SUMMARY
Adipose tissue inflammation is one pathway shown to mediate insulin resistance in obese humans and rodents. Obesity induces dynamic cellular changes in adipose tissue to increase proinflammatory cytokines and diminish anti-inflammatory cytokines. However, we have found that anti-inflammatory interleukin-13 (IL-13) is unexpectedly induced in adipose tissue of obese humans and high-fat diet (HFD)-fed mice, and the source of IL-13 is primarily the adipocyte. Moreover, HFD-induced proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and IL-1β mediate IL-13 production in adipocytes in an IKKβ-dependent manner. In contrast, adipocyte-specific IKKβ-deficient mice show diminished IL-13 expression and enhanced inflammation after HFD feeding, resulting in a worsening of the insulin-resistant state. Together these data demonstrate that although IKKβ activates the expression of proinflammatory mediators, in adipocytes, IKKβ signaling also induces the expression of the anti-inflammatory cytokine IL-13, which plays a unique protective role by limiting adipose tissue inflammation and insulin resistance.
INTRODUCTION
Inflammation is an important process for the development of insulin resistance in obese humans and rodents, and adipose tissue is a major site mediating the inflammatory response (Hotamisligil, 2006; Kwon and Pessin, 2013; Schenk et al., 2008; Shoelson et al., 2006). Adipose tissue consists of a variety of cell types, and recent studies have established that a high-fat diet (HFD) results in inflammation with dynamic cellular changes in adipose tissue (Feuerer et al., 2009; Liu et al., 2009; Lynch et al., 2012; Molofsky et al., 2013; Nishimura et al., 2009, 2013; Schipper et al., 2012; Talukdar et al., 2012; Winer et al., 2009, 2011; Wu et al., 2011). The proinflammatory Th1 cytokines such as interferon γ are increased in obese humans and rodents to induce the differentiation of classically activated macrophages (M1) and Th1 CD4+ T cells, whereas the anti-inflammatory Th2 cytokines, including IL-4-mediating differentiation of alternatively activated macrophages (M2) and Th2 CD4+ T cells, are decreased (Lumeng et al., 2007; Martinez et al., 2009; Molofsky et al., 2013; Winer et al., 2009; Wu et al., 2011). IL-10 also suppresses the inflammation in adipose tissue, and its expression was decreased due to depletion of Foxp3+CD4+ regulatory T cells (Treg) and regulatory B cells in obesity (Feuerer et al., 2009; Nishimura et al., 2013). Th2 CD4+ T cells and innate lymphoid type 2 cells (ILC2s) express another Th2 cytokine interleukin-13 (IL-13) to sustain anti-inflammatory M2 macrophages and eosinophils (Molofsky et al., 2013; Winer et al., 2009). However, Th2 CD4+ T cells and ILC2 cells are decreased in obese mice as part of the adipose tissue proinflammatory activation pathway.
Proinflammatory mediators such as tumor necrosis factor alpha (TNF-α), IL-1β, saturated free fatty acids (FFAs), and endotoxins stimulate nuclear factor-κB (NF-κB) activation through the IKK complex in obesity (Oeckinghaus et al., 2011). IKKβ is an established critical signaling molecule to modulate HFD-induced inflammation and insulin resistance and as a target for an anti-inflammatory therapy (Baker et al., 2011; Tornatore et al., 2012; Yuan et al., 2001). Expression of constitutively active IKKβ in hepatocytes enhances inflammation in liver, resulting in glucose intolerance and insulin resistance (Cai et al., 2005). Accordingly, hepatocyte-specific IKKβ-deficient mice show suppressed HFD-induced inflammation and liver insulin resistance (Arkan et al., 2005). Deletion of IKKβ in macrophages shows systemic suppression of HFD-induced inflammation, resulting in improved glucose tolerance and insulin sensitivity (Arkan et al., 2005). IKKβ also mediates inflammation in hypothalamus and heart. Deletion of IKKβ in hypothalamus suppresses HFD-induced inflammation and insulin resistance (Zhang et al., 2008). In addition, constitutively active IKKβ expression in cardiomyocytes enhances inflammation and cardiomyopathy (Maier et al., 2012). Thus, these data suggest that IKKβ in diverse cells behaves as a proinflammatory modulator to mediate HFD-induced inflammation and insulin resistance. However, the role of IKKβ in adipocytes has not been studied. We demonstrate here that the IKKβ signaling pathway in adipocytes, in contrast to most other tissues (Arkan et al., 2005; Cai et al., 2005; Maier et al., 2012; Yuan et al., 2001; Zhang et al., 2008), plays an anti-inflammatory modulatory role through an IKKβ-IL-13 axis to limit HFD-induced adipose tissue inflammation and insulin resistance.
RESULTS
HFD Induced the Expression of IL-13 in Adipocytes
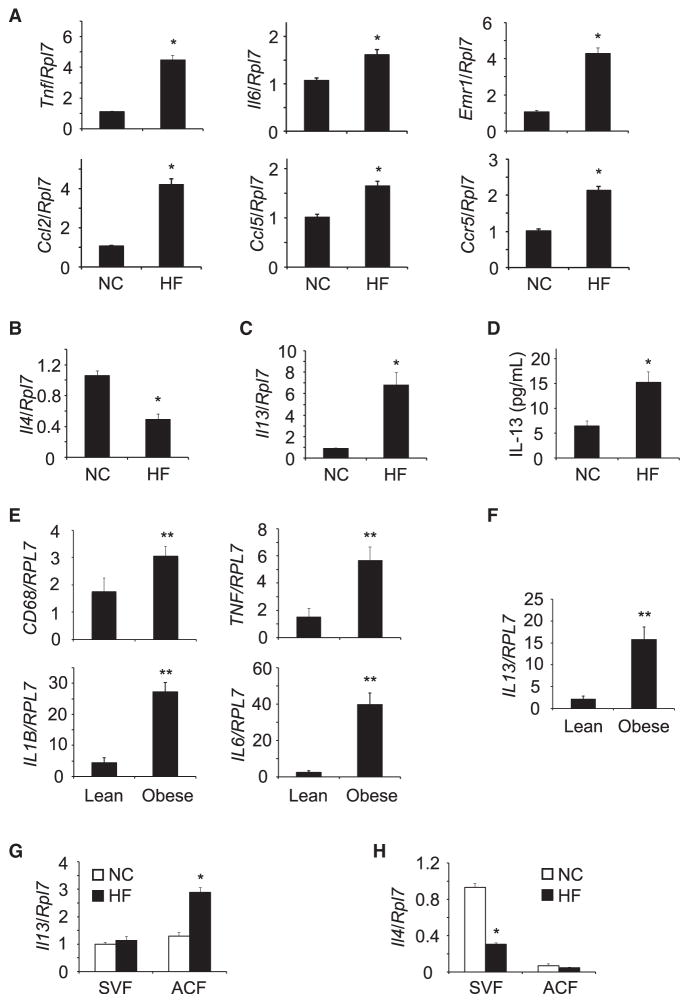
IL-4 and IL-13 are critical for the differentiation of anti-inflammatory immune subsets, M2 macrophages, and Th2 CD4+ T cells to suppress inflammation (Guo et al., 2012; Van Dyken and Locksley, 2013). Cytokine/chemokine profiling of mouse epididymal adipose tissue from normal chow diet (NCD) and HFD-fed mice demonstrated the expected increase in proinflammatory gene expression such as TNF-α, IL-6, CCL2, CCL5, and CCR5 and the enhanced infiltration of macrophages indicated by increased Emr1 (Figure 1A). In addition, the expression of anti-inflammatory Th2 cytokine IL-4 was diminished in epididymal adipose tissues of HFD-fed mice (Figure 1B). However, the expression of another typical anti-inflammatory Th2 cytokine IL-13 was significantly increased in adipose tissue of the HFD-fed mice (Figure 1C). In parallel, IL-13 protein levels were also increased in plasma of the HFD-fed mice (Figure 1D). To determine whether IL-13 expression was also enhanced in obese humans, omental adipose tissue was isolated from patients undergoing bariatric surgery. Similar to HFD-fed mice, obese humans demonstrated the increased expression of proinflammatory markers (Figure 1E) in omental adipose tissues. Omental adipose tissues prepared from obese humans also showed marked increase in IL-13 gene expression (Figure 1F). These results suggest that anti-inflammatory IL-13 expression in adipose tissue is increased in both obese mice and humans.
Figure 1. HFD Enhanced the Expression of IL-13 in Adipocytes.
(A) Expression of inflammatory mediators in epididymal adipose tissues of HFD-fed C57BL/6J mice (n = 7–8 per group).
(B and C) IL-4 and IL-13 expression in epididymal adipose tissues of normal chow (NC) and high-fat (HF) diet-fed C57BL/6J mice (n = 7–8 per group).
(D) IL-13 level in plasma of HFD-fed C57BL/6J mice (n = 8–10 per group).
(E) Expression of inflammatory cytokines in omental adipose tissues of lean and obese humans.
(F) IL-13 expression in omental adipose tissue of lean and obese humans (lean: BMI = 24.9 ± 1.3, n = 5; obese: BMI = 45.4 ± 2.1, n = 11).
(G and H) IL-13 and IL-4 expression in SVF and ACF of NCD- and HFD-fed mice (n = 4–6 per group).
All data are the mean ± SEM, *p < 0.005 compared with NC, **p < 0.035 compared with lean.
Th2 CD4+ T cells, eosinophils, NKT cells, and ILC2 cells have been shown as major sources of IL-4 and IL-13. However, Th2 CD4+ T cells, eosinophils, NKT cells, and ILC2 cells are decreased in adipose tissue of HFD-fed mice (Feuerer et al., 2009; Lynch et al., 2012; Molofsky et al., 2013; Nishimura et al., 2009; Winer et al., 2009; Wu et al., 2011), suggesting that a different cell population is responsible for IL-13 expression in adipose tissue of HFD-fed mice. Thus, we isolated adipocyte fraction (ACF) and stromal vascular fraction (SVF) and then compared the expression of IL-13. IL-13 expression in SVF was comparable in both NCD and HFD fed mice (Figure 1G). In contrast, IL-13 expression was significantly increased in the ACF of HFD-fed mice (Figure 1G). However IL-4 expression was decreased in the SVF of the HFD-fed mice and was relatively low and unchanged in the ACF (Figure 1H). These data are consistent with previous studies demonstrating that the two cell populations Th2 CD4+ T cells and eosinophils that secrete IL-4 are decreased in adipose tissue of HFD-fed mice (Winer et al., 2009; Wu et al., 2011), but in contrast indicate that HFD induces the expression of anti-inflammatory IL-13 in adipocytes.
IL-1β and TNF-α Synergistically Induced the Expression of IL-13 in Adipocytes
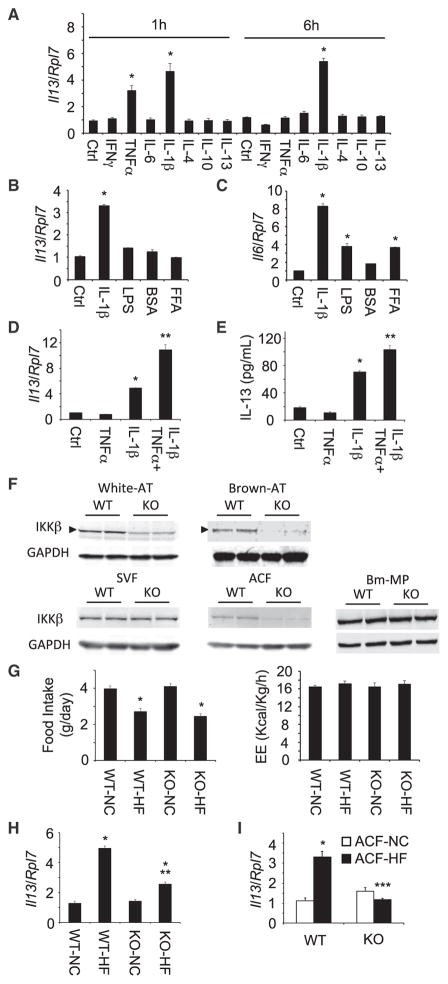
Previously it has been suggested that adipocytes have the capacity to express IL-13, and adipocytes culture conditioned media containing IL-13 induces M2 macrophage differentiation (Kang et al., 2008). We therefore examined the expression of IL-13 in 3T3-L1 adipocytes treated with diverse cytokines, which are expressed in adipose tissues (Figure 2A). TNF-α induced IL-13 expression in a transient manner following 1 hr of TNF-α treatment but not following 6 hr. In contrast, IL-1β induced IL-13 expression in a more persistent manner, being increased at both 1 and 6 hr (Figure 2A), suggesting that IL-1β was able to induce persistent IL-13 expression in 3T3-L1 adipocytes. In addition to increased inflammatory cytokines, hyperlipidemia and endotoxemia also cause inflammation in adipose tissues. Thus, we examined the expression of IL-13 with saturated FFA and endotoxin treatment in 3T3-L1 adipocytes. Similarly, IL-1β, LPS, and palmitate induced IL-6 expression (Figure 2C), but again, only IL-1β induced IL-13 expression in 3T3-L1 adipocytes (Figure 2B). As TNF-α transiently induced IL-13 expression, whereas IL-1β had a more persistent effect, we examined the potential synergy between TNF-α and IL-1β. The combination of TNF-α and IL-1β stimulation resulted in nearly an approximate 10-fold induction of IL-13 expression (Figure 2D). In parallel, there was a concomitant increase in IL-13 protein secretion (Figure 2E). These results indicate that IL-1β is a major driver of IL-13 expression and that TNF-α synergizes with IL-1β to ϕυρτηεr enhance IL-13 expression in adipocytes.
Figure 2. IL-1β and TNF-α Induced the Expression of IL-13 in Adipocytes, and HFD-Induced IL-13 Expression in Adipocytes Was Diminished in Adipocyte-Specific IKKβ Knockout Mice.
(A) TNF-α and IL-1β-induced IL-13 expression in 3T3-L1 adipocytes. Cytokines (20 ng/ml except IL-1β [10 ng/ml]) were treated for 1 or 6 hr (n = 4–6 per group).
(B and C) FFA and endotoxin did not induce IL-13 expression in 3T3-L1 adipocytes (n = 4). FFA (1 mM palmitate and BSA adduct) and endotoxin LPS (50 ng/ml) were treated for 6 hr.
(D and E) TNF-α and IL-1β-induced synergistic expression of IL-13 in 3T3-L1 adipocytes (n = 4–8 per group). Cytokines were treated for 6 hr. All data are the mean ± SEM, *p < 0.0007 compared with control (Ctrl), **p < 0.0003 compared with IL-1β.
(F) IKKβ expression in WT and Ad-IKKβKO (KO) mice. NCD-fed mice (5–7 weeks old) were used.
(G) Food intake and energy expenditure (EE) of WT and Ad-IKKβKO (KO) mice after NCD or HFD feeding for 12–14 weeks (n = 4–6 per group).
(H) IL-13 expression in epididymal adipose tissues of HFD-fed WT (WT-HF) and Ad-IKKβKO (KO-HF) mice (n = 5–7 per group).
(I) IL-13 expression in ACF prepared from HFD-fed WT and Ad-IKKβKO (n = 5–7 per group).
All data are the mean ± SEM, *p < 0.004 compared with NC, **p < 0.0034 compared with WT-HF, ***p < 0.0001 compared with WT ACF-HF.
Adipocyte-Specific IKKβ Deletion Abrogated HFD-Induced IL-13 Production
Since IL-1β as well as TNF-α activates NF-κB signaling pathway through IKKβ activation, we generated adipocyte specific IKKβ--deficient (Ad-IKKβKO) mice by crossing the Ikbkb floxed mice with adiponectin-Cre mice. As expected, there was an approximate 50% reduction in IKKβ protein in white adipose tissue with a greater decrease in IKKβ protein in brown adipose tissue (Figure 2F). We also examined IKKβ protein levels in the SVF and adipocytes in white adipose tissue. As readily apparent, there was no reduction in IKKβ protein in the SVF but a near complete loss of IKKβ in adipocytes. The specificity of the IKKβ knockout was further confirmed because there was no change in IKKβ protein levels in bone marrow-derived macrophages (Bm-MPs) (Figure 2F) and in IKKβ mRNA levels in muscle, pancreas, liver, brain, and splenocytes (Figure S1A), suggesting that IKKβ expression is specifically deleted in adipocytes.
Having demonstrated the adipocyte specific deletion of IKKβ, we characterized the metabolic and inflammatory phenotype of these mice. The WT and Ad-IKKβKO mice had similar body weights on a NCD. Similarly, the age-dependent increase body weight and relative proportions between fat mass and lean mass were not significantly different after NCD and HFD feeding of WT and Ad-IKKβKO mice (Figure S1B). Moreover, the extent of HFD-induced increase in white adipose tissue mass (epididymal and subcutaneous) was similar between the WT and Ad-IKKβKO mice (Figure S1C). We then examined the food intake and energy expenditure using Oxymax indirect calorimetry system. As we expected, there were no significant differences in food intake and energy expenditure in NCD and HFD-fed WT and Ad-IKKβKO mice (Figure 2G). These mice also showed similar respiratory exchange rate and spontaneous locomotor activity (Figure S1D).
As we found that adipocyte-specific IKKβ deletion did not affect body weight, fat accumulation, and energy expenditure, we next determined the effect of IKKβ on the IL-13 expression in epididymal adipose tissues of WT and Ad-IKKβKO mice. As previously observed, IL-13 expression in WT mice was increased in epididymal adipose tissue following HFD feeding. However, the HFD-induced IL-13 expression was significantly blunted in the Ad-IKKβKO mice (Figure 2H). Furthermore, in isolated adipocytes, the loss of IKKβ completely suppressed the HFD-induced induction of IL-13 expression (Figure 2I). As the isolated ACF was highly enriched for the adipocyte-specific marker (Lep) and de-enriched for the T cell (Cd3g) and macrophage (Itgma) markers (Figure S2A), these results confirm that IKKβ is critical for HFD-induced IL-13 expression in adipocytes.
Adipocyte-Specific IKKβ Deletion Enhanced Inflammatory Cytokine Production in Adipose Tissue
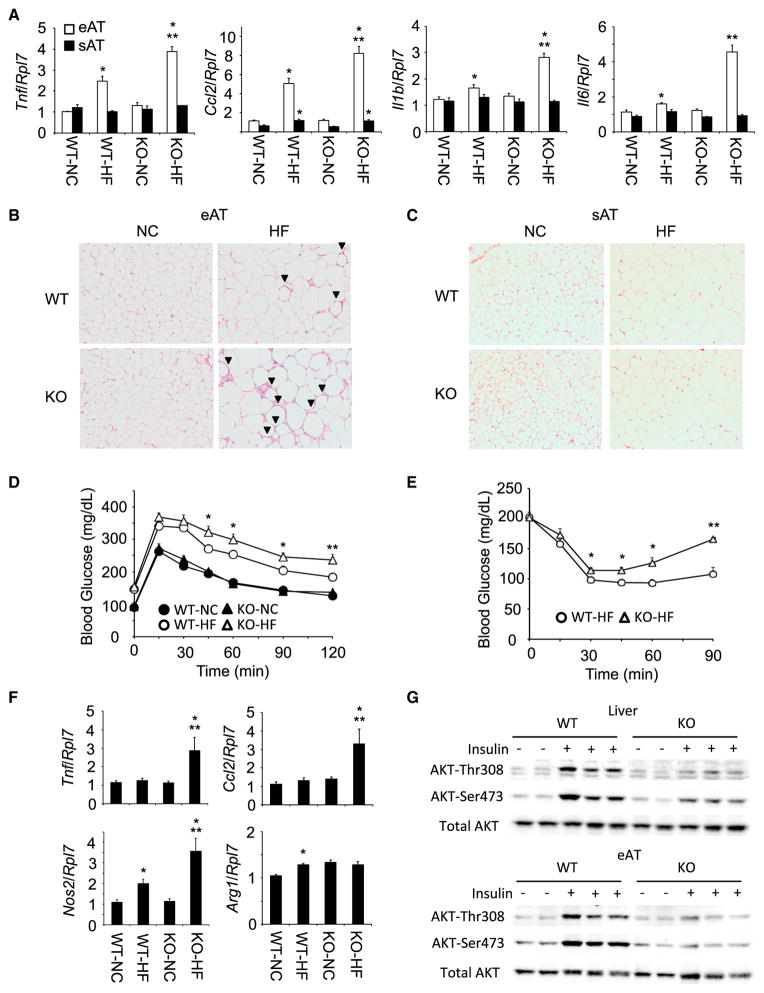
As adipocyte-specific IKKβ deletion reduced the HFD-induced IL-13 expression in adipocytes, we determined the effects of suppressed IL-13 expression on adipose tissue inflammation. The gene expression of typical proinflammatory cytokines was increased in HFD-fed WT mice (Figure 3A). Interestingly, the reduction in adipocyte-specific IKKβ signaling and IL-13 expression resulted in enhanced epididymal adipose tissue proinflammatory gene expression after HFD feeding with no significant change in NCD-fed mice. The increase in proinflammatory gene expression also directly correlated with increased secretion of the corresponding inflammatory cytokine proteins (Figure S2B). These data suggest that diminished HFD-induced IL-13 expression promotes the epididymal adipose tissue inflammation in Ad-IKKβKO mice.
Figure 3. Adipocyte-Specific IKKβ Knockout Mice Resulted in Enhanced Inflammation and Insulin Resistance.
(A) Expression of inflammatory cytokines and chemokines in epididymal and subcutaneous adipose tissues of HFD-fed WT and Ad-IKKβKO mice (n = 5–7 per group). All data are the mean ± SEM, *p < 0.034 compared with NC, **p < 0.0034 compared with WT-HF.
(B and C) H&E staining of epididymal (eAT) and subcutaneous (sAT) adipose tissues in WT and Ad-IKKβKO (KO) mice after HFD feeding for 12 weeks. The arrow head indicates crown-like structure.
(D and E) Glucose and insulin tolerance test in HFD-fed WT and Ad-IKKβKO mice (n = 7–10 per group). All data are the mean ± SEM, *p < 0.04 compared with WT-HF, **p < 0.012 compared with WT-HF.
(F) Expression of inflammatory mediators in liver of HFD-fed WT and Ad-IKKβKO mice (n = 5–6 per group). All data are the mean ± SEM, *p < 0.031 compared with NC, **p < 0.046 compared with WT-HF.
(G) Insulin-stimulated phosphorylation of AKT in liver and epididymal adipose tissue of HFD-fed WT and Ad-IKKβKO (KO) mice. Mice were fed HFD for 12–14 weeks and AKT-Thr308 and AKT-Ser473 phosphorylation were determined on the same membrane.
In contrast to epididymal adipose tissue, subcutaneous adipose tissue of WT mice did not display any significant increase in inflammatory cytokine expression under these conditions of HFD feeding. Moreover, there was no increased inflammatory cytokine marker expression in subcutaneous adipose tissue in the HFD-fed Ad-IKKβ KO mice. These data indicate that although epidiymal adipose tissue is sensitive to HFD-induced inflammation, subcutaneous adipose tissue is relatively resistant even in Ad-IKKβKO mice (Figure 3A). To confirm the enhanced immune cell infiltration in adipose tissues of HFD-fed Ad-IKKβKO mice, we stained epididymal adipose tissues with hematoxylin-eosin (H&E) (Figure 3B). NCD-fed WT and Ad-IKKβKO mice showed comparable adipocytes size and morphology with little infiltration and crown-like structure. In contrast, HFD-fed WT and Ad-IKKβKO mice showed enlarged adipocytes with HFD-induced crown-like structure, and Ad-IKKβKO mice have more severe cell infiltration and crown-like structure (Figure 3B). Consistent with inflammatory gene expression (Figure 3A), H&E staining of subcutaneous adipose tissue from WT or Ad-IKKβKO mice displayed little infiltration in either NCD or HFD-fed mice despite the marked increase in adipocyte cell size following HFD feeding (Figure 3C). To examine the consequences of inflammation in Ad-IKKβKO mice, we conducted glucose and insulin tolerance test. The greater proinflammatory gene expression of the HFD-fed Ad-IKKβKO compared with HFD-fed WT mice was also reflected by a further impairment of glucose tolerance and insulin sensitivity (Figures 3D and 3E).
The liver is relatively resistant to HFD-induced inflammation requiring longer periods of HFD feeding with a lesser extent of inflammatory gene marker expression compared to epididymal adipose tissue (Fontana et al., 2013). Although HFD had little effect on liver inflammation of the WT mice, there was a clear induction of liver proinflammatory markers in the HFD-fed Ad-IKKβKO mice (Figure 3F). The increase in tissue inflammation, along with the impairment of glucose tolerance and insulin sensitivity, was molecularly linked to reduction in insulin signaling assessed by a reduction in insulin-stimulated AKT-Thr308 and AKT-Ser473 phosphorylation (Figure 3G).
Adipocyte-Specific IKKβ Deletion Increased Infiltration of Proinflammatory Immune Cells in Adipose Tissue
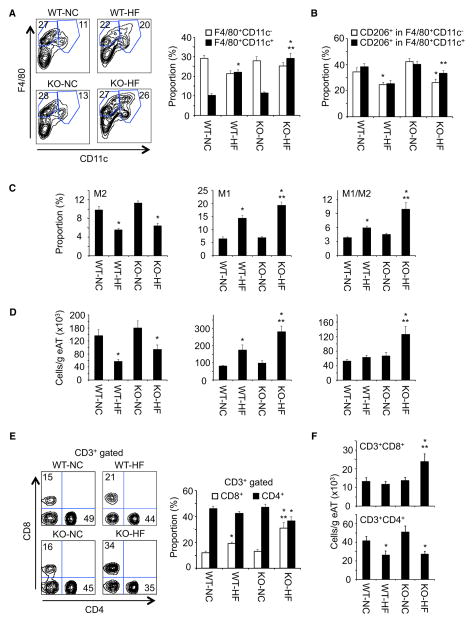
To identify the cell populations responsible for the increased inflammation in HFD-fed Ad-IKKβKO mice, we examine the macrophage profiles of adipose tissue using flow cytometry (Figure S3A). The Ad-IKKβKO mice displayed increased F4/80+CD11c+ macrophages and F4/80+CD11c+CD206+ macrophages compared with adipose tissue of WT mice on a HFD (Figures 4A and 4B). Using a generic definition of F4/80+CD11c−CD206+ as M2, F4/80+CD11c+CD206− as M1 and F4/80+CD11c+CD206+ as mixed M1/M2 macrophages (Shaul et al., 2010; Wentworth et al., 2010), the relative proportion of M2 macrophages were equally decreased in adipose tissue of HFD-fed WT and Ad-IKKβKO mice, whereas the proportion of M1 and M1/M2 macrophages was further increased in the Ad-IKKβKO mice (Figure 4C). When we analyzed for the total number of each macrophage subsets in whole pads of epididymal adipose tissue, all three populations were further increased in both HFD-fed WT and Ad-IKKβKO mice (Figure S3B). However, when we determined the number of each subset in the same mass of epididymal adipose tissue, proinflammatory M1 and M1/M2 macrophages were significantly increased in the Ad-IKKβKO mice, suggesting that HFD-fed Ad-IKKβKO mice showed increased accumulation of proinflammatory macrophages (Figure 4D).
Figure 4. Proinflammatory Immune Cells Were Accumulated in Adipose Tissue of Adipocyte-Specific IKKβ Knockout Mice, Resulting in Enhanced Inflammation.
(A) Proportion of F4/80+CD11c− and F4/80+CD11c+ macrophages in epididymal adipose tissues of HFD-fed WT and Ad-IKKβKO mice (n = 6–10 per group).
(B) Proportion of CD206 in F4/80+CD11c− and F4/80+CD11c+ macrophages in epididymal adipose tissues of HFD-fed WT and Ad-IKKβKO mice (n = 6–10 per group).
(C and D) Proportion and number of M2 (F4/80+CD11c−CD206+), M1 (F4/80+CD11c+CD206−), and M1/M2 (F4/80+CD11c+CD206+) macrophages in epididymal adipose tissues of HFD-fed WT and Ad-IKKβKO mice (n = 6–10 per group).
(E) Proportion of CD4+ and CD8+ T cells in epididymal adipose tissues of HFD-fed WT and Ad-IKKβKO mice (n = 6–9 per group).
(F) Cell number of CD4+ and CD8+ T cells in epididymal adipose tissues of HFD-fed WT and Ad-IKKβKO mice (n = 6–9 per group).
All data are the mean ± SEM, *p < 0.04 compared with NC, **p < 0.04 compared with WT-HF.
In addition to macrophages, other immune cells are also altered in adipose tissue of HFD-fed mice. Several studies have demonstrated that CD4+ T cells are decreased while CD8+ T cells are increased in adipose tissue following HFD feeding. We therefore examined T cell populations in adipose tissue (Figure S3C). Consistent with previous reports (Feuerer et al., 2009; Nishimura et al., 2009; Winer et al., 2009), within the CD3+ gated T cell population there was an marked increase in the proportion of CD8+ T cells and partial decrease in CD4+ T cells in epididymal adipose tissue of HFD-fed WT mice (Figure 4E). The increase in CD8+ T cells and decrease in CD4+ T cells were significantly augmented in HFD-fed Ad-IKKβKO mice (Figure 4E). Similar results were obtained when the numbers of CD4+ and CD8+ T cells were normalized per adipose tissue mass (Figure 4F) or by the total number of T cells present (Figure S3D). In all these cases, the ratios between CD4+/CD8+ T cells were consistently diminished in the HFD-fed Ad-IKKβKO mice. These data demonstrate that the loss of adipocyte-specific IKKβ signaling results in an enhanced adipose tissue inflammation in response to diet-induced obesity.
Adipocyte Differentiation and Cell Survival Is IKKβ Independent
In addition to the regulation of inflammatory gene expression, NF-κB signaling has been reported to play important roles in cell survival and cell fate determination (Oeckinghaus et al., 2011). Thus, it is important to determine whether the Ad-IKKβKO mice have an alteration in cell autonomous adipocyte differentiation and/or cell death. As shown in Figures 3C and S1C, there was no significant difference in adipose tissue cell mass and adipocytes morphology between WT and Ad-IKKβKO mice under normal chow feeding conditions. More specifically, there was no significant difference in the gene expression of the adipogenesis markers, including Cebpb, Pparg, Fabp2, and Lep (Figure S4A). In vitro adipocyte differentiation of isolated SVF from WT and Ad-IKKβ KO mice also demonstrated the same time course and extent of differentiation marker expression (Figure S4B). However, the differentiated adipocytes from the Ad-IKKβ mice displayed reduced cytokine-stimulated TNF-α, IL-6, and IL-13 expression (Figure S4C) consistent with a reduction in NF-κB signaling. Moreover, the enhanced HFD-induced inflammation of Ad-IKKβ mice was not a result of adipocyte cell autonomous apoptosis as there was no detectable induction of cleaved (activated) Caspase-3 and PARP1 (Figure S4D).
Exogenous IL-13 Suppressed HFD-Induced Inflammation in Adipocyte-Specific IKKβ-Deficient Mice
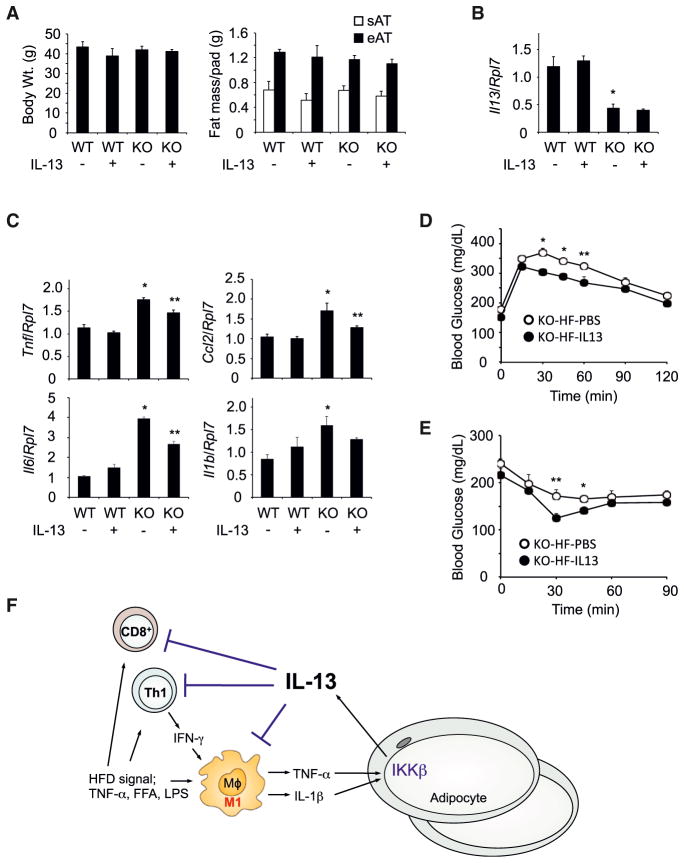
The data presented above demonstrate that HFD induces the expression of IL-13 in adipocytes through IKKβ dependent signaling. Thus, to determine whether exogenous IL-13 administration compensates for IL-13 deficiency in HFD-fed Ad-IKKβKO mice, HFD-fed WT, and Ad-IKKβKO mice were injected every other day for 2–4 weeks with exogenous IL-13. The body weight and adipose tissue mass were similar in WT and Ad-IKKβKO mice after IL-13 administration (Figure 5A). As previously observed, IL-13 expression in epididymal adipose tissue was suppressed in Ad-IKKβKO mice. However, IL-13 administration did not change the expression of IL-13 mRNA in epididymal adipose tissue (Figure 5B). In contrast, IL-13 administration significantly reduced the expression of inflammatory cytokines such as TNF-α, CCL2, and IL-6 in epididymal adipose tissue of HFD-fed Ad-IKKβKO mice (Figure 5C). Although IL-1β expression was not significantly suppressed after IL-13 administration, its expression showed similar trend with other proinflammatory cytokines. However, IL-13 administration significantly reduced IL-1β protein secretion as well as TNF-α secretion (Figure S5A). Although HFD induced macrophage proliferation in epididymal adipose tissue of Ad-IKKβKO mice (Amano et al., 2014), the IL-13-mediated suppression of proinflammatory marker levels occurred without any significant effect on macrophage proliferation (Figures S5B–S5D). Physiologically, the IL-13-mediated reduction in adipose tissue inflammation directly correlated with improvement in both glucose tolerance and insulin sensitivity in HFD-fed Ad-IKKβKO mice (Figures 5D and 5E). Together, these data directly demonstrate that exogenous IL-13 can compensates for the deficiency of IL-13 in HFD-fed Ad-IKKβKO mice, resulting in the suppression of the HFD-induced adipose tissue inflammation and partial restoration of glucose tolerance and insulin sensitivity.
Figure 5. Exogenous IL-13 Administration Diminished the Inflammation in Adipose Tissue of Adipocyte-Specific IKKβ Knockout Mice.
(A and B) Body weight, fat mass, and IL-13 expression in epididymal adipose tissues of HFD-fed WT and Ad-IKKβKO (KO) mice with exogenous IL-13 administration (n = 6–8 per group). sAT, subcutaneous adipose tissue; eAT, epididymal adipose tissue.
(C) Exogenous IL-13 administration suppressed inflammatory responses in epididymal adipose tissues of HFD-fed WT and Ad-IKKβKO (KO) mice (n = 6–8 per group). All data are the mean ± SEM, *p < 0.0054 compared with WT without IL-13, **p < 0.024 compared with KO without IL-13.
(D and E) Glucose and insulin tolerance test in HFD-fed Ad-IKKβKO mice with IL-13 administration (n = 4–6 per group). All data are the mean ± SEM, *p < 0.021 and **p < 0.05 compared to KO-HF-IL13.
(F) Schematic model of the pathway responsible for HFD-induced IL-13 expression in adipocytes as a feedback pathway to limit the inflammatory response.
DISCUSSION
IKKβ has diverse functions, and suppression of IKKβ signaling prevents inflammatory responses in diverse cells and protects HFD-induced insulin resistance (Arkan et al., 2005; Cai et al., 2005; Yuan et al., 2001). In addition, genetic inhibition of IKKβ signaling in specific target tissues such as liver, heart, and brain also protects against tissue dysfunction as well as insulin resistance (Arkan et al., 2005; Cai et al., 2004, 2005; Maier et al., 2012). Based on these data, it is generally accepted that IKKβ activation enhances intracellular inflammatory signaling pathways. However, the specific role of IKKβ in adipocytes has not been addressed. Here we demonstrate an additional function for IKKβ in adipocytes in the regulation of diet-induced inflammation and insulin resistance through an IKKβ-IL-13 axis (Figure 5F). HFD-induced proinflammatory cytokine signals such as IL-1β originating from adipose tissue infiltrating immune cells activate IKKβ in the adipocyte to express and secrete the anti-inflammatory IL-13 cytokine. In turn, IL-13 functions in a paracrine manner to suppress the expansion of proinflammatory immune cells within adipose tissue. These data reveal an active defense mechanism present in adipocytes that limits the extent of tissue inflammation and insulin resistance through an autoregulatory loop.
Although adipocytes express IL-13 to induce M2 macrophage differentiation (Kang et al., 2008), normal chow fed lean mice show little expression of IL-13 in adipocytes (Molofsky et al., 2013). We also found that IL-13 expression in adipocytes was dramatically induced in obese humans and rodents, suggesting that HFD-induced inflammatory signaling such as IL-1β triggers the expression of IL-13 in adipocytes. These data suggest that Th2 CD4+ T cells, eosinophils, and ILC2 cells produce IL-4 and IL-13 in lean mice to maintain anti-inflammatory immune response in the basal state. However, HFD results in a decreased Th2 CD4+ T cells, eosinophils, and ILC2 cells in adipose tissue, accounting for decreased expression of the anti-inflammatory cytokine IL-4 and IL-13, whereas adipocyte are now activated to express IL-13 limiting the proinflammatory state.
Constitutively active IKKβ has been expressed in adipocytes using murine adipocyte fatty acid binding protein (aP2) promoter. We expected constitutively active IKKβ transgenic mice showed enhanced systemic and adipose tissue inflammation (Jiao et al., 2012). However, those transgenic mice displayed insulin sensitivity after HFD feeding due to decreased body weight gain and increased energy expenditure. In contrast, adipocyte-specific IKKβ deletion using adiponectin-Cre mice interestingly showed enhanced inflammation and insulin resistance without any significant differences in body weight gain, food intake, and energy expenditure. These discrepancies may be caused by decreased body weight gain in transgenic mice after HFD feeding. Overexpression of constitutively active IKKβ may overwhelm inflammatory responses in adipocytes, resulting in cell death and lipolysis, and these consequences interfere the interpretation of the role of IKKβ in adipocytes. Although aP2 promoter has been used for adipocyte-specific gene expression and deletion, aP2 promoter activation is not specific for adipocytes (Lee et al., 2013a). In addition, conventional transgenic mice have potential to insert target genes randomly with multitarget genes, resulting in unexpected results. Thus, results from adipocyte-specific IKKβ deletion using adiponectin-Cre mice should be more reasonable to interpret the role of IKKβ in adipocytes of HFD-fed mice.
These data also beg the question why adipocytes express an anti-inflammatory adipokine during HFD-induced inflammation and insulin resistance. Numerous studies have documented that obesity and HFD create a chronic low-grade inflammation, but the molecular mechanisms have not been resolved. Not all obese individuals display inflammation and insulin resistance, and in fact, approximately 25% of obese individuals are metabolically normal without any evidence for adipose tissue or systemic inflammation (Primeau et al., 2011). These obese metabolically normal individuals tend to be substantially more overweight than insulin-resistant obese individuals with predominant expansion of subcutaneous adipose tissue. We therefore hypothesize that the maintenance of low-grade inflammation may serve to create a state of persistent insulin resistance to limit the amount of obesity. In this manner, adipose tissue inflammation that induces IKKβ-dependent IL-13 expression from adipocytes would serve as a factor to maintain a chronic low-grade inflammation and thereby limit the magnitude of obesity.
EXPERIMENTAL PROCEDURES
Animals
C57BL/6J mice were obtained from the Jackson Laboratory. Ikbkb floxed mice (Chen et al., 2003) and adiponectin-cre (Eguchi et al., 2011) mice were generously obtained from the Dr. Michael Karin (University of California) and Dr. Evan Rosen (Harvard University), respectively. Mice are housed in a facility equipped with a 12 hr light/dark cycle. Animals (8-week-old male mice) were fed either a NCD (Research Diets) containing 75.9% (kcal) carbohydrates, 14.7% protein, and 9.4% fat or a HFD containing 20% (kcal) carbohydrates, 20% protein, and 60% fat for 12–14 weeks. For IL-13 administration, HFD-fed mice for 10 weeks were treated with either 1 μg rIL-13 (PeproTech) or an equal volume of PBS using intraperitoneal injection every other day for 2–4 weeks. All studies were approved by and performed in compliance with the guidelines of the Albert Einstein College of Medicine Institutional Animal Care and Use Committee.
Cell Culture and Treatment
Murine 3T3-L1 adipocyte differentiation were conducted as previously described (Feng et al., 2011). Fully differentiated 3T3-L1 adipocytes were placed 2 days in Dulbecco’s modified Eagle’s medium (DMEM) in the presence of 10% serum and then stimulated with diverse cytokines. For FFA and lipopolysaccharide (LPS) treatment, 1 mM palmitate and 2% bovine serum albumin (BSA) adducts and 50 ng/ml LPS (Sigma-Aldrich) were used. Fibroblast culture from SVF and adipocytes differentiation were conducted as described (Ohno et al., 2012). For Bm-MPs, bone marrow cells were prepared from femurs and then treated with 10 ng/ml M-CSF (R&D Systems) in high-glucose DMEM with 10% serum for 7 days. Bm-MPs were rested in high glucose DMEM with 10% serum for 24 hr. For positive control of apoptosis, 3T3-L1 cells were treated with 1 μM staurosporine for 4 hr.
Quantitative RT-PCR
Quantitative RT-PCR was conducted as previously described(Kwon et al., 2009; Lee et al., 2013b). Epididymal adipose tissue (mouse), omental adipose tissue (human), andcells were homogenized into QIAzol Lysis Reagent (Quiagen). Total RNA (1 μg) was isolated using RNeasy Mini Kit (QIAGEN Sciences) and reverse transcribed to cDNA using the SuperScript VILO cDNA synthesis kit (Invitrogen). TaqMan (Applied Biosystems) RT-PCR was performed for measurement of mRNA using standard curves. Gene expression was adjusted by comparison with Rpl7 expression. Primer-probe mixtures for TNF-α and Rpl7 were customized, and other primer-probe mixtures were from Applied Biosystems.
Statistics
Results are expressed as mean ± SEM. The data were analyzed by a two-tailed Student’s t test. Differences were considered statistically significant at a level of p < 0.05.
Supplementary Material
Highlights.
IL-13 expression is enhanced in adipose tissue of obese humans and rodents
IL-1β and TNF-α induce the expression of IL-13 in adipocytes
Adipocyte-specific IKKβ deficiency suppresses diet-induced IL-13 expression
IL-13 production limits diet-induced inflammation and whole-body insulin resistance
Acknowledgments
We thank Flow Cytometry Core Facility and Histology Core Facility at the Albert Einstein College of Medicine for technical support. This work was supported in part by Grants DK033823, DK082694, and DK020541 from the NIH to J.E.P and the Pilot and Feasibility award from the Diabetes Research Center at the Albert Einstein College of Medicine (P60DK020541) to H.K.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2014.10.068.
AUTHOR CONTRIBUTIONS
H.K. and J.E.P. conceived and designed the project. H.K., S.L., Y.T., H.Z., and P.V. performed experiments. H.K. and J.E.P. wrote and edited the manuscript.
References
- Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wyn-shaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13:249–259. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Tang Y, Kwon H, Zong H, Hawkins M, Kitsis RN, Pessin JE. High-fat diet-induced adipocyte cell death occurs through a cyclophilin D intrinsic signaling pathway independent of adipose tissue inflammation. Diabetes. 2011;60:2134–2143. doi: 10.2337/db10-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Zhao E, Amir M, Dong H, Tanaka K, Czaja MJ. Aging promotes the development of diet-induced murine steatohepatitis but not steatosis. Hepatology. 2013;57:995–1004. doi: 10.1002/hep.26099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Junttila IS, Paul WE. Cytokine-induced cytokine production by conventional and innate lymphoid cells. Trends Immunol. 2012;33:598–606. doi: 10.1016/j.it.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Jiao P, Feng B, Ma J, Nie Y, Paul E, Li Y, Xu H. Constitutive activation of IKKβ in adipose tissue prevents diet-induced obesity in mice. Endocrinology. 2012;153:154–165. doi: 10.1210/en.2011-1346. [DOI] [PubMed] [Google Scholar]
- Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPAR-delta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne) 2013;4:71. doi: 10.3389/fendo.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, Carotta S, Donovan CE, Goldman ML, Tailor P, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Russell SJ, Ussar S, Boucher J, Vernochet C, Mori MA, Smyth G, Rourk M, Cederquist C, Rosen ED, et al. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013a;62:864–874. doi: 10.2337/db12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Kwon H, Zong H, Yamada E, Vatish M, Pessin JE, Bastie CC. Fyn deficiency promotes a preferential increase in subcutaneous adipose tissue mass and decreased visceral adipose tissue inflammation. Diabetes. 2013b;62:1537–1546. doi: 10.2337/db12-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O’Shea D, O’Farrelly C, Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier HJ, Schips TG, Wietelmann A, Krüger M, Brunner C, Sauter M, Klingel K, Böttger T, Braun T, Wirth T. Cardiomyocyte-specific IκB kinase (IKK)/NF-κB activation induces reversible inflammatory cardiomyopathy and heart failure. Proc Natl Acad Sci USA. 2012;109:11794–11799. doi: 10.1073/pnas.1116584109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Takaki S, Nagasaki M, Otsu M, Yamashita H, Sugita J, Yoshimura K, Eto K, Komuro I, et al. Adipose Natural Regulatory B Cells Negatively Control Adipose Tissue Inflammation. Cell Metab. 2013 doi: 10.1016/j.cmet.2013.09.017. Published online October 22, 2013 http://dx.doi.org/10.1016/j.cmet.2013.09.017. [DOI] [PubMed]
- Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15:395–404. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, Sladek R, Rabasa-Lhoret R. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35:971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R, Prop S, Meerding J, Hamers N, Besra G, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–3354. doi: 10.1172/JCI62739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet—induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Oh Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 2012;22:557–566. doi: 10.1016/j.tcb.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013;31:317–343. doi: 10.1146/annurev-immunol-032712-095906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O’Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.