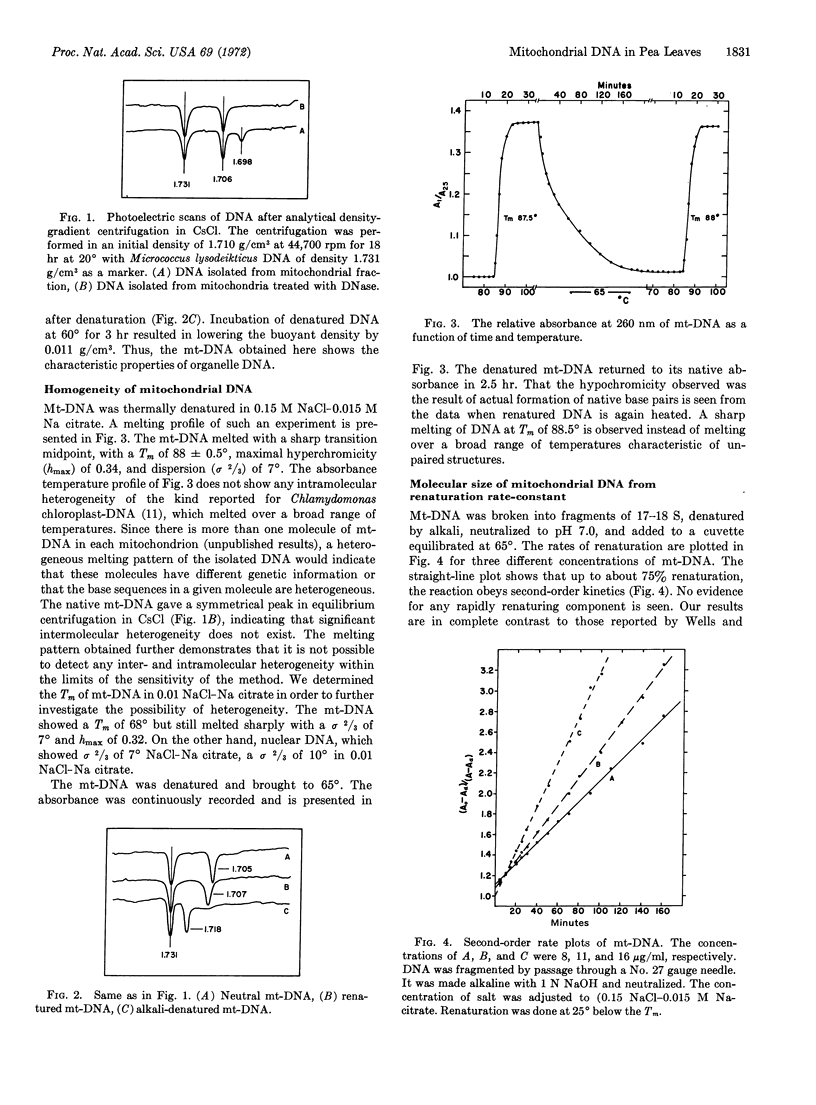
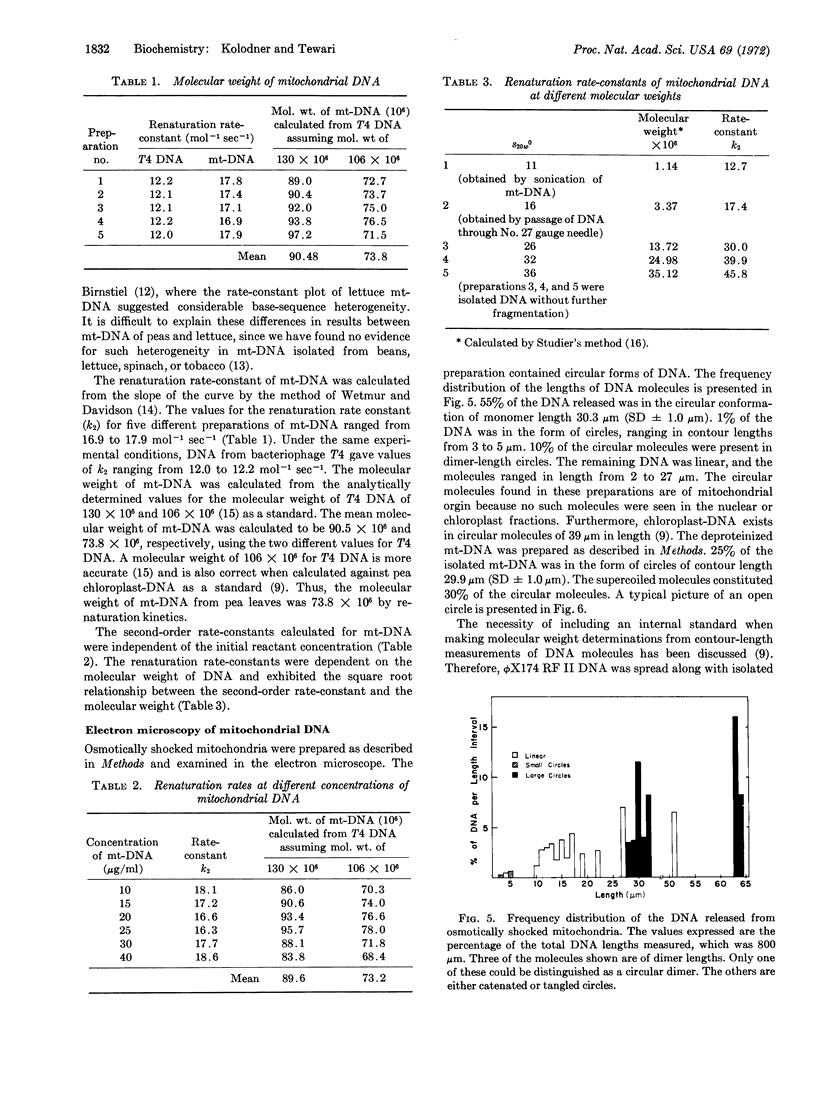
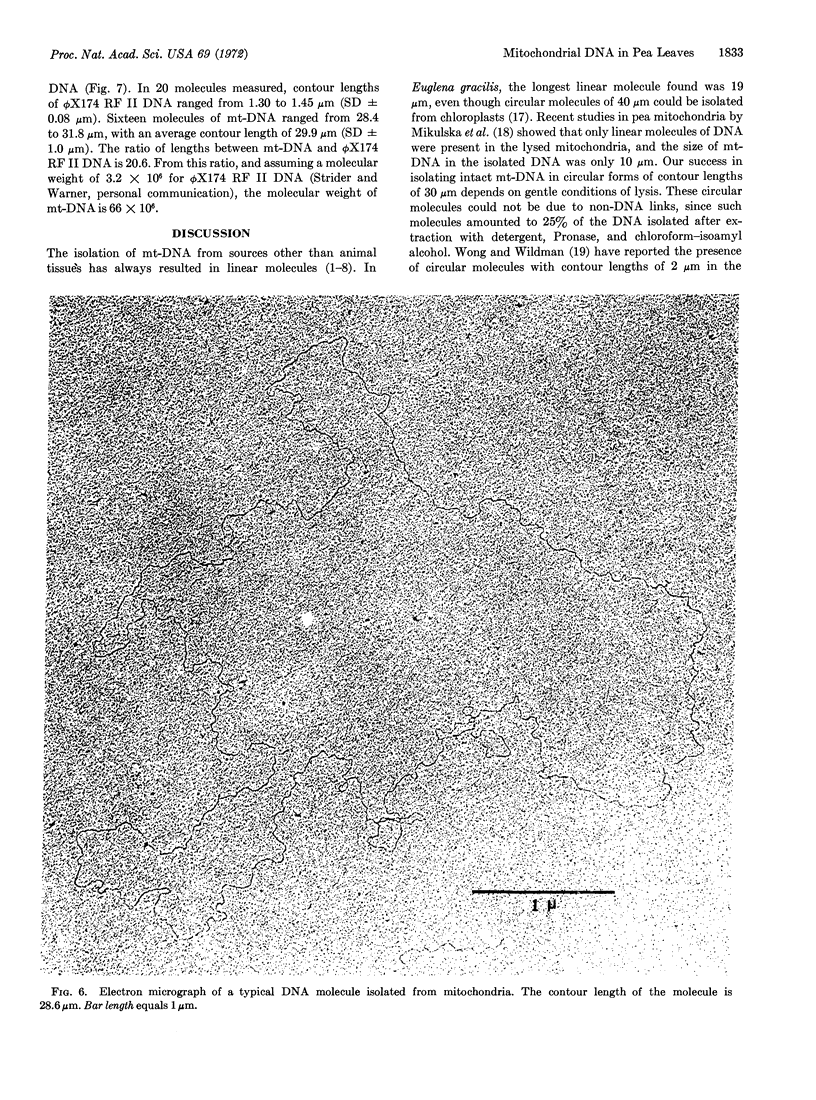
Abstract
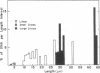
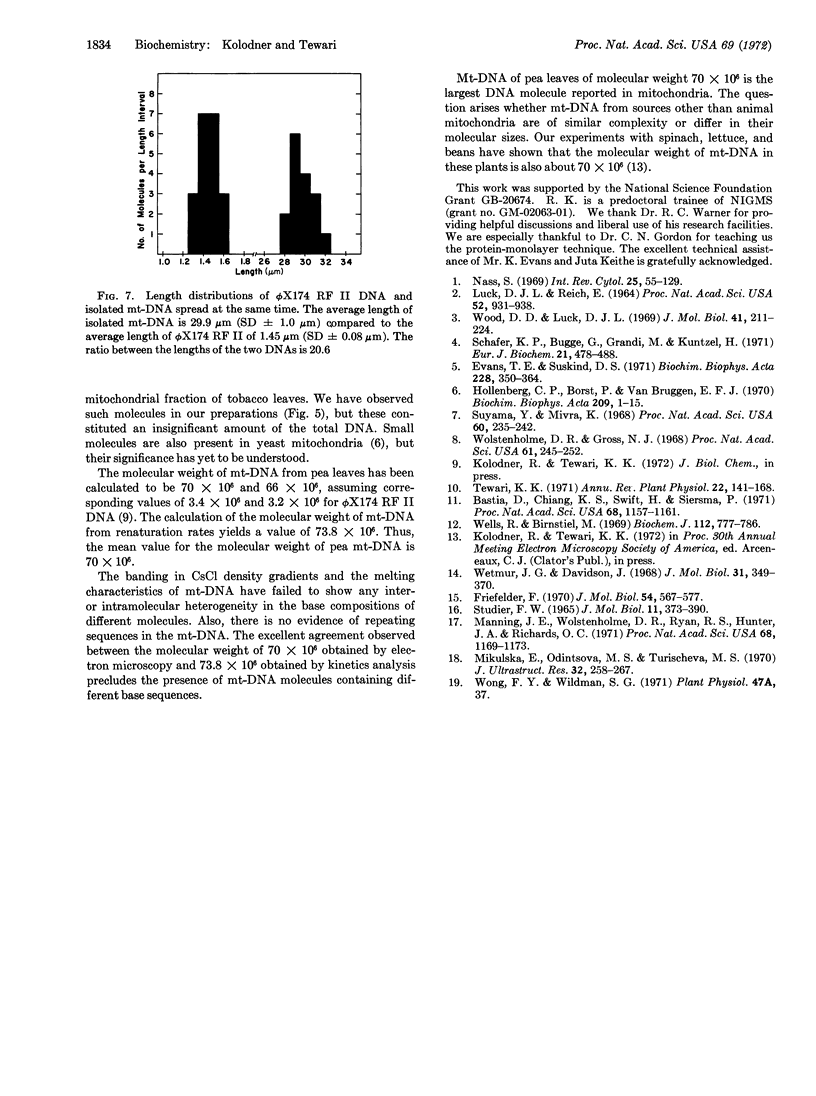
The mitochondrial DNA from pea leaves exists in a circular conformation. 25% of the circular molecules exist as supercoils, and 10% of the molecules are dimers. The molecular weight of mitochondrial DNA is about 66 to 70 × 106 by electron microscopy, and 74 × 106 from its renaturation kinetics. No evidence for inter- and intramolecular heterogeneity is found.
Keywords: circular conformation, molecular size, renaturation kinetics
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastia D., Chiang K. S., Swift H., Siersma P. Heterogeneity, complexity, and repetition of the chloroplast DNA of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1157–1161. doi: 10.1073/pnas.68.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. E., Suskind D. Characterization of the mitochondrial DNA of the slime mold Physarum polycephalum. Biochim Biophys Acta. 1971 Jan 28;228(2):350–364. doi: 10.1016/0005-2787(71)90043-8. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Hollenberg C. P., Borst P., van Bruggen E. F. Mitochondrial DNA. V. A 25 micron closed circular duplex DNA molecule in wild-type yeast mitochondria. Stucture and genetic complexity. Biochim Biophys Acta. 1970 May 21;209(1):1–15. [PubMed] [Google Scholar]
- LUCK D. J., REICH E. DNA IN MITOCHONDRIA OF NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1964 Oct;52:931–938. doi: 10.1073/pnas.52.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J. E., Wolstenholme D. R., Ryan R. S., Hunter J. A., Richards O. C. Circular chloroplast DNA from Euglena gracilis. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1169–1173. doi: 10.1073/pnas.68.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulska E., Odintsova M. S., Turischeva M. S. Electron microscopy of DNA in mitochondria of pea seedlings. J Ultrastruct Res. 1970 Aug;32(3):258–267. doi: 10.1016/s0022-5320(70)80006-5. [DOI] [PubMed] [Google Scholar]
- Nass S. The significance of the structural and functional similarities of bacteria and mitochondria. Int Rev Cytol. 1969;25:55–129. doi: 10.1016/s0074-7696(08)60201-6. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schäfer K. P., Bugge G., Grandi M., Küntzel H. Transcription of mitochondrial DNA in vitro from Neurospora crassa. Eur J Biochem. 1971 Aug 25;21(4):478–488. doi: 10.1111/j.1432-1033.1971.tb01493.x. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Miura K. Size and structural variations of mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):235–242. doi: 10.1073/pnas.60.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R., Birnstiel M. Kinetic complexity of chloroplastal deoxyribonucleic acid and mitochondrial deoxyribonucleic acid from higher plants. Biochem J. 1969 May;112(5):777–786. doi: 10.1042/bj1120777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Gross N. J. The form and size of mitochondrial DNA of the red bean, Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1968 Sep;61(1):245–252. doi: 10.1073/pnas.61.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. D., Luck D. J. Hybridization of mitochondrial ribosomal RNA. J Mol Biol. 1969 Apr;41(2):211–224. doi: 10.1016/0022-2836(69)90386-6. [DOI] [PubMed] [Google Scholar]