Abstract
We have recently described a genetic system, termed in vivo expression technology (IVET), that uses an animal as a selective medium to identify genes that pathogenic bacteria specifically express when infecting host tissues. Here, the potential utility of the IVET approach has been expanded with the development of a transcriptional-fusion vector, pIVET8, which uses antibiotics resistance as the basis for selection in host tissues. pIVET8 contains promoterless chloramphenicol acetyltransferase (cat) and lacZY genes. A pool of Salmonella typhimurium clones carrying random cat-lac transcriptional fusions, produced with pIVET8, was used to infect BALB/c mice that were subsequently treated with intraperitoneal injections of chloramphenicol. Strains that survived the selection by expressing the cat gene in the animal were then screened for those that had low-level lacZY expression on laboratory medium. These strains carry operon fusions to genes that are specifically induced in vivo (ivi genes). One of the ivi genes identified (fadB) encodes an enzyme involved in fatty acid oxidation, suggesting that this enzyme might contribute to the metabolism of bactericidal or proinflammatory host fatty acids. The pIVET8-based selection system was also used to identify S. typhimurium genes that are induced in cultured macrophages. The nature of ivi gene products will provide a more complete understanding of the metabolic, physiological, and genetic factors that contribute to the virulence of microbial pathogens.
Full text
PDF


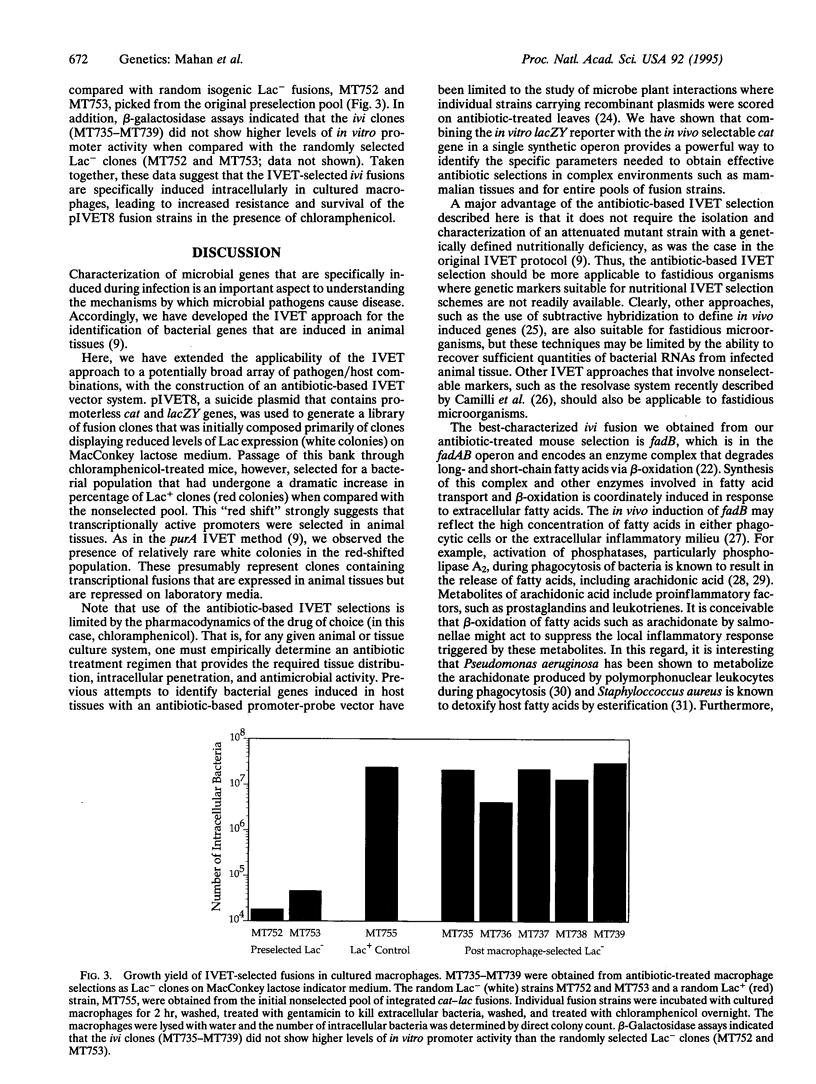

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abshire K. Z., Neidhardt F. C. Analysis of proteins synthesized by Salmonella typhimurium during growth within a host macrophage. J Bacteriol. 1993 Jun;175(12):3734–3743. doi: 10.1128/jb.175.12.3734-3743.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli A., Beattie D. T., Mekalanos J. J. Use of genetic recombination as a reporter of gene expression. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2634–2638. doi: 10.1073/pnas.91.7.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Botstein D., Watanabe T., Ogata Y. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology. 1972 Dec;50(3):883–898. doi: 10.1016/0042-6822(72)90442-4. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna P. C., Acosta D., Collier R. J. On the role of macrophages in anthrax. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10198–10201. doi: 10.1073/pnas.90.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Knapp H. R., Melly M. A. Bactericidal effects of polyunsaturated fatty acids. J Infect Dis. 1986 Jul;154(1):84–94. doi: 10.1093/infdis/154.1.84. [DOI] [PubMed] [Google Scholar]
- Kolter R., Inuzuka M., Helinski D. R. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell. 1978 Dec;15(4):1199–1208. doi: 10.1016/0092-8674(78)90046-6. [DOI] [PubMed] [Google Scholar]
- Mahan M. J. Revealing bacterial infection strategies. Lancet. 1994 Apr 9;343(8902):869–870. doi: 10.1016/s0140-6736(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Mahan M. J., Slauch J. M., Hanna P. C., Camilli A., Tobias J. W., Waldor M. K., Mekalanos J. J. Selection for bacterial genes that are specifically induced in host tissues: the hunt for virulence factors. Infect Agents Dis. 1993 Aug;2(4):263–268. [PubMed] [Google Scholar]
- Mahan M. J., Slauch J. M., Mekalanos J. J. Bacteriophage P22 transduction of integrated plasmids: single-step cloning of Salmonella typhimurium gene fusions. J Bacteriol. 1993 Nov;175(21):7086–7091. doi: 10.1128/jb.175.21.7086-7091.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan M. J., Slauch J. M., Mekalanos J. J. Selection of bacterial virulence genes that are specifically induced in host tissues. Science. 1993 Jan 29;259(5095):686–688. doi: 10.1126/science.8430319. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992 Jan;174(1):1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen J. E., Shryock T. R., Kapral F. A. Modification of bactericidal fatty acids by an enzyme of Staphylococcus aureus. J Med Microbiol. 1992 Apr;36(4):293–298. doi: 10.1099/00222615-36-4-293. [DOI] [PubMed] [Google Scholar]
- Osbourn A. E., Barber C. E., Daniels M. J. Identification of plant-induced genes of the bacterial pathogen Xanthomonas campestris pathovar campestris using a promoter-probe plasmid. EMBO J. 1987 Jan;6(1):23–28. doi: 10.1002/j.1460-2075.1987.tb04713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace J., Hayman M. J., Galán J. E. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell. 1993 Feb 26;72(4):505–514. doi: 10.1016/0092-8674(93)90070-7. [DOI] [PubMed] [Google Scholar]
- Plum G., Clark-Curtiss J. E. Induction of Mycobacterium avium gene expression following phagocytosis by human macrophages. Infect Immun. 1994 Feb;62(2):476–483. doi: 10.1128/iai.62.2.476-483.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Slauch J. M., Mahan M. J., Mekalanos J. J. Measurement of transcriptional activity in pathogenic bacteria recovered directly from infected host tissue. Biotechniques. 1994 Apr;16(4):641–644. [PubMed] [Google Scholar]
- Sorrell T. C., Muller M., Sztelma K. Bacterial metabolism of human polymorphonuclear leukocyte-derived arachidonic acid. Infect Immun. 1992 May;60(5):1779–1785. doi: 10.1128/iai.60.5.1779-1785.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson U., Holst E., Sundler R. Protein-kinase-C-independent activation of arachidonate release and prostaglandin E2 formation in macrophages interacting with certain bacteria. Eur J Biochem. 1991 Sep 15;200(3):699–705. doi: 10.1111/j.1432-1033.1991.tb16234.x. [DOI] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]





