Abstract
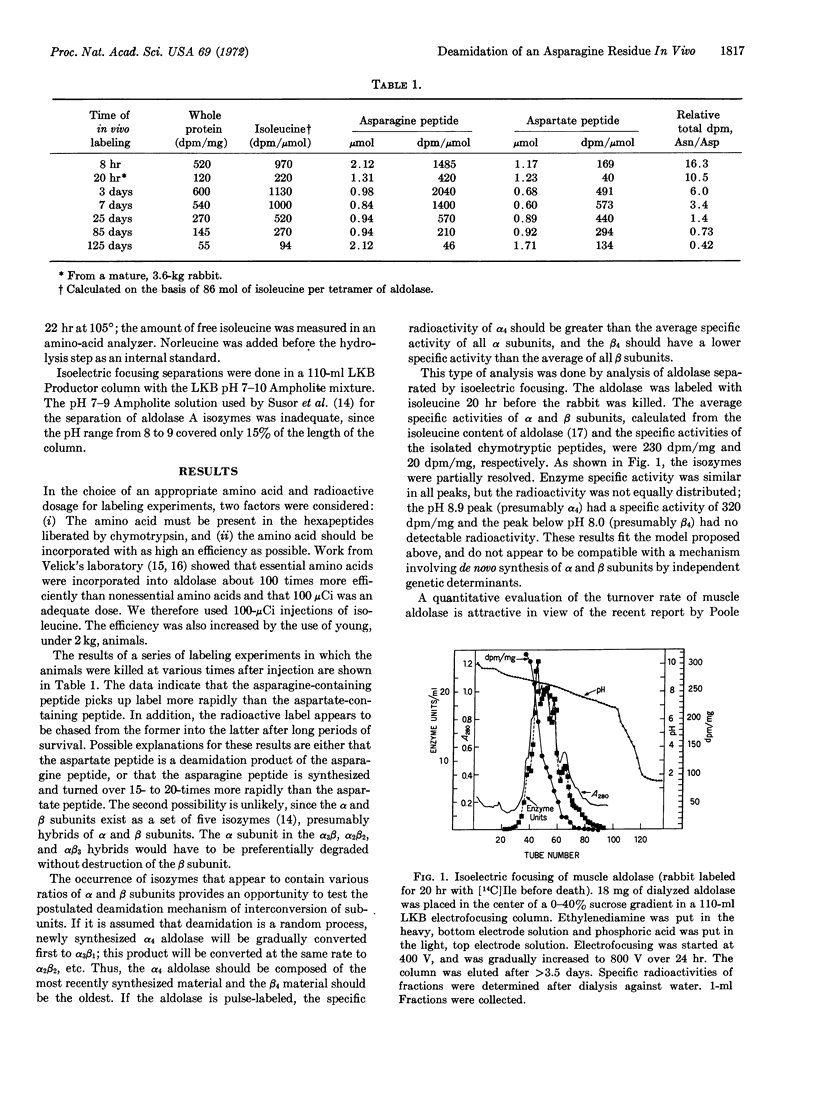
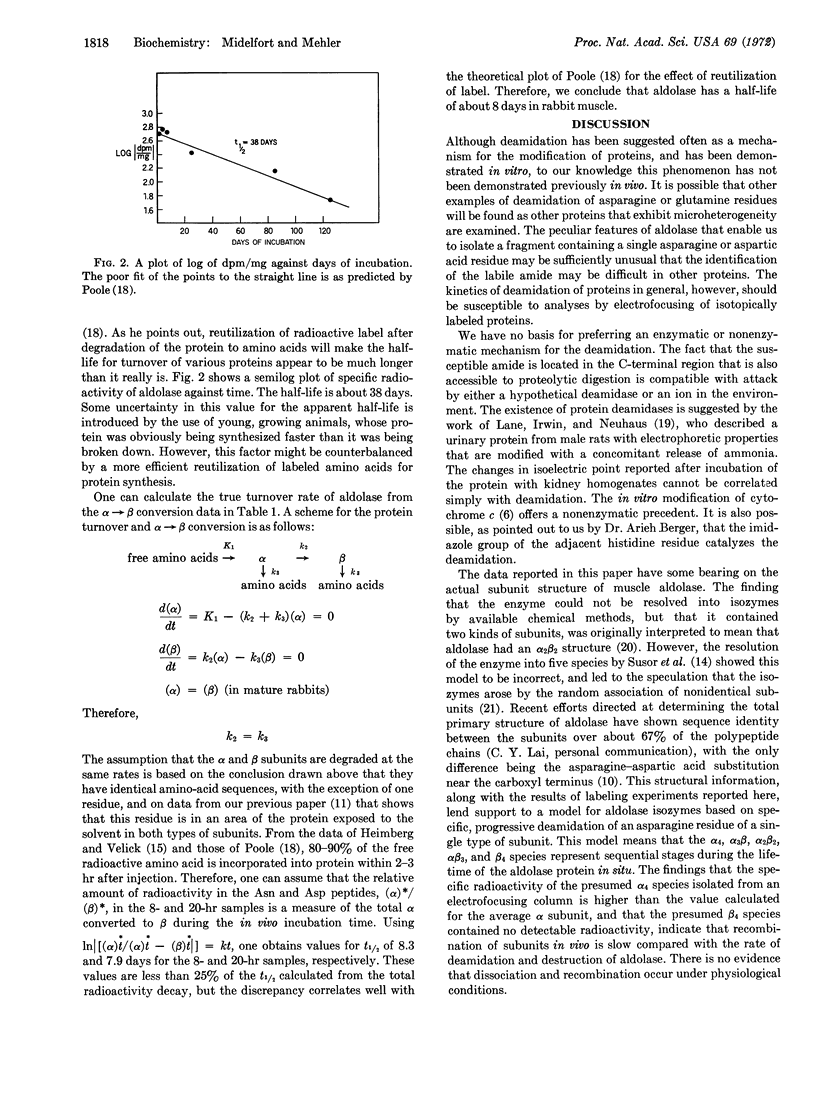
Microheterogeneity of rabbit muscle aldolase is caused by deamidation in vivo of an asparagine residue near the C-terminus of each subunit. Isotopic labeling of a peptide containing the asparagine residue at various time intervals before isolation of aldolase permits estimation of the half-time for the deamidation as about 8 days, which is about the time estimated for the half-life of the enzyme in vivo. It is concluded that the aldolase as genetically determined is a tetramer, designated α4, that undergoes random deamidation to form α3β, α2β2, and αβ3 species as intermediates in the formation of β4, the species in which all of the specific asparagine has been deamidated. Isoelectric focusing data indicate that the subunits do not exchange appreciably in vivo.
Keywords: isozymes, electrofocusing, subunits, protein structure
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan W., Morse D. E., Horecker B. L. Nonidentity of subunits of rabbit muscle aldolase. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1013–1020. doi: 10.1073/pnas.57.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards Y. H., Hopkinson D. A., Harris H. Inherited variants of human nucleoside phosphorylase. Ann Hum Genet. 1971 May;34(4):395–408. doi: 10.1111/j.1469-1809.1971.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Flatmark T., Sletten K. Multiple forms of cytochrome c in the rat. Precursor-product relationship between the main component Cy I and the minor components Cy II and Cy 3 in vivo. J Biol Chem. 1968 Apr 10;243(7):1623–1629. [PubMed] [Google Scholar]
- Funakoshi S., Deutsch H. F. Human carbonic anhydrases. II. Some physicochemical properties of native isozymes and of similar isozymes generated in vitro. J Biol Chem. 1969 Jul 10;244(13):3438–3446. [PubMed] [Google Scholar]
- HEIMBERG M., VELICK S. F. The synthesis of aldolase and phosphorylase in rabbits. J Biol Chem. 1954 Jun;208(2):725–730. [PubMed] [Google Scholar]
- Koida M., Lai C. Y., Horecker B. L. Subunit structure of rabbit muscle aldolase: extent of homology of the alpha and beta subunits and age-dependent changes in their ratio. Arch Biochem Biophys. 1969 Nov;134(2):623–631. doi: 10.1016/0003-9861(69)90326-9. [DOI] [PubMed] [Google Scholar]
- Lai C. Y., Chen C., Horecker B. L. Primary structure of two COOH-terminal hexapeptides from rabbit muscle aldolase: a difference in the structure of the alpha and beta subunits. Biochem Biophys Res Commun. 1970 Jul 27;40(2):461–468. doi: 10.1016/0006-291x(70)91031-4. [DOI] [PubMed] [Google Scholar]
- Lutstorf U. M., von Wartburg J. P. Subunit Composition of horse liver alcohol dehydrogenase isoenzymes. FEBS Lett. 1969 Nov 12;5(3):202–206. doi: 10.1016/0014-5793(69)80332-7. [DOI] [PubMed] [Google Scholar]
- Midelfort C. F., Mehler A. H. A chymotrypsin-catalyzed modification of rabbit muscle aldolase. J Biol Chem. 1972 Jun 10;247(11):3618–3621. [PubMed] [Google Scholar]
- Monn E. Relation between blood cell phosphoglucomutase isoenzymes and age of cell population. Scand J Haematol. 1969;6(2):133–138. doi: 10.1111/j.1600-0609.1969.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Chan W., Horecker B. L. The subunit structure and carboxy-terminal sequence of rabbit muscle aldolase. Proc Natl Acad Sci U S A. 1967 Aug;58(2):628–634. doi: 10.1073/pnas.58.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Horecker B. L. The mechanism of action of aldolases. Adv Enzymol Relat Areas Mol Biol. 1968;31:125–181. doi: 10.1002/9780470122761.ch4. [DOI] [PubMed] [Google Scholar]
- Poole B. The kinetics of disappearance of labeled leucine from the free leucine pool of rat liver and its effect on the apparent turnover of catalase and other hepatic proteins. J Biol Chem. 1971 Nov;246(21):6587–6591. [PubMed] [Google Scholar]
- SIMPSON M. V., VELICK S. F. The synthesis of aldolase and glyceraldehyde-3-phosphate dehydrogenase in the rabbit. J Biol Chem. 1954 May;208(1):61–71. [PubMed] [Google Scholar]
- Shimizu H., Ozawa H. Studies on aldolases. I. Amino acid composition and subunit structure of rabbit-muscle aldolase. Biochim Biophys Acta. 1967 Feb 21;133(2):195–205. doi: 10.1016/0005-2795(67)90059-1. [DOI] [PubMed] [Google Scholar]
- Susor W. A., Kochman M., Rutter W. J. Heterogeneity of presumably homogeneous protein preparations. Science. 1969 Sep 19;165(3899):1260–1262. doi: 10.1126/science.165.3899.1260. [DOI] [PubMed] [Google Scholar]



