Abstract
This review critically discusses the most recent advances on the role of Notch signaling in liver development, homeostasis and disease. It is now clear that the significance of Notch in determining mammalian cell fates and functions extends beyond development, and Notch is a major regular of organ homeostasis. Moreover, Notch signaling is reactivated upon injury and regulates the complex interactions between the distinct cellular types involved in the repair process. Notch is also involved in the regulation of liver metabolism, inflammation and cancer. The net effects of Notch signaling are highly variable and finely regulated at multiple levels, but also depend on the specific cellular context in which Notch is activated. Persistent activation of Notch signaling is associated with liver malignancies, such as hepatocellular carcinoma with stem cell features and intrahepatic cholangiocarcinoma. The complexity of the pathway provides several possible targets for agents able to inhibit Notch. However, further cell- and context-specific in depth understanding of Notch signaling in liver homeostasis and disease will be essential to translate these concepts into the clinical practice and be able to predict benefits and risks of evolving therapies.
Keywords: Notch signaling, liver development, liver disease, liver regeneration, liver repair, liver fibrosis, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, hepatic progenitor cells
The liver is composed of diverse cell types that serve complex functions. An array of highly conserved morphogenetic signaling mechanisms, including Wnt, TGFβ, sonic hedgehog (Shh), PI3K/AKT, and Notch, control cell fate decisions, morphogenesis, proliferation, and apoptosis during developmental and reparative liver morphogenesis. The Notch signaling pathway is unique because it enables cells to communicate with their direct neighbors by cell-cell ligand-receptor interaction to convey the signal into a transcriptional response.
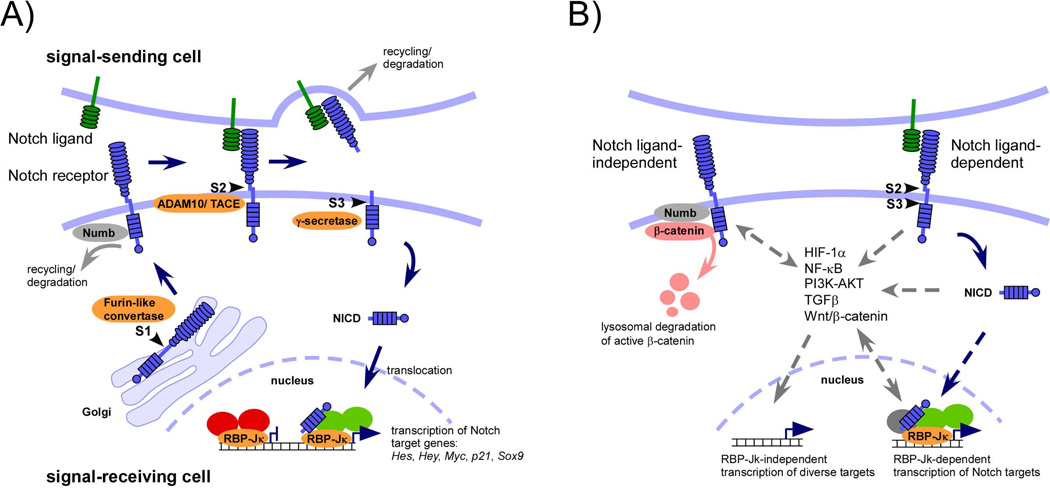
In mammals, there are four known Notch receptors (Notch 1–4) and five ligands belonging to the Jagged (Jagged1, 2) and Delta-like (Delta-like, Dll1, 3, and 4) family. Signaling upon ligand-receptor binding leads to sequential proteolytic cleavage processes in the Notch receptor extracellular and transmembrane domain to release the Notch intracellular domain (NICD). NICD translocates into the nucleus where it binds and converts the DNA-binding recombination signal binding protein (RBP)-Jκ from a transcriptional repressor into an activator resulting in transcription of Notch target genes (Fig. 1A). Notch signaling is rapidly switched off by proteasomal NICD degradation and turnover of the activator complex. This core signaling pathway is nominated ‘canonical’ pathway and is most commonly used in Notch-dependent processes (see Fig. 1B legend for ‘non-canonical’ Notch signaling).
Figure 1. The core ‘canonical’ Notch signaling pathway (A), ‘non-canonical’ Notch signaling, and cross-talk with other signaling pathways (B).
(A) After synthesis Notch receptors are cleaved by a Furin-like convertase in the trans-Golgi (S1 cleavage) to produce heterodimeric receptors containing an intra- and extracellular domain. Notch receptors are exocytosed to the cell surface of the signal-receiving cell where Notch signaling is initiated upon binding to Notch ligands belonging to the Delta or Jagged family expressed on neighboring signal-sending cells. Steady state levels of non-ligand bound Notch receptors are controlled by E3 ligases and several proteins such as Numb and α-Adaptin that control Notch turnover by recycling or lysosomal degradation. Notch ligand-receptor binding enables proteolytic cleavage of the Notch extracellular domain by ADAM10/TACE metalloprotease (S2 cleavage) that remains bound to its ligand and is further subjected to lysosomal degradation in the signal-sending cell. The remnant receptor is then cleaved by the γ-secretase complex within its transmembrane domain at site 3 (S3 cleavage) to allow release and nuclear translocation of the Notch intracellular domain (NICD) where it associates with RBP-Jκ, a DNA-binding adaptor protein mediating the corpus of canonical Notch signals. In the absence of NICD, RBP-Jκ forms a co-repressor complex (red) with many proteins including histone deacetylases (HDACs) and SHARP. NICD binding to RBP-Jκ enables displacement of the co-repressor complex and binding of the adaptor protein Mastermind-like (MAML) that recruits other proteins to form a co-activator complex (green) resulting in the transcriptional activation of Notch target genes.
(B) Some effects of Notch signaling were found to be independent from canonical ligands and/or from RBP-Jκ-mediated transcription that may or may not require cleavage of Notch (‘non-canonical’ Notch signaling). Furthermore, at virtually all stages of the Notch signaling cascade, interactions of components of multiple other signaling pathways are found that may influence Notch turnover, non-nuclear signaling, or modulate RBP-Jκ-dependent or –independent transcriptional pathway activity (3, 59). The best-characterized ligand/RBP-Jκ-independent Notch pathway involves the regulation of Wnt/β-catenin signaling. The Notch and Wnt/β-catenin pathway have long been known to interact in multiple and even opposing ways. In a very simplified view, the synergistic interactions between Notch and Wnt/β-catenin depend on RBP-Jκ-mediated canonical signaling, whereas the antagonistic effects are mediated by ligand/RBP-Jκ-independent Notch signaling. Several hypotheses have been proposed to explain this non-canonical mechanism, including binding and degradation of activated β-catenin by plasma membrane-bound Notch involving interaction with proteins such as Numb, Numb-like, and α-Adaptin.
Despite its simple design, Notch signaling can mount diverse and even opposite biological effects that are highly context-specific in a time-, gene dose-, and cell type-dependent manner. There are no second messengers involved to amplify Notch signal transduction, resulting in a 1:1 transmission of signal input to output, thus sensitizing cells to small changes in Notch activity. The diversity in cellular responses may be generated through several means. For example Notch ligands and receptors are subject to extensive posttranslational modifications that affect their synthesis, secretion, endocytic trafficking, and degradation (1, 2). Signaling diversity is also generated at the transcriptional level, where gene sets produced from Notch activation may greatly vary. There are only a handful classical Notch targets genes, among which the best characterized belong to the HES or HEY family, which execute most biological processes and partially underlie target specificity of different Notch receptor paralogs. However, countless other genes can be also regulated in parallel of these direct Notch target genes. This may be accomplished by intense cross-talk with other signaling pathways such as the Wnt/β-catenin, TGFβ, PI3K/AKT, Shh, HIF-1α, or NF-κB pathway, through direct or indirect interactions with Notch signaling components (3) (Fig. 1B).
Notch signaling is reiteratively used during development of organs and tissues determining the lineage segregation of progenitor cells. Unsurprisingly, mutations in Notch genes can result in a variety of hereditary disease syndromes affecting many organs including the liver (4). However, the significance of Notch in determining mammalian cell fates (and functions) during development extends beyond birth and it is now clear that in adult tissues Notch is also required for tissue homeostasis in self-renewing organs. Notch signaling is frequently reactivated together with other developmental and morphogenic signaling pathways upon organ injury to orchestrate the interplay, differentiation, and proliferation of distinct cell types and adult progenitors for tissue repair, deregulation of which may ultimately result in carcinogenesis.
In mammalian livers, all Notch ligands and receptors are transcriptionally expressed. However, only for few of them there is unequivocal consent about their cell-specific localization. Their functional significance can only be guessed, because of the context-specific, unpredictable nature of Notch signaling. Mice homozygous for null mutations in most Notch ligand and receptor genes are of limited use, because of mostly embryonic lethality. However, availability of conditional cell/tissue-specific Notch loss/gain-of-function animal models has added immensely to our understanding of the diversity of Notch functions in liver development, homeostasis, injury repair, and carcinogenesis (summarized in Fig. 2). There is growing evidence that Notch may also modulate key processes of liver vascular biology, liver metabolism, and inflammation, but the overall effects may greatly differ in distinct liver cell compartments. Consequently, modulating Notch signaling by Notch agonistic or antagonistic therapeutic strategies in liver disease may be beneficial in one compartment but may have detrimental side effects in others. Here, we summarize recent findings and critically discuss evolving concepts of Notch signaling in the liver.
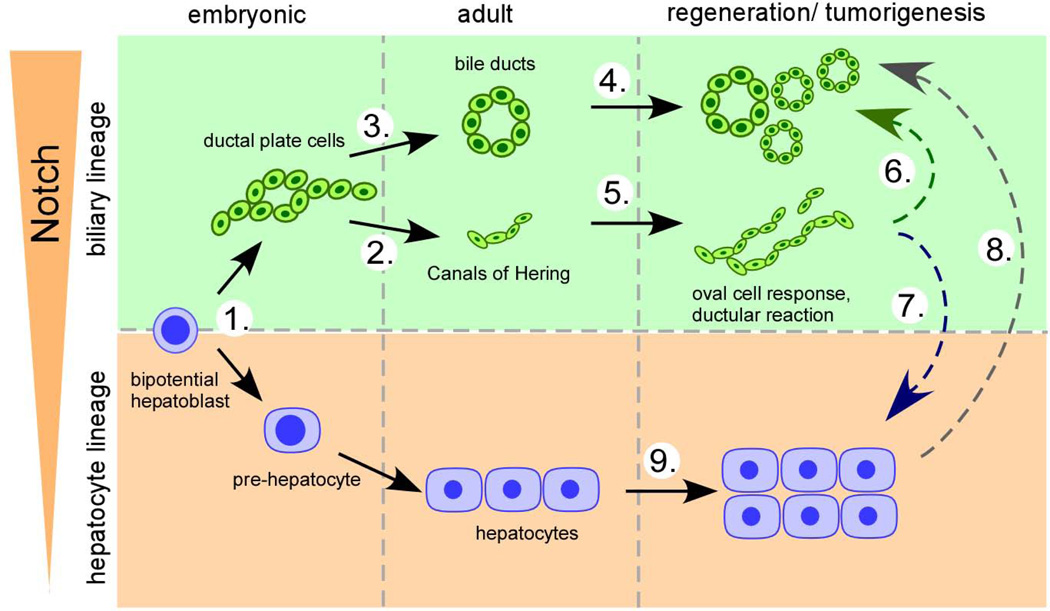
Figure 2. Putative multiple functions of Notch in epithelial lineage control in liver development, homeostasis, and disease.
Notch signaling may act at multiple stages in the embryonic and adult liver to control cell fate decisions and cell growth in development, homeostasis, regeneration, and disease. (1.) During embryonic development Notch converts bipotential hepatoblast to the biliary lineage (12, 20, 21) and (2./3.) is required for proper morphogenesis and maturation of the intrahepatic biliary tree (7, 10–12, 14). (4./5.) Notch signaling may also contribute to biliary injury repair by regulating expansion and morphogenesis of ductular reactions and bile ducts (30, 31). (6./7.) Further, Notch has been proposed to function as the key signaling pathway to destine the cellular fate of adult liver progenitor cells either towards the biliary (Notch activation) or hepatocyte lineage (Notch suppression) both, in liver regeneration (30) and carcinogenesis (46). (8.) Notch has also been shown to be capable to reprogram adult hepatocytes to biliary cells (17, 35) that may give rise to intrahepatic cholangiocarcinoma (37, 38). (9.) Further, data support a role for Notch in hepatocyte regeneration after partial hepatectomy (24) and hepatocellular carcinoma development arising from the hepatocyte compartment (47, 48).
Notch in liver Development
A role for the Notch signaling pathway in liver development was first established in 1997 when genetic studies uncovered mutations in the JAGGED1 (JAG1) gene in most patients with ‘Alagille Syndrome’ (ALGS) (5, 6). Paucity of intrahepatic bile ducts (IHBD) is considered the pathological hallmark, however, as with extrahepatic manifestations of ALGS, liver phenotypes may show a remarkably variable expressivity ranging from subclinical liver enzyme elevation to severe cholestasis, to cirrhosis requiring liver transplant. A series of genetic studies in mice and zebrafish have clearly established an arbitrative role of Notch in determining biliary cell fates and guide proper morphogenesis of the developing biliary tree (7–15), (Table 1, please refer to Fig. 3 legend for a description of IHBD development).
Table 1.
Selected genetic mouse models investigating the role of Notch and associated pathway in liver development.
| Genotype | Phenotype |
|---|---|
| Jag1+/− | Normal liver and IHBD development (7). |
|
Jag1F/F;AlfpCre* Jag1F/F;Notch2del1/+;AlfpCe* |
Normal liver and IHBD development (9). |
| Jag1F/−;AlfpCre* | Impaired postnatal IHBD tubulogenesis and maturation (9). |
| Jag1F/F;VECadCre | Embryonic Deletion of Jagged1 in endothelial cells at ≅ E8.5: Normal development of IHBDs (14). |
| Jag1F/F;Sm22Cre | Embryonic deletion in periportal mesenchymal cells at ≅ E12.5: Severely impaired ductal plate maturation and IHBD morphogenesis (14). |
| Jag1+/−;Notch2+/del1 | Jaundice, IHBD paucity, growth retardation. Additional defect in heart, eye, and kidney development typical for ALGS (7, 10). |
| Notch1F/F;AlbCre* | Normal liver and IHBD development (11, 26). |
| Notch2del1/del1 | Homozygous for hypomorphic Notch2 allele. Perinatal lethal due to kidney glomerular development. Lack of mature IHBDs at birth (7). |
| Notch2F/F;AlbCre* | Impaired ductal plate remodeling and perinatal IHBD morphogenesis. Specification of first ductal plate layer largely unaffected (11, 13). |
|
Notch2F/−;AlbCre* Notch2F/del1;AlbCre* |
Impaired ductal plate remodeling and perinatal IHBD morphogenesis. Specification of first ductal plate layer largely unaffected. Phenotype more severe than in Notch2F/F;AlbCre animals (10, 11). |
|
RbpjF/F;AlbCre* RbpjF/FAlfpCre* |
Formation/ specification of first ductal plate layer preserved. Impaired perinatal ductal plate remodeling and IHBD morphogenesis (12, 13, 17). |
| RbpjF/F;Foxa3Cre* | Reduced Sox9+ ductal plate cells and reduced CK19+ bile ducts at birth (12). |
| Hes1−/− | Perinatal lethality at E18.5-P0 due to neural tube defects. Gallbladder agenesia, hypoplastic extrahepatic biliary tree, conversion of extrahepatic bile ducts to pancreatic tissue (8). |
| Hes1F/F;AlbCre* | Normal liver and IHBD development (17). |
| Sox9F/F;AlfpCre* | Mildly delayed maturation of IHBD development (60). |
In AlbCre, AlfpCre, and Foxa3Cre mice Cre expression in the liver occurs specifically in embryonic bipotential hepatoblasts. Cre expression in AlbCre and AlfpCre animals starts at ≅ E14.5, in Foxa3Cre animals at ≅ E10.5.
Figure 3. Normal embryonic development of the intrahepatic biliary tree and consequences of alterations in Notch signaling.
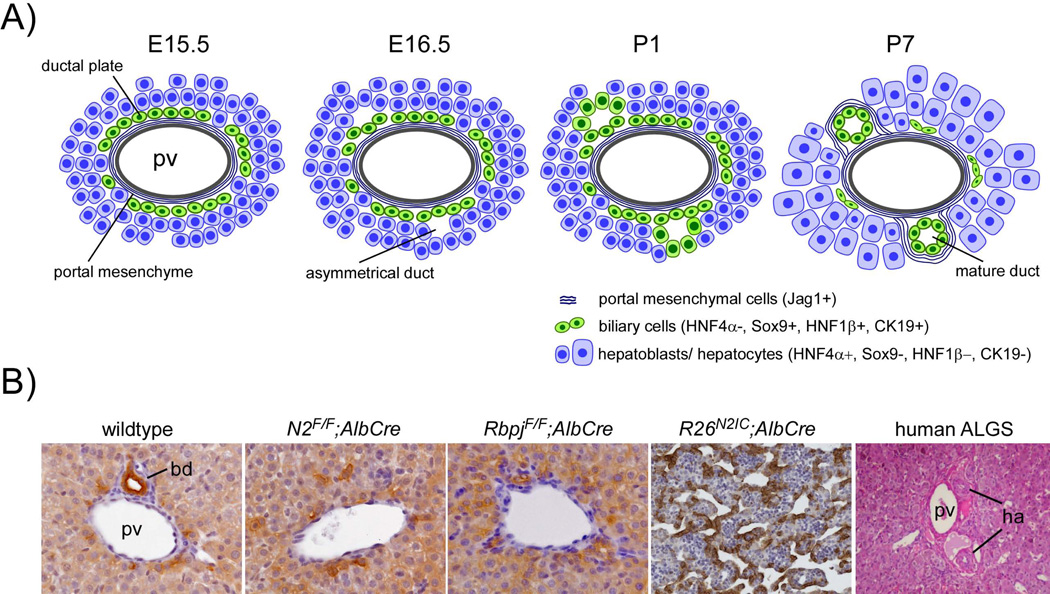
(A) Embryonic development of the intrahepatic biliary tree starts with the differentiation of a subset of Notch2+ periportal hepatoblasts expressing biliary lineage defining proteins such as Sox9, HNF1β, OPN, or CK19. These cells, committed to the biliary fate, encircle the Jag1+ portal mesenchyme in a mono-layered ring, called the ‘ductal plate’, for review see (22, 23). In a second step, lumina arise at distinct sites of the ductal plate that are lined asymmetrically by biliary cells of the first ductal plate layer (portal side) and hepatoblasts (parenchymal side). Later, during development, this asymmetry of immature ducts resolves when the duct-lining hepatoblasts maturate to biliary cells (second ductal plate layer) by loosing HNF4α and gradually expressing Hes1, OPN, SOX9, HNF1β and CK19. This two-step specification process in IHBD tubulogenesis is observed in mice and humans and starts at around E15.5 and W12 respectively. Because IHBD development proceeds from the hilum to the periphery, mature symmetric ducts are first observed at the hilar region when more peripheral IHBDs may still display asymmetric tubules beyond birth before completing their remodeling program by around P2 (12). Thus, differentiation of hepatoblasts to biliary cells represents the main mechanism for intrahepatic biliary morphogenesis in the embryonic and early postnatal period.
(B) Bile duct development is disturbed in Notch mutants. At P10, panCytokeratin+ mature bile ducts are well incorporated in the mesenchyme of medium size portal tracts in wildtype mice. In contrast, P10 mice with conditional deletion of Notch2 (N2F/F;AlbCre) or RBP-Jκ (RbpjF/F;AlbCre) lack mature bile ducts due to impaired perinatal IHBD morphogenesis/elongation. Conversely, conditional expression of the intracellular domain of Notch2 in embryonic hepatoblasts (R26N2IC;AlbCre) directs commitment of embryonic hepatoblasts to the biliary lineage with ectopic formation of biliary-like tubular-cystic structures expressing biliary-specific DBA at P0. In human ALGS portal tracts typically lack mature bile ducts. pv = portal vein; bd = bile duct; ha = hepatic artery.
Compound heterozygous mice for Jagged1 and a hypomorphic Notch2 allele (Jag1+/−;Notch2+/del1) or mice homozygous for hypomorphic Notch2 (Notch2del1/del1) display several features of ALGS, including postnatal IHBD paucity (7). Consistent with the hypothesis that Jagged1/Notch2 interactions are crucial for normal IHBD development, NOTCH2 mutations have been identified in ALGS patients negative for known JAG1 mutations (16). Experimental inactivation of both Notch2 alleles in embryonic hepatoblasts using the AlbCre mouse strain (N2F/F;AlbCre, N2F/−;AlbCre, or N2F/del1;AlbCre) did not affect biliary specification of the single layer ductal plate (DP) (10, 11, 13). However, further perinatal IHBD morphogenesis is impaired leading to paucity of mature bile ducts at the lobe periphery. Similar phenotypes are observed after hepatoblast-specific deletion of Rbp-Jκ, the master transcriptional downstream mediator of canonical Notch, using AlbCre or AlfpCre animals (12, 13, 17). These Notch-defective mice display periportal irregular ductular structures that appear to arise from some degree of biliary conversion of hepatocytes rather than from the DP (17) and likely represent a rescue attempt when developmental tubulogenesis of the biliary tree is impaired. Ductular reactions (DRs) are variably observed in ALGS patients (18). It is tempting to speculate that the degree of biliary transdifferentiation of hepatocytes is one of the factors responsible for the variable clinical outcome of ALGS. Accordingly, in a series of severe ALGS cases requiring liver transplantation, ductular structures were absent with an abundance of CK19-/CK7+ hepatocytes likely stuck in the transdifferentiation process (19).
These initial studies showed that Notch is important for IHBD morphogenesis and elongation but its role in biliary specification of the single layer DP remained unclear, possibly because of the incomplete recombination of floxed Notch alleles in hepatoblasts, when using AlbCre or AlfpCre animals (10–12). Using the Foxa3Cre mouse strain to inactivate Rbp-Jκ well before DP specification, Zong et al. found a reduced number of Sox9+ DP cells and less CK19+ bile ducts at P0 (12), demonstrating that Notch also determines initial lineage commitment of hepatoblasts to biliary cells.
In support of this view, N1ICD- or N2ICD-expressing gain-of-function models demonstrated conversion of bipotential hepatoblasts to the biliary lineage (12, 13, 17, 20, 21). Notably, N1ICD and N2ICD exert largely redundant functions in hepatoblasts when overexpressed; however, Notch2 appears to be the predominant receptor expressed in developing biliary epithelia and clearly mediates the bulk of Notch effects during normal IHBD development (11, 13). The portal mesenchyme is likely the critical compartment harboring the Jagged1 signal-sending cells instructing hepatoblasts into biliary cells and guiding IHBD tubulogenesis (14). Remarkably, however, biliary cell specification and morphogenesis are never blocked completely in Notch mutant models; even after inactivation of all canonical Notch early in development (RbpjF/F;Foxa3Cre mice) specification of DP cells takes place, albeit reduced by about 50% (12). There are several candidate genes regulated by Notch that are critical for IHBD development, including Sox9, HNF1β, and Hes1; however, it is increasingly appreciated that there is no single or few transcription factor(s) in chief downstream of Notch that mediate all biological effects on IHBD development. Rather, Notch is embedded in an intensely cross-communicating signaling network, requiring the common action of the TGFβ, Wnt, FGF, and multiple other signaling pathways (22, 23) to define a transcriptome that allows hepatoblasts to enter the biliary lineage and maturate to biliary ducts.
Notch in liver repair
A role of Notch signaling in post-natal liver homeostasis and liver disease is becoming evident. Recent studies propose that Notch plays a key role in progenitor cell-mediated liver repair and in reparative morphogenesis of the biliary tree. Liver repair mechanisms are intended to regenerate the structures of the hepatic lobules and biliary tree, however, in chronic damage conditions, these set of tools may actually promote a pathologic repair process that leads to liver fibrosis, architectural distortion and liver cancer.
Liver regeneration
The liver’s capacity to regenerate in response to injury is best observed after acute loss of liver mass, i.e. by partial hepatectomy (PH), when a sequence of highly orchestrated cellular events induce quiescent hepatocytes to proliferate and restore liver mass and function. Earlier studies showed up-regulation of Notch factors early after PH and a reduction in post-PH proliferative capacity of hepatocytes after siRNA-meditated gene silencing of Jagged1 or Notch1 (24). However, recent studies found that inactivation of Notch1 in all liver cell compartments (Notch1F/F;MxCre mice) caused lesions similar to nodular regenerative hyperplasia (NRH). Closer analysis revealed that disruption of Notch signaling within liver sinusoidal epithelial cells (LSECs) rather than in hepatocytes generated this intriguing phenotype. Inactivation of Notch1 or Rbp-Jκ caused LSEC dedifferentiation and proliferation with neovessel formation/malformation and deregulation of several angiocrine signals that proved essential not only for angiogenesis, but also for inductive paracrine signaling to hepatocytes in liver regeneration after PH (25, 26) (for further discussion of the role of Notch signaling on angiogenesis see supplementary material). In line with these observations, genetic inactivation of Notch1, Notch2, Rbp-Jκ, or Hes1 in the hepatoblast-derived compartments (hepatocytes and BEC), but not in LSECs, failed to reproduce the spontaneous proliferation phenotype of hepatocytes (11, 13, 17) observed in MxCre-based mouse models (25–27). Furthermore, hepatocyte proliferation appears normal after PH in Notch1F/F;AlbCre or Hes1F/F;AlbCre animals (F. Geisler, unpublished observation). Thus, there is no conclusive evidence that Notch signaling within hepatocytes is critical for liver mass regeneration after PH. Rather, these studies highlight the context and cell-type specificity of Notch signaling in the liver and underscore that altering Notch activity levels in the vascular compartment may have multiple indirect effects on other liver cell compartments.
Progenitor cell-mediated regeneration/repair
Unlike liver regeneration after resection, dominated by division of mature epithelial cells, in many cases of liver injury the proliferative ability of hepatocytes and cholangiocytes may be impaired, calling into action a population of cells commonly designated ‘hepatic progenitor cells’ (HPCs), which niche most likely resides in the canals of Hering (CoH). HPCs expand in response to injury within ‘ductular reactions’ (DRs) in virtually all forms of human liver disease (28) and it is widely believed that they contain ‘real’ progenitors able to generate both, hepatocytes and cholangiocytes, when their self-renewing capacity is impaired. In many adult tissues, progenitor cell-dependent regeneration recapitulates embryonic differentiation programs and uses identical signaling pathways and morphogens. Several studies of human DRs found liver injury type-dependent activation of embryonic pathways, such as the Notch, Wnt/β-catenin and/or Shh pathways (29, 30). These pathways instruct embryonic hepatoblasts towards the biliary or hepatocyte lineage during development and they also appear engaged in growth regulation, morphogenesis, and/or lineage commitment of adult HPCs. Indeed, several groups demonstrated impaired expansion of DRs in various rodent injury models when pretreated with a γ-secretase inhibitor (GSI) to block Notch signaling (30, 31). In line with these observations, genetic Notch activation by expression of N2IC specifically in the biliary/HPC compartment resulted in spontaneous appearance of DRs (17), suggesting that Notch is embedded in the complex signaling network regulating HPC activation and expansion.
Boulter et al. suggested that the cellular niche surrounding HPCs, and its paracrine signals determine Notch activity levels and specification of HPCs towards cholangiocytes or hepatocytes, dependent on the type of injury and the morphogenetic signals that are primarily and differentially activated (30). In biliary damage models, DRs are surrounded by Jag1-expressing myofibroblasts and show high expression levels of Notch target genes, whereas in parenchymal damage DRs radiate into the parenchyma among macrophages and display a blunted expression of Notch targets and biliary markers. In fact, hepatocyte damage leads to Wnt3a release from macrophages and activation of canonical Wnt/β-catenin signaling in HPCs, up-regulation of the Notch repressor Numb, decrease Notch signaling and consequent commitment towards the hepatocyte lineage. This highly attractive hypothesis awaits validation from lineage tracing approaches, as it relies mainly on morphological data and in vitro gene expression analysis.
Strictly speaking, beyond in vitro culture systems and transplantation studies, there is no firm evidence that HPCs contribute in a quantitatively relevant way to the neogenesis of hepatocytes in liver injury (32, 33). Furthermore, the histogenesis of HPCs is unclear, as several studies suggest also a possible origin from hepatocytes through a process of biliary transdifferentiation. Most likely, the liver possesses several different “reparative tools and protocols” that are differentially activated in response to different damages. It will be of great importance, to reappraise current concepts of HPC lineage allocations using compartment-specific genetic mouse models for signaling pathway modulation combined with well-controlled lineage tracing approaches.
Biliary repair
Liver repair in chronic diseases requires the concerted action of epithelial, mesenchymal and inflammatory cells. Ductular reactive cholangiocytes and HPCs (see above) are central to the cross-talk between these cell types. DRs express several inflammatory mediators, cytokines and receptors that help establish the cellular interactions needed for epithelial healing. Continuous expansion of this reactive cell population is associated with persistent inflammation, mesenchymal cell activation, and portal fibrosis (34), leading to the typical architectural distortion of progressive liver diseases. Liver morphogenetic pathways, including Notch are activated in HPCs during liver repair to restore liver architecture and function. In ALGS, paucity of bile ducts is associated with impaired biliary differentiation of HPCs, consistent with the hypothesis that Notch is a default inducer of biliary specification (19). Recent data derived from liver-specific Rbp-Jκ and Notch2 mouse mutants indicate that canonical Notch is essential to mount an effective HPC response after liver damage where Notch2 mediates proper biliary tubular morphogenesis (31). Tubule formation is a fundamental aspect of biliary repair, and failure to regenerate a proper branching structure results in parenchymal necrosis or vanishing bile duct syndrome and fibrosis, as it happens in end-stage cholangiopathies. Cell-cell interactions between Notch-expressing HPCs and Jag1-expressing portal fibroblasts regulate biliary specification of HPCs, and, likely, tubule formation. As mentioned above, the histogenesis of HPCs is not completely understood. Recent studies add further credit to the hypothesis that, depending on the type of liver injury, HPCs/DRs may derive a Notch-dependent reprogramming of hepatocytes (17, 35, 36). This is consistent with reports showing that intrahepatic cholangiocarcinoma may also derive from hepatocytes (37, 38).
Fibrosis/ Inflammation
Pathologic repair leads to liver fibrosis, the main determinant of liver disease progression. Notch signaling components are expressed both in epithelial and mesenchymal liver cells, where they appear to be up-regulated during repair. In fact, Jag1 is expressed by DRs, hepatocytes, and activated hepatic stellate cells (HSC), and is strongly up-regulated in injured livers. The role of Jag1 in hepatic HSC biology is unclear. Jag1 may be important to maintain the stem cell niche and HSC quiescence, however, more recent data suggest that exposure of HSC to Jag1 stimulates αSMA and collagen production (39) (Strazzabosco, unpublished observation). It is intriguing that in patients with ALGS (in which Jag1 is defective) there is limited deposition of fibrotic tissue and a slow progression to cirrhosis (19). Notch activation and upregulation of Notch3 in myofibroblasts has been described in an experimental rat model of CCl4-induced liver fibrosis. In this model, pharmacological Notch inhibition reduced the extent of liver fibrosis (40).
Notch signaling may also be involved in liver fibrosis by modulating the inflammatory response and the function of macrophages. Notch signaling regulates macrophage function by controlling genes involved in the M1 polarization. In fact Rbp-Jκ and TLR4 have been shown to cooperate to induce the translation of key transcription factors (such as IRF8) associated to M1 activation of macrophages (43). Furthermore, macrophage recruitment and macrophage cytokine secretion in response to LPS/IFNγ are decreased in Notch1+/− mice (41). Thus, in Notch1+/− mice, reduced M1 polarization is accompanied by a reduction in TLR4-triggered inflammatory responses (42). Although available data do not permit to solve the role of Notch signaling in inflammation it is safe to conclude that Notch plays a role in the modulation of innate immune responses and also it is regulated by immune stimuli.
Notch in liver carcinogenesis
The role of Notch signaling in hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) is being actively investigated. The key features of cirrhosis, necroinflammation, fibrosis and HPC-driven hepatic reparative processes may favor the reprogramming of HPCs into cancer stem cells (44). In fact, a subset of tumors that exhibit characteristics of both ICC and HCC may arise from the HPCs compartment, and show gene expression signatures of Notch activation (see also ref (45)). Gain-of-function mutations of Notch receptors have not been reported yet in solid tumors, however there is increasing evidence that inappropriate Notch pathway activation occurs in several tumors, including liver cancers, and that Notch signaling may promote oncogenesis by activating a subset of Sox9 and K19-positive progenitors.
Several mouse models have been created to characterize the role(s) of Notch in liver cancer (Table 2), and are mostly consistent with the concept of Notch acting as an oncogene, however, uncertainties remain on its tumor-suppressive vs. tumor-promoting role. Furthermore, mouse models supporting an oncogenic function of Notch have yielded unexpected or mixed histological phenotypes. This is not surprising, given the role of Notch as a master regulator of cell fate determination (37, 38, 46–48). Constitutive activation of Notch1 in embryonic hepatoblasts (N1IC;AlfpCre mice) promoted HCC development with 100% penetrance (47). These tumors recapitulated all stages and differentiation patterns of human hepatocarcinogenesis and were associated with IGF2 co-activation due to reactivation of Igf2 promoters. Most interestingly, a Notch gene signature obtained from these tumors was also found in 1/3 of human HCCs from different etiology (47). Similarly, in mice with constitutive expression of N2IC in hepatoblasts (N2IC;AlbCre) HCC development was dramatically accelerated and accompanied by the appearance of mixed HCC/ICC tumors upon treatment with the carcinogenic diethylnitrosamine (48). In both studies, HCCs showed high Sox9 expression and were surrounded or intermingled with HPC-like ductular cells. Similarly, mice with constitutive hepatoblast-specific activation of N1IC (N1IC;AlbCre), developed undifferentiated tumors classified as ICCs; in this model cyclin E was identified as a Notch-regulated downstream effector of tumorigenesis. Tumors in N1IC;AlbCre animals frequently displayed progenitor-like features and consequently, N1IC overexpression in a HPC cell line (derived from embryonic hepatoblasts) led to cholangiocarcinoma formation in orthotopic transplantation experiments; furthermore, the additional inactivation of p53 was reported to increase tumor burden (49).
Table 2.
Selected genetic mouse models investigating the role of Notch in liver carcinogenesis.
| Genotype/ mouse model | Phenotype |
|---|---|
| RbF/F;p130F/F;p107−/−;Notch1F/F(Cre expression in liver by intrasplenic delivery of Adeno-Cre) | Inactivation of Notch1 accelerates HCC development in mice with inactivation of the Rb pathway (61). |
| N1F/F;MxCre | Inducible inactivation of Notch1 in all liver cells in adult mice. LSEC/hepatocyte proliferation, portal hypertension, nodular regenerative hyperplasia (3 months) (27), and angiosarcoma after 12 months (48). |
| N1IC;AlfpCre | Embryonic expression of N1IC in hepatoblasts. Formation of ectopic biliary structures during embryogenesis (12). Development of HCC with high penetrance within 12 months (47). |
|
N1IC;AlbCre N1IC;p53F/F;AlbCre |
Embryonic expression of N1IC in hepatoblasts. Formation of ectopic biliary structures during embryogenesis (13). Development of undifferentiated ICCs within 12 months, believed to arise from HPCs (46). Accelerated ICC development after concomitant inactivation of p53 (49). |
| N1IC/ ± AKT expression in hepatocytes (N1IC/AKT encoding plasmids delivered to adult livers by HDTVI combined with hepatocyte lineage tracing) | Delivery of N1IC to adult livers. Development of biliary cysts and cystadenocarcinoma within 5–6 months. Occurrence of ICC within 4–5 weeks after NI1C/AKT delivery. ICCs shown to be hepatocyte-derived (38). |
| N1IC;AlbCreERT | Tamoxifen-inducible expression of N1IC in hepatocytes. Acceleration of ICC formation in thioacetamide tumor model (37). |
| N2IC;AlbCre (+/− diethylnitrosamine) | Embryonic expression of N2IC in hepatoblasts. Aberrant formation of biliary structures (17, 20) with high perinatal lethality in one study (17). HCC development within 12 months with high penetrance. Accelerated by DEN treatment shifting histologic phenotype to HCC/ICC (48). |
| N2IC;MxCre | Inducible expression of N2IC in adult livers: Hepatomegaly, conversion of entire parenchyma to proliferative biliary-like tubular-cystic structures within 10 days (17). |
It remains puzzling why N1IC expression led to either HCC or ICC formation in nearly identical models (46, 47) and why additional diethylnitrosamine treatment in N2IC-expressing animals resulted in a phenotypic shift from HCC to ICC (48). Because all models with persistent Notch activation displayed varying histological features of adult HPC expansion or tumors with biphenotypic/stem cell characteristics, HPCs are the likely cellular compartment prone to undergo Notch-induced malignant transformation (46–48). Unfortunately, the use of AlbCre or AlfpCre animals precludes such a conclusion as all hepatoblast-derived lineage cells, hepatocytes, cholangiocytes, and adult HPCs, are equally subjected to Notch-activation in these models. In fact, hepatocytes can adopt a ductular biliary-like morphology with the expression of biliary/HPC markers (Sox9, OPN, CK19, A6) and concomitant downregulation of hepatocyte markers (Albumin, HNF4α) in response to chronic injury (35, 36, 50) or after over-expression of N1IC or N2IC (12, 17). Recent studies provided strong evidence that adult hepatocytes can indeed be ICC precursors. While hydrodynamic tail vein injection of AKT plasmids caused HCC development, the additional delivery of N1IC (AKT/N1IC) led to the rapid emergence of invasive ICC that in a combined lineage tracing approach were shown to arise from hepatocytes (38). Sekiya et al. selectively fate-traced the adult CK19+ biliary or the Albumin+ hepatocyte compartment in the thioacetamide (TAA)-induced tumor mouse model and found ICCs to arise from transdifferentiated hepatocytes (37). Inducible hepatocyte-specific overexpression of N1IC accelerated ICC development in the TAA model (37). Beyond the TAA model, a recent study using in vivo electroporation of oncogenic Kras into p53 deficient livers without genetic Notch activation also identified hepatocytes as potential precursors of ICC (51). It is likely that, similar to biliary specification during embryogenesis, the signaling network regulating hepatocyte dedifferentiation/conversion to biliary cells involves several other signaling pathways besides Notch, that may act independent or in concert with Notch. In light of these studies (12, 17, 35, 36, 51, 52), hepatocytes at least equally qualify as candidate cells of origin for all types of epithelial liver cancer where Notch may act as both, tumor promoter and/or signaling pathway to fate change phenotypical lineage identities. Whether adult HPCs residing in the biliary compartment in the CoH can act as cancer stem cells and give rise to HCCs/ICCs in liver tumor models (53, 54) and, if so, whether carcinogenesis from adult HPCs may underlie Notch regulation, remains to be proven. Moreover, inflammatory mediators (i.e. inducible nitric oxide synthase)-stimulated N1ICD expression was reported in human ICC (55), further indicating that persistent activation of Notch signaling may play an oncogenic role depending on modifier factors, such as the inflammatory field or the presence of other carcinogenetic conditions, potentially giving rise to either HCC with stem cell features or to ICC.
Metabolic aspects of Notch
Recent data show that Notch participates in liver glucose and lipid homeostasis (56, 57). Pajvani et al demonstrated that Notch regulates both hepatic glucose metabolism and lipid production through FoxO1 and AKT/mTORC1. Combined haploinsufficiency of FoxO1 and Notch1 in diet-induced insulin resistance, as well as liver-specific knockout of Rbp-Jκ increased insulin sensitivity, whereas Notch1 gain-of-function caused insulin resistance in a FoxO1-dependent manner and induced glucose-6-phosphatase expression (56). Increased hepatic lipid content is a consequence of insulin-resistance and can be induced by activation of mTOR. Inhibition of Notch with several strategies blocked mTOR activity and prevented hepatosteatosis. Conversely, Notch gain-of-function caused fatty liver through constitutive activation of mTorc1 and of Srebp1c-mediated lipogenesis. Pharmacological blockade of Notch signaling with GSIs increased insulin sensitivity and hepatosteatosis in vivo (57). Thus, Notch signaling could be a target for therapeutic modulation of liver metabolism in diabetes and hepatosteatosis. Preliminary data indicate that pharmacologic Notch inhibition also reduces steatohepatitis in a model not associated to insulin-resistance. Furthermore, Notch inhibition is able to reduce the associated HPC/DRs expansion and fibrosis, thereby targeting the metabolic defect and the pathologic repair in NASH (Strazzabosco, unpublished observation).
Translational perspective
First described about 5 decades ago, as the Notch locus in Drosophila, Notch is now recognized as a major player to steer developmental interactions and in liver biology and pathophysiology. Notch controls important aspects of liver homeostasis, metabolism, and vascular physiology and also regulates HPC specification and orchestrates the reparative remodeling of the biliary tree. Furthermore, persistent activation of Notch may lead to HCC and/or ICC.
Although several aspects of these functions remain to be fully understood, these findings provide an intriguing rationale for investigating Notch-based therapies in patients with liver diseases and cancers. GSIs efficiently inhibit Notch signaling and are effective in mouse models of fibrosis, however, GSIs are not cell-selective, neither system-specific and possess a considerable toxicity profile. General inhibition of Notch signaling may have deleterious side effects (58), thus a more precise identification of the potentially relevant Notch receptor(s) and factors is needed. More selective monoclonal antibodies against Notch receptors and ligands are being developed and may potentially be effective in a subset of liver cancers. However, there is no data available that prove the efficacy of pharmacological Notch inhibition in HCC/ICC animal models. Moreover, the chances of success of Notch-targeted strategies depend on a variety of factors, context-, cell type-dependent, and disease-specific; in addition, interactions with other pathways and post-transcriptional Notch modifications will likely determine the biological outcome of Notch-targeted treatments. Finally, identification of the tumor-initiating cellular compartment(s) may have major impact for treatment. Currently, treatment decisions in case of ICC are largely based on histological features, likely intermingling hepatocyte- and biliary-/HPC-derived ICCs. However, though phenotypically indistinguishable, these entities derived from different cellular compartments with different molecular background may well require different therapy regimens. Nevertheless, as considerable gaps of understanding of Notch signaling in adult liver disease remain, the effects of therapeutic modulation of Notch activation status in liver repair and carcinogenesis are largely speculative at the current stage. In contrast, the cell- and time-specific mechanisms of Notch action shown in ALGS should allow the testing of the therapeutic of gene complementation of cells therapy, aslo considering that bile duct morphogenesis and maturation extends beyond birth.
Supplementary Material
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG grants GE 2289/1-2) to FG and, by NIH NIDDK Award Number R01DK079005 and by a NIH Grant DK34989: Silvio O. Conte Digestive Diseases Research Core Centers, and by CARIPLO 2011-0470 and PRIN 2009ARYX4T_005 to MS.
Abbreviations
- ALGS
Alagille syndrome
- BEC
biliary epithelial cell
- CoH
canals of Hering
- CCl4
carbon tetrachloride
- CDE diet
choline-deficient ethionine-supplemented diet
- DDC diet
3,5-diethoxycarbonyl-1,4-dihydrocollidine diet
- Dll
delta-like ligand
- DP
ductal plate
- DR
ductular reaction
- DRC
ductular reactive cells (found in DRs)
- GSI
γ-secretase inhibitor
- HCC
hepatocellular carcinoma
- HSC
hepatic stellate cell
- HES
hairy/Enhancer of split
- HEY
hairy/Enhancer-of-split-related with YRPW motif
- HNF
hepatocyte nuclear factor
- HPC
hepatic progenitor cell
- ICC
intrahepatic cholangiocarcinoma
- IHBD
intrahepatic bile ducts
- LSEC
liver sinusoidal epithelial cell
- NIC/ NICD
Notch intracellular domain
- NRH
nodular regenerative hyperplasia
- PH
partial hepatectomy
- RBP-Jκ
DNA-binding recombination signal binding protein Jκ
- SOX9
SRY-related HMG box transcription factor 9
- TAA
thioacetamide
- TGFβ
transforming growth factor β
References
- 1.Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 4.Gridley T. Notch signaling and inherited disease syndromes. Hum Mol Genet. 2003;12(Spec No 1):R9–R13. doi: 10.1093/hmg/ddg052. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 6.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 7.McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129:1075–1082. doi: 10.1242/dev.129.4.1075. [DOI] [PubMed] [Google Scholar]
- 8.Kodama Y, Hijikata M, Kageyama R, Shimotohno K, Chiba T. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. 2004;127:1775–1786. doi: 10.1053/j.gastro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Loomes KM, Russo P, Ryan M, Nelson A, Underkoffler L, Glover C, Fu H, et al. Bile duct proliferation in liver-specific Jag1 conditional knockout mice: effects of gene dosage. Hepatology. 2007;45:323–330. doi: 10.1002/hep.21460. [DOI] [PubMed] [Google Scholar]
- 10.Lozier J, McCright B, Gridley T. Notch signaling regulates bile duct morphogenesis in mice. PLoS ONE. 2008;3:e1851. doi: 10.1371/journal.pone.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geisler F, Nagl F, Mazur PK, Lee M, Zimber-Strobl U, Strobl LJ, Radtke F, et al. Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology. 2008;48:607–616. doi: 10.1002/hep.22381. [DOI] [PubMed] [Google Scholar]
- 12.Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, Stanger BZ. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparks EE, Huppert KA, Brown MA, Washington MK, Huppert SS. Notch signaling regulates formation of the three-dimensional architecture of intrahepatic bile ducts in mice. Hepatology. 2010;51:1391–1400. doi: 10.1002/hep.23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann JJ, Zovein AC, Koh H, Radtke F, Weinmaster G, Iruela-Arispe ML. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137:4061–4072. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorent K, Moore JC, Siekmann AF, Lawson N, Pack M. Reiterative use of the notch signal during zebrafish intrahepatic biliary development. Dev Dyn. 2010;239:855–864. doi: 10.1002/dvdy.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79:169–173. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeliazkova P, Jors S, Lee M, Zimber-Strobl U, Ferrer J, Schmid RM, Siveke JT, et al. Canonical Notch2 signaling determines biliary cell fates of embryonic hepatoblasts and adult hepatocytes independent of Hes1. Hepatology. 2013;57:2469–2479. doi: 10.1002/hep.26254. [DOI] [PubMed] [Google Scholar]
- 18.Novotny NM, Zetterman RK, Antonson DL, Vanderhoof JA. Variation in liver histology in Alagille's syndrome. Am J Gastroenterol. 1981;75:449–450. [PubMed] [Google Scholar]
- 19.Fabris L, Cadamuro M, Guido M, Spirli C, Fiorotto R, Colledan M, Torre G, et al. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am J Pathol. 2007;171:641–653. doi: 10.2353/ajpath.2007.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tchorz JS, Kinter J, Muller M, Tornillo L, Heim MH, Bettler B. Notch2 signaling promotes biliary epithelial cell fate specification and tubulogenesis during bile duct development in mice. Hepatology. 2009 doi: 10.1002/hep.23048. [DOI] [PubMed] [Google Scholar]
- 21.Tanimizu N, Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci. 2004;117:3165–3174. doi: 10.1242/jcs.01169. [DOI] [PubMed] [Google Scholar]
- 22.Zong Y, Stanger BZ. Molecular mechanisms of liver and bile duct development. Wiley Interdiscip Rev Dev Biol. 2012;1:643–655. doi: 10.1002/wdev.47. [DOI] [PubMed] [Google Scholar]
- 23.Raynaud P, Carpentier R, Antoniou A, Lemaigre FP. Biliary differentiation and bile duct morphogenesis in development and disease. Int J Biochem Cell Biol. 2011;43:245–256. doi: 10.1016/j.biocel.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Kohler C, Bell AW, Bowen WC, Monga SP, Fleig W, Michalopoulos GK. Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology. 2004;39:1056–1065. doi: 10.1002/hep.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Wang CM, Hou LH, Dou GR, Wang YC, Hu XB, He F, et al. Disruption of the transcription factor recombination signal-binding protein-Jkappa (RBP-J) leads to veno-occlusive disease and interfered liver regeneration in mice. Hepatology. 2009;49:268–277. doi: 10.1002/hep.22579. [DOI] [PubMed] [Google Scholar]
- 26.Dill MT, Rothweiler S, Djonov V, Hlushchuk R, Tornillo L, Terracciano L, Meili-Butz S, et al. Disruption of Notch1 induces vascular remodeling, intussusceptive angiogenesis, and angiosarcomas in livers of mice. Gastroenterology. 2012;142:967–977. e962. doi: 10.1053/j.gastro.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 27.Croquelois A, Blindenbacher A, Terracciano L, Wang X, Langer I, Radtke F, Heim MH. Inducible inactivation of Notch1 causes nodular regenerative hyperplasia in mice. Hepatology. 2005;41:487–496. doi: 10.1002/hep.20571. [DOI] [PubMed] [Google Scholar]
- 28.Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54:1853–1863. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
- 29.Spee B, Carpino G, Schotanus BA, Katoonizadeh A, Vander Borght S, Gaudio E, Roskams T. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 2010;59:247–257. doi: 10.1136/gut.2009.188367. [DOI] [PubMed] [Google Scholar]
- 30.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiorotto R, Raizner A, Morell CM, Torsello B, Scirpo R, Fabris L, Spirli C, et al. Notch signaling regulates tubular morphogenesis during repair from biliary damage in mice. J Hepatol. 2013;59:124–130. doi: 10.1016/j.jhep.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malato Y, Naqvi S, Schurmann N, Ng R, Wang B, Zape J, Kay MA, et al. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J Clin Invest. 2011;121:4850–4860. doi: 10.1172/JCI59261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Espanol-Suner R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, Jacquemin P, et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–1575. e1567. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146:349–356. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 35.Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanimizu N, Nishikawa Y, Ichinohe N, Akiyama H, Mitaka T. Sry HMG box protein 9-positive (Sox9+) epithelial cell adhesion molecule-negative (EpCAM−) biphenotypic cells derived from hepatocytes are involved in mouse liver regeneration. J Biol Chem. 2014 doi: 10.1074/jbc.M113.517243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–3918. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–2915. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawitza I, Kordes C, Reister S, Haussinger D. The niche of stellate cells within rat liver. Hepatology. 2009;50:1617–1624. doi: 10.1002/hep.23184. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Zheng S, Qi D, Guo J, Zhang S, Weng Z. Inhibition of Notch signaling by a gamma-secretase inhibitor attenuates hepatic fibrosis in rats. PLoS One. 2012;7:e46512. doi: 10.1371/journal.pone.0046512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Outtz HH, Wu JK, Wang X, Kitajewski J. Notch1 deficiency results in decreased inflammation during wound healing and regulates vascular endothelial growth factor receptor-1 and inflammatory cytokine expression in macrophages. J Immunol. 2010;185:4363–4373. doi: 10.4049/jimmunol.1000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Wang C, Liu Z, Liu X, Han C, Cao X, Li N. Notch signal suppresses Toll-like receptor-triggered inflammatory responses in macrophages by inhibiting extracellular signal-regulated kinase 1/2-mediated nuclear factor kappaB activation. J Biol Chem. 2012;287:6208–6217. doi: 10.1074/jbc.M111.310375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu H, Zhu J, Smith S, Foldi J, Zhao B, Chung AY, Outtz H, et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nature immunology. 2012;13:642–650. doi: 10.1038/ni.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 45.Strazzabosco M, Fabris L. Notch signaling in hepatocellular carcinoma: guilty in association! Gastroenterology. 2012;143:1430–1434. doi: 10.1053/j.gastro.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zender S, Nickeleit I, Wuestefeld T, Sorensen I, Dauch D, Bozko P, El-Khatib M, et al. A critical role for notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell. 2013;23:784–795. doi: 10.1016/j.ccr.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 47.Villanueva A, Alsinet C, Yanger K, Hoshida Y, Zong Y, Toffanin S, Rodriguez-Carunchio L, et al. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology. 2012;143:1660–1669. e1667. doi: 10.1053/j.gastro.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dill MT, Tornillo L, Fritzius T, Terracciano L, Semela D, Bettler B, Heim MH, et al. Constitutive Notch2 signaling induces hepatic tumors in mice. Hepatology. 2013;57:1607–1619. doi: 10.1002/hep.26165. [DOI] [PubMed] [Google Scholar]
- 49.El Khatib M, Bozko P, Palagani V, Malek NP, Wilkens L, Plentz RR. Activation of Notch signaling is required for cholangiocarcinoma progression and is enhanced by inactivation of p53 in vivo. PLoS ONE. 2013;8:e77433. doi: 10.1371/journal.pone.0077433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gurlevik E, Fleischmann-Mundt B, Armbrecht N, Longerich T, Woller N, Kloos A, Hoffmann D, et al. Adjuvant gemcitabine therapy improves survival in a locally induced, R0-resectable model of metastatic intrahepatic cholangiocarcinoma. Hepatology. 2013;58:1031–1041. doi: 10.1002/hep.26468. [DOI] [PubMed] [Google Scholar]
- 52.Holczbauer A, Factor VM, Andersen JB, Marquardt JU, Kleiner DE, Raggi C, Kitade M, et al. Modeling pathogenesis of primary liver cancer in lineage-specific mouse cell types. Gastroenterology. 2013;145:221–231. doi: 10.1053/j.gastro.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dumble ML, Croager EJ, Yeoh GC, Quail EA. Generation and characterization of p53 null transformed hepatic progenitor cells: oval cells give rise to hepatocellular carcinoma. Carcinogenesis. 2002;23:435–445. doi: 10.1093/carcin/23.3.435. [DOI] [PubMed] [Google Scholar]
- 54.Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest. 2013;123:1911–1918. doi: 10.1172/JCI66024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates Notch-1 in mouse cholangiocytes: implications for carcinogenesis. Gastroenterology. 2005;128:1354–1368. doi: 10.1053/j.gastro.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 56.Pajvani UB, Shawber CJ, Samuel VT, Birkenfeld AL, Shulman GI, Kitajewski J, Accili D. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nat Med. 2011;17:961–967. doi: 10.1038/nm.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pajvani UB, Qiang L, Kangsamaksin T, Kitajewski J, Ginsberg HN, Accili D. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med. 2013;19:1054–1060. doi: 10.1038/nm.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morell CM, Strazzabosco M. Notch signaling and new therapeutic options in liver disease. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 59.Heitzler P. Biodiversity and noncanonical Notch signaling. Curr Top Dev Biol. 2010;92:457–481. doi: 10.1016/S0070-2153(10)92014-0. [DOI] [PubMed] [Google Scholar]
- 60.Antoniou A, Raynaud P, Cordi S, Zong YW, Tronche F, Stanger BZ, Jacquemin P, et al. Intrahepatic Bile Ducts Develop According to a New Mode of Tubulogenesis Regulated by the Transcription Factor SOX9. Gastroenterology. 2009;136:2325–2333. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Viatour P, Ehmer U, Saddic LA, Dorrell C, Andersen JB, Lin C, Zmoos AF, et al. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med. 2011;208:1963–1976. doi: 10.1084/jem.20110198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.