and B): Striatum (caudate and putamen) on the T2-weighted structural images of a pig-tailed adult macaque monkey. C) Representative proton MR spectrum in PGM with fitted by the LCModel software (post SIV inoculation) with the residual shown on the top.
Abbreviations: Cr, total creatine; Glx, glutamate/glutamine; mI, myo-inositol; NAA, N-acetylaspartate; Cho, choline.
CD4+ and CD8+ T-cell monitoring
Blood samples were collected from anesthetized animals for quantitation of circulating CD4+ and CD8+ T-cell subsets, one day before each MRI scan and a complete blood (CBC) count with differential and lymphocyte phenotype analysis was performed. Absolute numbers and percentages of CD3+/CD4+ and CD3+/CD8+ T lymphocytes were measured, in whole blood, using four-color flow cytometry and gating on the lymphocyte population. All monoclonal antibodies (CD3, CD20, CD4 and CD8) used here were purchased from BD company as previously described (Novembre et al, 1998; O’Neil et al, 2004).
Data analysis
In vivo MRS data were processed using the LC-Model software (Provencher, 1993). Total creatine (Cr, plus phosphocreatine) levels were used as an internal reference (Fuller et al, 2004; Greco et al, 2002; Haga et al, 2009; Herndon et al, 1998; Kaiser et al, 2005; Ratai et al, 2009; Wiebenga et al, 2014). Metabolite ratios including NAA/Cr, Cho/Cr, mI/Cr and glutamate/glutamine (Glx)/Cr were calculated. Representative spectra of the post- inoculation proton MRS in PGM and striatum were shown (Fig 1c). Also, the pre-inoculation data of each animal were processed separately and the metabolite ratios with smallest standard deviation percentage in the LC-model fitting were used as the reference baseline for the following data analysis of that animal.
Statistical Analysis
Analysis of variance (ANOVA) with post hoc testing was employed for the statistical analysis of repeated measurements across different time points by using SPSS 20.0. Student’s t-test was applied to compare the results between the baseline and that in the post-inoculation. Relationships between the percentages of T cells (or CD4/CD8 ratio) and metabolite changes were examined using linear mixed regression analysis (Blackwell et al, 2006). P-values less than 0.05 were considered statistically significant. All the p-values were adjusted for multiple comparisons by means of the false discovery rate (FDR) procedure (Benjamini, 1995).
Results
CD4+ and CD8+ T cell percentage changes post SIV infection
Longitudinal changes in circulating CD4+ and CD8+ T-cell percentages and numbers are illustrated in Fig. 2. CD4+ T cell percentage declined progressively as expected, differing significantly from baseline at 12, 16, 20 and 24 wpi. In the acute phase (<1 month), the CD8+ T cell percentages increased significantly and then became steady and declined slightly in the chronic phase after SIV infection (Fig 2).
Fig. 2.
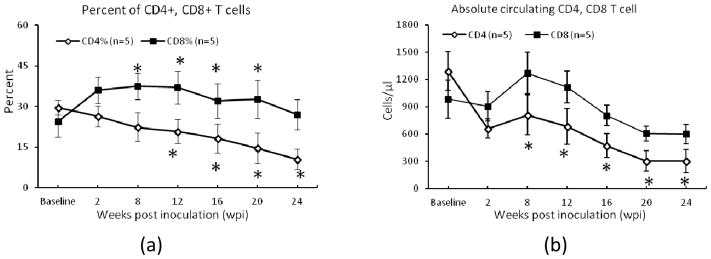
CD4+ and CD8+ T-cell percentages (a) and absolute numbers (b) of the lymphocyte changes post SIV inoculation. Error bars represent Standard Error of the Mean (SEM). *: P < 0.05, compared with the baseline (pre SIV inoculation).
Cerebral Metabolite ratio changes post SIV inoculation
Compared to the baselines, mean NAA/Cr and Glx/Cr ratio (averaged cross time post-inoculation) were reduced 13.6% ± 6.5% (SD) and 13.1% ± 9.3% (SD), respectively in PGM and 13.2% ± 8.0% (SD) and 9.6% ± 1.5% (SD) in striatum, although these declines were not statistically significant (Fig 3). Also, in comparison with the baselines, the mean Cho/Cr and mI/Cr ratios remained almost unchanged in PGM and striatum (Fig 3).
Fig. 3.
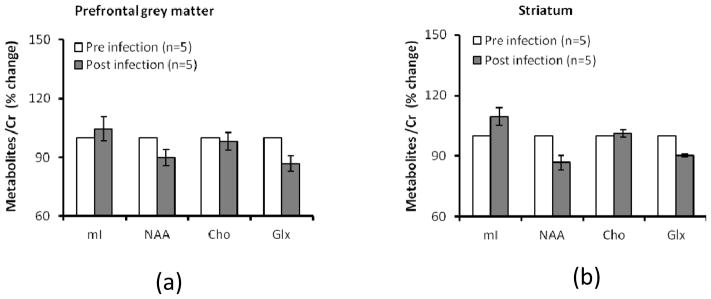
Cerebral metabolite ratio changes (averaged across time) in prefrontal grey matter (PGM) (a) and striatum (b) after SIV inoculation. Error bars represent Standard Error of the Mean (SEM). *: P <0.05, compared with baseline. Metabolites include: N-acetylasparlate (NAA/Cr), total creatine (Cr), total choline (Cho), myo-inositol (mI), Glutmate/Glutamin (Glx). Total creatine (Cr) is used as internal reference.
In the longitudinal examination, ANOVA analysis indicated there were no significant changes observed in all interested metabolites in PGM and striatum at most time points after the SIV infection except in the acute stage. Specifically, significant changes of the NAA/Cr ratio in PGM and Glx/Cr and mI/Cr ratios in striatum were observed at 2 wpi (Fig 4a and 4b). Significant reduction of Cho/Cr and Glx/Cr ratios in PGM occurred at 4 and 12 wpi (Fig 4a). The Glx/Cr ratio in PGM continued to decrease over the time, while the Glx/Cr ratio in striatum had a transient drop at 2 wpi. The NAA/Cr ratio in PMG and striatum correlated with the Glx/Cr ratio in striatum (r = 0.36, p =0.03).
Fig. 4.
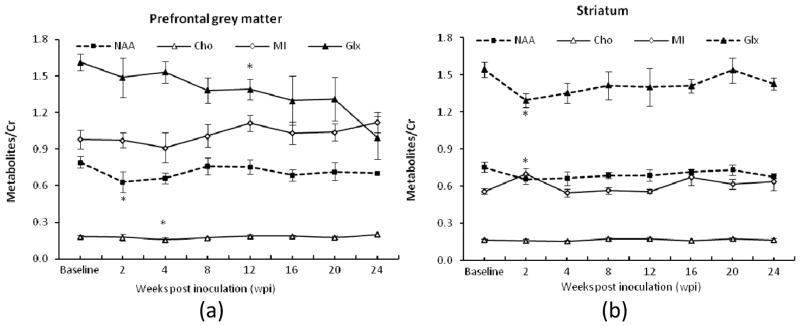
Longitudinal cerebral metabolite ratio changes in prefrontal grey matter (PGM) (a) and striatum (b) of pig-tailed macaques after SIV inoculation. Error bars represent Standard Error of the Mean (SEM). *: P <0.05, compared with the baseline; “----”: the temporal changes of metabolites/Cr significantly negatively correlated with CD8+ T-cell percentages change.
Relationships of the metabolite alterations with immunologic dysfunction
As seen in Fig 4 and Table 1, the progressive changes of the NAA/Cr ratios in PGM and striatum and the Glx/Cr ratio in striatum were correlated with the percentage of CD8+ T cells during SIV infection, as indicated by linear mixed effects regression analyses (Table 1). Standardized beta values (slopes) were significantly greater than 0 for the relationship between the NAA/Cr ratio and CD8 percentage in PGM (F(1,13.87)=14.55, P=.002), and for the relationship between the NAA/Cr ratio and CD8 percentage in striatum (F(1,15.19)= 9.57, p=0.007), and for the relationship between the Glx/Cr ratio and CD8 percentage in striatum (F(1,18.85)= 16.85, p=0.001). No significant correlation between any metabolite ratios and the CD4+ T cell depletion (or CD4/CD8 ratio) was observed.
Table 1.
Standardized β value between CD4+, CD8+ T-cell percentages and CD4/CD8 with cerebral metabolites in prefrontal grey matter (PGM) and striatum, *: P<0.05 after FDR correction.
| Location | % | mI/Cr | NAA/Cr | Cho/Cr | Glx/Cr |
|---|---|---|---|---|---|
| PGM | CD4 | −0.37 | −0.02 | 0.33 | 0.18 |
| CD8 | 0.24 | *−0.51 | 0.16 | −0.20 | |
| CD4/CD8 | −0.26 | 0.29 | 0.11 | 0.29 | |
| Striatum | CD4 | −0.04 | −0.27 | 0.12 | −0.09 |
| CD8 | −0.06 | *−0.43 | 0.13 | *−0.52 | |
| CD4/CD8 | 0.05 | 0.18 | 0.09 | 0.37 |
F(1,13.87) NAA:CD8(PGM)=14.55, p=0.002
F(1,15.19) NAA:CD8(striatum)=9.57, p=0.007
F(1,18.85) GLX:CD8(striatum)=16.85, p=0.001
Discussion
The present in vivo MRS study reveals that the cerebral metabolites in prefrontal grey matter and striatum of pig-tailed macaques are altered progressively during the SIVsmmFGb infection and largely in agreement with those seen in SIVmac251-infected rhesus macaques as well as in HIV-infected patients. Notably, the temporal metabolite changes are associated with the progressive immune dysfunction. In addition, the Glx/Cr ratio changed with different temporal profiles in striatum vs. prefrontal grey matter, indicating that the glutamatergic system might be affected differently in these two regions following SIV infection. As SIVsmmFGb enters the brain in about 90% of the infected animals, we believe that SIVsmmFGb has advantages over SIVmac251 for neuroimaging studies designed to characterize CNS injury following SIV infection of macaque monkeys.
Brain metabolite changes in PGM and striatum
N-acetyl-asparate (NAA)
NAA is a neuronal marker widely used to assess brain injury. Previous studies have demonstrated that no significant changes in NAA/Cr were seen in basal ganglia, frontal cortex and centrum semiovale at any time point after 2 weeks in rhesus monkeys (n=8) with SIVmac251 infection (Fuller et al, 2004). In contrast, the present study revealed significant reduction in NAA/Cr of PGM at 2 wpi (Fig 4a), consistent with the NAA reduction in frontal cortical grey matter seen in the prior studies of HIV+ patients in the early stages of infection (Lentz et al, 2009) and the NAA/Cr decrease in frontal cortex at 13 days post inoculation in SIVmac251-infected macaques (n=10) (Greco et al, 2004) and those seen at 12 days (n=3) and 14 days (n=3) post inoculation in an ex vivo NMR study (Lentz et al, 2005). Such transient reduction of NAA/Cr correlated with the changes in synaptophysin and might reflect the reversible neuronal injury during the primary SIV infection (Lentz et al, 2005). Also, the NAA/Cr alteration is in agreement with previous finding of whole brain fractional anisotropy (FA) in SIVsmmFGb-infected macaque brains examined with diffusion tensor imaging (DTI) (Li et al, 2011).
It is of use to compare the present macaque experiment done at 3T with previous ones at 1.5T. Even though current 3T offers higher (nearly two-fold) sensitivity than 1.5T in in vivo MRS and a dedicated surface coil was used, the voxel size used in the present study was 6mm × 6mm × 6 mm (0.22ml), much smaller (15-fold) than that (15mm × 15mm × 15mm, or 3.4ml) used in previous rhesus macaque studies. In consideration of the spectrum SNR and the sample size difference, the NAA/Cr reduction at 2 weeks post inoculation was seen at the present study with n=5 and previous study with n=10 (Greco et al, 2004) but not with n=8 (Fuller et al, 2004), the present MRS results suggest that SIVsmmFGb in pig-tailed macaques is highly neuropathogenic in comparison with SIVmac251 in rhesus macaques.
Glutamate and glutamine (Glx)
Neurons and glial cells are believed to be the primary sources of glutamate and glutamine in which glutamate is considered as the principal excitatory neurotransmitter in brain. Disturbance of these substances could reflect dysfunction in one or both of these cell types (Lentz et al, 2009). Significant Glx reduction has been observed in the frontal cortical gray matter of HIV patients at early and chronic stages (Lentz et al, 2009) and in basal ganglia at chronic stage (Lentz et al, 2011). Also, no changes in Glx or Glx/Cr were reported in the frontal cortex and centrum semiovale but the reduction was nearly significant in basal ganglia in previous macaque study (Ratai et al, 2009). In contrast, Glx/Cr in PGM demonstrated continuous decline, and significant reduction at 12 wpi, consistent with the changes seen in HIV patients with early infection (Lentz et al, 2009). Also, significant Glx/Cr reduction in striatum was seen in 2 wpi in the present study while such change was also observed in basal ganglia in HIV patients at chronic stage (Lentz et al, 2011), consistent with that seen in the SIVmac251 infected macaques with CD8 depletion (Ratai et al, 2009). In addition, the difference of Glx/Cr evolutions in PGM and striatum of the SIVsmmFGb infected macaques suggests that the glutamatergic system may be affected differently in these regions probably due to the higher viral load in PGM than in striatum in the unique SIV model of neuroAIDS (O’Neil et al, 2004).
Choline (Cho)
As a marker for inflammation and membrane disruption, increased Cho levels, seen in patients with chronic HIV infection, are typically thought to represent increased lipid membrane turnover due to glial activation in response to the HIV infection (Lentz et al, 2011). This interpretation is supported by the observation of pathologic changes during early HIV and SIV infection (Gray et al, 1996; Kim et al, 2005).
Elevation in Cho has been observed in the frontal white matter of chronic HIV infection (Chang et al, 1999; Meyerhoff et al, 1999) and in Cho/Cr of the frontal cortex and white matter of HIV patients in early and chronic stage (Lentz et al, 2011). Ratai and colleagues reported Cho and Cho/Cr increased at 2 wpi and decrease at 4 wpi in the frontal cortex, basal ganglia during SIV infection (Ratai et al, 2009). In contrast, reduction of Cho/Cr in PGM was only seen at 4 wpi in the present study.
myo-inositol (mI)
Because mI exists primarily in glia, its elevation is considered to reflect increased glial cell activity and a marker of inflammation (Brand et al, 1993). Higher mI has been commonly observed in the white matter of asymptomatic HIV+ patients (Chang et al, 2004). Also, such mI increase was seen in frontal cortex in SIVmac251-infected macaques (n=10) during acute infection (Ratai et al, 2009). In contrast, no changes in mI/Cr were reported in the frontal cortex and basal ganglia of SIVmac251-infected macaques in (n=8) in early and chronic stage (Fuller et al, 2004). In the present study, mI/Cr was stable in PGM in acute stage but significantly elevated in striatum at 2 wpi and then reduced at 4 wpi, different from that seen in the SIVmac251 infected macaques (Ratai et al, 2009).
Correlation between temporal changes of brain metabolite ratios in PGM and striatum
Region-specific metabolic responses have been reported previously in SIV-infected macaques (Ratai et al, 2009). The temporal changes in NAA/Cr and Glx/Cr ratios of striatum, and NAA/Cr in PGM were correlated with each other, suggesting that the temporal association of neuronal injury in the frontal cortex and basal ganglia during SIVsmmFGb infection. In contrast, significant correlation was observed in Glx but not NAA in frontal cortex and basal ganglia in Ratai et al’s study.
T cell phenotypes and their association with brain metabolite alterations
Immune system responses to HIV or SIV infection have been reported previously in both SIV and HIV immunology literature (Marcondes et al, 2001; von Geldern et al, 2007). Depletion of CD4+ T cell and accumulation of CD8+ T lymphocytes are the most significant predictor of the clinical disease severity (Chang et al, 2000; Grassi et al, 1999; Langford et al, 2007). Down-regulation of viral replication in the brain coincides with increased CD8+ T cell infiltration and reduced glial and macrophage activation have been observed in SIV infected macaques (Clements et al, 2002). The results of the temporal correlations between NAA/Cr in PGM and striatum and Glx/Cr in striatum and the percentage of CD8+ T cells during the SIV infection is consistent with the findings in patients with early HIV+ infection (Lentz et al, 2009), suggesting that recruited CD8+ T cells may damage bystander neurons during down-regulated virus expression, as indicated by reduced NAA/Cr and Glx/Cr probably due to neuronal loss or dysfunction (Crews et al, 2008; De Stefano et al, 1995; Guimaraes et al, 1995; Urenjak et al, 1993).
Previous MRS studies of HIV patients showed such relationship was driven only by the early infection cohort, and no correlation between any CD8+ T-cell population and the NAA or Glx levels in chronic infection cohort were reported (Lentz et al, 2009). However, in the present study of SIV-infected monkeys, it is seen that NAA/Cr and Glx/Cr correlated with the percentage of CD8+ T cells, but not with the percentage of CD4+ T cells. The difference in MRS findings between the SIV monkeys and HIV patients may be attributed to the unknown or variable factors including the mode and time course of infection and the medication in HIV patients.
Macaque models are widely used in HIV/AIDS studies and therapeutic development due to the difficulty of studying the disease in humans. The neurochemical alteration during SIV infection has previously been investigated extensively in macaques infected with SIVmac251. Here we report similar findings in SIVsmmFGb infected macaques. However, as demonstrated in our previous studies (Reeve et al, 2009; Reeve et al, 2010), SIVsmmFGb induces neuropathology in over 90% of the infected pig-tailed macaques, a much higher infection rate than seen in macaques infected with SIVmac251 and other strains (Westmoreland et al, 1998). Although the sample size (n=5) in the present study was smaller than those used in previous MRS studies of SIVmac251-infected macaques (n= 8–18) (Fuller et al, 2004; Greco et al, 2004; Ratai et al, 2009), we were still able to detect significant metabolite changes, as described above. Further, these changes were detected in a shorter time course than seen in other models (6-month vs. 24-month endpoint) (Fuller et al, 2004). Therefore, our results suggest that SIVsmmFGb-infected macaques are a more sensitive and responsive model than others for characterizing neuronal injury during SIV infection.
HIV-infection leads to immune deficiency and a range of neurological disorders due to the infection of central nervous system and associated dementia. MRI holds great potential to evaluate the physiological, microstructural, neurochemical, and functional changes in HIV patients (Chang, 1995; Chang et al, 2004; Offiah and Turnbull, 2006; Shyam babu et al, 2013; Tucker et al, 2004; Zhang and Li, 2013). However, to date, MRI is still not a sensitive approach in screening HIV patients without neurological symptoms (Nishijima et al, 2014). Our previous study has demonstrated the sensitivity of DTI and perfusion techniques in SIVsmmFGb-infected brains using high-field MRI (Li et al, 2011). Currently, high-field (3T) and ultra-high field (7T and above) MRI techniques exhibit improved image quality and expanded range of applications in preclinical and clinical studies (Duyn, 2012; Oz et al, 2013). In particular, some novel diffusion, perfusion, and multi-parameter MRI methods may be used to facilitate better detection of brain injury in SIV-infected macaques (Li et al, 2014; Meng and Zhang, 2014; Zhang et al, 2014). Taken together with the present MRS results at 3T, our findings suggest that SIVsmmFGb infected macaques could serve as an excellent platform for exploring advanced imaging techniques in HIV/AIDS studies.
Conclusions
The temporal cerebral metabolite changes during the SIVsmmFGb infection are evaluated with in vivo MRS at 3T. The MRS results demonstrate the metabolite alterations are largely consistent with previous findings of neuronal injury in SIVmac251-infected rhesus monkeys and HIV patients, and, in particular, associated with progressive immune dysfunction. The findings suggest that the SIVsmmFGb infected pig-tailed macaques are an excellent model for characterizing CNS infection using novel neuroimaging approaches and exploring advanced neuroimaging techniques as well.
Acknowledgments
The authors thank the vet team of Yerkes Imaging Center and the Animal Resources of Yerkes National Primate Research Center for their assistance in this study. This project was in part funded by the National Center for Research Resources P51RR000165 and is currently supported by the Office of Research Infrastructure Programs/OD P51OD011132, and by NIH grant MH067769 (FJN).
Footnotes
Disclosure: The authors have no conflict of interest to disclose.
References
- Apetrei C, Pandrea I, Mellors JW. Nonhuman primate models for HIV cure research. PLoS Pathog. 2012;8:e1002892. doi: 10.1371/journal.ppat.1002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini YH, Yosef Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Blackwell E, de Leon CF, Miller GE. Applying mixed regression models to the analysis of repeated-measures data in psychosomatic medicine. Psychosom Med. 2006;68:870–8. doi: 10.1097/01.psy.0000239144.91689.ca. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–98. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Chang L. In vivo magnetic resonance spectroscopy in HIV and HIV-related brain diseases. Rev Neurosci. 1995;6:365–78. doi: 10.1515/revneuro.1995.6.4.365. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Leonido-Yee M, Speck O. Perfusion MRI detects rCBF abnormalities in early stages of HIV-cognitive motor complex. Neurology. 2000;54:389–96. doi: 10.1212/wnl.54.2.389. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology. 1999;52:100–8. doi: 10.1212/wnl.52.1.100. [DOI] [PubMed] [Google Scholar]
- Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, Kolson D, Schifitto G, Jarvik JG, Miller EN, Lenkinski R, Gonzalez G, Navia BA. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23:1336–47. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Clements JE, Babas T, Mankowski JL, Suryanarayana K, Piatak M, Jr, Tarwater PM, Lifson JD, Zink MC. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J Infect Dis. 2002;186:905–13. doi: 10.1086/343768. [DOI] [PubMed] [Google Scholar]
- Crews L, Lentz MR, Gonzalez RG, Fox HS, Masliah E. Neuronal injury in simian immunodeficiency virus and other animal models of neuroAIDS. J Neurovirol. 2008;14:327–39. doi: 10.1080/13550280802132840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med. 1995;34:721–7. doi: 10.1002/mrm.1910340511. [DOI] [PubMed] [Google Scholar]
- Duyn JH. The future of ultra-high field MRI and fMRI for study of the human brain. Neuroimage. 2012;62:1241–8. doi: 10.1016/j.neuroimage.2011.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, Arnold S. Increased glial metabolites predict increased working memory network activation in HIV brain injury. Neuroimage. 2003;19:1686–93. doi: 10.1016/s1053-8119(03)00232-5. [DOI] [PubMed] [Google Scholar]
- Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging. 2010;32:1045–53. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RA, Westmoreland SV, Ratai E, Greco JB, Kim JP, Lentz MR, He J, Sehgal PK, Masliah E, Halpern E, Lackner AA, Gonzalez RG. A prospective longitudinal in vivo 1H MR spectroscopy study of the SIV/macaque model of neuroAIDS. BMC Neurosci. 2004;5:10. doi: 10.1186/1471-2202-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez RG, Cheng LL, Westmoreland SV, Sakaie KE, Becerra LR, Lee PL, Masliah E, Lackner AA. Early brain injury in the SIV-macaque model of AIDS. AIDS. 2000;14:2841–9. doi: 10.1097/00002030-200012220-00005. [DOI] [PubMed] [Google Scholar]
- Grassi MP, Perin C, Borella M, Mangoni A. Assessment of cognitive function in asymptomatic HIV-positive subjects. Eur Neurol. 1999;42:225–9. doi: 10.1159/000008112. [DOI] [PubMed] [Google Scholar]
- Gray F, Scaravilli F, Everall I, Chretien F, An S, Boche D, Adle-Biassette H, Wingertsmann L, Durigon M, Hurtrel B, Chiodi F, Bell J, Lantos P. Neuropathology of early HIV-1 infection. Brain Pathol. 1996;6:1–15. doi: 10.1111/j.1750-3639.1996.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Greco JB, Sakaie KE, Aminipour S, Lee PL, Chang LL, He J, Westmoreland S, Lackner AA, Gonzalez RG. Magnetic resonance spectroscopy: an in vivo tool for monitoring cerebral injury in SIV-infected macaques. J Med Primatol. 2002;31:228–36. doi: 10.1034/j.1600-0684.2002.02009.x. [DOI] [PubMed] [Google Scholar]
- Greco JB, Westmoreland SV, Ratai EM, Lentz MR, Sakaie K, He J, Sehgal PK, Masliah E, Lackner AA, Gonzalez RG. In vivo 1H MRS of brain injury and repair during acute SIV infection in the macaque model of neuroAIDS. Magn Reson Med. 2004;51:1108–14. doi: 10.1002/mrm.20073. [DOI] [PubMed] [Google Scholar]
- Guimaraes AR, Schwartz P, Prakash MR, Carr CA, Berger UV, Jenkins BG, Coyle JT, Gonzalez RG. Quantitative in vivo 1H nuclear magnetic resonance spectroscopic imaging of neuronal loss in rat brain. Neuroscience. 1995;69:1095–1101. doi: 10.1016/0306-4522(95)00300-8. [DOI] [PubMed] [Google Scholar]
- Haga KK, Khor YP, Farrall A, Wardlaw JM. A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiol Aging. 2009;30:353–63. doi: 10.1016/j.neurobiolaging.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Haigwood NL. Predictive value of primate models for AIDS. AIDS Rev. 2004;6:187–98. [PubMed] [Google Scholar]
- Herndon JG, Constantinidis I, Moss MB. Age-related brain changes in rhesus monkeys: a magnetic resonance spectroscopic study. Neuroreport. 1998;9:2127–30. doi: 10.1097/00001756-199806220-00040. [DOI] [PubMed] [Google Scholar]
- Hu SL. Non-human primate models for AIDS vaccine research. Curr Drug Targets Infect Disord. 2005;5:193–201. doi: 10.2174/1568005054201508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol Aging. 2005;26:665–72. doi: 10.1016/j.neurobiolaging.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JP, Lentz MR, Westmoreland SV, Greco JB, Ratai EM, Halpern E, Lackner AA, Masliah E, Gonzalez RG. Relationships between astrogliosis and 1H MR spectroscopic measures of brain choline/creatine and myo-inositol/creatine in a primate model. AJNR Am J Neuroradiol. 2005;26:752–9. [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Mori K, Kawahara T, Ringler DJ, Desrosiers RC. Analysis of simian immunodeficiency virus sequence variation in tissues of rhesus macaques with simian AIDS. J Virol. 1993;67:6522–34. doi: 10.1128/jvi.67.11.6522-6534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford SE, Ananworanich J, Cooper DA. Predictors of disease progression in HIV infection: a review. AIDS Res Ther. 2007;4:11. doi: 10.1186/1742-6405-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz MR, Kim JP, Westmoreland SV, Greco JB, Fuller RA, Ratai EM, He J, Sehgal PK, Halpern EF, Lackner AA, Masliah E, Gonzalez RG. Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology. 2005;235:461–8. doi: 10.1148/radiol.2352040003. [DOI] [PubMed] [Google Scholar]
- Lentz MR, Kim WK, Kim H, Soulas C, Lee V, Venna N, Halpern EF, Rosenberg ES, Williams K, Gonzalez RG. Alterations in brain metabolism during the first year of HIV infection. J Neurovirol. 2011;17:220–9. doi: 10.1007/s13365-011-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz MR, Kim WK, Lee V, Bazner S, Halpern EF, Venna N, Williams K, Rosenberg ES, Gonzalez RG. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology. 2009;72:1465–72. doi: 10.1212/WNL.0b013e3181a2e90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhang X, Komery A, Li Y, Novembre FJ, Herndon JG. Longitudinal diffusion tensor imaging and perfusion MRI investigation in a macaque model of neuro-AIDS: a preliminary study. Neuroimage. 2011;58:286–92. doi: 10.1016/j.neuroimage.2011.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CX, Patel S, Wang DJ, Zhang X. Effect of high dose isoflurane on cerebral blood flow in macaque monkeys. Magn Reson Imaging. 2014;32:956–60. doi: 10.1016/j.mri.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes MC, Burudi EM, Huitron-Resendiz S, Sanchez-Alavez M, Watry D, Zandonatti M, Henriksen SJ, Fox HS. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol. 2001;167:5429–38. doi: 10.4049/jimmunol.167.9.5429. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang X. In vivo diffusion spectrum imaging of non-human primate brain: initial experience in transcallosal fiber examination. Quantitative imaging in medicine and surgery. 2014;4:129–35. doi: 10.3978/j.issn.2223-4292.2014.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, Weiner MW, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology. 1999;52:995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- Nishijima T, Gatanaga H, Teruya K, Tajima T, Kikuchi Y, Hasuo K, Oka S. Brain magnetic resonance imaging screening is not useful for HIV-1-infected patients without neurological symptoms. AIDS Res Hum Retroviruses. 2014 doi: 10.1089/aid.2014.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novembre FJ, De Rosayro J, O’Neil SP, Anderson DC, Klumpp SA, McClure HM. Isolation and characterization of a neuropathogenic simian immunodeficiency virus derived from a sooty mangabey. J Virol. 1998;72:8841–51. doi: 10.1128/jvi.72.11.8841-8851.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil SP, Suwyn C, Anderson DC, Niedziela G, Bradley J, Novembre FJ, Herndon JG, McClure HM. Correlation of acute humoral response with brain virus burden and survival time in pig-tailed macaques infected with the neurovirulent simian immunodeficiency virus SIVsmmFGb. Am J Pathol. 2004;164:1157–72. doi: 10.1016/S0002-9440(10)63204-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offiah CE, Turnbull IW. The imaging appearances of intracranial CNS infections in adult HIV and AIDS patients. Clin Radiol. 2006;61:393–401. doi: 10.1016/j.crad.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Oz G, Tkac I, Ugurbil K. Animal models and high field imaging and spectroscopy. Dialogues Clin Neurosci. 2013;15:263–78. doi: 10.31887/DCNS.2013.15.3/goz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Ratai EM, Bombardier JP, Joo CG, Annamalai L, Burdo TH, Campbell J, Fell R, Hakimelahi R, He J, Autissier P, Lentz MR, Halpern EF, Masliah E, Williams KC, Westmoreland SV, Gonzalez RG. Proton magnetic resonance spectroscopy reveals neuroprotection by oral minocycline in a nonhuman primate model of accelerated NeuroAIDS. PLoS One. 2010;5:e10523. doi: 10.1371/journal.pone.0010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratai EM, Pilkenton SJ, Greco JB, Lentz MR, Bombardier JP, Turk KW, He J, Joo CG, Lee V, Westmoreland S, Halpern E, Lackner AA, Gonzalez RG. In vivo proton magnetic resonance spectroscopy reveals region specific metabolic responses to SIV infection in the macaque brain. BMC Neurosci. 2009;10:63. doi: 10.1186/1471-2202-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve AB, Patel K, Pearce NC, Augustus KV, Domingues HG, O’Neil SP, Novembre FJ. Reduced genetic diversity in lymphoid and central nervous system tissues and selection-induced tissue-specific compartmentalization of neuropathogenic SIVsmmFGb during acute infection. AIDS Res Hum Retroviruses. 2009;25:583–601. doi: 10.1089/aid.2008.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve AB, Pearce NC, Patel K, Augustus KV, Novembre FJ. Neuropathogenic SIVsmmFGb genetic diversity and selection-induced tissue-specific compartmentalization during chronic infection and temporal evolution of viral genes in lymphoid tissues and regions of the central nervous system. AIDS Res Hum Retroviruses. 2010;26:663–79. doi: 10.1089/aid.2009.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvan AM, Vion-Dury J, Confort-Gouny S, Nicoli F, Lamoureux S, Cozzone PJ. Brain proton magnetic resonance spectroscopy in HIV-related encephalopathy: identification of evolving metabolic patterns in relation to dementia and therapy. AIDS Res Hum Retroviruses. 1997;13:1055–66. doi: 10.1089/aid.1997.13.1055. [DOI] [PubMed] [Google Scholar]
- Shyam babu C, Satishchandra P, Mahadevan A, Pillai Shibu V, Ravishankar S, Sidappa N, Udaykumar R, Ravi V, Shankar SK. Usefulness of stereotactic biopsy and neuroimaging in management of HIV-1 Clade C associated focal brain lesions with special focus on cerebral toxoplasmosis. Clin Neurol Neurosurg. 2013;115:995–1002. doi: 10.1016/j.clineuro.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans SI, Feinberg MB. The roles of nonhuman primates in the preclinical evaluation of candidate AIDS vaccines. Expert Rev Vaccines. 2004;3:S5–32. doi: 10.1586/14760584.3.4.s5. [DOI] [PubMed] [Google Scholar]
- Strickland SL, Gray RR, Lamers SL, Burdo TH, Huenink E, Nolan DJ, Nowlin B, Alvarez X, Midkiff CC, Goodenow MM, Williams K, Salemi M. Significant genetic heterogeneity of the SIVmac251 viral swarm derived from different sources. AIDS Res Hum Retroviruses. 2011;27:1327–32. doi: 10.1089/aid.2011.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Carr CA, Guimaraes AR, Worth JL, Navia BA, Gonzalez RG. Brain choline-containing compounds are elevated in HIV-positive patients before the onset of AIDS dementia complex: A proton magnetic resonance spectroscopic study. Neurology. 1996;46:783–8. doi: 10.1212/wnl.46.3.783. [DOI] [PubMed] [Google Scholar]
- Tucker KA, Robertson KR, Lin W, Smith JK, An H, Chen Y, Aylward SR, Hall CD. Neuroimaging in human immunodeficiency virus infection. J Neuroimmunol. 2004;157:153–62. doi: 10.1016/j.jneuroim.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13:981–9. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rompay KK. The use of nonhuman primate models of HIV infection for the evaluation of antiviral strategies. AIDS Res Hum Retroviruses. 2012;28:16–35. doi: 10.1089/aid.2011.0234. [DOI] [PubMed] [Google Scholar]
- Vion-Dury J, Salvan AM, Confort-Gouny S, Cozzone PJ. Brain proton magnetic resonance spectroscopy. Indications for diagnosis and follow-up of HIV-related encephalopathy in the adult. Presse Med. 1998;27:1398–405. [PubMed] [Google Scholar]
- von Geldern G, Cepok S, Nolting T, Du Y, Grummel V, Adams O, Hartung HP, Arendt G, Hemmer B. CD8 T-cell subsets and viral load in the cerebrospinal fluid of therapy-naive HIV-infected individuals. AIDS. 2007;21:250–3. doi: 10.1097/QAD.0b013e328011ec76. [DOI] [PubMed] [Google Scholar]
- Westmoreland SV, Halpern E, Lackner AA. Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J Neurovirol. 1998;4:260–8. doi: 10.3109/13550289809114527. [DOI] [PubMed] [Google Scholar]
- Wiebenga OT, Klauser AM, Nagtegaal GJ, Schoonheim MM, Barkhof F, Geurts JJ, Pouwels PJ. Longitudinal absolute metabolite quantification of white and gray matter regions in healthy controls using proton MR spectroscopic imaging. NMR Biomed. 2014;27:304–11. doi: 10.1002/nbm.3063. [DOI] [PubMed] [Google Scholar]
- Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–45. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Bokhari S, Silverstein P, Pinson D, Kumar A, Buch S. Nonhuman primate models of NeuroAIDS. J Neurovirol. 2008;14:292–300. doi: 10.1080/13550280802074539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WE, Tal A, Kirov, Rusinek H, Charytonowicz D, Babb JS, Ratai EM, Gilberto Gonzalez R, Gonen O. Global gray and white matter metabolic changes after simian immunodeficiency virus infection in CD8-depleted rhesus macaques: proton MRS imaging at 3 T. NMR Biomed. 2013a doi: 10.1002/nbm.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WE, Tal A, Zhang K, Babb JS, Ratai EM, Gonzalez RG, Gonen O. Structure-specific glial response in a macaque model of neuroAIDS: multivoxel proton magnetic resonance spectroscopic imaging at 3 Tesla. AIDS. 2013b;27:2519–28. doi: 10.1097/01.aids.0000433244.32105.96. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li C. Quantitative MRI Measures in SIV-Infected Macaque Brains. J Clin Cell Immunol Suppl. 2013;7:S7–005. doi: 10.4172/2155-9899.S7-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tong F, Li CX, Yan Y, Nair G, Nagaoka T, Tanaka Y, Zola S, Howell L. A fast multiparameter MRI approach for acute stroke assessment on a 3T clinical scanner: preliminary results in a non-human primate model with transient ischemic occlusion. Quantitative imaging in medicine and surgery. 2014;4:112–22. doi: 10.3978/j.issn.2223-4292.2014.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]